Seed Treatment Potential for the Improvement of Lucerne Seed Performance and Early Field Growth
Abstract
1. Introduction
2. Materials and Methods
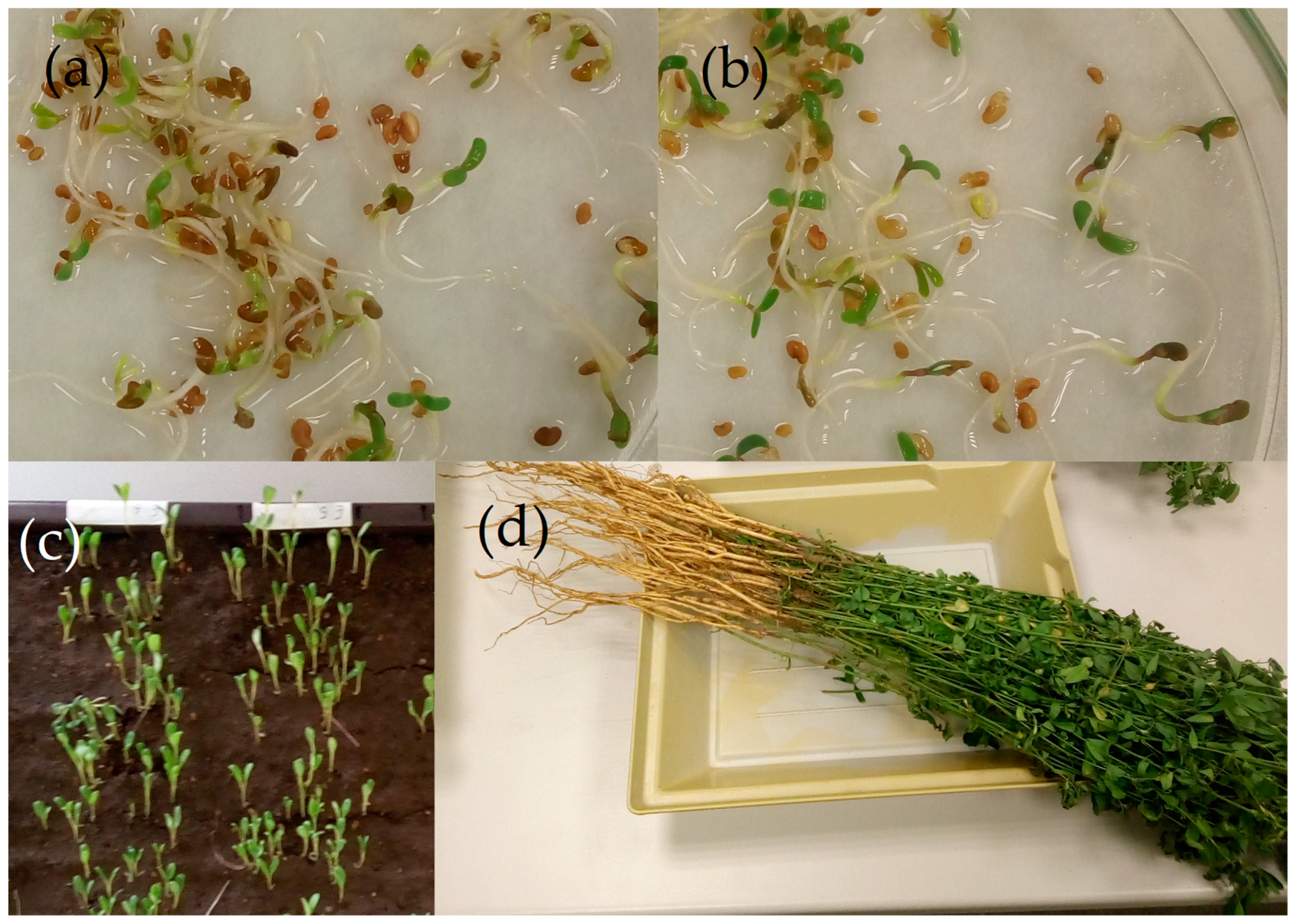
2.1. Germination Experiments
2.2. Seed Emergence Experiments
2.3. Small-Scale Field Experiment
2.4. Statistical Analysis
3. Results
3.1. Germination Experiments
3.2. Emergence Experiments
3.3. Small-Scale Field Experiment
4. Discussion
4.1. Lucerne Seed Priming
4.2. Lucerne Seed Coating
4.3. Relationships of Simple Laboratory Tests to Field Condition and Root Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrzejewska, J.; Albrecht, K.A.; Ignaczak, S.; Skinder, Z. Method and time of alfalfa sowing when climate is changing. Acta Sci. Pol. 2015, 14, 3–13. [Google Scholar]
- Shi, S.; Nan, L.; Smith, K.F. The current status, problems, and prospects of alfalfa (Medicago sativa L.) breeding in China. Agronomy 2017, 7, 1. [Google Scholar] [CrossRef]
- Lanini, W.T.; Orloff, S.B.; Vargas, R.N.; Orr, J.P.; Marble, V.L.; Grattan, S.R. Oat companion crop seeding rate effect on alfalfa establishment, yield, and weed control. Agron. J. 1991, 83, 330–333. [Google Scholar] [CrossRef]
- Matteau, C.; Seguin, P.; Baurhoo, B.; Mustafa, A.F. Sudangrass as companion crop to establish alfalfa. Crop Forage Turfgrass Manag. 2020, 6, e20006. [Google Scholar] [CrossRef]
- Hakl, J.; Pisarčik, M.; Fuksa, P.; Šantrůček, J. Potential of lucerne sowing rate to influence root development and its implications for field stand productivity. Grass Forage Sci. 2021, 76, 378–389. [Google Scholar] [CrossRef]
- Morrow, G.E.; Hunt, T.F. Field experiments in oats, 1888. Germination of grass and clover seeds. Bull. Univ. Ill. Urbana-Champaign Campus Agric. Exp. Stn. 1888, 3, 25–35. [Google Scholar]
- Hall, M.H.; Hebrock, N.S.; Pierson, P.E.; Caddel, J.L.; Owens, V.N.; Sulc, R.M.; Undersander, D.J.; Whitesides, R.E. The effects of glyphosate-tolerant technology on reduced alfalfa seeding rates. Agron. J. 2010, 102, 911–916. [Google Scholar] [CrossRef]
- Bellamkonda, M.; Madaraju, S.; Kola, S.; Tummanepally, S.; Ravipati, S. Enhancement of seed setting in Lucerne through foliar spray of nutrients and growth regulators. Pharma Innov. J. 2021, 10, 1225–1229. [Google Scholar]
- Hakl, J.; Fuksa, P.; Konečná, J.; Pacek, L.; Tlustoš, P. Effect of applied cultivation technology and environmental conditions on lucerne farm yield in the Central Europe. Plant Soil Environ. 2014, 60, 475–480. [Google Scholar] [CrossRef]
- Hughes, H.D. Making legumes grow. Farm Fireside 1915, 38, 7. [Google Scholar]
- Ellis, T.J.; Palmer, T.P. Heat treatment of hard seed in lucerne. N. Z. J. Exp. Agric. 1973, 1, 44–45. [Google Scholar] [CrossRef]
- Harrington, G.T. Agricultural value of impermeable seeds. J. Agric. Res. 1916, 6, 761–796. [Google Scholar]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the Concept and Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; pp. 11–41. [Google Scholar]
- Basra, S.M.A.; Farooq, M.; Tabassam, R.; Ahmad, N. Physiological and biochemical aspects of pre-sowing seed treatments in fine rice (Oryza sativa L.). Seed Sci. Technol. 2005, 33, 623–628. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Gull, S.; Ali, A.; Khalid, E.; Abbas, A.N.; Tariq, M. Rice seedling establishment as influenced by cultivars and seed priming with potassium nitrate. J. Appl. Res. Plant Sci. 2020, 1, 65–75. [Google Scholar]
- Sivasubramaniam, K.; Geetha, R.; Sujatha, K.; Raja, K.; Sripunitha, A.; Selvarani, R. Seed priming: Triumphs and tribulations. Madras Agric. J. 2011, 98, 197–209. [Google Scholar]
- Benazzouk, S.; Djazouli, Z.E.; Lutts, S. Vermicompost leachate as a promising agent for priming and rejuvenation of salt-treated germinating seeds in Brassica napus. Commun. Soil Sci. Plant Anal. 2019, 50, 1344–1357. [Google Scholar] [CrossRef]
- Dhivya, R.S.; Ray, L.I.; Behera, U. Organic amendments on soil nutrient balance under mid hills of Meghalaya. E-Planet 2020, 18, 29–38. [Google Scholar]
- Suleiman, H.; Rorat, A.; Grobelak, A.; Grosser, A.; Milczarek, M.; Płytycz, B.; Kacprzak, M.; Vandenbulcke, F. Determination of the performance of vermicomposting process applied to sewage sludge by monitoring of the compost quality and immune responses in three earthworm species: Eisenia fetida, Eisenia andrei and Dendrobaena veneta. Bioresour. Technol. 2017, 241, 103–112. [Google Scholar] [CrossRef]
- Dickerson, G.W. Vermicomposting, Guide H-164, 1st ed.; College of Agriculture and Home Economics, New Mexico State University: Las Cruces, NM, USA, 2001. [Google Scholar]
- Simeon, L.B.; Bugawisan, E.P. Potential of vermitea and nutrient solution under non-circulating hydroponic system on production performance of pechay (Brassica rapa L.). EPRA Int. J. Agric. Rural. Econ. Res. (ARER) 2023, 11, 40–51. [Google Scholar]
- Aslam, Z.; Ahmad, A.; Bellitürk, K.; Iqbal, N.; Idrees, M.; Ur Rehman, W.U.; Akbar, G.; Tariq, M.; Raza, M.; Riasat, S.; et al. Effects of vermicompost, vermi-tea and chemical fertilizer on morpho-physiological characteristics of tomato (Solanum lycopersicum) in Suleymanpasa District, Tekirdag of Turkey. Pure Appl. Biol. (PAB) 2020, 9, 1920–1931. [Google Scholar] [CrossRef]
- El-Goud, A.; Amal, K. Efficiency response of vermicompost and vermitea levels on growth and yield of eggplant (Solanum melongena L.). Alex. Sci. Exch. J. 2020, 41, 69–75. [Google Scholar]
- Lim, L.S.; Tan, K.S.; Fu, M.Y.; Au, H.L.; Ebi, I.; Lal, M.T.M.; Kawamura, G.; Shapawi, R.; Lam, S.S. Valorization of Bokashi leachate as feed additive in tilapia farming. Environ. Res. 2021, 198, 110472. [Google Scholar] [CrossRef] [PubMed]
- Samarah, N.H.; AL-Quraan, N.A.; Massad, R.S.; Welbaum, G.E. Treatment of bell pepper (Capsicum annuum L.) seeds with chitosan increases chitinase and glucanase activities and enhances emergence in a standard cold test. Sci. Hortic. 2020, 269, 109393. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed and crop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Kowalska, J.; Zbytek, Z. Microbiological dressing of pea seeds as a form of increase resistance and plant development. In Proceedings of the Sixth International Scientific Agricultural Symposium “Agrosym 2015”, Jahorina, Bosnia and Herzegovina, 15–18 October 2015. [Google Scholar]
- Samac, D.; Scraber, S.; Blosberg, J.; Barclay, S. A mineral seed treatment for control of seedling diseases of alfalfa suitable for organic production systems. Phytopathology 2014, 104, 103. [Google Scholar]
- Scott, D. Effects of seed coating on establishment. N. Z. J. Agric. Res. 1975, 18, 59–67. [Google Scholar] [CrossRef]
- Sousa, P.G.; Vieira, H.D.; Acha, A.J. Coating with different doses of fertilizer in Vinhático seeds. Am. J. Plant Sci. 2017, 8, 2554–2568. [Google Scholar] [CrossRef]
- Cooper, R.M.; Williams, J.S. Elemental sulphur as an induced antifungal substance in plant defence. J. Exp. Bot. 2004, 55, 1947–1953. [Google Scholar] [CrossRef]
- Scott, D.; Archie, W.J. Sulphur, phosphate, and molybdenum coating of legume seed. N. Z. J. Agric. Res. 1978, 21, 643–649. [Google Scholar] [CrossRef]
- Eriksson, J. Dissolution of Hardened Wood Ash in Forest Soils. Studies in a Column Experiment, 1st ed.; Swedish National Board for Industrial and Technical Development (NUTEK): Upsala, Sweden, 1996. [Google Scholar]
- Mortensen, L.H.; Rønn, R.; Vestergård, M. Bioaccumulation of cadmium in soil organisms–With focus on wood ash application. Ecotoxicol. Environ. Saf. 2018, 156, 452–462. [Google Scholar] [CrossRef]
- Nabeela, F.; Murad, W.; Khan, I.; Mian, I.A.; Rehman, H.; Adnan, M.; Azizullah, A. Effect of wood ash application on the morphological, physiological and biochemical parameters of Brassica napus L. Plant. Physiol. Biochem. 2015, 95, 15–25. [Google Scholar]
- Nottfige, D.O.; Ojeniyi, S.O.; Asawalam, D.O. Comparative effect of plant residues and NPK fertilizer on nutrient status and yield of maize (Zea mays L.) in a humid ultisol. Niger. J. Soil Sci. 2005, 15, 1–8. [Google Scholar]
- Mbah, C.N.; Nwite, J.N.; Njoku, C.; Nweke, I.A. Response of maize (Zea mays L.) to different rates of wood-ash application in acid ultisol in Southeast Nigeria. Afr. J. Agric. Res. 2010, 5, 580–583. [Google Scholar]
- Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Gorzelany, J.; Migut, D. Effect of Fertilisation with Ash from Biomass Combustion on the Mechanical Properties of Potato Tubers (Solanum tuberosum L.) Grown in Two Types of Soil. Agronomy 2022, 12, 379. [Google Scholar] [CrossRef]
- Owolabi, O.; Ojeniyi, S.O.; Amodu, A.O.; Hazzan, K. Response of cowpea, okra and tomato sawdust ash manure. Moor J. Agric. Res. 2003, 4, 178–182. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited, 1st ed.; Press syndicate of Cambridge University: Cambridge, UK, 1989. [Google Scholar]
- Cadena, M.B.; Preston, G.M.; Van der Hoorn, R.A.; Flanagan, N.A.; Townley, H.E.; Thompson, I.P. Enhancing cinnamon essential oil activity by nanoparticle encapsulation to control seed pathogens. Ind. Crops Prod. 2018, 124, 755–764. [Google Scholar] [CrossRef]
- Perina, F.J.; Lage De Andrade, C.C.; Intra Moreira, S.; Nery, E.M.; Ogoshi, C.; Alves, E. Cinnamomun zeylanicum oil and transcinnamaldehyde against alternaria brown spot in tangerine: Direct effects and induced resistance. Phytoparasitica 2019, 47, 575–589. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Jakubowska, M.; Łukaszyk, J.; Krzymińska, J. Cinnamon as a useful preventive substance for the care of human and plant health. Molecules 2021, 26, 5299. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lee, S.; Manohar, M.; Chen, J. Efficacy of Ascaroside #18 Treatments in Control of Salmonella enterica on Alfalfa and Fenugreek Seeds and Sprouts. J. Food Prot. 2023, 86, 100064. [Google Scholar] [PubMed]
- Song, W.J.; Kang, D.H. Inactivation of Salmonella Typhimurium, Escherichia coli O157: H7 and Listeria monocytogenes on alfalfa seeds by the combination treatment of vacuumed hydrogen peroxide vapour and vacuumed dry heat. Lett. Appl. Microbiol. 2022, 74, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Šerá, B.; Scholtz, V.; Jirešová, J.; Khun, J.; Julák, J.; Šerý, M. Effects of non-thermal plasma treatment on seed germination and early growth of leguminous plants—A review. Plants 2021, 10, 1616. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mayookha, V.P.; Kothakota, A.; Sharmila, L.; Ramesh, S.V.; Bharathi, C.P.; Gomathy, K.; Srikanth, V. Im-pact of ozone treatment on seed germination–A systematic review. Ozone Sci. Eng. 2020, 42, 331–346. [Google Scholar] [CrossRef]
- Mohammad, Z.; Kalbasi-Ashtari, A.; Riskowski, G.; Juneja, V.; Castillo, A. Inactivation of Salmonella and Shiga toxin-producing Escherichia coli (STEC) from the surface of alfalfa seeds and sprouts by combined antimicrobial treatments using ozone and electrolyzed water. Food Res. Int. 2020, 136, 109488. [Google Scholar] [CrossRef] [PubMed]
- Fuksa, P.; Hrevušová, Z.; Szabó, O.; Hakl, J. Effect of Row Spacing and Plant Density on Silage Maize Growth, Dry Matter Distribution and Yield. Agronomy 2023, 13, 1117. [Google Scholar] [CrossRef]
- Hakl, J.; Pisarčik, M.; Hrevušová, Z.; Šantrůček, J. In-field lucerne root morphology traits over time in relation to forage yield, plant density, and root disease under two cutting managements. Field Crops Res. 2017, 213, 109–117. [Google Scholar] [CrossRef]
- StatSoft, Inc. Statistica for Windows; StatSoft: Tulsa, OK, USA, 2012. [Google Scholar]
- Chaturvedi, R.S.; Rai, P.K.; Bara, B.M.; Kumar, S.; Pradhan, V. Effect of priming on germination and seed vigour in Wheat (Triticum aestivum L.) Seeds. J. Pharmacogn. Phytochem. 2017, 6, 605–608. [Google Scholar]
- Chauhan, P.; Pandey, G.; Pandey, P.K. Priming with potassium solutions improves seedling growth and vigor in forage sorghum (Sorghum bicolor L.). J. Appl. Nat. Sci. 2016, 8, 1937–1940. [Google Scholar] [CrossRef]
- Mustafa, G.; Shehzad, M.A.; Tahir, M.H.N.; Nawaz, F.; Akhtar, G.; Bashir, M.A.; Ghaffar, A. Pretreatment with chitosan arbitrates physiological processes and antioxidant defense system to increase drought tolerance in alfalfa (Medicago sativa L.). J. Soil Sci. Plant Nutr. 2022, 22, 2169–2186. [Google Scholar] [CrossRef]
- Hinojosa-Dávalos, J.; Arias-Rios, E.V.; Varela-Hernández, J.J.; Cardona-López, M.A.; Orozco-Muñiz, R.; Cabrera-Diaz, E. Thermal and Chemical Treatments To Reduce Salmonella on Alfalfa (Medicago sativa) and Broccoli (Brassica oleracea var. italica) Seeds before and during the Sprouting Process: A Hurdle Approach. J. Food Prot. 2020, 83, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Fiutak, G.; Tarko, T. Effect of hot water treatment of seeds on quality indicators of alfalfa sprouts. LWT 2019, 113, 108270. [Google Scholar] [CrossRef]
- Amooaghaie, R. The effect of hydro and osmopriming on alfalfa seed germination and antioxidant defenses under salt stress. Afr. J. Biotechnol. 2011, 10, 6269–6275. [Google Scholar]
- Sepehri, A.; Najari, S.; Rouhi, H.R. Seed priming to overcome salinity stress in Persian cultivars of alfalfa (Medicago sativa L.). Not. Sci. Biol. 2015, 7, 96–101. [Google Scholar] [CrossRef][Green Version]
- Jorjandi, M.; GR, S.S. The effect of priming on germination and seedling growth of alfalfa (Medicago sativa L.) under salinity stress. J. Stress Physiol. Biochem. 2012, 8, 234–239. [Google Scholar]
- Wilder, F.A. Gypsum: Its Occurrence, Origin, Technology and Uses. Iowa Geol. Surv. Annu. Rep. 1919, 28, 246–472. [Google Scholar] [CrossRef][Green Version]
- Seim, E.C.; Caldwell, A.C.; Rehm, G.W. Sulfur Response by Alfalfa (Medicago sativa L.) on a Sulfur-Deficient Soil 1. Agron. J. 1969, 61, 368–371. [Google Scholar] [CrossRef]
- Talley, B. Advances in Alfalfa Seed Coatings. In Proceedings of the Kentucky Alfalfa and Stored Forage Conference, Lexington, KY, USA, 20–25 February 2010. [Google Scholar]
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopath. J. 2010, 25, 441–451. [Google Scholar]
- Christian, E.J. Plant Extracted Essential Oils as a Contact Fungicide Seed Treatment for Organic Corn. Master’s Thesis, Iowa State University, Ames, IA, USA, 2007. [Google Scholar]
- Kowalska, J.; Tyburski, J.; Krzymińska, J.; Jakubowska, M. Cinnamon powder: An in vitro and in vivo evaluation of antifungal and plant growth promoting activity. Eur. J. Plant Pathol. 2020, 156, 237–243. [Google Scholar] [CrossRef]
- O’Leary, J.W.; Prisco, J.T. Response of osmotically stressed plants to growth regulators. Adv. Front. Plant Sci. 1970, 25, 129–139. [Google Scholar]
- Hakl, J.; Pisarčik, M.; Šantrůček, J. Effect of seed pelleting on lucerne seed emergence. In Proceedings of the 13th Scientific and Technical Seminar on Seeds and Seedings, Prague, Czech Republic, 2 February 2017. [Google Scholar]
- Giri, G.S.; Schillinger, W.F. Seed priming winter wheat for germination, emergence, and yield. Crop Sci. 2003, 43, 2135–2141. [Google Scholar] [CrossRef]
- Bradford, K.J.; Steiner, J.J.; Trawatha, S.E. Seed priming influence on germination and emergence of pepper seed lots. Crop Sci. 1990, 30, 718–721. [Google Scholar] [CrossRef]
- Pisarčik, M.; Hakl, J.; Szabó, O.; Nerušil, P. Efficacy of Pythium oligandrum on improvement of lucerne yield, root development and disease score under field conditions. Front. Plant Sci. 2022, 13, 1045225. [Google Scholar] [CrossRef]

| Temperature, Concentration (w/v%), Duration | Proportion of Additional Binders | |
|---|---|---|
| Seed priming/heat | ||
| Control | ||
| Heat treatment | 41 °C/100 h | |
| Hydro-priming (water) | 6 h | |
| Vermitea | 10%/6 h | |
| Bokashi juice | 10%/6 h | |
| Potassium permanganate | 0.2%/6 h | |
| Chitosan | 1%/6 h | |
| Seed coating | ||
| Bentonite (B) | 100% Bentonite or 50% Gypsum/50% Bentonite | |
| Gypsum (G) | 2.0% | 50% Bentonite |
| Leaf-wood ash | 0.2% | 50% Bentonite or 50% Gypsum |
| Picea abies ash | 0.2% | 50% Bentonite |
| Tannin quebracho | 0.2% | 50% Bentonite |
| Cinnamon powder | 2.0% | 50% Bentonite or 33% Bentonite + 33% Gypsum |
| Cocoa powder | 0.5% | 50% Bentonite or 50% Gypsum |
| Time (Days) | 3rd | 4th | 5th | 6th | 7th | Seedling Length |
|---|---|---|---|---|---|---|
| Suspensions experiment | ||||||
| Control | 12.8 af | 41.8 a | 65.8 ae | 72.6 ae | 75.0 | 56.2 a |
| Leaf-wood ash | 15.8 fg | 47.2 a | 69.4 ae | 73.4 ae | 76.8 | 48.0 b |
| Picea abies ash | 11.2 abf | 38.8 abe | 62.0 ab | 69.0 abd | 73.4 | 50.0 b |
| Tannin quebracho | 5.4 cd | 21.0 c | 45.4 bc | 60.0 c | 75.6 | 26.3 d |
| Cocoa powder | 6.2 bc | 33.2 be | 61.6 ab | 73.2 ae | 77.6 | 49.1 b |
| Gypsum | 3.0 cde | 22.4 c | 51.2 bc | 66.4 abcd | 74.6 | 56.3 a |
| Cinnamon powder | 0.4 de | 20.6 c | 46.8 c | 61.4 bc | 71.4 | 39.5 e |
| Priming experiment | ||||||
| Control | 22.2 ab | 54.8 | 66.0 | 75.6 | 75.6 | 55.0 a |
| Heat treatment | 16.8 b | 49.8 | 65.4 | 75.4 | 75.4 | 53.0 ab |
| Chitosan | 33.6 c | 55.4 | 64.4 | 72.8 | 72.8 | 61.6 c |
| Bokashi juice | 24.0 a | 52.8 | 66.8 | 76.2 | 76.2 | 52.0 b |
| Hydro-priming | 41.6 d | 55.4 | 64.4 | 75.6 | 75.6 | 53.6 ab |
| Potassium permanganate | 32.4 c | 53.2 | 63.4 | 72.0 | 72.0 | 59.4 c |
| Vermitea | 25.0 a | 53.8 | 66.8 | 73.5 | 73.5 | 52.2 b |
| Time (Days) | 3rd | 5th | 7th | 10th | Seedling Length (mm) |
|---|---|---|---|---|---|
| Coating experiment | |||||
| Control | 0.0 | 54.0 ab | 68.0 | 70.4 abc | 69.2 a |
| Vermitea priming | 0.4 | 59.4 a | 69.8 | 73.6 bc | 69.0 a |
| Bentonite | 0.0 | 51.2 abc | 67.8 | 72.6 abc | 68.4 ab |
| Gypsum/Bentonite | 0.0 | 54.0 ab | 72.0 | 74.0 c | 70.3 a |
| Leaf-wood ash/Bentonite | 0.4 | 51.4 abc | 68.4 | 70.8 abc | 68.7 ab |
| Picea abies ash/Bentonite | 0.0 | 42.0 c | 64.2 | 64.2 a | 66.2 b |
| Cocoa/Bentonite | 0.0 | 40.0 c | 63.6 | 65.4 ab | 68.4 ab |
| Cocoa/Gypsum | 0.0 | 43.2 bc | 66.2 | 69.4 abc | 70.8 a |
| Priming experiment | |||||
| Control | 7.8 ab | 66.0 ab | 67.2 abc | 68.2 abc | 67.0 ab |
| Heat treatment | 11.2 abc | 67.4 abc | 68.6 abc | 69.2 abc | 67.0 ab |
| Chitosan | 15.2 abc | 74.2 cd | 75.0 cd | 75.6 cd | 65.6 b |
| Bokashi juice | 8.8 ab | 62.8 a | 62.8 a | 63.2 a | 63.2 e |
| Hydro-priming | 17.8 bc | 67.8 abc | 68.4 abc | 69.2 abc | 70.3 cd |
| Potassium permanganate | 22.6 c | 66.4 ab | 67.0 ab | 67.2 ab | 68.7 ac |
| Tannin quebracho/Bentonite | 10.0 ab | 70.8 bcd | 70.8 bcd | 71.0 bcd | 69.2 acd |
| Cinnamon powder/Bentonite | 5.6 a | 75.6 d | 77.6 d | 77.6 d | 71.3 d |
| Treatment | SL | TD | LRN | DPW |
|---|---|---|---|---|
| Control | 27.5 a | 2.98 bc | 1.13 abcd | 44.0 ab |
| Hydro-priming | 28.4 abcd | 2.91 ab | 1.06 ab | 51.3 ab |
| KMnO4 | 28.3 abcd | 3.03 bcd | 0.97 a | 47.1 ab |
| Chitosan | 30.0 cdef | 2.93 abc | 1.30 cdefg | 49.1 ab |
| Vermitea | 28.0 abc | 2.98 bc | 1.24 bcdefg | 51.6 ab |
| Cinnamon powder/Gypsum/Bentonite | 31.2 ef | 3.13 cde | 1.17 abcdef | 48.8 ab |
| Gypsum/Bentonite | 29.8 bcdef | 2.74 a | 1.10 abc | 50.7 ab |
| KMnO4 priming + Gypsum/Bentonite | 30.7 ef | 3.08 bcde | 1.38 efg | 46.1 ab |
| KMnO4 priming + Cinnamon powder/Gypsum/Bentonite | 30.2 def | 3.2 de | 1.39 fg | 45.6 ab |
| KMnO4 priming+ Leaf-wood ash/Gypsum | 31.7 f | 3.12 cde | 1.42 g | 57.3 b |
| Chitosan priming + Gypsum/Bentonite | 29.7 bcde | 3.25 e | 1.38 fg | 50.0 ab |
| Chitosan priming + Cinnamon powder/Gypsum/Bentonite | 29.3 abcde | 3.01 bcd | 1.15 abcde | 40.1 a |
| Vermitea priming + Gypsum/Bentonite | 30.3 def | 3.04 bcde | 1.32 cdefg | 51.3 ab |
| Vermitea priming + Cinnamon powder/Gypsum/Bentonite | 28.3 abcd | 2.98 bc | 1.26 bcdefg | 53.2 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, O.; Pisarčik, M.; Hrevušová, Z.; Hakl, J. Seed Treatment Potential for the Improvement of Lucerne Seed Performance and Early Field Growth. Agronomy 2023, 13, 2207. https://doi.org/10.3390/agronomy13092207
Szabó O, Pisarčik M, Hrevušová Z, Hakl J. Seed Treatment Potential for the Improvement of Lucerne Seed Performance and Early Field Growth. Agronomy. 2023; 13(9):2207. https://doi.org/10.3390/agronomy13092207
Chicago/Turabian StyleSzabó, Ondřej, Martin Pisarčik, Zuzana Hrevušová, and Josef Hakl. 2023. "Seed Treatment Potential for the Improvement of Lucerne Seed Performance and Early Field Growth" Agronomy 13, no. 9: 2207. https://doi.org/10.3390/agronomy13092207
APA StyleSzabó, O., Pisarčik, M., Hrevušová, Z., & Hakl, J. (2023). Seed Treatment Potential for the Improvement of Lucerne Seed Performance and Early Field Growth. Agronomy, 13(9), 2207. https://doi.org/10.3390/agronomy13092207





