Spectroscopy Techniques for Monitoring the Composting Process: A Review
Abstract
:1. Introduction
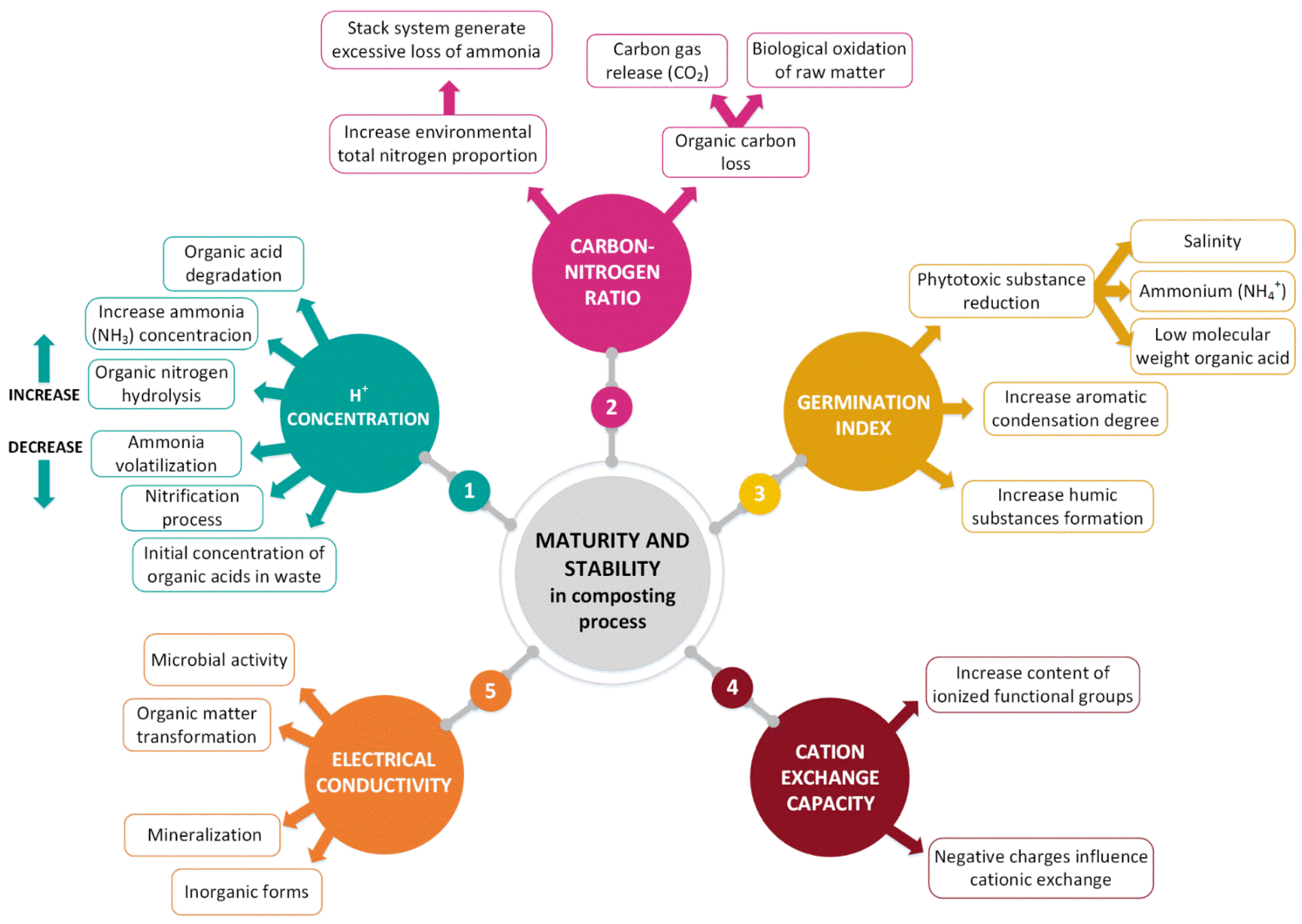
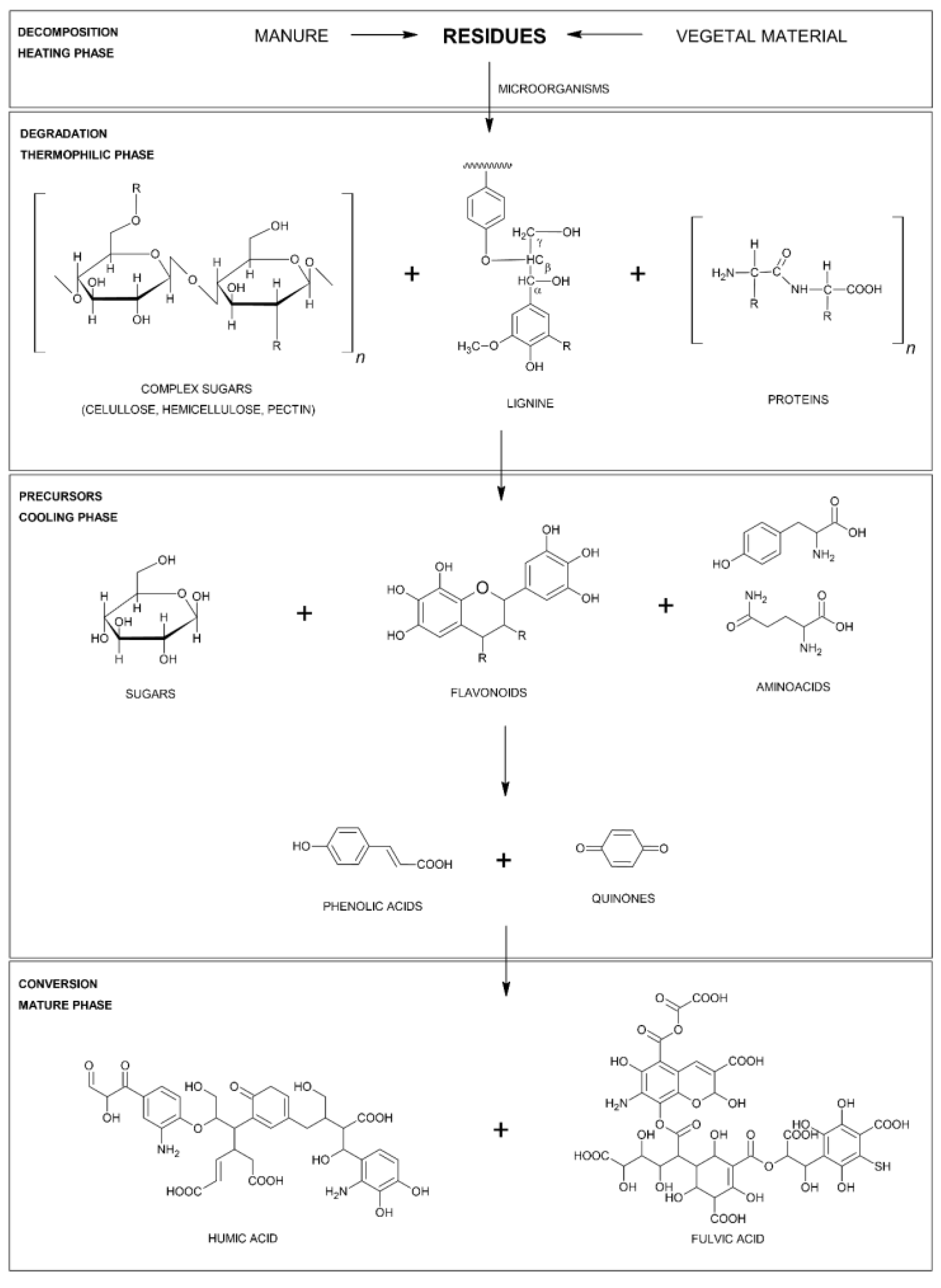
2. Composting Process
3. Ultraviolet-Visible Spectroscopy (UV-Vis)
| Indicator | Description | References |
|---|---|---|
| Absorption region (nm) | ||
| 250 | Absorption of aromatic C=C and ketone groups (C=O). | [49] |
| 280 | Evaluate the beginning of the transformation of aliphatic compounds and lignin—absorption of aromatic groups. | [51] |
| 365 | Related to fluorescent humic-like and fulvic-like substances. | [28] |
| 465 | Indicates the beginning of the process of humification and depolymerization of complex molecules. | [47,48] |
| 665 | Indicates process humification, oxygen content, and aromatic groups. | |
| SUVA254 | Related to the percentage of aromaticity of humic substances and their molecular weight. | [59] |
| SUVA280 | Describes the amount of aromaticity present at the stages evaluated. | [60] |
| Ratios | ||
| E250/E203 | Substitution measure of aromatic rings. The high degree indicates a greater presence of polar groups. | [53,54] |
| E250/E365 | Evaluates the degree of aromaticity of organic molecules and inverse to the degree of humification. | [55] |
| E250/E436 | Associated with terrestrial origin of humic substances (allochthonous or autochthonous). | [51] |
| E280/E665 | Indicates the transformation process of humic substances, maintaining a decreasing trend over time. | [43,52] |
| E465/E665 | Related to the degree of condensation and aromaticity of humic compounds. |
4. Infrared Spectroscopy (IR)
| Wavenumber (cm−1) | Description | Reference |
|---|---|---|
| 3437–3263 | This occurs due to stretching vibration produced by OH groups of alcohols, phenols, and organic acids. | [28,52,53,57,66] |
| 2964–2930 | Bands corresponding to C-H stretching and asymmetric vibrations in aliphatic structures. | |
| 1652–1642 | Bands produced by stretching vibrations of C=C bonds in aromatic structures by ketone groups such as quinones and amide groups (C-N). | |
| 1590–1500 | Obtained due to the deformation and vibration stretching of amide II groups (N-H) and (C-N) of secondary amides, respectively. | |
| 1408 | Intensity assigned to the vibration asymmetric stretching of carboxyl groups (C-O). | |
| 1387 | Described by the deformation of phenolic OH groups and aromatic alcohols, by the asymmetric stretching of carboxyl ions (COO−) of disubstituted aromatic rings, and by the presence of inorganic nitrogen as nitrates. | |
| 1116–1003 | Characteristics of C-O bond stretching vibrations in structures such as polysaccharides, ethers, and secondary alcohols. |
5. Fluorescence Spectroscopy
5.1. Emission and Excitation Spectra
5.2. Synchronous-Scan Spectra
| Region (nm) | Description | Reference |
|---|---|---|
| 250–308 | Describes the presence of protein-like substances and mono-aromatic compounds, which tend to decrease during composting. | [28,72] |
| 308–365 | Attributed to the presence of fulvic-like acid and polycyclic aromatics with fused benzene rings and conjugated systems in unsaturated aliphatic structures. | [84,86] |
| 363–595 | This region is associated with polycyclic aromatic compounds with fused benzene rings, which increase in proportion to the maturation of the compost. | [14,28] |
5.3. Excitation-Emission Matrix
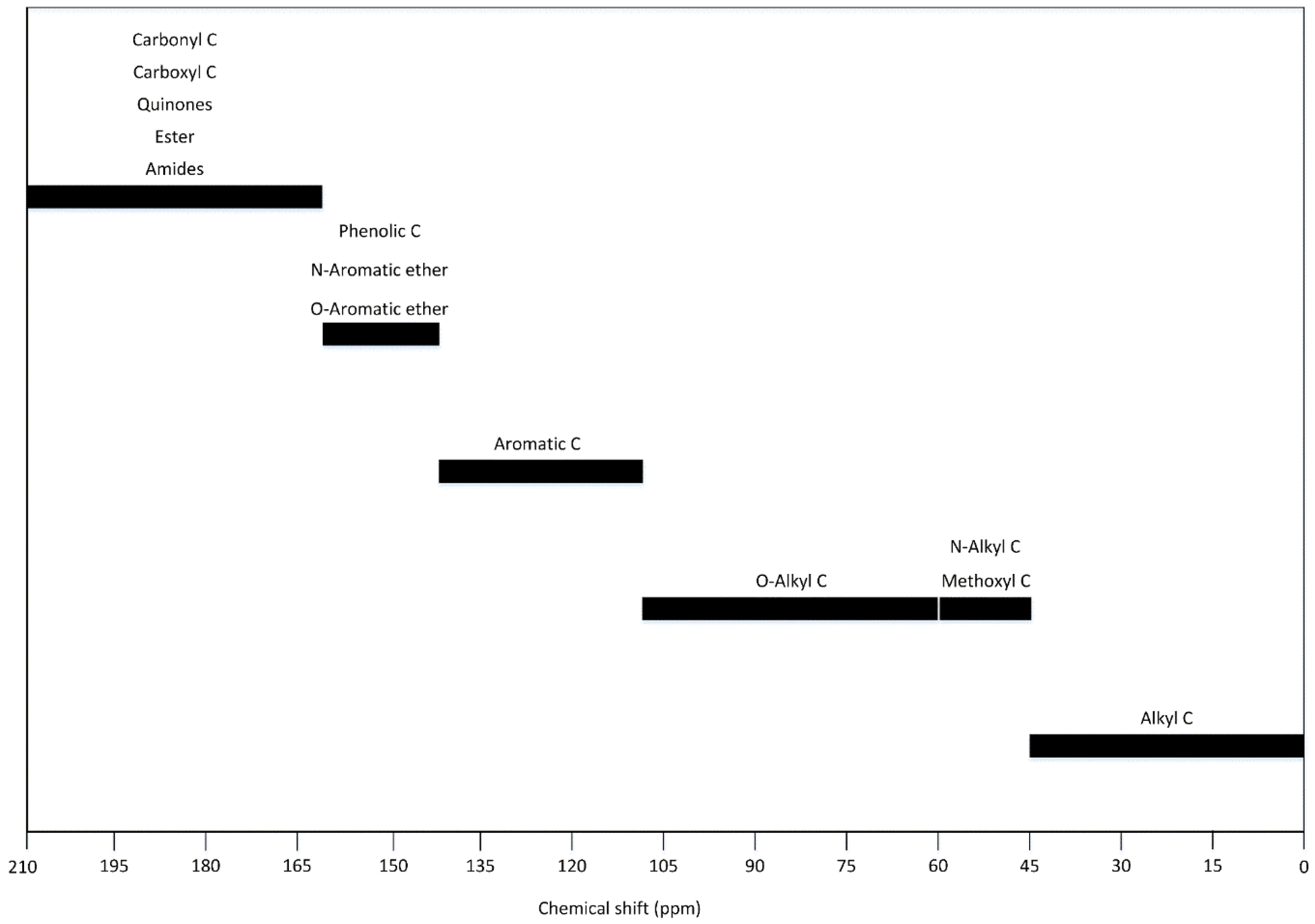
6. Nuclear Magnetic Resonance (13C NMR)
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018; ISBN 9781464813290. [Google Scholar]
- Hunter, M.C.; Smith, R.G.; Schipanski, M.E.; Atwood, L.W.; Mortensen, D.A. Agriculture in 2050: Recalibrating Targets for Sustainable Intensification. Bioscience 2017, 67, 386–391. [Google Scholar] [CrossRef]
- Pergola, M.; Persiani, A.; Palese, A.M.; Di Meo, V.; Pastore, V.; D’Adamo, C.; Celano, G. Composting: The Way for a Sustainable Agriculture. Appl. Soil Ecol. 2018, 123, 744–750. [Google Scholar] [CrossRef]
- Sharma, N.; Singhvi, R. Effects of Chemical Fertilizers and Pesticides on Human Health and Environment: A Review. Int. J. Agric. Environ. Biotechnol. 2017, 10, 675–680. [Google Scholar] [CrossRef]
- Wang, R.; Yao, Z.; Lei, Y. Modeling of Soil Available Phosphorus Surplus in an Intensive Wheat–Maize Rotation Production Area of the North China Plain. Agric. Ecosyst. Environ. 2019, 269, 22–29. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental Challenges Impeding the Composting of Biodegradable Municipal Solid Waste: A Critical Review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Ospina, D.A.; Montoya, S. Compost Supplementation with Nutrients and Microorganisms in Composting Process. Waste Manag. 2017, 69, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Hirai, H.; Mimoto, H.; Quyen, T.N.M.; Koyama, M.; Takeda, K. Succession of Microbial Community during Vigorous Organic Matter Degradation in the Primary Fermentation Stage of Food Waste Composting. Sci. Total Environ. 2019, 671, 1237–1244. [Google Scholar] [CrossRef]
- Sanasam, S.D.; Talukdar, N.C. Quality Compost Production from Municipality Biowaste in Mix with Rice Straw, Cow Dung, and Earthworm Eisenia Fetida. Compost Sci. Util. 2017, 25, 141–151. [Google Scholar] [CrossRef]
- Curaqueo, G.; Riquelme, P.; Carmona, E.; Pérez-San Martín, A.; González, A. Composting with Industrial and Domiciliary Ashes in Temuco, Chile. IOP Conf. Ser. Earth Environ. Sci. 2020, 503, 012026. [Google Scholar] [CrossRef]
- Akratos, C.S.; Tekerlekopoulou, A.G.; Vasiliadou, I.A.; Vayenas, D.V. Cocomposting of Olive Mill Waste for the Production of Soil Amendments; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128053140. [Google Scholar]
- Torres-Climent, A.; Gomis, P.; Martín-Mata, J.; Bustamante, M.A.; Marhuenda-Egea, F.C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Paredes, C.; Moral, R. Chemical, Thermal and Spectroscopic Methods to Assess Biodegradation of Winery-Distillery Wastes during Composting. PLoS ONE 2015, 10, e0138925. [Google Scholar] [CrossRef]
- Ma, C.; Hu, B.; Wei, M.-B.; Zhao, J.-H.; Zhang, H.-Z. Influence of Matured Compost Inoculation on Sewage Sludge Composting: Enzyme Activity, Bacterial and Fungal Community Succession. Bioresour. Technol. 2019, 294, 122165. [Google Scholar] [CrossRef]
- He, X.; Xi, B.; Wei, Z.; Guo, X.; Li, M.; An, D.; Liu, H. Spectroscopic Characterization of Water Extractable Organic Matter during Composting of Municipal Solid Waste. Chemosphere 2011, 82, 541–548. [Google Scholar] [CrossRef]
- Biyada, S.; Merzouki, M.; Elkarrach, K.; Benlemlih, M. Spectroscopic Characterization of Organic Matter Transformation during Composting of Textile Solid Waste Using UV–Visible Spectroscopy, Infrared Spectroscopy and X-Ray Diffraction (XRD). Microchem. J. 2020, 159, 105314. [Google Scholar] [CrossRef]
- Guo, X.; Li, C.; Zhu, Q.; Huang, T.; Cai, Y.; Li, N.; Liu, J.; Tan, X. Characterization of Dissolved Organic Matter from Biogas Residue Composting Using Spectroscopic Techniques. Waste Manag. 2018, 78, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Aranganathan, L.; Rajasree, S.R.R.; Suman, T.Y.; Remya, R.R.; Gayathri, S.; Jayaseelan, C.; Karthih, M.G.; Gobalakrishnan, M. Comparison of Molecular Characteristics of Type A Humic Acids Derived from Fish Waste and Sugarcane Bagasse Co-Compost Influenced by Various Alkaline Extraction Protocols. Microchem. J. 2019, 149, 104038. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Wen, Q.; Ma, J.; He, Z. Assessment of Maturity during Co-Composting of Penicillin Mycelial Dreg via Fluorescence Excitation-Emission Matrix Spectra: Characteristics of Chemical and Fluorescent Parameters of Water-Extractable Organic Matter. Chemosphere 2016, 155, 358–366. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Huang, H.; Sun, E.; Butterly, C.; Xu, Y.; He, H.; Zhang, J.; Chang, Z. Spectroscopic Evidence for Hyperthermophilic Pretreatment Intensifying Humification during Pig Manure and Rice Straw Composting. Bioresour. Technol. 2019, 294, 122131. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced Turnover of Organic Matter Fractions by Microbial Stimulation during Lignocellulosic Waste Composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef]
- Guo, X.-X.; Liu, H.-T.; Wu, S.-B. Humic Substances Developed during Organic Waste Composting: Formation Mechanisms, Structural Properties, and Agronomic Functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Khadra, A.; Pinelli, E.; Ezzariai, A.; Mohamed, O.; Merlina, G.; Lyamlouli, K.; Kouisni, L.; Hafidi, M. Assessment of the Genotoxicity of Antibiotics and Chromium in Primary Sludge and Compost Using Vicia Faba Micronucleus Test. Ecotoxicol. Environ. Saf. 2019, 185, 109693. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Simpson, A.J. Humic Substances in Soils: Are They Really Chemically Distinct? Environ. Sci. Technol. 2006, 40, 4605–4611. [Google Scholar] [CrossRef]
- Idrovo-Novillo, J.; Gavilanes-Terán, I.; Bustamante, M.A.; Paredes, C. Composting as a Method to Recycle Renewable Plant Resources Back to the Ornamental Plant Industry: Agronomic and Economic Assessment of Composts. Process Saf. Environ. Prot. 2018, 116, 388–395. [Google Scholar] [CrossRef]
- Chiarelotto, M.; Restrepo, J.C.P.S.; Lorin, H.E.F.; Damaceno, F.M. Composting Organic Waste from the Broiler Production Chain: A Perspective for the Circular Economy. J. Clean. Prod. 2021, 329, 129717. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within Composting: A Review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.; Shaheen, S.M.; Rinklebe, J.; Bolan, N.S.; Ali, E.F.; Li, G.; Tsang, D.C.W. Effects of Microorganism-Mediated Inoculants on Humification Processes and Phosphorus Dynamics during the Aerobic Composting of Swine Manure. J. Hazard. Mater. 2021, 416, 125738. [Google Scholar] [CrossRef]
- Song, C.; Li, M.; Xi, B.; Wei, Z.; Zhao, Y.; Jia, X.; Qi, H.; Zhu, C. Characterisation of Dissolved Organic Matter Extracted from the Bio-Oxidative Phase of Co-Composting of Biogas Residues and Livestock Manure Using Spectroscopic Techniques. Int. Biodeterior. Biodegrad. 2015, 103, 38–50. [Google Scholar] [CrossRef]
- Wu, S.; Shen, Z.; Yang, C.; Zhou, Y.; Li, X.; Zeng, G.; Ai, S.; He, H. Effects of C/N Ratio and Bulking Agent on Speciation of Zn and Cu and Enzymatic Activity during Pig Manure Composting. Int. Biodeterior. Biodegrad. 2017, 119, 429–436. [Google Scholar] [CrossRef]
- Khaing, T.; Win, S.S.; Win, N.N. Physical and Chemical Properties of Compost Made from Agricultural Wastes. Int. J. Environ. Rural. Dev. 2019, 10, 61–66. [Google Scholar] [CrossRef]
- Soobhany, N. Insight into the Recovery of Nutrients from Organic Solid Waste through Biochemical Conversion Processes for Fertilizer Production: A Review. J. Clean. Prod. 2019, 241, 118413. [Google Scholar] [CrossRef]
- Ezzariai, A.; Hafidi, M.; Khadra, A.; Aemig, Q.; El Fels, L.; Barret, M.; Merlina, G.; Patureau, D.; Pinelli, E. Human and Veterinary Antibiotics during Composting of Sludge or Manure: Global Perspectives on Persistence, Degradation, and Resistance Genes. J. Hazard. Mater. 2018, 359, 465–481. [Google Scholar] [CrossRef]
- Wang, G.; Kong, Y.; Liu, Y.; Li, D.; Zhang, X.; Yuan, J.; Li, G. Evolution of Phytotoxicity during the Active Phase of Co-Composting of Chicken Manure, Tobacco Powder and Mushroom Substrate. Waste Manag. 2020, 114, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Milinković, M.; Lalević, B.; Jovičić-Petrović, J.; Golubović-Ćurguz, V.; Kljujev, I.; Raičević, V. Biopotential of Compost and Compost Products Derived from Horticultural Waste—Effect on Plant Growth and Plant Pathogens’ Suppression. Process Saf. Environ. Prot. 2019, 121, 299–306. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Zhang, F.; Tan, W.; Fan, Y.; Xi, B. How Do Fungal Communities and Their Interaction with Bacterial Communities Influence Dissolved Organic Matter on the Stability and Safety of Sludge Compost? Environ. Sci. Pollut. Res. 2019, 26, 4141–4146. [Google Scholar] [CrossRef]
- Cheung, H.N.B.; Huang, G.H.; Yu, H. Microbial-Growth Inhibition during Composting of Food Waste: Effects of Organic Acids. Bioresour. Technol. 2010, 101, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Hirai, H. Temperature Control Strategy to Enhance the Activity of Yeast Inoculated into Compost Raw Material for Accelerated Composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.H.; Marschner, P. Respiration, Available N and Microbial Biomass N in Soil Amended with Mixes of Organic Materials Differing in C/N Ratio and Decomposition Stage. Geoderma 2018, 319, 167–174. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wong, J.W.C.; Kumar, S.; Awasthi, S.K.; Wang, Q.; Wang, M.; Ren, X.; Zhao, J.; Chen, H.; Zhang, Z. Biodegradation of Food Waste Using Microbial Cultures Producing Thermostable A-Amylase and Cellulase under Different PH and Temperature. Bioresour. Technol. 2018, 248, 160–170. [Google Scholar] [CrossRef]
- Sharma, A.; Weindorf, D.C.; Wang, D.; Chakraborty, S. Characterizing Soils via Portable X-Ray Fluorescence Spectrometer: 4. Cation Exchange Capacity (CEC). Geoderma 2015, 239, 130–134. [Google Scholar] [CrossRef]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of Humic Substances and Water Extracts from Compost Made by Ligno-Cellulose Wastes from Biorefinery. Sci. Total Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef]
- Hart, K.M. Assessing the Role of Soil Chemoautotrophs in Carbon Cycling: An Investigation into Isotopically Labelled Soil Microorganisms. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2011. [Google Scholar]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Characterization of PH-Fractionated Humic Acids Derived from Chinese Weathered Coal. Chemosphere 2017, 166, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhao, Y.; Zhao, X.; Yang, T.; Dang, Q.; Wu, J.; Lv, P.; Wang, H.; Wei, Z. Effect of Manganese Dioxide on the Formation of Humin during Different Agricultural Organic Wastes Compostable Environments: It Is Meaningful Carbon Sequestration. Bioresour. Technol. 2020, 299, 122596. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, X.; Li, M.; Zhu, Q.; Li, G.; Ma, C.; Li, Q.; Meng, J.; Liu, Y.; Li, Q. Impacts of Red Mud on Lignin Depolymerization and Humic Substance Formation Mediated by Laccase-Producing Bacterial Community during Composting. J. Hazard. Mater. 2021, 410, 124557. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.L.C.; Sarraguça, M.C.; Saraiva, M.L.M.F.S.; Rao, T.P.; Biju, V.M. Spectrophotometry|Organic Compounds. Encycl. Anal. Sci. 2018, 9, 236–243. [Google Scholar] [CrossRef]
- Sellami, F.; Hachicha, S.; Chtourou, M.; Medhioub, K.; Ammar, E. Maturity Assessment of Composted Olive Mill Wastes Using UV Spectra and Humification Parameters. Bioresour. Technol. 2008, 99, 6900–6907. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, A.; Richel, A.; Destain, J.; Druart, P.; Thonart, P.; Ongena, M. Comprehensive Comparison of the Chemical and Structural Characterization of Landfill Leachate and Leonardite Humic Fractions. Anal. Bioanal. Chem. 2016, 408, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Eshwar, M.; Srilatha, M.; Rekha, K.B.; Sharma, S.H.K. Characterization of Humic Substances by Functional Groups and Spectroscopic Methods. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1768–1774. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular Weight, Polydispersity, and Spectroscopic Properties of Aquatic Humic Substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring Changes in the Structure and Properties of Humic Substances Following Ozonation Using UV-Vis, FTIR and 1H NMR Techniques. Sci. Total Environ. 2016, 541, 623–637. [Google Scholar] [CrossRef]
- Zittel, R.; da Silva, C.P.; Domingues, C.E.; Seremeta, D.C.H.; Estrada, R.A.; de Campos, S.X. Composting of Smuggled Cigarettes Tobacco and Industrial Sewage Sludge in Reactors: Physicochemical, Phytotoxic and Spectroscopic Study. Waste Manag. 2018, 79, 537–544. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Jiang, Y.-H.; He, L.-S.; Li, D.; Pan, H.-W.; Bai, S.-G. Structural Transformation Study of Water-Extractable Organic Matter during the Industrial Composting of Cattle Manure. Microchem. J. 2013, 106, 160–166. [Google Scholar] [CrossRef]
- Morán Vieyra, F.E.; Palazzi, V.I.; Sanchez de Pinto, M.I.; Borsarelli, C.D. Combined UV–Vis Absorbance and Fluorescence Properties of Extracted Humic Substances-like for Characterization of Composting Evolution of Domestic Solid Wastes. Geoderma 2009, 151, 61–67. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Zhao, J.; Ren, X.; Wang, M.; Li, R.; Wang, Z.; Zhang, Z. Utilization of Medical Stone to Improve the Composition and Quality of Dissolved Organic Matter in Composted Pig Manure. J. Clean. Prod. 2018, 197, 472–478. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Implementation of Spectroscopic Parameters for Practical Monitoring of Natural Organic Matter. Desalination 2005, 176, 47–55. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Li, J.; Li, G.; Zhang, B. Evaluation of Humic Substances during Co-Composting of Sewage Sludge and Corn Stalk under Different Aeration Rates. Bioresour. Technol. 2017, 245, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Abid, W.; Mahmoud, I.B.; Masmoudi, S.; Triki, M.A.; Mounier, S.; Ammar, E. Physico-Chemical and Spectroscopic Quality Assessment of Compost from Date Palm (Phoenix Dactylifera L.) Waste Valorization. J. Environ. Manag. 2020, 264, 110492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.-M.; Lee, H.-S.; Lee, S.-Y.; Hur, J.; Shin, H.-S. Changes in Structural Characteristics of Humic and Fulvic Acids under Chlorination and Their Association with Trihalomethanes and Haloacetic Acids Formation. Sci. Total Environ. 2021, 790, 148142. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, Q.; Chen, X.; Zhang, Y.; Sun, Y.; Li, R.; Li, J.; Zhang, Z. Elucidating the Optimum Added Dosage of Diatomite during Co-Composting of Pig Manure and Sawdust: Carbon Dynamics and Microbial Community. Sci. Total Environ. 2021, 777, 146058. [Google Scholar] [CrossRef] [PubMed]
- Soobhany, N.; Gunasee, S.; Rago, Y.P.; Joyram, H.; Raghoo, P.; Mohee, R.; Garg, V.K. Spectroscopic, Thermogravimetric and Structural Characterization Analyses for Comparing Municipal Solid Waste Composts and Vermicomposts Stability and Maturity. Bioresour. Technol. 2017, 236, 11–19. [Google Scholar] [CrossRef]
- Fialho, L.L.; da Silva, W.T.L.; Milori, D.M.B.P.; Simões, M.L.; Martin-Neto, L. Characterization of Organic Matter from Composting of Different Residues by Physicochemical and Spectroscopic Methods. Bioresour. Technol. 2010, 101, 1927–1934. [Google Scholar] [CrossRef]
- Marhuenda-Egea, F.C.; Martínez-Sabater, E.; Jordá, J.; Sánchez-Sánchez, A.; Moral, R.; Bustamante, M.A.; Paredes, C.; Pérez-Murcia, M.D. Evaluation of the Aerobic Composting Process of Winery and Distillery Residues by Thermal Methods. Thermochim Acta 2007, 454, 135–143. [Google Scholar] [CrossRef]
- Smidt, E.; Lechner, P.; Schwanninger, M.; Haberhauer, G.; Gerzabek, M.H. Characterization of Waste Organic Matter by FT-IR Spectroscopy: Application in Waste Science. Appl. Spectrosc. 2002, 56, 1170–1175. [Google Scholar] [CrossRef]
- El Fels, L.; Zamama, M.; Hafidi, M. Advantages and Limitations of Using FTIR Spectroscopy for Assessing the Maturity of Sewage Sludge and Olive Oil Waste Co-Composts. In Biodegradation and Bioremediation of Polluted Systems–New Advances and Technologies; InTech: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Grube, M.; Lin, J.G.; Lee, P.H.; Kokorevicha, S. Evaluation of Sewage Sludge-Based Compost by FT-IR Spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.V.; Gómez, X.; González-Andrés, F.; Morán, A. Characterization of Different Compost Extracts Using Fourier-Transform Infrared Spectroscopy (FTIR) and Thermal Analysis. Biodegradation 2008, 19, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, J.; Shen, L.; Wu, X.; Amanze, C.; Zeng, W. Effect of Bamboo Sphere Amendment on the Organic Matter Decomposition and Humification of Food Waste Composting. Waste Manag. 2021, 133, 19–27. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Lee, C.-H.; Hsieh, C.-Y.; Chen, T.-C.; Jien, S.-H. Using Fluorescence Spectroscopy to Assess Compost Maturity Degree during Composting. Agronomy 2023, 13, 1870. [Google Scholar] [CrossRef]
- Chirayil, C.J.; Abraham, J.; Mishra, R.K.; George, S.C.; Thomas, S. Instrumental Techniques for the Characterization of Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 3, ISBN 9780323461450. [Google Scholar]
- Wei, Z.; Zhao, X.; Zhu, C.; Xi, B.; Zhao, Y.; Yu, X. Assessment of Humification Degree of Dissolved Organic Matter from Different Composts Using Fluorescence Spectroscopy Technology. Chemosphere 2014, 95, 261–267. [Google Scholar] [CrossRef]
- He, X.-S.; Xi, B.-D.; Jiang, Y.-H.; Li, M.-X.; Yu, H.-B.; An, D.; Yang, Y.; Liu, H.-L. Elemental and Spectroscopic Methods with Chemometric Analysis for Characterizing Composition and Transformation of Dissolved Organic Matter during Chicken Manure Composting. Environ. Technol. 2012, 33, 2033–2039. [Google Scholar] [CrossRef]
- Dos Santos, L.M.; Simões, M.L.; de Melo, W.J.; Martin-Neto, L.; Pereira-Filho, E.R. Application of Chemometric Methods in the Evaluation of Chemical and Spectroscopic Data on Organic Matter from Oxisols in Sewage Sludge Applications. Geoderma 2010, 155, 121–127. [Google Scholar] [CrossRef]
- Liu, D.; Yu, H.; Yang, F.; Liu, L.; Gao, H.; Cui, B. Characterizing Humic Substances from Native Halophyte Soils by Fluorescence Spectroscopy Combined with Parallel Factor Analysis and Canonical Correlation Analysis. Sustainability 2020, 12, 9787. [Google Scholar] [CrossRef]
- Xie, Z.; Guan, W. Research on Fluorescence Spectroscopy Characteristics of Dissolved Organic Matter of Landfill Leachate in the Rear Part of Three Gorges Reservoir. J. Spectrosc. 2015, 2015, 9787. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; An, T.; Wei, D.; Chi, F.; Zhou, B. Effects of Long-Term Fertilization on Soil Humic Acid Composition and Structure in Black Soil. PLoS ONE 2017, 12, e0186918. [Google Scholar] [CrossRef]
- Řezáčová, V.; Gryndler, M. Fluorescence Spectroscopy: A Tool to Characterize Humic Substances in Soil Colonized by Microorganisms? Folia Microbiol. 2006, 51, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, Y.; Hu, S.; Huang, Q.; Wei, C.; Zhang, W.; Kang, L.; Huang, Z.; Hao, A. Fluorescent Probes for “off–on” Sensitive and Selective Detection of Mercury Ions and l-Cysteine Based on Graphitic Carbon Nitride Nanosheets. J. Mater. Chem. C 2015, 3, 2093–2100. [Google Scholar] [CrossRef]
- Provenzano, M.R.; D’Orazio, V.; Jerzykiewicz, M.; Senesi, N. Fluorescence Behaviour of Zn and Ni Complexes of Humic Acids from Different Sources. Chemosphere 2004, 55, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Egea, F.C.; Martínez-Sabater, E.; Jordá, J.; Moral, R.; Bustamante, M.A.; Paredes, C.; Pérez-Murcia, M.D. Dissolved Organic Matter Fractions Formed during Composting of Winery and Distillery Residues: Evaluation of the Process by Fluorescence Excitation-Emission Matrix. Chemosphere 2007, 68, 301–309. [Google Scholar] [CrossRef]
- Shao, Z.-H.; He, P.-J.; Zhang, D.-Q.; Shao, L.-M. Characterization of Water-Extractable Organic Matter during the Biostabilization of Municipal Solid Waste. J. Hazard. Mater. 2009, 164, 1191–1197. [Google Scholar] [CrossRef]
- Sunuwar, S.; Manzanares, C.E. Excitation, Emission, and Synchronous Fluorescence for Astrochemical Applications: Experiments and Computer Simulations of Synchronous Spectra of Polycyclic Aromatic Hydrocarbons and Their Mixtures. Icarus 2021, 370, 114689. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, L.; Bi, J.; Li, Y.; Zhang, H. Study on Spectral Characteristics of Fulvic Acid in the Process of Mixed Composting of Bioleach Deep Dehydrated Sludge and Rice Straw. J. Phys. Conf. Ser. 2021, 2021, 012094. [Google Scholar] [CrossRef]
- Guo, X.; He, X.; Zhang, H.; Deng, Y.; Chen, L.; Jiang, J. Characterization of Dissolved Organic Matter Extracted from Fermentation Effluent of Swine Manure Slurry Using Spectroscopic Techniques and Parallel Factor Analysis (PARAFAC). Microchem. J. 2012, 102, 115–122. [Google Scholar] [CrossRef]
- Peuravuori, J.; Koivikko, R.; Pihlaja, K. Characterization, Differentiation and Classification of Aquatic Humic Matter Separated with Different Sorbents: Synchronous Scanning Fluorescence Spectroscopy. Water Res. 2002, 36, 4552–4562. [Google Scholar] [CrossRef]
- Yang, Y.; Du, W.; Cui, Z.; Zhao, T.; Wang, X.; Lv, J. Spectroscopic Characteristics of Dissolved Organic Matter during Pig Manure Composting with Bean Dregs and Biochar Amendments. Microchem. J. 2020, 158, 105226. [Google Scholar] [CrossRef]
- Santín, C.; González-Pérez, M.; Otero, X.L.; Vidal-Torrado, P.; Macías, F.; Álvarez, M.Á. Characterization of Humic Substances in Salt Marsh Soils under Sea Rush (Juncus Maritimus). Estuar. Coast Shelf Sci. 2008, 79, 541–548. [Google Scholar] [CrossRef]
- Gao, J.; Dou, S.; Wang, Z. Structural Analysis of Humic Acid in Soil at Different Corn Straw Returning Modes through Fluorescence Spectroscopy and Infrared Spectroscopy. Int. J. Anal. Chem. 2019, 2019, 1086324. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Song, F.; Wu, F.; Feng, W.; Tang, Z.; Giesy, J.P.; Guo, F.; Shi, D.; Liu, X.; Qin, N.; Xing, B.; et al. Fluorescence Regional Integration and Differential Fluorescence Spectroscopy for Analysis of Structural Characteristics and Proton Binding Properties of Fulvic Acid Sub-Fractions. J. Environ. Sci. 2018, 74, 116–125. [Google Scholar] [CrossRef]
- Huang, J.; Han, L.; Huang, G. Characterization of Digestate Composting Stability Using Fluorescence EEM Spectroscopy Combining with PARAFAC. Waste Manag. Res. J. A Sustain. Circ. Econ. 2019, 37, 486–494. [Google Scholar] [CrossRef]
- Duan, H.; Ji, M.; Chen, A.; Zhang, B.; Shi, J.; Liu, L.; Li, X.; Sun, J. Evaluating the Impact of Rice Husk on Successions of Bacterial and Fungal Communities during Cow Manure Composting. Environ. Technol. Innov. 2021, 24, 102084. [Google Scholar] [CrossRef]
- Li, X.; Shi, Z.; Wang, J.; Jiang, R. The Quality of Dissolved Organic Matter Extracted at Different Times from Pig Compost and Its Copper Binding Capacity Based on EEM-PARAFAC. Ecotoxicol. Environ. Saf. 2021, 207, 111545. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Yu, D.; Hou, R.; Ma, X.; Liu, J.; Cao, Z.; Cheng, K.; Yan, G.; Zhang, C.; et al. Combined Addition of Biochar and Garbage Enzyme Improving the Humification and Succession of Fungal Community during Sewage Sludge Composting. Bioresour. Technol. 2022, 346, 126344. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Zhao, M.; Zhao, W.; Liu, J.; Tang, J.; Liao, H.; Chen, Z.; Zhou, S. Hyperthermophilic Composting Accelerates the Humification Process of Sewage Sludge: Molecular Characterization of Dissolved Organic Matter Using EEM–PARAFAC and Two-Dimensional Correlation Spectroscopy. Bioresour. Technol. 2019, 274, 198–206. [Google Scholar] [CrossRef]
- Liu, H.-T.; Guo, H.-N.; Guo, X.-X.; Wu, S. Probing Changes in Humus Chemical Characteristics in Response to Biochar Addition and Varying Bulking Agents during Composting: A Holistic Multi-Evidence-Based Approach. J. Environ. Manag. 2021, 300, 113736. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Q.; Myers, M.; Chen, Q.; Li, X. Application of Nuclear Magnetic Resonance Technology to Carbon Capture, Utilization and Storage: A Review. J. Rock Mech. Geotech. Eng. 2019, 11, 892–908. [Google Scholar] [CrossRef]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of Biochar Addition on the Humic Substances of Composting Manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Spaccini, R.; De Martino, A.; Scognamiglio, F.; di Meo, V. Soil Washing with Solutions of Humic Substances from Manure Compost Removes Heavy Metal Contaminants as a Function of Humic Molecular Composition. Chemosphere 2019, 225, 150–156. [Google Scholar] [CrossRef]
- Rodríguez, L.; Cerrillo, M.I.; García-Albiach, V.; Villaseñor, J. Domestic Sewage Sludge Composting in a Rotary Drum Reactor: Optimizing the Thermophilic Stage. J. Environ. Manag. 2012, 112, 284–291. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A. Molecular Characteristics of Humic Acids Extracted from Compost at Increasing Maturity Stages. Soil Biol. Biochem. 2009, 41, 1164–1172. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Hamdi, H.; Revel, J.-C. Elemental Analysis, FTIR and 13C-NMR of Humic Acids from Sewage Sludge Composting. Agronomie 2004, 24, 13–18. [Google Scholar] [CrossRef]
- Albrecht, R.; Ziarelli, F.; Alarcón-Gutiérrez, E.; Le Petit, J.; Terrom, G.; Perissol, C. 13C Solid-State NMR Assessment of Decomposition Pattern during Co-Composting of Sewage Sludge and Green Wastes. Eur. J. Soil Sci. 2008, 59, 445–452. [Google Scholar] [CrossRef]
- Wang, C.; Tu, Q.; Dong, D.; Strong, P.J.; Wang, H.; Sun, B.; Wu, W. Spectroscopic Evidence for Biochar Amendment Promoting Humic Acid Synthesis and Intensifying Humification during Composting. J. Hazard Mater. 2014, 280, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Du, C.; Zeng, Y.; Ma, F.; Zhou, J. Characterizing Typical Farmland Soils in China Using Raman Spectroscopy. Geoderma 2016, 268, 147–155. [Google Scholar] [CrossRef]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, F.N.D.; Calderón, F.J. Soil Chemical Insights Provided through Vibrational Spectroscopy; Elsevier:: Amsterdam, The Netherlands, 2014; Volume 126, ISBN 9780128001325. [Google Scholar]

| Spectroscopy | Description | Advantages | Disadvantage |
|---|---|---|---|
| UV-Vis | An electronic transition effect occurs due to the absorption of energy by the electrons (transmittance). |
|
|
| IR | The energy absorbed by the functional groups produces different types of molecular vibrations (reflectance). |
|
|
| Fluorescence | Based on the signal interpretation by the effect of light emission in the process of electronic de-excitation of conjugated systems (emission). |
|
|
| NMR | Produced by the reorganization of nuclear spin due to the application of a magnetic field to the nuclei (emission). |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, A.P.-S.; Marhuenda-Egea, F.C.; Bustamante, M.A.; Curaqueo, G. Spectroscopy Techniques for Monitoring the Composting Process: A Review. Agronomy 2023, 13, 2245. https://doi.org/10.3390/agronomy13092245
Martín AP-S, Marhuenda-Egea FC, Bustamante MA, Curaqueo G. Spectroscopy Techniques for Monitoring the Composting Process: A Review. Agronomy. 2023; 13(9):2245. https://doi.org/10.3390/agronomy13092245
Chicago/Turabian StyleMartín, Andrés Pérez-San, Frutos C. Marhuenda-Egea, Maria Angeles Bustamante, and Gustavo Curaqueo. 2023. "Spectroscopy Techniques for Monitoring the Composting Process: A Review" Agronomy 13, no. 9: 2245. https://doi.org/10.3390/agronomy13092245
APA StyleMartín, A. P.-S., Marhuenda-Egea, F. C., Bustamante, M. A., & Curaqueo, G. (2023). Spectroscopy Techniques for Monitoring the Composting Process: A Review. Agronomy, 13(9), 2245. https://doi.org/10.3390/agronomy13092245






