Abstract
Food insecurity has become a pressing issue on a worldwide scale as the globe plows through a food crisis. The disastrous impact of this menace has been exacerbated by climate change, frequent conflicts, pandemic outbreaks, and the global economic recession, which have been prevalent in recent years. Although food insecurity prevails globally, it is especially critical in some regions in Africa, East and Southeast Asia, and South America. Several efforts have been made to curb food insecurity; however, none have been able to curtail it sufficiently. Genetic engineering of crops is a fast-growing technology that could be a viable tool for mitigating food insecurity. Crop varieties resistant to pests and diseases, abiotic stress, spoilage, or specific herbicides have been developed using this technology. Crops have been modified for increased yield, nutritional content, essential vitamins, and micro-mineral fortification. More intriguing is the advent of plant-derived edible vaccines, which prove equally effective and significantly affordable. However, in many countries, government policies pose a limiting factor for the acceptance of this technology. This article discusses the genetic modification of crops, highlighting its origins, methods, applications, achievements, impact, acceptance, distribution, and potential as a viable antidote to global food insecurity.
1. Introduction
1.1. Food Insecurity: A Global Menace
Food insecurity persists all around the world [1]. According to Bickel et al. [2], the United States Department of Agriculture (USDA) elucidated that food insecurity pertains to a situation whereby access to safe, healthy, and nutritious meals is restricted, uncertain, limited, or unpredictable, as well as the capacity to acquire acceptable foods through socially acceptable means. This is a disheartening reality in many regions of the world.
Currently, there are about 8 billion people on planet Earth. It is unfortunate that about 1 billion people languish through undernutrition, living daily in hunger, and that 2 billion more people suffer from essential micronutrient deficiencies [3]. Since 2019, the number of people affected by severe hunger has surged from 135 million to 345 million. Recent statistics reveal that about 49 million people across 49 countries are at risk of famine [4], and a large proportion of the affected population is in sub-Saharan Africa and Asia.
Undernourishment, a tragic impact of food insecurity, is equally ravaging the world [5]. It affects 20% of developing nations, contributing to infant mortality in 50% of cases. Globally, children are the hardest hit by food insecurity. Hundreds of millions of newborns and mothers are vitamin A and iodine deficient. Micronutrient deficiencies, such as iron and vitamin A, affect a substantially larger proportion of people, resulting in anemia. It has been estimated that 2 billion individuals (one out of every three) are anemic [1]. Anaemia, a disease caused by a lack of iron, accounts for approximately 20% of maternal deaths in Asia and Africa. In developing nations, in particular, food insecurity and malnutrition pose major challenges to public health [6,7]. The world is indeed in a food crisis [1]. Globally, more than one-third of children under 5 years of age are affected by malnutrition or undernutrition, not getting enough nutrients needed for optimum growth from their daily diet [8]. Also, more than 400 million mothers have stillborn or underweight infants owing to iron insufficiency. Energy and protein malnutrition affects over 160 million preschool children, resulting in the deaths of more than 5 million children (<5 years) each year [9,10].
The International Food Policy Research Institute [11] further revealed that more than one in every four children is affected by stunting, and wasting is also prevalent, affecting 9% of children. The situation is aggravated by natural disasters, wars, and pandemic outbreaks. For instance, the recent COVID-19 pandemic caused a severe increase in the world population affected by food insecurity, and the number of households experiencing this menace surged drastically. Figure 1 shows the rise in food insecurity in the USA and Africa, respectively. According to a United Nations Report, it is disheartening to note that global efforts toward achieving the eradication of hunger, food insecurity, and nutritional deficiency in all forms by 2030 do not appear to be getting close to the intended goals [12].
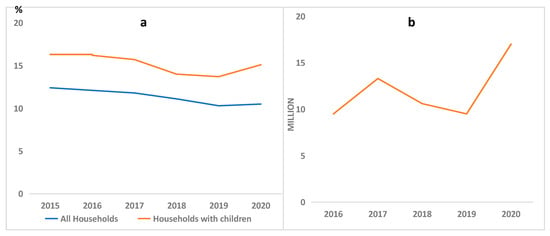
Figure 1.
Food insecurity tipped up during the COVID-19 pandemic in (a) the USA [13]; (b) Africa [14].
In the face of diminishing land availability for agriculture and food production due to industrial and construction activities, the world is left with no alternative but to seek means to maximize the limited resources available and produce more food from the fast-depleting farmlands and the small amount of irrigation water. As a result, the requirement for additional food must be addressed by increasing yields per unit of resource input (land, water, energy, and time). It becomes imperative to examine how science can be deployed to increase productivity ceilings with no further harm to the ecosystem [15,16], a need that biotechnology duly meets.
Achieving food security is a matter of global urgency and all hands must be on deck. Several international agreements and institutions have been formed to this end. The Sustainable Development Goals (SDGs) are the main worldwide policy for reducing hunger and poverty. The second goal of the SDG, tagged ‘The Zero Hunger Initiative’, aims to achieve a set of universally agreed-upon goals that will put an end to hunger, ensure food security and improve nutrition, and promote sustainable agriculture by the year 2030. A question still needing an answer is how this can be achieved within a limited time and with limited resources. Biotechnology is a promising solution! As asked by Habibi-Najafi [17], is it achievable without the use of innovative technology and methods capable of increasing agricultural productivity to minimize crop loss owing to attacks by pests, producing foods with superior nutrient content, and ultimately achieving global food security and sustainable agriculture with an expanding population? Is there an alternative to genetic engineering and biotechnology?
1.2. Defining a Food-Secure World
Food security is an overly broad concept. It is, therefore, difficult to capture the totality of this concept in a few words. Several attempts have been made by global bodies in recent decades before arriving at the current definition [18]. At the World Food Conference (1974), the concept of ‘food security’ was introduced, and it was defined as the consistent availability of sufficient global food supplies, including essential nutrients, to enable a steady increase in food consumption and counteract fluctuations in production and prices [19]. The definition was dynamic, as it reflected the prevalent protein–energy deficiency in 1970, which affected more than 25% of the global population. However, the definition has been consistently revised to broaden its coverage. In 1983, the Food and Agriculture Organisation (FAO) [20] introduced a new concept that emphasized the importance of guaranteeing the universal availability and affordability of basic food requirements for all individuals. Similarly, in 1986, the World Bank [21] incorporated a new notion of ensuring that everyone has access to adequate food to lead a healthy and productive life. These concepts highlight the critical role of equitable and sustainable food systems in promoting human well-being and development. A broader definition was achieved by the FAO in 1996 [22], which was revised again in “The State of Food Insecurity in the World 2001” with a social emphasis. Food security was then described as a situation in which all people always have sufficient, affordable, and nutritious food to satisfy their dietary demands for an active and healthy life [12]. However, the definition was deemed inadequate and was revised, adding another component, ‘food stability’, which emphasizes the ability of food systems to withstand alterations or declines, whether caused by natural or man-induced factors [23].
Food security is commonly associated with the level of assurance that a population has sufficient access to the required amounts of food from available sources to fulfill their dietary needs at the national or regional level. This is commonly linked to the adequacy of the national food balance. The degree of food security in a country is measured largely by the minimum per-capita dietary calories required by the lowest nutritional group, assuming that all locations and socioeconomic groups can equally access the available food supply for a prolonged period [24]. To this effect, the world aims to achieve high food security for all people regardless of social status, race, or location [25].
Food security is comprehensively defined across four dimensions, namely food availability, food accessibility, food utilization, and food stability, as illustrated in Figure 2.
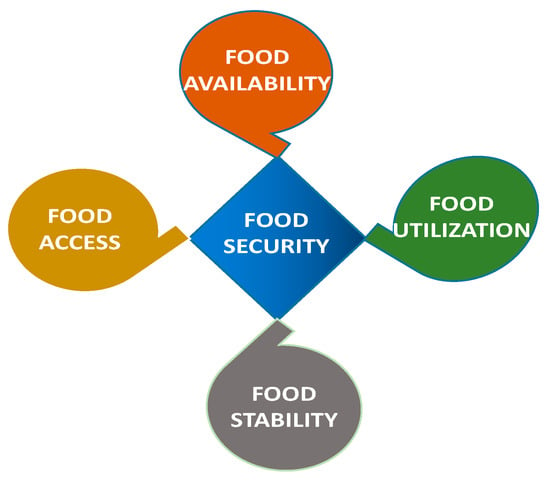
Figure 2.
Essential components of food security [12,18].
Food availability refers to the availability of high-quality food in adequate amounts, whether produced domestically or imported. Food accessibility, on the other hand, ensures that food of acceptable quality is obtainable by the consumer at affordable costs. Food utilization focuses on “safe and nutritious food that fits individuals’ dietary demands,” or the body’s capacity to efficiently absorb food nutrients, enabling a person to live and function optimally. The fourth entity, food stability, emphasizes that a population, household, or individual must always have access to adequate food to be considered food secure. Food access should not be threatened by economic or climatic disasters or cyclical events (such as seasonal food insecurity) [12,18,24]. The concept of stability can apply to both the accessibility and availability of food.
Meeting the world’s rising food demand is a global challenge, particularly in Africa, and agricultural sustainability is indispensable for achieving this [26]. Current agricultural production and conventional technologies are grossly incapable of meeting the increasing food demand of the expanding population, which is a critical concern [26,27]. There is a sense of urgency around the world to curtail the menace and ensure food security, but how can this be achieved? Any viable approach to ameliorating the situation by boosting food production in both quality and quantity should be considered [28]. To this effect, biotechnology is a promising panacea for world food insecurity [29]. Although genetic engineering and other biotechnological approaches available for improving the desirable traits of plants and animals offer massive benefits for combating food insecurity, debate is ongoing on their biosafety and unlikely aftermath.
2. Biotechnology: A Viable Approach to Curbing Food Insecurity
Biotechnology is an integration of numerous technologies that comprises several fields of science with wide applications. In modern agriculture, biotechnology plays key roles in the advancement of animal husbandry, cropping systems, soil science, soil conservation, plant physiology, seed technology, and crop management, all of which are pivotal to improving the quality and quantity of food production. It has also helped in the production of recombinant proteins, crops that are resistant to pests and pathogens and varieties with high nutrient content, and animals that produce more milk, among other applications [30].
Although biotechnology is not the only means of crop genetic improvement, it offers a faster and more accurate technology with broader applications to improve crops compared to its alternative, i.e., conventional plant breeding. While genetic modification (GM) targets the introduction of a foreign gene into the plant genome to produce the desired traits, conventional breeding interbreeds plants with the desired characteristics over many generations and then selects individuals with such traits among the progenies [31]. In addition, genetic engineering offers opportunities for improving crops that are sterile or asexually propagated [32]. Although the use of GM is becoming increasingly popular in crop improvement, it is not aimed at completely replacing conventional breeding; both approaches are indispensable, depending on the breeder’s goal and selected crop [33].
Conventional breeding is very affordable, simple, and unrestricted by the government. The main constraint of conventional methods is the sexual procedure, especially the sexual compatibility of the organisms of interest; the desired gene might be in an unrelated species that cannot be effectively cross-pollinated with the recipient or from a non-plant species, such as genes from bacteria or insects. Another limitation is that the conventional approach cannot be used for improving sterile crops. If a valuable gene is identified in a wild relative, the conventional approach to improvement may not be suitable because performing a cross between the high-yield line and its wild relative creates a ‘mix’ of the parents’ genomes, altering the carefully chosen and delicate assemblage of desirable genes present in the high-yield line [32].
Genetic Modification of Crops: A Silver Bullet to Combat Food Insecurity
As stated in [34], among the numerous possibilities for crop improvement, genetic modification of crops deserves special attention and could be a ‘life jacket’ in this ocean of food insecurity. Genetically engineered crops have the potential to reduce food insecurity to a minimum. Genetic modification of crops emerged with considerably lofty expectations and anticipations of enabling farmers to achieve a greater and better agricultural yield, counting on its limitless potential for crop improvement. Although it is still a subject of public debate, “super plants” offer a feasible alternative for addressing world hunger [31].
Plant genetic modification has occurred in distinct phases throughout history. The earliest crops were modified for resistance or tolerance to pests, diseases, abiotic stress, spoilage, or herbicides. The second generation of modified crops targeted an increase in the yield quality or nutritional content, e.g., fortifying crops with essential vitamins and micro-minerals. The third generation of genetically modified crops will have a wider scope and limitless applications. It will be used for non-food purposes like pharmaceuticals (edible vaccines), biofuel production, and bioremediation, as well as other critical areas. Many of these are already in use today [35,36].
3. Genetically Modified Crops; A Masterpiece
According to [37], the first instance of a genetically modified crop was reported in 1983. Specifically, a tobacco plant was the first crop to undergo genetic modification with the introduction of an antibiotic-resistance gene into its genome. Generally, tobacco plants dominated early developments in plant genetic modification in the 1980s and early 1990s, with several successes reported using Agrobacterium-mediated gene transfer. In 1994, the ‘Flavr Savr’ tomato became the inaugural genetically modified crop authorized for commercial sale in the United States. It was developed for extended shelf life [38], and over time, many GM crops have been produced [39].
The development of plant-genome editing tools has revolutionized the area of plant genetic engineering by making it possible for greater precision and effective modification of plants’ genetic compositions [40]. Scientists employ genome editing as a method to explore potential genes and genetic variations that impact favorable characteristics, more effectively utilize genetic variability, and gain insight into the functioning of genes [41] (Figure 3). The technology allows the precise alteration of plant traits to introduce new characteristics into crops, such as disease resistance, drought tolerance, and improved nutritional content.
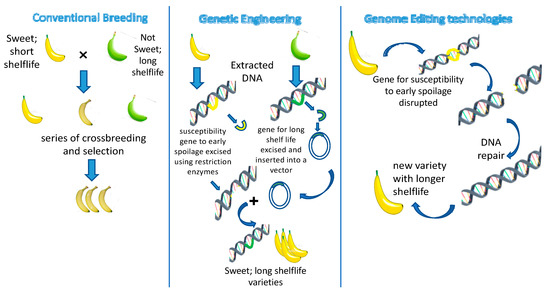
Figure 3.
The concept of conventional breeding, genetic engineering, and genome editing technologies.
Plant gene editing uses an array of novel techniques, including CRISPR/Cas9, TALENs, and Zinc-Finger Nucleases [42,43]. The CRISPR/Cas system is the most widely used gene-editing tool in plants due to its simplicity, efficiency, and versatility. Its ease of use and cost-effectiveness have opened up gene editing to a much wider range of researchers, including those in developing countries [44]. Since the first successful application of CRISPR/Cas9 in plant gene editing in 2013 [42,45], CRISPR/Cas-based gene editing has been successfully used to generate crops with improved yields, increased resistance to pests and diseases, enhanced nutrient content (Table 1), and altered plant architecture across a wide range of crops [42], including soybean [46], rice [47,48,49], wheat [50,51], maize [52], tomato [53,54,55], cassava [56], tobacco, and potato [57,58], among others. Some achievements of gene editing in crop improvement are listed in Table 1.

Table 1.
CRISPR-CAS technologies used in crop improvement.
Additionally, gene editing can be used to reduce the use of pesticides and other harmful chemicals in agriculture by introducing traits that confer natural pest and disease resistance (Table 1) [43]. Another important application of plant gene editing is the reduction or elimination of allergens in crops. Food allergies affect millions of people worldwide and can cause severe and sometimes life-threatening reactions. Gene editing offers a promising solution to this problem by allowing the targeted removal of allergenic proteins from crops without affecting their nutritional value [99,100]. Researchers have used CRISPR/Cas-based gene editing to create hypoallergenic peanuts, wheat, and soybeans, among other crops [99,101,102,103].
Despite the potential benefits of plant gene editing, there are also concerns about its safety and ethical implications [104]. Critics argue that the technology could have unintended consequences, such as off-target effects or the creation of new allergens or toxins [105]. With continued research and development, plant gene-editing technologies could help transform the future of agriculture and ensure food security for generations to come.
3.1. Developing Crop Varieties with Higher Yield and Extended Shelf Life
Plants genetically modified for enhanced yield have greater productivity and faster growth in the face of limited growth resources and adverse climatic conditions compared to their unmodified relatives. According to a meta-analysis conducted between 1996 and 2013, the yield of genetically modified crops increased by 20%. Furthermore, in developing nations, yields and producer gains are higher than in developed countries [26,35]. Pellegrino et al. [106] discovered that genetically modified maize increased yields by up to 25% and significantly reduced dangerous food contaminants in their critical analysis of over 6000 peer-reviewed publications over 21 years (1996–2016). The findings further favor the global cultivation and consumption of GM maize owing to its improved quality and grain yield, as well as reduced human exposure to mycotoxins. Researchers have estimated the increase in food production attributed to the contributions of GM crops to be about $133 billion [107,108]. More is achievable if scientists are given the liberty to modify crops in this regard.
Extending the shelf life of agricultural products is another laudable achievement of plant biotechnology, as it can significantly reduce post-harvest losses due to spoilage, which claims a sizeable percentage of agricultural products every year. Since the commencement of this endeavor, researchers have successfully released new varieties of important crops with extended shelf life, many of which are still under development [31]. This has been achieved with tomatoes and bananas. For example, the antisense targeting of genes involved in ethylene generation and sensing in tomato plants has proven to be particularly successful in delaying fruit ripening [109]. Scientists from Israel’s Agricultural Research Organization created transgenic banana plants with increased shelf life by lowering the expression of two transcriptional regulators, MaMADS1 and MaMADS2 (MADS-box genes) [110]. Furthermore, the researchers discovered that the flavor and quality of the genetically modified bananas remained unchanged. This technology can be applied to other crops to reduce food shortages caused by post-harvest spoilage, especially in countries without suitable technologies for fruit storage.
3.2. Insect Tolerance and Herbicide Resistance
Several crops have been genetically modified to resist specific insects (Table 1 and Table 2). This is mainly achieved through the heterologous expression of proteins from Bacillus thuringiensis (Bt) that exhibit toxicity toward insects possessing alkaline digestive systems. When insects consume Bt protein, their digestive systems are interrupted, resulting in slower development and, eventually, death.

Table 2.
Genetically modified crops approved by FDA for nutritional purposes in the last decade.
This observation has led to a growing interest in exploring the potential of transferring this Bt gene into plants [121,122,123]. Sweet corn, cotton, and potatoes are among the most commonly grown commercial crops that possess Bt insect resistance. This technology has helped to reduce the devastating impact of the Asian corn borer (Wilson), which causes approximately 40% yield loss in Indonesia’s maize plantations [121,123]. The introduction of new varieties of cotton that are insect-resistant has brought great relief to cotton farmers globally. For instance, according to [29], five million farmers in India are growing Bt cotton on more than 7.6 million hectares of land. This improved variety has significantly promoted cotton production in India, as well as other parts of the world. The variety protects itself from insects without the use of an external pesticide. The switch to Bt cotton has resulted in a 31% increase in output, a 39% decrease in pesticide use, and an increased profit for farmers equivalent to USD 250 per hectare. In 2017, about 100 million hectares of farmland were cultivated with Bt crops, producing Bt toxins [124].
To broaden the impact range of GE crops, further pest-tolerance techniques against a wider range of devastating insects, using innovative technologies like RNAi and other non-Bt approaches, have been introduced [125]. Plant genetic modification for insect resistance has evolved from directed immunity against a single insect order to a wider immune response with various mechanisms to combat similar or multiple insect species (Table 1 and Table 2).
Among the various genetic engineering technologies for combating insect pests, sequence-specific gene silencing using RNA interference (RNAi) has proven to be outstanding for successful pest management in agriculture. RNAi is a naturally occurring, conserved mechanism that is important for gene control and pathogen resistance. Crops are designed with a hairpin RNAi vector to generate dsRNA against the target gene of an insect pest in the plant-mediated or host-induced RNAi (HI-RNAi) technique. Upon eating plant components, dsRNA enters the insect gut, activating RNAi machinery and silencing the target gene in the insect pest. Many major successes have been recorded since its pioneering success in corn and Nicotiana tobacum in 2007 [126,127]. Jin et al. [128] engineered chloroplast dsRNA to silence target genes in the gut of Helicoverpa armigera (cotton bollworm) and impair larval development. Mamta and Rajam [129] used the HI-RNAi method to induce H. armigera resistance in tobacco and tomato plants. The host-induced RNAi (HI-RNAi) approach has gained increasing attention from researchers [130,131,132,133].
The application of genetic engineering in tackling these pests (insects) not only prevents production loss but also saves farmers money on insecticides. Klümper and Qaim [35] conducted an in-depth meta-analysis of approximately 147 published biotech crop research studies in which genetically modified (GM) crops were used to tackle insects, diseases, and weeds. The study examined the impact of GM crops from 1995 to 2014. Based on their findings, the opportunities and vast benefits of the genetic modification of crops in this regard are evident. The study also established that not only does it boost plant immunity and insect tolerance but it also reduces the usage of chemical pesticides (the majority of which are not environmentally friendly) by 37% and increases farmers’ profits by 68 percent, a position corroborated by [26]. Also, as observed in some cases where insect-resistant varieties have been implemented, for example, Bt maize and Bt cotton in the US [134,135,136] and China [137], key target pests have been significantly suppressed across the region, thus bringing relief to all farmers, including non-adopters of the GM variety.
Engineering crops for herbicide resistance (HR) allows the plants to tolerate herbicides that would otherwise kill them. In 2015, genetic engineering produced herbicide-resistant (HR) traits in crops for nine herbicides. These traits are available in several commonly cultivated crops, e.g., maize, sugar beets, cotton, soybeans, and alfalfa (Table 1 and Table 2). However, not all of these improved varieties are available for commercial production. For instance, glufosinate-resistant sugar beets have been created but have not yet been commercialized [138]. Generally, from 1996 to 2015, HR crops that were released were predominantly developed to resist a single, targeted herbicide, usually glyphosate. Glyphosate is a broad-spectrum, non-selective herbicide that suppresses the EPSPS gene, which is vital in the synthesis of aromatic amino acids [139]. Thus, glyphosate and glyphosate-resistant crops were the most prevalent herbicide–HR crop combinations that farmers employed. However, in 2015, certain crop varieties with stacked herbicide-resistance characteristics (for example, glyphosate and 2,4-D or glyphosate and dicamba) were already under development. Glyphosate resistance is now available in several other crops [140].
Transgenic technology has produced the majority of commercialized herbicide-resistant crops [141]. However, CRISPR/Cas-mediated genome editing is increasingly gaining prominence, as it provides a more efficient approach by permitting precise modifications of DNA sequences associated with herbicide resistance without the introduction of exogenous genes, thus being deemed safer [141]. Since its emergence, it has been applied to induce herbicide resistance in several crops, such as rice [142,143,144], maize [145], watermelon [146], rapeseed [147,148], wheat [149], tomato [58], and potato [58], among others (Table 1 and Table 2).
3.3. Enhanced Nutritional Content
The development of transgenic crop varieties with higher nutritional content, for example, increased protein, essential vitamins, iron content, or higher amounts of folate, can offer adequate quantities of essential micronutrients that are typically deficient in diets in the developing world [150]. These improved varieties must be made readily available and accessible to local farmers to have a meaningful effect on the nutritional well-being of a community [151,152]. In recent years, there have been notable advancements in the utilization of biotechnology to create food crops with enhanced nutritional value, such as reduced amylose, phytic acid, and gluten contents, and increased amylose, oleic acid, and Gamma-Aminobutyric Acid (GABA) contents, among others (Table 1 and Table 2). These advancements aim to reduce detrimental nutrient elements and provide essential nutrient elements, such as iron and vitamin A to consumers, especially poor populations across the globe, who are the worst hit by food insecurity [153].
Let us consider a well-known example: Golden Rice. Every year, an estimated three million children under the age of five (30% of children within the age group) are affected by vitamin A deficiency [154]. Golden Rice was created by Ingo Potyrus’ research team and provides a viable solution. This novel variety was designed using two genes harvested from two unrelated species: one from the ‘daffodil’ plant and another from the ‘Erwinia uredovoia’ bacterium [155]. Transgenic biofortified rice offers a low-cost solution to folate deficiency. Phytic acid (PA) is a recognized zinc-absorption inhibitor that is found in many grains. Today, new rice varieties, as well as a couple of cereals, have been developed with an extremely low content of this acid. These varieties were created by altering the phytic acid biosynthesis pathway. Although beneficial, these tactics are occasionally linked to some degree of reduction in yield and vigor [152,156].
As enhancing rice Fe content has proved challenging [157], significant achievements have been made using transgenic techniques [158,159]. RNAi silencing-based methods have also been used to create rice varieties with notable lysine content [160]. Another accomplishment in plant biotechnology is the development of triple-vitamin-fortified maize that has several times the usual amount of beta-carotene, ascorbate, and folate (Table 1 and Table 2) [161]. The BioCassava Plus initiative was launched to focus on cassava, a nutritionally inadequate staple crop consumed in sub-Saharan Africa. These innovations suggest fortifying maize with vitamin A [162]. Crops like this can provide considerably more nutritionally balanced meals to malnourished populations in Africa [162]. In recent years, more and more genetic modification events to improve the nutritional value, quality, and marketability of food from crops have continued to emerge (Table 2).
3.4. Plant-Derived Edible Vaccines
Edible vaccines are a novel type of vaccine produced using transgenic plants and animals. They are created by introducing genes encoding antigens or immunostimulants into the genome of plants or animals, which activate the immune system and induce an immune response [163]. Edible vaccines are easy and relatively cost-friendly to produce since they require nothing more than the plant’s basic growth requirements. They are administered orally, eliminating the occurrence of injection-related complications [164,165]. Like standard vaccines, edible vaccines include antigens rather than whole pathogen-forming genes. Antigens derived from several diseases can be produced in large quantities and their natural forms in plants. Plants can provide many antigens for recurrent inoculations since they can generate more than one transgene [165].
Edible vaccines have been fostered by the success of oral vaccines [166]. Edible vaccines require less refrigeration and can be stored at room temperature, making them more readily accessible and significantly more affordable, especially for developing countries. The difficulties in developing an edible vaccine include dosing, antigen selection, and distinguishing it from non-engineered varieties to prevent the wrong vaccination [165]. Plant-derived vaccines have been developed to protect against diseases such as diarrhea, enteritis, measles, hepatitis, cholera, malaria, and Newcastle disease, among others (Table 3).

Table 3.
A sample of achievements in GM plant-derived vaccines.
The idea of edible vaccines was first featured in 1990 in a patent application after the discovery of a surface protein from Streptococcus in tobacco plants that could help the body develop antibodies against the pathogen [181]. Shortly afterward, Arntzen and his team developed the first successful edible vaccine using genetically modified tomatoes, which expressed a protein from the virus that causes diarrhea in pigs [182]. Since then, several significant achievements have been reported in potatoes, rice, spinach, carrots, and bananas, among others (Table 3). In 1995, researchers at the John Innes Centre in the UK developed an edible vaccine for Hepatitis B using transgenic potatoes [183]. In 1999, a team of researchers developed an edible vaccine for the Norwalk virus, which causes gastroenteritis, using transgenic potatoes [184]. Another significant achievement was made in 2005 when scientists at the University of Tokyo developed an edible vaccine for Influenza A using genetically modified rice [185]. These accomplishments demonstrate the potential of edible vaccines as an alternative to traditional vaccines.
Embracing plant-derived vaccines will contribute immensely to public health by making vaccine production cheaper and more accessible, especially in underdeveloped nations. For the same cost, 1000 times more individuals would be reached with edible vaccines than would be through usual vaccination. Moreover, syringes and medical kits are not required [165].
4. Global Acceptance and Perspectives on Biotechnologically Developed Crops
The year 2023 marks the 28th year of biotech food commercialization. About 17 million farmers in 45 nations with GM crop approvals, largely in poor countries, cultivate GM crops throughout the world [186]. Almost every sector of the food industry is adopting this technology. Since 1994, at least 45 nations have received regulatory clearance for GM crops. Among these nations, Japan and the United States have had the most GM events approved [125,152,186]. Several genetically modified crops are currently being commercially cultivated around the world. These include potatoes and alfalfa in the USA, aubergine in Bangladesh, sugar beet in the USA and Canada, and papaya in the USA and China. Other crops that have been genetically modified and are being grown commercially in multiple countries include rapeseed oil in four countries, maize in seventeen countries, soybeans in eleven countries, and cotton in fifteen countries. Among these, the top five biotech plants that are cultivated on the largest areas of land are soybeans, covering 95.9 million hectares; maize, covering 58.9 million hectares; cotton, covering 24.9 million hectares; canola, covering 10.1 million hectares; and alfalfa, covering 1.3 million hectares. Between 1992 and 2016, significant success has been achieved in the genetic modification of crops, especially maize, which is a global staple food. A total of 148 improved varieties of maize have been developed, followed by cotton (58 events), potato (45 events), soybean (34 events), canola (38 events), and dozens of other crops (Figure 4). These crops have been significantly improved for abiotic stress tolerance, better yield, and resistance to diseases, herbicides, insects, and nematodes [125,186]. Over two decades since the introduction of GM crops, many countries across North America, Asia, Africa, and South America have begun commercial cultivation of several GM crop varieties to enhance their food production (Table 4). However, only one event (maize) has been commercially cultivated across Europe, reflecting the continent’s cold receptivity to GM varieties.
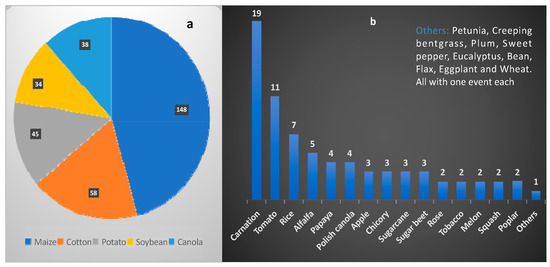
Figure 4.
(a,b) Approved biotech crop events globally (1992–2016). (a) Crops with more than 30 events. (b) Crops with less than 30 events [125,186].

Table 4.
Commercial cultivation of genetically modified crops by country (2015) [125,187].
4.1. Europe and Africa
As described in [188], the European Union has resisted GM technology since its inception, raising concerns about possible ecological and health impacts. The EU enacted stringent regulations and legislation to restrict the use of genetically modified organisms (GMOs) within its borders. Public acceptance of genetically modified crops in Europe is also relatively low. As of 2018, of the 4349 GM events registered and approved across countries around the globe, only 4.6 percent were allowed in the EU (199 events for food and feed and 3 maize events for cultivation). In 2018, only two countries (Spain and Portugal) cultivated GM maize varieties. It is also worth mentioning that plants that are transformed using wild-type Agrobacterium sp. (the so-called ‘naturally transgenic plants’) could potentially not be subject to EU GMO legislation because they do not contain recombinant DNA elements.
In Africa, genetically modified crops designed to tackle inadequate food production and macronutrient deficiencies are needed to help the continent overcome its nutrition challenges and make agriculture more profitable for farmers [31]. Nevertheless, African nations exercise caution in accepting GM varieties for commercial production owing to ethical issues about the crops. However, the number of nations cultivating developed varieties doubled from an initial three in 2018 to six a year later. Three African nations have approved GM cotton and maize for commercial production (South Africa, Burkina Faso, and Sudan), while several nations are conducting field experiments on selected GM varieties for their commercialization. According to [125,189], South Africa is among the top developing nations cultivating GM crops, with almost 6.7 million acres planted in 2019 with GM maize, soybeans, and cotton. Every year, there is a constant increase in the number of hectares of land cultivated with GM cultivars in South Africa. GM varieties are becoming increasingly popular in the country, accounting for more than 80% of the total crops planted.
Approximately 150,000 Sudanese farmers planted over 580,000 acres of GM cotton during the same year. Eleven additional nations—Burkina Faso, Ethiopia, Ghana, Kenya, Malawi, Mozambique, Nigeria, South Africa, Swaziland, Tanzania, and Uganda—have been undertaking field experiments on ten GM crops, with sixteen features related to abiotic stress tolerance (drought and salinity), nitrogen utilization efficiency, and increased nutritional content [152]. New GM crops, such as pest-resistant cowpea and cotton, are also being researched and developed to benefit poor Nigerian farmers [31].
Despite Africa’s increasing openness to GMOs, they remain outlawed in Algeria and Madagascar, with strict laws against their cultivation and importation. Although GM crop cultivation, research, and development are permitted in Asia to address food and nutrient shortages, Turkey, Kyrgyzstan, Bhutan, and Saudi Arabia have implemented stringent regulations against GM crops. In the Americas, the USA is a strong voice for GMOs, with significant support for their development; however, GMOs are banned in Peru, Belize, Venezuela, and Ecuador.
4.2. GMO-Free CRISPR/Cas9 Crops—Global Thoughts and Acceptance
CRISPR/Cas9 technology has emerged as a potent genome-editing tool, with diverse applications for crop improvement and agricultural sustainability (Table 1). Global thoughts on GMO-free CRISPR/Cas9 crops range from appreciating the enormous prospects for crop enhancement and agricultural sustainability to addressing concerns about regulation, safety, and public perception.
The regulatory framework for CRISPR/Cas9 crops varies by country and region. Different jurisdictions have used different methods to regulate genetically modified organisms (GMOs) and genetically modified crops [190]. Some nations, such as the United States, have adopted a product-based strategy, emphasizing the end product’s characteristics rather than the process adopted to develop it. In such regions, if the modified crop does not contain foreign DNA or has qualities that could not be acquired through traditional breeding, it may not be subject to the same regulatory restrictions as transgenic GMOs [191].
In contrast, other countries, such as those in the European Union, have adopted a more stringent process-based regulatory framework that treats gene-edited crops as GMOs, subjecting them to similar regulations and safety assessments as transgenic crops. This approach considers the process of genome editing as a form of genetic modification, irrespective of the presence of foreign DNA [190,192]. However, in recent times, there have been calls for a review of the current regulations in Europe to differentiate between genome-edited crops and transgenic GMOs, considering the precise and targeted nature of the former. This is now being debated in the EU scientific community [193,194,195,196,197].
4.3. Opposition to Global Acceptance of Biotech Crops
The adoption of GM crops for commercial production is highly disputed due to their uncertain consequences [26,27]. Nonetheless, GMO technology has expanded enormously across the world, and many poor countries see GM foods as an answer to increasing agricultural yield and attaining national food security. The environmental effect of genetically modified organisms should also be assessed in their adoption for achieving food security in poor nations. Opposition to GM crops is centered on concerns about biosafety and the possibility of altering the delicate balance of the ecosystem [189,198]. Thus, all GM crops must go through a rigorous and detailed risk assessment to evaluate their potential detrimental effects on humans and the environment. These restrictions are based on a thorough examination of the crop’s molecular characterization, an evaluation of the crop’s potential for toxicity and its potential allergen response, and a nutritional study of the crop. Researchers often disagree on the long-term consequences of GMO crops on human health and the environmental [26]. It was suggested in [5] that it appears that some businesses in the West are abusing the technology, using it to enhance crop appearances to the detriment of essential nutrients and safety for human consumption.
It has also been argued that using genetically modified crops could have a deteriorating impact on the economy of a nation, subjecting local farmers to predation by corporate monopolies because companies that produce the seeds will sell them at high prices that peasant farmers cannot afford, encouraging large-scale farmers (who can afford the expensive seeds) to dominate over the diversity contributed by small farmers who cannot afford the technology. Also, small-scale farmers could have limited access to seeds due to the patenting of the engineered varieties. However, several studies have proved this wrong. More than 90% of farmers who planted genetically modified crops were small-scale, impoverished farmers in developing nations [199]. Governments can still subsidize the price of GM seeds and put a price ceiling on the seeds to reach more peasant farmers.
Another unforeseen environmental consequence is the possibility of the transmission of herbicide-resistance genes to weeds or other wild plants. This is possible if the weeds are closely related and could cross-pollinate. These wild crops could acquire the herbicide-resistance gene over time, becoming ‘super-weeds’, thus creating more problems for farmers. Genetically modified crops may fail to germinate, kill microorganisms that are helpful to plants, deplete soil fertility, and potentially impart insecticidal or viral resistance to wild relatives of the crop species [29]. An instance of the unpredictable aftermath of GM varieties on the environment is Bt maize, which was engineered with a new gene to produce pollen that has toxins against the European corn borer; however, over time, pollen from Bt maize was dispersed by the wind, landing on milkweed on which the monarch butterfly feeds, and killing the caterpillars that feed on it. This accounted for the obvious decline in the Monarch butterfly population [200].
The acceptance of GM crops remains controversial due to varying regulatory approaches, public perceptions, and cultural factors. As technology advances and global conversations continue, it is essential to engage stakeholders, bridge the gap in public understanding, and establish regulatory frameworks that balance innovation, safety, and public trust.
4.4. Contributions to Food Security and Human Health
GM crops have proven their significance in increasing food production and quality. They have also enhanced farmers’ incomes, especially smallholder farmers, who constitute the vast majority of undernourished people globally, thus improving their standard of living [201]. A survey conducted in 2018 revealed that farmers in underdeveloped nations earned USD 4.42 in return for each USD 1 spent on procuring engineered seeds [202]. GM crops are a great relief to farmers worldwide, not only in economic terms but also by contributing to their psychological and physiological well-being. With the introduction of GM crops, farmers can now farm with more confidence that their crops will not fail due to insect attacks, unpredictable climatic conditions, or weeds. Moreover, they are assured of optimum yields at reduced farming costs. This was substantiated by Gruère and Sengupta [203] in their research on the impact of Bt cotton in India, which showed a 25% decrease in the annual suicide rate among Indian farmers only a year after it was commercialized. GM crops are beneficial not only to farmers but also to the entire population. Biofortified staple varieties have significantly improved nutrient availability, promoting public health and preventing or treating several killer diseases, e.g., diabetes, cancer, hypertension, and immune disorders. Moreover, plant-derived vaccines strengthen their force in disease prevention. Bt maize has been proven to contain significantly lower concentrations of mycotoxins, which are toxic and carcinogenic to humans [106]. The consumption of foods with a balanced nutrient composition provides health benefits that last a lifetime. It may yet be too early to quantify these benefits until several decades from now, especially in developing countries where the majority of their nutrient intake is plant-derived [204].
5. Conclusions
Farmers throughout the world have enjoyed major benefits from growing GM crops, including improved yields and reduced production costs. The replanting rate for genetically modified cultivars in fields where they were introduced is about 100%, which indicates that this innovative technology is a top choice, and its performance is satisfactory. GM crops significantly aid in the alleviation of poverty, improving the livelihood of agricultural families across the globe (estimated at 65 million people). Giving more farmers access to genetically modified seeds is necessary for a country that aims to expand its domestic crop production. Higher agricultural yields and plant-mediated vaccinations can contribute to food and health security in developing nations. Edible vaccines have proven to have several advantages over usual vaccinations, including easy administration, decreased manufacturing costs, and the absence of injection-related complications. Embracing plant-derived vaccines would revolutionize global immunization and the vaccine delivery system, with endless prospects. Also, with the development of GM crops with higher agricultural yields, green revolutions may become a reality.
However, regional policies pose an obstacle to the global acceptance of GM crops. Governments with GM bans should consider removing restrictions on GM imports to meet their populations’ nutritional (quality) and food-quantity demands. GM foods will help reduce our dependency on food imports from other countries while also greatly increasing domestic agricultural production. Biotech crops are promising, and much more could be achieved if the limiting policies and metered bans are lifted on the commercialization of biotech varieties. Indeed, biotech crops are the pill the world needs to overcome food insecurity!
Author Contributions
Conceptualization, P.A.A., L.B. and D.A.A.; validation, D.A.A., Y.S. and L.B.; formal analysis, Y.S.; investigation, P.A.A., D.A.A., Y.S. and L.B.; resources, D.A.A., F.O.E.; writing—original draft preparation, P.A.A.; writing—review and editing, P.A.A., D.A.A., Y.S. and L.B.; funding acquisition, L.T., Y.S. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation within the framework of the federal program “School of Advanced Engineering Studies”, № 075-15-2022-1143 from 7 July 2022.
Data Availability Statement
The datasets are contained within the article.
Acknowledgments
We wish to thank the Russian Academy of Sciences, Far Eastern Branch, Vladivostok, for their support throughout the study. The authors are also grateful to the Instrumental Center for Biotechnology and Gene Engineering at the Federal Scientific Center of East Asia Terrestrial Biodiversity of the Far East Branch of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Basonde, R.; Andhare, P. GM crops is a solution for world food crisis? Asian J. Microbiol. Biotechnol. Environ. Sci. 2014, 17, 163–166. [Google Scholar]
- Bickel, G.; Nord, M.; Price, C.; Hamilton, W.; Cook, J. Guide to Measuring Household Food Security; USDA Food and Nutrition Service: Alexandria, VA, USA, 2000; p. 82. [Google Scholar]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- World Food Programme. A Global Food Crisis. 2022. Available online: www.wfp.org/global-hunger-crisis (accessed on 26 June 2023).
- Habibi-Najafi, M.B.; Lee, B.H. Biotechnology and its Impact on Food Security and Safety. Curr. Nutr. Food Sci. 2014, 10, 94–99. [Google Scholar] [CrossRef]
- Webb, P.; Stordalen, G.A.; Singh, S.; Wijesinha-Bettoni, R.; Shetty, P.; Lartey, A. Hunger and malnutrition in the 21st century. BMJ 2018, 361, k2238. [Google Scholar] [CrossRef]
- Militao, E.M.A.; Salvador, E.M.; Uthman, O.A.; Vinberg, S.; Macassa, G. Food Insecurity and Health Outcomes Other than Malnutrition in Southern Africa: A Descriptive Systematic Review. Int. J. Environ. Res. Public. Health. 2022, 21, 5082. [Google Scholar] [CrossRef]
- GNAFC; FSIN. 2020 Global Report on Food Crisis. 2020. Available online: https://www.fsinplatform.org/sites/default/files/resources/files/GRFC_2020_KM_200420.pdf (accessed on 6 July 2023).
- Clark, H.; Coll-Seck, A.M.; Banerjee, A.; Peterson, S.; Dalglish, S.L.; Ameratunga, S.; Balabanova, D.; Bhan, M.K.; Bhutta, Z.A.; Borrazzo, J.; et al. A future for the world’s children? A WHO–UNICEF–Lancet Commission. Lancet 2020, 395, 605–658. [Google Scholar] [CrossRef]
- Govender, I.; Rangiah, S.; Kaswa, R.; Nzaumvila, D. Malnutrition in children under the age of 5 years in a primary health care setting. S. Afr. Fam. Pract. 2021, 63, e1–e6, Erratum in S. Afr. Fam. Pract. 2021, 63, 5416. [Google Scholar] [CrossRef]
- IFPRI. 2015 Annual Report: International Food Policy Research Institute (IFPRI). 2016. Available online: http://ebrary.ifpri.org/cdm/ref/collection/p15738coll2/id/130442 (accessed on 1 July 2023).
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022: Re-Purposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022; 260p, ISBN 978-92-5-136499-4. [Google Scholar] [CrossRef]
- Coleman-Jensen, A.; Rabbitt, M.P.; Gregory, C.A.; Singh, A. Household Food Security in the United States in 2020; USDA, Economic Research Service: Washington, DC, USA, 2021; 56p. Available online: https://www.ers.usda.gov/webdocs/publications/102076/err-298.pdf (accessed on 2 July 2023).
- SWAC/OECD. Food and Nutrition Crisis 2020: Analyses & Responses; Maps & Facts; OECD: Paris, France, 2020; No. 3; 56p, Available online: https://www.oecd.org/swac/maps/Food-nutrition-crisis-2020-Sahel-West-Africa_EN.pdf (accessed on 22 June 2023).
- Swaminathan, M.S. Science in response to basic human needs. Science 2000, 284, 425. [Google Scholar]
- Tonukari, N.J.; Omotor, D.G. Biotechnology and food security in developing countries. Biotechnol. Mol. Biol. Rev. 2010, 5, 13–23. [Google Scholar]
- Habibi-Najafi, M.B. Food Biotechnology and its impact on our food supply. J. Biochem. Biotechnol. 2006, 1, 22–27. [Google Scholar]
- Peng, W.; Berry, E.M. The Concept of Food Security. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Cambridge, MA, USA, 2019; Volume 2, pp. 1–7. ISBN 9780128126875. [Google Scholar]
- United Nations. Report of the World Food Conference, Rome, 5–16 November 1974. 1975. Available online: https://digitallibrary.un.org/record/701143?ln=en (accessed on 30 June 2023).
- FAO. World Food Security: A Reappraisal of the Concepts and Approaches: Director General’s Report. 1983. Available online: https://www.fao.org/3/AK626E/ak626e08.htm (accessed on 30 June 2023).
- World Bank. Poverty and Hunger: Issues and Options for Food Security in Developing Countries. 1986. Available online: https://documents1.worldbank.org/curated/en/166331467990005748/pdf/multi-page.pdf (accessed on 30 June 2023).
- FAO. Rome Declaration on Food Security and World Food Summit Plan of Action. 1996. Available online: https://www.fao.org/3/w3548e/w3548e00.htm (accessed on 22 June 2023).
- FAO. Declaration of the World Food Summit on Food Security. 2009. Available online: https://www.fao.org/fileadmin/templates/wsfs/Summit/Docs/Final_Declaration/WSFS09_Declaration.pdf (accessed on 30 June 2023).
- Chen, R.S.; Kates, R.W. World food security: Prospects and trends. Food Policy 1994, 19, 192–208. [Google Scholar] [CrossRef]
- Gil, R. Addressing Food Insecurity in SLP with a Food Security Task Force. Seed Feeds. 2021. Available online: https://seedsfeeds.org/programs-resources-news/addressing-food-insecurity (accessed on 30 June 2023).
- Khan, F.; Hasan, A. Genetically Modified Organisms: A Solution to Food Security and Environment. Int. J. Soc. Sci. 2016, 6, 1–12. [Google Scholar]
- Lamichhane, S.A. Genetically Modified Foods- Solution for food security. Int. J. Genet. Eng. Biotechnol. 2014, 5, 43–48. [Google Scholar]
- Reddy, P.B. Framing GM crops as a solution for Global Food Security. Int. J. Res. Granthaalayah 2015, 3, 1–5. [Google Scholar]
- Jamil, K. Biotechnology, a Solution to Hunger: UN Chronicle. 2012. Available online: www.un.org/en/chronicle/article/biotechnology-solution-hunger (accessed on 20 June 2023).
- Verma, A.S.; Agrahari, S.; Rastogi, S.; Singh, A. Biotechnology in the Realm of History. J. Pharm. Bioallied Sci. 2011, 3, 321–323. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Azeez, M.A.; Adubi, A.O.; Durodola, F.A.; Morakinyo, J.A. Trends in genetically modified crops development in Nigeria: Issues and challenges. In Genetically Modified and Irradiated Food Controversial Issues: Facts Versus Perceptions; Andersen, V., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2020; pp. 131–150. [Google Scholar]
- Then, C.; Österreicher, A. Differences between Conventional Breeding and Genetic Engineering: An Assessment of the Statement Made by the Group of Chief Scientific Advisors’ (SAM). TESTBIOTECH. 8pp. 2019. Available online: https://www.testbiotech.org/en/content/differences-between-conventional-breeding-and-genetic-engineering-sam (accessed on 15 June 2023).
- Manshardt, R. Crop Improvement by Conventional Breeding or Genetic Engineering: How Different Are They? Biotechnology 2004, 5, 3. [Google Scholar]
- Jamil, S.; Shahzad, R.; Ahmad, S.; Fatima, R.; Zahid, R.; Anwar, M.; Iqbal, M.Z.; Wang, X. Role of Genetics, Genomics, and Breeding Approaches to Combat Stripe Rust of Wheat. Front. Nutr. 2020, 7, 580715. [Google Scholar] [CrossRef]
- Klümper, W.; Qaim, M. A meta-analysis of the impacts of genetically modified crops. PLoS ONE 2014, 9, e111629. [Google Scholar] [CrossRef]
- Animasaun, D.A.; Adedibu, P.A.; Joseph, G.G. Exploring the potentials of microalgae as an alternative source of renewable energy. Niger. J. Biotechnol. 2021, 38, 24–39. [Google Scholar] [CrossRef]
- Lemaux, P.G. Genetically Engineered Plants and Foods: A Scientist’s Analysis of the Issues (Part I). Annu. Rev. Plant Biol. 2008, 59, 771–812. [Google Scholar]
- Bruening, G.; Lyons, J.M. The case of the FLAVR SAVR tomato. Calif. Agric. 2000, 54, 6–7. [Google Scholar] [CrossRef]
- Shetty, M.J.; Chandan, K.; Krishna, H.C.; Aparna, G.S. Genetically Modified Crops: An Overview. J. Pharmacogn. Phytochem. 2018, 7, 2405–2410. [Google Scholar]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision genome editing in plants: State-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Ahmad, M. Plant breeding advancements with “CRISPR-Cas” genome editing technologies will assist future food security. Front. Plant Sci. 2023, 14, 1133036. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Xu, J.; Sui, C.; Wei, J. Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 2017, 7, 292–302. [Google Scholar] [CrossRef]
- Yin, K.; Qiu, J. Genome editing for plant disease resistance: Applications and perspectives. Phil. Trans. R. Soc. 2019, 374, 2018032220180322. [Google Scholar] [CrossRef]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 21, 353–361. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, K.; Wang, C.; Zhang, Z.; Zheng, C.; Zhao, Y.; Zheng, Y.; Liu, C.; An, Y.; Shi, L.; et al. Multistage delivery nanoparticle facilitates efficient CRISPR/dCas9 activation and tumor growth suppression in vivo. Adv. Sci. 2019, 6, 1801423. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.B.; Xing, A.; Moon, B.P.; Koellhoffer, J.P.; Huang, L.; Ward, R.T.; Clifton, E.; Falco, S.C.; Cigan, A.M. Cas9-Guide RNA directed genome editing in soybean. Plant Physiol. 2015, 169, 960–970. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Y.; Qin, R.; Li, H.; Qiu, C.; Li, L.; Wei, P.; Yang, J. Rapid improvement of grain weight via highly efficient CRISPR/Cas9-mediated multiplex genome editing in rice. J. Genet. Genom. 2016, 43, 529. [Google Scholar]
- Dong, O.X.; Yu, S.; Jain, R.; Zhang, N.; Duong, P.Q.; Butler, C.; Li, Y.; Lipzen, A.; Martin, J.A.; Barry, K.W.; et al. Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR-Cas9. Nat Commun. 2020, 11, 1178. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.; Zhang, C.; Chu, R.; Gu, Z.; Tan, H.; Zhao, D.; Fan, X.; Liu, Q. Creating novel Wx alleles with fine-tuned amylose levels and improved grain quality in rice by promoter editing using CRISPR/Cas9 system. Plant Biotechnol. J. 2020, 18, 2164–2166. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; He, F.; Akhunova, A.; Chao, S.; Trick, H.; Akhunov, E. Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Tian, B.; He, F.; Chen, Y.; Bai, G.; Akhunova, A.; Trick, H.N.; Akhunov, E. Gene editing of the wheat homologs of TONNEAU1-recruiting motif encoding gene affects grain shape and weight in wheat. Plant J. 2019, 100, 251–264. [Google Scholar] [CrossRef]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genom. 2016, 43, 25–36. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Stigliani, A.L.; Giorio, G. CRISPR/Cas9 editing of carotenoid genes in tomato. Transgenic Res. 2018, 27, 367–378. [Google Scholar] [CrossRef]
- Wang, D.; Samsulrizal, N.H.; Yan, C.; Allcock, N.S.; Craigon, J.; Blanco-Ulate, B.; Ortega-Salazar, I.; Marcus, S.E.; Bagheri, H.M.; Fons, L.P. Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 2019, 179, 544–557. [Google Scholar]
- Yuste-Lisbona, F.J.; Fernández-Lozano, A.; Pineda, B.; Bretones, S.; Ortíz-Atienza, A.; García-Sogo, B.; Müller, N.A.; Angosto, T.; Capel, J.; Moreno, V.; et al. ENO regulates tomato fruit size through the floral meristem development network. Proc. Natl. Acad. Sci. USA 2020, 117, 8187–8195. [Google Scholar] [CrossRef]
- Bull, S.E.; Seung, D.; Chanez, C.; Mehta, D.; Kuon, J.-E.; Truernit, E.; Hochmuth, A.; Zurkirchen, I.; Zeeman, S.C.; Gruissem, W. Accelerated ex situ breeding of GBSS-and PTST1-edited cassava for modified starch. Sci. Adv. 2018, 4, eaat6086. [Google Scholar] [CrossRef]
- Tuncel, A.; Corbin, K.R.; Ahn-Jarvis, J.; Harris, S.; Hawkins, E.; Smedley, M.A.; Harwood, W.; Warren, F.J.; Patron, N.J.; Smith, A.M. Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol. J. 2019, 17, 2259–2271. [Google Scholar] [CrossRef]
- Veillet, F.; Chauvin, L.; Kermarrec, M.-P.; Sevestre, F.; Merrer, M.; Terret, Z.; Szydlowski, N.; Devaux, P.; Gallois, J.-L.; Chauvin, J.-E. The solanum tuberosum GBSSI gene: A target for assessing gene and base editing in tetraploid potato. Plant Cell Rep. 2019, 38, 1065–1080. [Google Scholar] [CrossRef]
- Zsögön, A.; Čermák, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L.E.P. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar]
- Rodríguez-Leal, D.; Lemmon, Z.H.; Man, J.; Bartlett, M.E.; Lippman, Z.B. Engineering quantitative trait variation for crop improvement by genome editing. Cell 2017, 171, 470–480. [Google Scholar] [PubMed]
- Lemmon, Z.H.; Reem, N.T.; Dalrymple, J.; Soyk, S.; Swartwood, K.E.; Rodriguez-Leal, D.; Van Eck, J.; Lippman, Z.B. Rapid improvement of domestication traits in an orphan crop by genome editing. Nat. Plants 2018, 4, 766–770. [Google Scholar]
- Zhou, J.; Xin, X.; He, Y.; Chen, H.; Li, Q.; Tang, X.; Zhong, Z.; Deng, K.; Zheng, X.; Akher, S.A. Multiplex QTL editing of grain-related genes improves yield in elite rice varieties. Plant Cell Rep. 2019, 38, 475–485. [Google Scholar]
- Shen, L.; Wang, C.; Fu, Y.; Wang, J.; Liu, Q.; Zhang, X.; Yan, C.; Qian, Q.; Wang, K. QTL editing confers opposing yield performance in different rice varieties. J. Integr. Plant Biol. 2018, 60, 89–93. [Google Scholar]
- Zhao, D.-S.; Li, Q.-F.; Zhang, C.-Q.; Zhang, C.; Yang, Q.-Q.; Pan, L.-X.; Ren, X.-Y.; Lu, J.; Gu, M.-H.; Liu, Q.-Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Commun. 2018, 9, 1240. [Google Scholar]
- Vu, T.V.; Sivankalyani, V.; Kim, E.-J.; Doan, D.T.H.; Tran, M.T.; Kim, J.; Sung, Y.W.; Park, M.; Kang, Y.J.; Kim, J.-Y. Highly efficient homology-directed repair using CRISPR/Cpf1-geminiviral replicon in tomato. Plant Biotechnol. J. 2020, 18, 2133–2143. [Google Scholar]
- Ben Shlush, I.; Samach, A.; Melamed-Bessudo, C.; Ben-Tov, D.; Dahan-Meir, T.; Filler-Hayut, S.; Levy, A.A. CRISPR/Cas9 induced somatic recombination at the CRTISO locus in tomato. Genes 2021, 12, 59. [Google Scholar]
- Deng, L.; Wang, H.; Sun, C.; Li, Q.; Jiang, H.; Du, M.; Li, C.-B.; Li, C. Efficient generation of pink-fruited tomatoes using CRISPR/Cas9 system. J. Genet. Genom. 2018, 45, 51–54. [Google Scholar]
- Watanabe, K.; Oda-Yamamizo, C.; Sage-Ono, K.; Ohmiya, A.; Ono, M. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4. Transgenic Res. 2018, 27, 25–38. [Google Scholar]
- Yu, J.; Tu, L.; Subburaj, S.; Bae, S.; Lee, G.-J. Simultaneous targeting of duplicated genes in Petunia protoplasts for flower color modification via CRISPR-Cas9 ribonucleoproteins. Plant Cell Rep. 2020, 40, 1037–1045. [Google Scholar] [PubMed]
- Zhong, Y.; Blennow, A.; Kofoed-Enevoldsen, O.; Jiang, D.; Hebelstrup, K.H. Protein Targeting to Starch 1 is essential for starchy endosperm development in barley. J. Exp. Bot. 2019, 70, 485–496. [Google Scholar] [PubMed]
- Sun, Y.; Jiao, G.; Liu, Z.; Zhang, X.; Li, J.; Guo, X.; Du, W.; Du, J.; Francis, F.; Zhao, Y.; et al. Generation of high-amylose rice through CRISPR/Cas9-mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. 2017, 8, 298. [Google Scholar] [PubMed]
- Abe, K.; Araki, E.; Suzuki, Y.; Toki, S.; Saika, H. Production of high oleic/low linoleic rice by genome editing. Plant Physiol. Biochem. 2018, 131, 58–62. [Google Scholar]
- Okuzaki, A.; Ogawa, T.; Koizuka, C.; Kaneko, K.; Inaba, M.; Imamura, J.; Koizuka, N. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol. Biochem. 2018, 131, 63–69. [Google Scholar]
- Jiang, W.Z.; Henry, I.M.; Lynagh, P.G.; Comai, L.; Cahoon, E.B.; Weeks, D.P. Significant enhancement of fatty acid composition in seeds of the allohexaploid, Camelina sativa, using CRISPR/Cas9 gene editing. Plant Biotechnol. J. 2017, 15, 648–657. [Google Scholar]
- Sashidhar, N.; Harloff, H.J.; Potgieter, L.; Jung, C. Gene editing of three BnITPK genes in tetraploid oilseed rape leads to significant reduction of phytic acid in seeds. Plant Biotechnol. J. 2020, 18, 2241–2250. [Google Scholar]
- Khan, M.S.S.; Basnet, R.; Islam, S.A.; Shu, Q. Mutational analysis of OsPLDα1 reveals its involvement in phytic acid biosynthesis in rice grains. J. Agric. Food Chem. 2019, 67, 11436–11443. [Google Scholar]
- Sanchez-Leon, S.; Gil-Humanes, J.; Ozuna, C.V.; Gimenez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar]
- Nonaka, S.; Arai, C.; Takayama, M.; Matsukura, C.; Ezura, H. Efficient increase of ɣ-aminobutyric acid (GABA) content in tomato fruits by targeted mutagenesis. Sci. Rep. 2017, 7, 7057. [Google Scholar]
- Akama, K.; Akter, N.; Endo, H.; Kanesaki, M.; Endo, M.; Toki, S. An in vivo targeted deletion of the calmodulin-binding domain from rice glutamate decarboxylase 3 (OsGAD3) increases γ-aminobutyric acid content in grains. Rice 2020, 13, 20. [Google Scholar] [PubMed]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.S.; Huang, S.; Liu, S.; Vera Cruz, C.; Frommer, W.B.; et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015, 82, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum Resistance to Bacterial Blight in Rice Using Genome Editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Santillan Martinez, M.I.; Bracuto, V.; Koseoglou, E.; Appiano, M.; Jacobsen, E.; Visser, R.G.F.; Wolters, A.M.A.; Bai, Y. CRISPR/Cas9-targeted Mutagenesis of the Tomato Susceptibility Gene PMR4 for Resistance against Powdery Mildew. BMC Plant Biol. 2020, 20, 284. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Solano, R. Design of a Bacterial Speck Resistant Tomato by CRISPR/Cas9-mediated Editing of SlJAZ2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef]
- Gomez, M.A.; Lin, D.Z.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous CRISPR/Cas9-Mediated Editing of Cassava eIF4E Isoforms nCBP-1 and nCBP-2 Reduces Cassava Brown Streak Disease Symptom Severity and Incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef]
- Baltes, N.J.; Hummel, A.W.; Konecna, E.; Cegan, R.; Bruns, A.N.; Bisaro, D.M.; Voytas, D.F. Conferring resistance to geminiviruses with the CRISPR–Cas prokaryotic immune system. Nat. Plants 2015, 1, 15145. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, H.; Zhang, Y.; Wang, Y.; Gao, C. Establishing a CRISPRCas-like immune system conferring DNA virus resistance in plants. Nat. Plants 2015, 1, 15144. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Liu, W.; Gao, W.; Liu, C.; Song, G.; Li, W.X.; Mao, L.; Chen, B.; Xu, Y.; et al. An alternative strategy for targeted gene replacement in plants using a dual-sgRNA/Cas9 design. Sci. Rep. 2016, 6, 23890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced drought tolerance by CRISPR/Cas9-mediated SlMAPK3 mutagenesis in tomato plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Yin, Y.; Qin, K.; Song, X.; Zhang, Q.; Zhou, Y.; Xia, X.; Yu, J. BZR1 Transcription Factor Regulates Heat Stress Tolerance through FERONIA Receptor-like Kinase-Mediated Reactive Oxygen Species Signaling in Tomato. Plant Cell Physiol. 2018, 59, 2239–2254. [Google Scholar] [CrossRef]
- Oz, M.T.; Altpeter, A.; Karan, R.; Merotto, A.; Altpeter, F. CRISPR/Cas9-Mediated Multi-Allelic Gene Targeting in Sugarcane Confers Herbicide Tolerance. Front. Genome Ed. 2021, 3, 673566. [Google Scholar] [CrossRef]
- Mao, X.; Zheng, Y.; Xiao, K.; Wei, Y.; Zhu, Y.; Cai, Q.; Chen, L.; Xie, H.; Zhang, J. OsPRX2 contributes to stomatal closure and improves potassium deficiency tolerance in rice. Biochem. Biophys. Res. Commun. 2018, 495, 461–467. [Google Scholar] [CrossRef]
- Cordones, M.N.; Mohamed, S.; Tanoi, K.; Natsuko Kobayashi, N.I.; Takagi, K.; Vernet, A.; Guiderdoni, E.; Perin, C.; Sentenac, H.; Very, A.A. Production of low-Cs + rice plants by inactivation of the K + transporter OsHAK1 with the CRISPR-Cas system. Plant J. 2017, 92, 43–56. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, T.; Chen, C.H.; Li, W.; Meyer, C.A.; Wu, Q.; Wu, D.; Cong, L.; Zhang, F.; Liu, J.S.; et al. Sequence determinants of improved CRISPR sgRNA design. Genome Res. 2015, 25, 1147–1157. [Google Scholar] [CrossRef]
- Brackett, N.F.; Pomés, A.; Chapman, M.D. New Frontiers: Precise Editing of Allergen Genes Using CRISPR. Front. Allergy 2022, 17, 821107. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, F.; Zhang, J.; Liu, H.; Rahman, S.; Islam, S.; Ma, W.; She, M. Application of CRISPR/Cas9 in Crop Quality Improvement. Int. J. Mol. Sci. 2021, 22, 4206. [Google Scholar] [CrossRef] [PubMed]
- Camerlengo, F.; Frittelli, A.; Sparks, C.; Doherty, A.; Martignago, D.; Larré, C.; Lupi, R.; Sestili, F.; Masci, S. CRISPR-Cas9 multiplex editing of the α-amylase/trypsin inhibitor genes to reduce allergen proteins in durum wheat. Front. Sustain. Food Syst. 2020, 4, 104. [Google Scholar] [CrossRef]
- Sugano, S.; Hirose, A.; Kanazashi, Y.; Adachi, K.; Hibara, M.; Itoh, T.; Mikami, M.; Endo, M.; Hirose, S.; Maruyama, N.; et al. Simultaneous induction of mutant alleles of two allergenic genes in soybean by using site-directed mutagenesis. BMC Plant Biol. 2020, 20, 513. [Google Scholar] [CrossRef]
- Dodo, H. SBIR Phase II: Development of an Allergen-Free Peanut Using Genome Editing Technology; National Science Foundation: Alexandria, VA, USA, 2021. [Google Scholar]
- Sarah, B. Beyond Risk Considerations: Where and How Can a Debate About Non-safety Related Issues of Genome Editing in Agriculture Take Place? Front. Plant Sci. 2018, 9, 1724. [Google Scholar] [CrossRef]
- Ayanoğlu, F.B.; Elçin, A.E.; Elçin, Y.M. Bioethical issues in genome editing by CRISPR-Cas9 technology. Turk. J. Biol. 2020, 44, 110–120. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S.; Nuti, M.; Ercoli, L. Impact of genetically engineered maize on agronomic, environmental and toxicological traits: A meta-analysis of 21 years of field data. Sci. Rep. 2018, 8, 3113. [Google Scholar] [CrossRef]
- Brookes, G.; Barfoot, P. Economic impact of GM crops: The global income and production effects 1996-2012. GM Crops Food 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Brookes, G. Genetically Modified (GM) Crop Use 1996-2020: Environmental Impacts Associated with Pesticide Use change. GM Crops Food 2022, 13, 262–289. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Elitzur, T.; Yakir, E.; Quansah, L.; Zhangjun, F.; Vrebalov, J.; Khayat, E.; Giovannoni, J.J.; Friedman, H. Banana MaMADS Transcription Factors Are Necessary for Fruit Ripening and Molecular Tools to Promote Shelf-Life and Food Security. Plant Physiol. 2016, 171, 380–391. [Google Scholar] [PubMed]
- Haun, W.; Coffman, A.; Clasen, B.M.; Demorest, Z.L.; Lowy, A.; Ray, E.; Retterath, A.; Stoddard, T.; Juillerat, A.; Cedrone, F.; et al. Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 2014, 12, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Gianotto, A.C.; Rocha, M.S.; Cutri, L.; Lopes, F.C.; Dal’Acqua, W.; Hjelle, J.J.; Lirette, R.P.; Oliveira, W.S.; Sereno, M.L. The insect-protected CTC91087-6 sugarcane event expresses Cry1Ac protein preferentially in leaves and presents compositional equivalence to conventional sugarcane. GM Crops Food 2019, 10, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Ligon, J.; Lessard, P.; Raab, R. Petition for the Determination of Nonregulated Status of Maize Event PY203; Agrivida Inc.: Woburn, MA, USA, 2019; 114p. [Google Scholar]
- Yu, X.; Sun, Y.; Lin, C.; Wang, P.; Shen, Z.; Zhao, Y. Development of Transgenic Maize Tolerant to Both Glyphosate and Glufosinate. Agronomy 2023, 13, 226. [Google Scholar] [CrossRef]
- Anderson, J.A.; Hong, B.; Moellring, E.; TeRonde, S.; Walker, C.; Wang, Y.; Maxwell, C. Composition of forage and grain from genetically modified DP202216 maize is equivalent to non-modified conventional maize (Zea mays L.). GM Crops Food 2019, 10, 77–89. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, S.C.; Shaw, M.; Connelly, M.; Yao, Z. Food and Feed Safety of NS-B5ØØ27-4 Omega-3 Canola (Brassica napus): A New Source of Long-Chain Omega-3 Fatty Acids. Front. Nutr. 2021, 8, 716659. [Google Scholar] [CrossRef]
- Ayala, F.; Fedrigo, G.V.; Burachik, M.; Miranda, P.V. Compositional equivalence of event IND-ØØ412-7 to non-transgenic wheat. Transgenic Res. 2019, 28, 165–176. [Google Scholar] [CrossRef]
- Mullins, E.; Bresson, J.; Dalmay, T.; Dewhurst, I.; Epstein, M.; Firbank, L.; Guerche, P.; Hejatko, J.; Moreno, F.J.; Naegeli, H.; et al. Assessment of genetically modified Maize MON 87429 for food and feed uses, under Regulation (EC) No 1829/2003 (application EFSA-GMO-NL-2019-161). EFSA J. 2022, 20, e07589. [Google Scholar] [CrossRef]
- Smith, B.; Zimmermann, C.; Carlson, A.; Mathesius, C.; Mukerji, P.; McNaughton, J.; Walker, C.; Roper, J. Evaluation of the safety and nutritional equivalency of maize grain with genetically modified event DP-Ø23211-2. GM Crops Food 2021, 12, 396–408. [Google Scholar] [CrossRef]
- Horikoshi, R.; Ferrari, G.; Dourado, P.; Climaco, J.; Vertuan, H.; Evans, A.; Pleau, M.; Morrell, K.; José, M.; Anderson, H.; et al. MON 95379 Bt maize as a new tool to manage sugarcane borer (Diatraea saccharalis) in South America. Pest Manag. Sci. 2022, 78, 3456–3466. [Google Scholar] [CrossRef]
- Webber, G.D. Insect-Resistant Crops through Genetic Engineering. North Central Regional Publication, University of Missouri Extension. 1995. Available online: https://mospace.umsystem.edu/xmlui/bitstream/handle/10355/7598/InsectResistantCropsGeneticEngineering.pdf?sequence=1&isAllowed=y (accessed on 12 June 2023).
- Koch, M.S.; Ward, J.M.; Levine, S.L.; Baum, G.A.; Vicini, J.L.; Hammond, B.G. The food and environmental safety of Bt crops. Front. Plant Sci. 2015, 6, 283–336. [Google Scholar] [PubMed]
- Abbas, M.S.T. Genetically engineered (modified) crops (Bacillus thuringiensis crops) and the world controversy on their safety. Egypt. J. Biol. Pest Control 2018, 28, 5. [Google Scholar]
- Brookes, G.; Barfoot, P. Global income and production impacts of using GM crop technology 1996–2014. GM Crops Food 2016, 7, 38–77. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. Global Status of Commercialized Biotech/GM Crops: 2019. ISAAA Brief 55. 2019. Available online: https://www.isaaa.org/resources/publications/briefs/55/default.asp (accessed on 2 July 2023).
- Baum, J.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Jin, S.; Singh, N.D.; Li, L.; Zhang, X.; Daniell, H. Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa armigera larval development and pupation. Plant Biotechnol. J. 2015, 13, 435–446. [Google Scholar] [CrossRef]
- Mamta, B.; Rajam, M.V. RNAi technology: A new platform for crop pest control. Physiol. Mol. Biol. Plants. 2017, 23, 487–501. [Google Scholar] [CrossRef]
- Malik, H.J.; Raza, A.; Amin, I.; Scheffler, J.A.; Scheffler, B.E.; Brown, J.K.; Mansoor, S. RNAi-mediated mortality of the whitefly through transgenic expression of double-stranded RNA homologous to acetylcholinesterase and ecdysone receptor in tobacco plants. Sci. Rep. 2016, 6, 38469. [Google Scholar] [CrossRef]
- Thakur, N.; Upadhyay, S.K.; Verma, P.C.; Chandrashekar, K.; Tuli, R.; Singh, P.K. Enhanced whitefly resistance in transgenic tobacco plants expressing double stranded RNA of v-ATPase A gene. PLoS ONE 2014, 9, e87235. [Google Scholar] [CrossRef]
- Mao, J.; Zeng, F. Plant-mediated RNAi of a gap gene-enhanced tobacco tolerance against the Myzus persicae. Transgenic Res. 2014, 23, 145–152. [Google Scholar] [CrossRef]
- Yu, R.; Xu, X.; Liang, Y.; Tian, H.; Pan, Z.; Jin, S.; Wang, N.; Zhang, W. The insect ecdysone receptor is a good potential target for RNAi-based pest control. Int. J. Biol. Sci. 2014, 10, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Carrière, Y.; Ellers-Kirk, C.; Sisterson, M.; Antilla, L.; Whitlow, M.; Dennehy, T.J.; Tabashnik, B.E. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proc. Natl. Acad. Sci. USA 2003, 100, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, W.D.; Burkness, E.C.; Mitchell, P.D.; Moon, R.D.; Leslie, T.W.; Fleischer, S.J.; Abrahamson, M.; Hamilton, K.L.; Steffey, K.L.; Gray, M.E.; et al. Area wide Suppression of European Corn Borer with Bt Maize Reaps Savings to Non-Bt Maize Growers. Science 2010, 330, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Genetically Engineered Crops: Experiences and Prospects—Stats. 2016. Available online: https://www.nap.edu/catalog/23395/genetically-engineered-crops-experiences-and-prospects (accessed on 7 July 2023).
- Achary, V.; Sheri, V.; Manna, M.; Panditi, V.; Borphukan, B.; Ram, B.; Agarwal, A.; Fartyal, D.; Teotia, D.; Masakapalli, S.K.; et al. Overexpression of improved EPSPS gene results in field level glyphosate tolerance and higher grain yield in rice. Plant Biotechnol. J. 2020, 18, 2504–2519. [Google Scholar] [CrossRef]
- Anderson, J.A.; Ellsworth, P.C.; Faria, J.C.; Head, G.P.; Owen, M.D.K.; Pilcher, C.D.; Shelton, A.M.; Meissle, M. Genetically Engineered Crops: Importance of Diversified Integrated Pest Management for Agricultural Sustainability. Front. Bioeng. Biotechnol. 2019, 7, 14. [Google Scholar]
- Dong, H.; Huang, Y.; Wang, K. The Development of Herbicide Resistance Crop Plants Using CRISPR/Cas9-Mediated Gene Editing. Genes 2021, 12, 912. [Google Scholar] [CrossRef]
- Butt, H.; Rao, G.S.; Sedeek, K.; Aman, R.; Kamel, R.; Mahfouz, M. Engineering herbicide resistance via prime editing in rice. Plant Biotechnol. J. 2020, 18, 2370–2372. [Google Scholar] [CrossRef]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar]
- Liu, X.; Qin, R.; Li, J.; Liao, S.; Shan, T.; Xu, R.; Wu, D.; Wei, P. A CRISPR-Cas9-mediated domain-specific base-editing screen enables functional assessment of ACCase variants in rice. Plant Biotechnol. J. 2020, 18, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Wu, H.; Liu, C.; Huang, C.; Lan, J.; Zhao, Y.; Xie, C. Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize. Crop J. 2020, 8, 449–456. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell. Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, C.; Xian, G.; Liu, D.; Lin, L.; Yin, S.; Sun, Q.; Fang, Y.; Zhang, H.; Wang, Y.; et al. Engineering herbicide-resistant oilseed rape by CRISPR/Cas9-mediated cytosine base-editing. Plant Biotechnol. J. 2020, 18, 1857–1859. [Google Scholar] [CrossRef]
- Wang, Z.; Wan, L.; Xin, Q.; Zhang, X.; Song, Y.; Wang, P.; Hong, D.; Fan, Z.; Yang, G. Optimising glyphosate tolerance in rapeseed (Brassica napus L.) by CRISPR/Cas9-based geminiviral donor DNA replicon system with Csy4-based single-guide RNA processing. J. Exp. Bot. Bot. 2021, 72, 4796–4808. [Google Scholar] [CrossRef]
- Li, C.; Zong, Y.; Wang, Y.; Jin, S.; Zhang, D.; Song, Q.; Zhang, R.; Gao, C. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 2018, 19, 59. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar]
- Al-Babili, S.; Beyer, P. Golden Rice Five years on the road Five years to go? Trends Plant Sci. 2005, 10, 565–573. [Google Scholar] [CrossRef]
- Hefferon, K.L. Nutritionally enhanced food crops; progress and perspectives. Int. J. Mol. Sci. 2015, 16, 3895–3914. [Google Scholar] [CrossRef]
- Pérez-Massot, E.; Banakar, R.; Gómez-Galera, S.; Zorrilla-López, U.; Sanahuja, G.; Arjó, G.; Miralpeix, B.; Vamvaka, E.; Farré, G.; Rivera, S.M. The contribution of transgenic plants to better health through improved nutrition: Opportunities and constraints. Genes. Nutr. 2013, 8, 29–41. [Google Scholar] [CrossRef]
- Rajaeieh, G.; Takian, A.; Kalantari, N.; Mohammadi-Nasrabadi, F. Analysis for policy to overcome barriers to reducing the prevalence of vitamin a deficiency among children (15–23 months) in Iran. BMC Public Health 2021, 21, 1234. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Grusak, M.A. Golden Rice is an effective source of vitamin A. Am. J. Clin. Nutr. 2009, 89, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Howe, J.A.; Tanumihardjo, S.A. Carotenoid-biofortified maize maintains adequate vitamin a status in Mongolian gerbils. J. Nutr. 2006, 136, 2562–2567. [Google Scholar] [CrossRef]
- Banakar, R.; Alvarez Fernandez, A.; Díaz-Benito, P.; Abadia, J.; Capell, T.; Christou, P. Phytosiderophores determine thresholds for iron and zinc accumulation in biofortified rice endosperm while inhibiting the accumulation of cadmium. J. Exp. Bot. 2017, 68, 4983–4995. [Google Scholar] [CrossRef]
- Matres, J.M.; Arcillas, E.; Cueto-Reaño, M.F.; Sallan-Gonzales, R.; Trijatmiko, K.R.; Slamet-Loedin, I. Biofortification of Rice Grains for Increased Iron Content. In Rice Improvement; Ali, J., Wani, S.H., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Senguttuvel, P.; Padmavathi, G.; Jasmine, C.; Sanjeeva Rao, D.; Neeraja, C.N.; Jaldhani, V.; Beulah, P.; Gobinath, R.; Aravind Kumar, J.; Sai Prasad, S.V.; et al. Rice biofortification: Breeding and genomic approaches for genetic enhancement of grain zinc and iron contents. Front. Plant Sci. 2023, 14, 1138408. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Yu, W.H.; Wu, H.Y.; Zhang, C.Q.; Sun, S.S.; Liu, Q.Q. Lysine biofortification in rice by modulating feedback inhibition of aspartate kinase and dihydrodipicolinate synthase. Plant Biotechnol. J. 2021, 19, 490–501. [Google Scholar] [CrossRef]
- Mugode, L.; Há, B.; Kaunda, A.; Sikombe, T.; Phiri, S.; Mutale, R.; Davis, C.; Tanumihardjo, S.; de Moura, F.F. Carotenoid retention of biofortified provitamin a maize (Zea mays L.) after Zambian traditional methods of milling, cooking and storage. J. Agric. Food Chem. 2014, 62, 6317–6325. [Google Scholar] [CrossRef]
- Jeong, J.; Guerinot, M.L. Biofortified and bioavailable: The gold standard for plant-based diets. Proc. Natl. Acad. Sci. USA 2008, 105, 1777–1778. [Google Scholar] [CrossRef]
- Patel, P.; Patel, R.; Patel, S.; Patel, Y.; Patel, M.; Trivedi, R. Edible Vaccines: A Nutritional Substitute for Traditional Immunization. Pharmacogn. Rev. 2022, 16, 62–69. [Google Scholar] [CrossRef]
- Stander, J.; Mbewana, S.; Meyers, A.E. Plant-derived human vaccines: Recent developments. Biol. Drugs 2022, 36, 573–589. [Google Scholar] [CrossRef]
- Qureshi, M.B. Arif, S.; Rathore, S.S. Edible Plant Vaccines: A Step Towards Revolution in the Field of Immunology. Int. J. Agric. Biol. 2023, 29, 361–369. [Google Scholar]
- Phoolcharoen, W.; Dye, J.M.; Kilbourne, J.; Piensook, K.; Pratt, W.D.; Arntzen, C.J.; Chen, Q.; Mason, H.S.; Herbst-Kralovetz, M.M. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc. Natl. Acad. Sci. USA 2011, 108, 20695–20700. [Google Scholar] [CrossRef] [PubMed]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef] [PubMed]
- Mason, H.S.; Haq, T.A.; Clements, J.D.; Arntzen, C.J. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): Potatoes expressing a synthetic LT-B gene. Vaccine 1998, 16, 1336–1343. [Google Scholar] [CrossRef]
- Mason, H.S.; Ball, J.M.; Shi, J.J.; Jiang, X.; Estes, M.K.; Arntzen, C.J. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5335–5340. [Google Scholar] [CrossRef]
- Chowdhury, K.; Bagasra, O. An edible vaccine for malaria using transgenic tomatoes of varying sizes, shapes and colors to carry different antigens. Med. Hypotheses 2007, 68, 22–30. [Google Scholar] [CrossRef]
- Abdurakhmonov, I.; Buriev, Z.; Shermatov, S.; Usmanov, D.; Mirzakhmedov, M.; Ubaydullaeva, K.; Kamburova, V.; Rakhmanov, B.; Ayubov, M.; Abdullaev, A.; et al. The edible tomato COVID-19 vaccine, TOMAVAC, induces neutralising IgGs. Researchsquare 2023. [Google Scholar] [CrossRef]
- Oszvald, M.; Kang, T.J.; Tomoskozi, S.; Tamas, C.; Tamas, L.; Kim, T.G.; Yang, M.S. Expression of a synthetic neutralizing epitope of porcine epidemic diarrhea virus fused with synthetic B subunit of Escherichia coli heat labile enterotoxin in rice endosperm. Mol. Biotechnol. 2007, 35, 215–223. [Google Scholar] [CrossRef]
- Huy, N.X.; Kim, S.H.; Yang, M.S.; Kim, T.G. Immunogenicity of a neutralizing epitope from porcine epidemic diarrhea virus: M cell targeting ligand fusion protein expressed in transgenic rice calli. Plant Cell Rep. 2012, 31, 1933–1942. [Google Scholar] [CrossRef]
- Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Srinivas, L.; Bapat, V.A. Expression of hepatitis B surface antigen in transgenic banana plants. Planta 2005, 222, 484–493. [Google Scholar] [CrossRef]
- Kim, T.G.; Kim, M.Y.; Kim, B.G.; Kang, T.J.; Kim, Y.S.; Jang, Y.S.; Arntzen, C.J.; Yang, M.S. Synthesis and assembly of Escherichia coli heat-labile enterotoxin B subunit in transgenic lettuce (Lactuca sativa). Protein Expr. Purif. 2007, 51, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Huy, N.X.; Yang, M.S.; Kim, T.G. Expression of a cholera toxin B subunit-neutralizing epitope of the porcine epidemic diarrhea virus fusion gene in transgenic lettuce (Lactuca sativa L.). Mol. Biotechnol. 2011, 48, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Karasev, A.V.; Foulke, S.; Wellens, C.; Rich, A.; Shon, K.J.; Zwierzynski, I.; Hone, D.; Koprowski, H.; Reitz, M. Plant based HIV-1 vaccine candidate: Tat protein produced in spinach. Vaccine 2005, 23, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.S.; Jeon, I.S.; Jung, Y.J.; Kim, J.B.; Park, J.S.; Ha, S.H.; Kim, K.H.; Kim, H.M.; Yang, J.S.; Kim, Y.H. Expression of hemagglutinin-neuraminidase protein of Newcastle disease virus in transgenic tobacco. Plant Biotechnol. Rep. 2007, 1, 85–92. [Google Scholar] [CrossRef]
- Tacket, C.O. Plant-based vaccines against diarrheal diseases. Trans. Am. Clin. Climatol. Assoc. 2007, 118, 79–87. [Google Scholar]
- Issaro, N.; Wang, D.; Liu, M.; Tassaneetrithep, B.; Phawong, C.; Rattanarojpong, T.; Jiang, C. Transgenic carrot plant-made edible vaccines against human infectious diseases. J. Innov. Pharm. Biol. Sci. 2018, 5, 43–48. [Google Scholar]
- Mishra, N.; Gupta, P.N.; Khatri, K.; Goyal, A.K.; Vyas, S.P. Edible vaccines: A new approach to oral immunization. Ind. J. Biotechnol. 2008, 7, 283–294. [Google Scholar]
- Kurup, V.M.; Thomas, J. Edible Vaccines: Promises and Challenges. Mol. Biotechnol. 2020, 62, 79–90. [Google Scholar] [CrossRef]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Letellier, M.; Lisowa, O.; Yusibov, V.; Koprowski, H.; Plucienniczak, A.; Legocki, A.B. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999, 13, 1796–1799, Erratum in FASEB J. 1999, 13, 2339. [Google Scholar] [CrossRef]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Estes, M.K.; Levine, M.M.; Arntzen, C.J. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 2000, 182, 302–305. [Google Scholar] [CrossRef]
- Kurokawa, S.; Nakamura, R.; Mejima, M.; Kozuka-Hata, H.; Kuroda, M.; Takeyama, N.; Oyama, M.; Satoh, S.; Kiyono, H.; Masumura, T.; et al. MucoRice-cholera toxin B-subunit, a rice-based oral cholera vaccine, down-regulates the expression of a-amylase/trypsin inhibitor-like protein family as major rice allergens. J. Proteome Res. 2013, 12, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. ISAAA’s GM Approval Database. 2022. Available online: www.isaaa.org/gmapprovaldatabase (accessed on 28 December 2022).
- James, C. 20th Anniversary (1996 to 2015) of the Global Commercialization of Biotech Crops and Biotech Crop Highlights in 2015; ISAAA Brief. No. 51; ISAAA: Ithaca, NY, USA, 2015; ISBN 978-1-892456-65-6. [Google Scholar]
- Ichim, M.C. The more favorable attitude of the citizens toward GMOs supports a new regulatory framework in the European Union. GM Crops Food 2021, 12, 18–24. [Google Scholar] [CrossRef]
- Rabbanee, F.K.; Afroz, T.; Naser, M.M. Are consumers loyal to genetically modified food? Evidence from Australia. Brit. Food J. 2020, 123, 803–819. [Google Scholar] [CrossRef]
- Kuzma, J.; Kokotovich, A. Renegotiating GM crop regulation: Targeted gene-modification technology raises new issues for the oversight of genetically modified crops. EMBO Rep. 2011, 12, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Sprink, T.; Eriksson, D.; Schiemann, J.; Hartung, F. Regulatory hurdles for genome editing: Process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep. 2016, 35, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Nerkar, G.; Devarumath, S.; Purankar, M.; Kumar, A.; Valarmathi, R.; Devarumath, R.; Appunu, C. Advances in Crop Breeding Through Precision Genome Editing. Front. Genet. 2022, 13, 880195. [Google Scholar] [CrossRef]
- Lassoued, R.; Phillips, P.W.P.; Macall, D.M.; Hesseln, H.; Smyth, S.J. Expert opinions on the regulation of plant genome editing. Plant Biotechnol. J. 2021, 19, 1104–1109. [Google Scholar] [CrossRef]
- Voigt, B. EU regulation of gene-edited plants—A reform proposal. Front. Genome Ed. 2023, 14, 1119442. [Google Scholar] [CrossRef]
- Escajedo San-Epifanio, L.; Filibi, I.; Lasa López, A.; Puigdomènech, P.; Uncetabarrenechea Larrabe, J. Possible EU futures for CRISPR-edited plants: Little margin for optimism? Front. Plant Sci. 2023, 14, 1141455. [Google Scholar] [CrossRef]
- Zimny, T. Regulation of GMO field trials in the EU and new genomic techniques: Will the planned reform facilitate experimenting with gene-edited plants? BioTechnologia 2023, 104, 75–83. [Google Scholar] [CrossRef]
- Davison, J.; Ammann, K. New GMO regulations for old: Determining a new future for EU crop biotechnology. GM Crops Food 2017, 8, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Sven, W.; Jacobsen, E. Feeding the world. Genetically Modified Crops Versus Agricultural Biodiversity. Agron. Sustain. Dev. 2013, 33, 651–662. [Google Scholar]
- ISAAA. Executive Summary Global Status of Commercialized Biotech/GM Crops; Brief 46; ISAAA: Ithaca, NY, USA, 2013. [Google Scholar]
- Young, S. GMO and the Nutritional Content of Food. Discovery Eye Foundation. 2015. Available online: https://discoveryeye.org/gmo-and-nutritional-content-of-food/ (accessed on 28 June 2023).
- Qaim, M.; Kouser, S. Genetically modified crops and food security. PLoS ONE 2013, 8, e64879. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G. Crop Biotechnology Continues to Provide Higher Farmer Income and Significant Environmental Benefits. PG Economics. 2020. Available online: https://pgeconomics.co.uk/press+releases/25/ (accessed on 11 June 2023).
- Gruère, G.; Sengupta, D. Bt Cotton and farmer suicides in India: An evidence-based assessment. J. Dev. Stud. 2011, 47, 316–337. [Google Scholar] [CrossRef]
- Smyth, S.J. The human health benefits from GM crops. Plant Biotechnol. J. 2020, 18, 887–888. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).