Perspectives and Advances in Organic Formulations for Agriculture: Encapsulation of Herbicides for Weed Control
Abstract
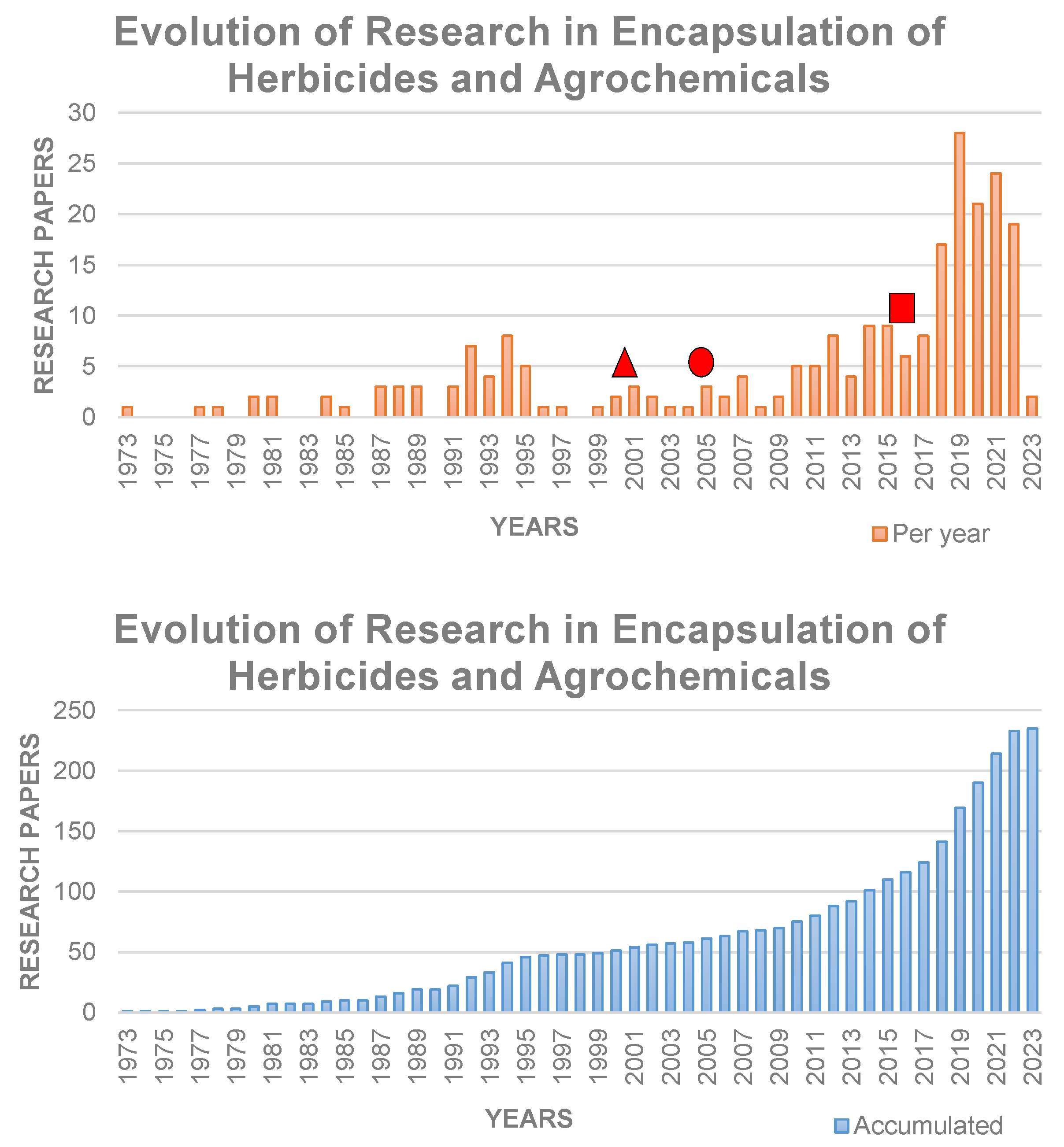
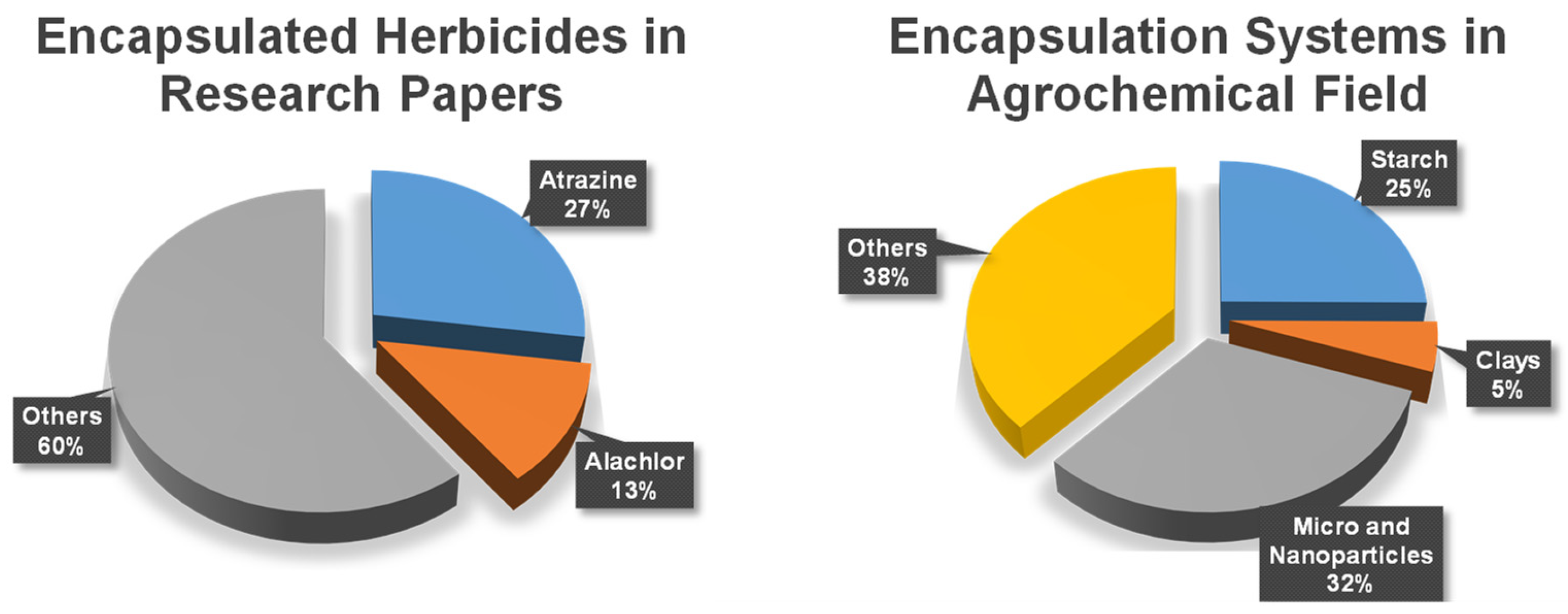
1. Introduction
2. Perspectives and Analysis of Organic Encapsulation Systems Employed for Weed Control
2.1. Cyclodextrins and Macrocycles
2.2. Clays
2.3. Matrices from Starch to Hybrids
2.4. Micro- and Nanoparticles
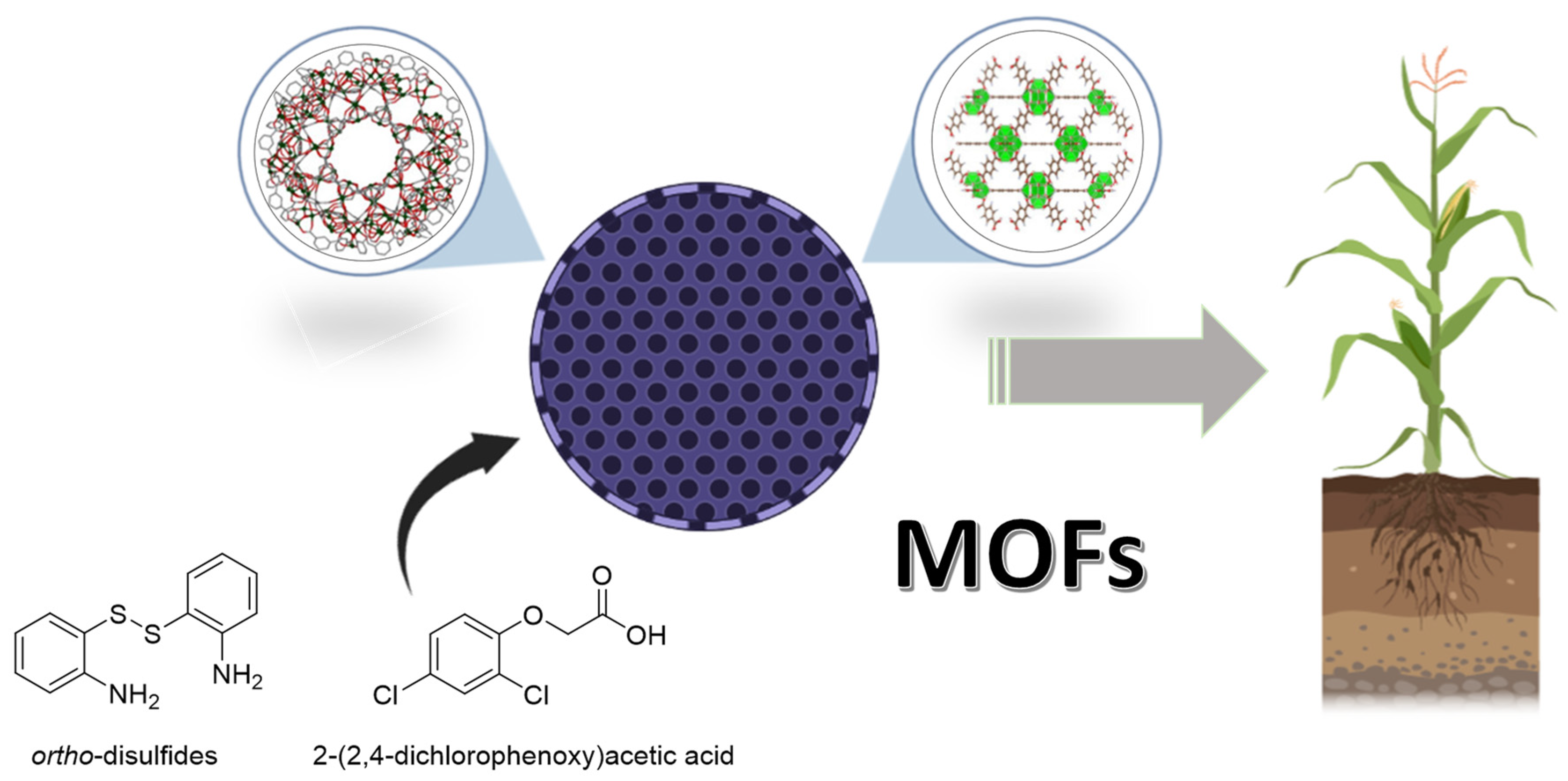
2.5. Metal–Organic Systems
2.6. New Trends
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinclair, R.G. Slow-Release Pesticide System. Polymers of Lactic and Glycolic Acids as Ecologically Beneficial, Cost-Effective Encapsulating Materials. Environ. Sci. Technol. 1973, 7, 955–956. [Google Scholar] [CrossRef]
- Pennwalt to Test Encapsulated Pesticide. Chem. Eng. News Arch. 1972, 50, 68–69. [CrossRef]
- Jaga, K.; Dharmani, C. Methyl Parathion: An Organophosphate Insecticide Not Quite Forgotten. Rev. Environ. Health 2006, 21, 57–68. [Google Scholar] [CrossRef] [PubMed]
- CNN News Monsanto Pleads Guilty to Illegally Spraying Banned Pesticide in Maui. Available online: https://edition.cnn.com/2019/11/22/us/monsanto-maui-pesticide-guilty-plea/index.html (accessed on 2 June 2023).
- Gilliom, R.J.; Barbash, J.E.; Crawford, C.G.; Hamilton, P.A.; Martin, J.D.; Nakagaki, N.; Nowell, L.H.; Scott, J.C.; Stackelberg, P.E.; Thelin, G.P.; et al. Occurrence and Distribution in Streams and Ground Water. In Pesticides in the Nation’s Streams and Ground Water, 1992–2001; Geological Survey (USGS): Reston, VA, USA, 2001; pp. 41–66. [Google Scholar]
- Law, E.U. Commission Decision of 10 March 2004 Concerning the Non-Inclusion of Atrazine in Annex I to Council Directive 91/414/EEC and the Withdrawal of Authorisations for Plant Protection Products Containing This Active Substance. Available online: https://eur-lex.europa.eu/eli/dec/2004/248/oj (accessed on 12 June 2023).
- Hedlund, B. Flemish Government Approves Belgium Glyphosate Ban for Individuals. Available online: https://www.baumhedlundlaw.com/belgium-glyphosate-ban-individuals/ (accessed on 12 June 2023).
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The Challenge of Herbicide Resistance around the World: A Current Summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Weed Resistance to Different Herbicide Modes of Action Is Driven by Agricultural Intensification. Field Crops Res. 2023, 292, 108819. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Macías, F.A.; Durán, A.G.; Molinillo, J.M.G. Allelopathy: The Chemical Language of Plants. In Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2020; Volume 112, pp. 1–84. [Google Scholar]
- Harding, D.P.; Raizada, M.N. Controlling Weeds with Fungi, Bacteria and Viruses: A Review. Front. Plant Sci. 2015, 6, 659. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Fernando, W.G.D.; Tennakoon, K.U. Achievements, Developments and Future Challenges in the Field of Bioherbicides for Weed Control: A Global Review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef]
- Gámiz, B.; Celis, R. S-Carvone Formulation Based on Granules of Organoclay to Modulate Its Losses and Phytotoxicity in Soil. Agronomy 2021, 11, 1593. [Google Scholar] [CrossRef]
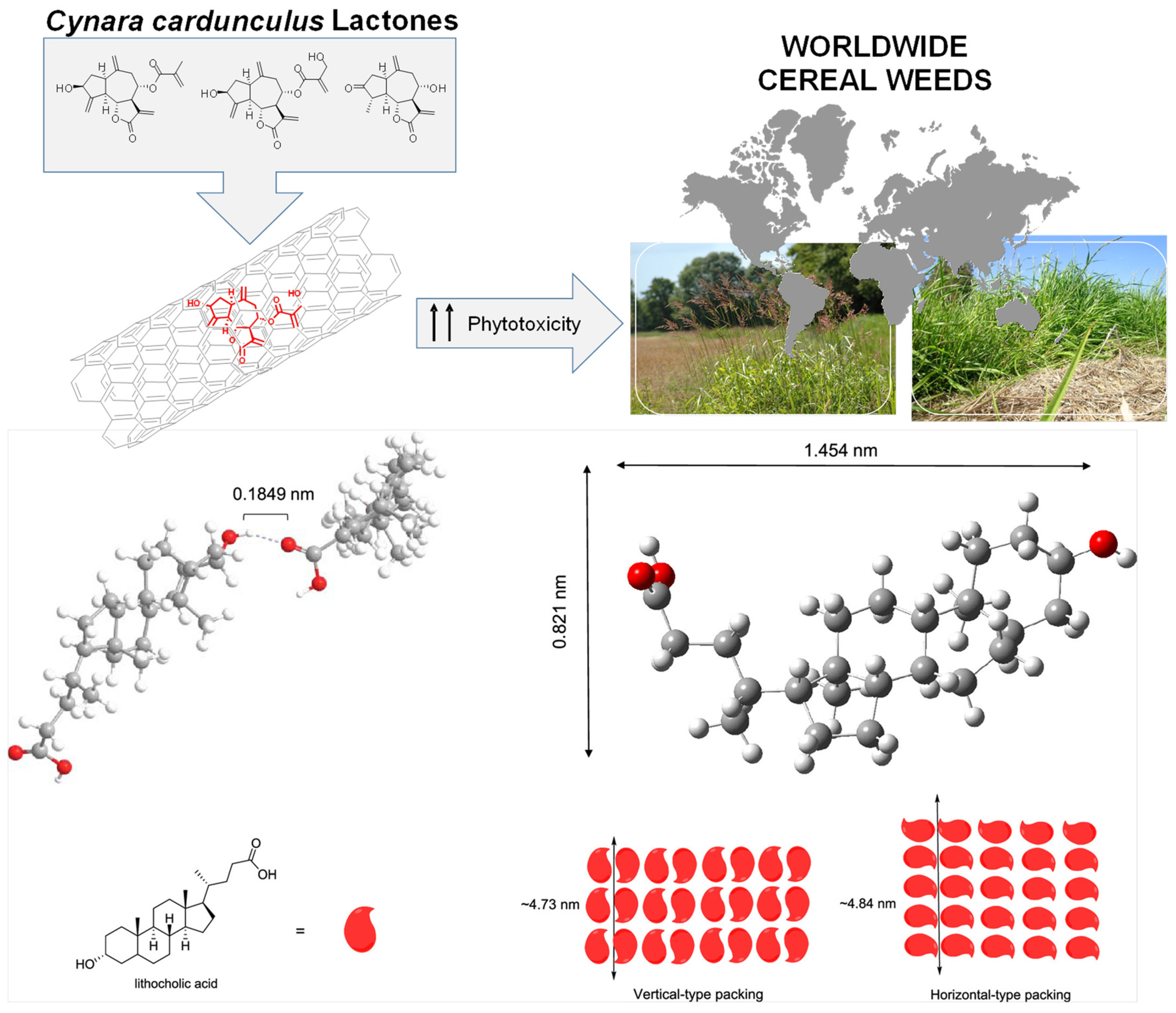
- Mejías, F.J.R.; Fernández, I.P.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Trasobares, S.; Macías, F.A. Encapsulation of Cynara Cardunculus Guaiane-Type Lactones in Fully Organic Nanotubes Enhances Their Phytotoxic Properties. J. Agric. Food Chem. 2022, 70, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef]
- Scavo, A.; Mejías, F.J.R.; Chinchilla, N.; Molinillo, J.M.G.; Schwaiger, S.; Lombardo, S.; Macías, F.A.; Mauromicale, G. Wheat Response and Weed-Suppressive Ability in the Field Application of a Nanoencapsulated Disulfide (DiS-NH2) Bioherbicide Mimic. Agronomy 2023, 13, 1132. [Google Scholar] [CrossRef]
- Szejtli, J. Physiological Effects of Cyclodextrins on Plants. Starch 1983, 35, 433–438. [Google Scholar] [CrossRef]
- Szejtli, J. Cyclodextrins in Pesticides. Starch 1985, 37, 382–386. [Google Scholar] [CrossRef]
- Gines, J.M.; Perez-Martinez, I.; Arias, M.J.; Moyano, J.R.; Morillo, E.; Ruiz-Conde, A.; Sachez-Soto, P.J. Inclusion of the Herbicide 2,4-Dichlorophenoxyacetic Acid (2,4-D) with β-Cyclodextrin by Different Processing Methods. Chemosphere 1996, 33, 321–334. [Google Scholar] [CrossRef]
- Hosangadi, B.; Asgaonkar, A. Inclusion of Acaricides by Complexation with β-Cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 23, 35–39. [Google Scholar] [CrossRef]
- Lezcano, M.; Novo, M.; Al-Soufi, W.; Rodríguez-Núñez, E.; Tato, J.V.; Vázquez Tato, J. Complexation of Several Fungicides with β-Cyclodextrin: Determination of the Association Constants and Isolation of the Solid Complexes. J. Agric. Food Chem. 2003, 51, 5036–5040. [Google Scholar] [CrossRef]
- Lezcano, M.; Al-Soufi, W.; Novo, M.; Rodríguez-Núñez, E.; Vázquez Tato, J. Complexation of Several Benzimidazole-Type Fungicides with α- and β-Cyclodextrins. J. Agric. Food Chem. 2002, 50, 108–112. [Google Scholar] [CrossRef]
- Benfeito, S.; Rodrigues, T.; Garrido, J.; Borges, F.; Garrido, E.M. Host-Guest Interaction between Herbicide Oxadiargyl and Hydroxypropyl-β-Cyclodextrin. Sci. World J. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Perez-Martinez, J.I.; Morillo, E.; Gines, J.M. β-CD Effect on 2,4-D Soil Adsorption. Chemosphere 1999, 39, 2047–2056. [Google Scholar] [CrossRef]
- Cai, X.; Liu, W.; Chen, S. Environmental Effects of Inclusion Complexation between Methylated β-Cyclodextrin and Diclofop-Methyl. J. Agric. Food Chem. 2005, 53, 6744–6749. [Google Scholar] [CrossRef] [PubMed]
- Cala, A.; Molinillo, J.M.G.G.; Fernández-Aparicio, M.; Ayuso, J.; Álvarez, J.A.; Rubiales, D.; Macías, F.A.; Delavault, P. Complexation of Sesquiterpene Lactones with Cyclodextrins: Synthesis and Effects on Their Activities on Parasitic Weeds. Org. Biomol. Chem. 2017, 15, 6500–6510. [Google Scholar] [CrossRef]
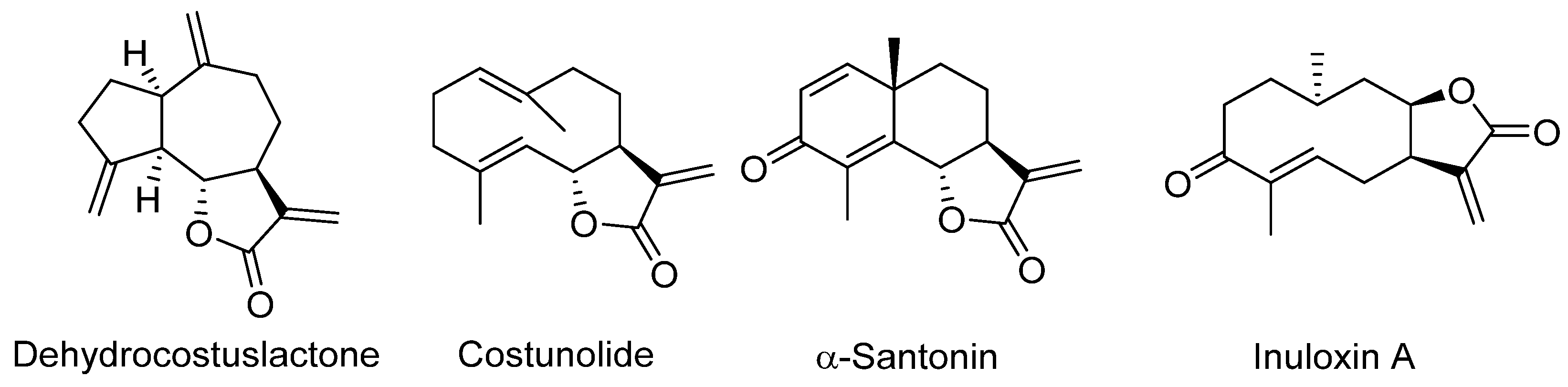
- Moeini, A.; Masi, M.; Zonno, M.C.; Boari, A.; Cimmino, A.; Tarallo, O.; Vurro, M.; Evidente, A. Encapsulation of Inuloxin A, a Plant Germacrane Sesquiterpene with Potential Herbicidal Activity, in β-Cyclodextrins. Org. Biomol. Chem. 2019, 17, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, B.; Bi, H.; Zhang, P. Synthesis of Atrazine-HPCD Inclusion and Its Bioactivity. Trans. Tianjin Univ. 2014, 20, 350–357. [Google Scholar] [CrossRef]
- Geng, Q.; Xie, J.; Wang, X.; Cai, M.; Ma, H.; Ni, H. Preparation and Characterization of Butachlor/(2-Hydroxypropyl)-β-Cyclodextrin Inclusion Complex: Improve Soil Mobility and Herbicidal Activity and Decrease Fish Toxicity. J. Agric. Food Chem. 2018, 66, 12198–12205. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jiang, J.; Li, X.; Liu, Y.; Zhao, L.; Fu, Y.; Ye, F. Enhanced Physicochemical Properties and Herbicidal Activity of an Environment-Friendly Clathrate Formed by β-Cyclodextrin and Herbicide Cyanazine. J. Mol. Liq. 2020, 305, 112858. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.Y.; Liu, Y.Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers 2019, 11, 1396. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; Carrasco, Á.; Durán, A.G.; Molinillo, J.M.G.; Macías, F.A.; Chinchilla, N. On the Formulation of Disulfide Herbicides Based on Aminophenoxazinones: Polymeric Nanoparticle Formulation and Cyclodextrin Complexation to Combat Crop Yield Losses. Pest Manag. Sci. 2022, 79, 1547–1556. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.C.; et al. Re-Evaluation of β-Cyclodextrin (E 459) as a Food Additive. EFSA J. 2016, 14, e04628. [Google Scholar] [CrossRef]
- Mokhtar, M.S.; Suliman, F.O.; Elbashir, A.A. Investigation of Inclusion Complexes of Ametryne and Atrazine with Cucurbit[n]Urils (n = 6–8) Using Experimental and Theoretical Techniques. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 31–43. [Google Scholar] [CrossRef]
- Mokhtar, M.S.; Suliman, F.O.; Elbashir, A.A. The Binding Interaction of Imazapyr with Cucurbit[n]Uril (n = 6–8): Combined Experimental and Molecular Modeling Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 194, 67–75. [Google Scholar] [CrossRef]
- Mokhtar, M.S.; Elbashir, A.A.; Suliman, F.O. Spectroscopic and Molecular Simulation Studies on the Interaction of Imazaquin Herbicide with Cucurbiturils (n = 6–8). J. Mol. Struct. 2023, 1274, 134444. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; He, S.; Varela, R.M.; Molinillo, J.M.G.; Barba-Bon, A.; Nau, W.M.; Macías, F.A. Stability and PKa Modulation of Aminophenoxazinones and Their Disulfide Mimics by Host–Guest Interaction with Cucurbit[7]Uril. Direct Applications in Agrochemical Wheat Models. J. Agric. Food Chem. 2023, 71, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Connick, W.J.; Bradow, J.M.; Wells, W.; Steward, K.K.; Van, T.K. Preparation and Evaluation of Controlled-Release Formulations of 2,6-Dichlorobenzonitrile. J. Agric. Food Chem. 1984, 32, 1199–1205. [Google Scholar] [CrossRef]
- Qasem, J.R. Nutrient Accumulation by Weeds and Their Associated Vegetable Crops. J. Hortic. Sci. 1992, 67, 189–195. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Germination Ecology of Goosegrass (Eleusine indica): An Important Grass Weed of Rainfed Rice. Weed Sci. 2008, 56, 699–706. [Google Scholar] [CrossRef]
- Zhang, S.-Z.; Li, Y.-H.; Kong, C.-H.; Xu, X.-H. Interference of Allelopathic Wheat with Different Weeds. Pest Manag. Sci. 2016, 72, 172–178. [Google Scholar] [CrossRef]
- Zhou, B.; Kong, C.H.; Li, Y.H.; Wang, P.; Xu, X.H. Crabgrass (Digitaria sanguinalis) Allelochemicals That Interfere with Crop Growth and the Soil Microbial Community. J. Agric. Food Chem. 2013, 61, 5310–5317. [Google Scholar] [CrossRef]
- Carr, M.E.; Wing, R.E.; Doane, W.M. Clay as a Carrier in Starch Encapsulated Herbicides Prepared by Extrusion Processing. Starch-Stärke 1994, 46, 9–13. [Google Scholar] [CrossRef]
- Flores Céspedes, F.; Villafranca Sánchez, M.; Pérez García, S.; Fernández Pérez, M. Modifying Sorbents in Controlled Release Formulations to Prevent Herbicides Pollution. Chemosphere 2007, 69, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Rivoira, L.; Frassati, S.; Cordola, S.; Castiglioni, M.; Onida, B.; Ronchetti, S.; Ingrando, I.; Bruzzoniti, M.C. Encapsulation of the Glyphosate Herbicide in Mesoporous and Soil-Affine Sorbents for Its Prolonged Release. Chem. Eng. J. 2022, 431, 134225. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, S.-Y.; Yang, J.-H.; Soo Hwang, H.; Park, I. Paraquat Release Control Using Intercalated Montmorillonite Compounds. J. Phys. Chem. Solids 2010, 71, 460–463. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A.; Hejazi, M.J. Controlled Release Systems Based on Intercalated Paraquat onto Montmorillonite and Clinoptilolite Clays Encapsulated with Sodium Alginate. Adv. Polym. Technol. 2017, 36, 177–185. [Google Scholar] [CrossRef]
- Marco-Brown, J.L.; Undabeytia, T.; Torres Sánchez, R.M.; dos Santos Afonso, M. Slow-Release Formulations of the Herbicide Picloram by Using Fe–Al Pillared Montmorillonite. Environ. Sci. Pollut. Res. 2017, 24, 10410–10420. [Google Scholar] [CrossRef]
- Khatem, R.; Celis, R.; Hermosín, M.C. Cationic and Anionic Clay Nanoformulations of Imazamox for Minimizing Environmental Risk. Appl. Clay Sci. 2019, 168, 106–115. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Spychaj, T.; Paździoch, W. Carboxymethyl Starch/Montmorillonite Composite Microparticles: Properties and Controlled Release of Isoproturon. Carbohydr. Polym. 2016, 136, 101–106. [Google Scholar] [CrossRef]
- Giroto, A.S.; De Campos, A.; Pereira, E.I.; Ribeiro, T.S.; Marconcini, J.M.; Ribeiro, C. Photoprotective Effect of Starch/Montmorillonite Composites on Ultraviolet-Induced Degradation of Herbicides. React. Funct. Polym. 2015, 93, 156–162. [Google Scholar] [CrossRef]
- Cabrera, A.; Celis, R.; Hermosín, M.C. Imazamox-Clay Complexes with Chitosan- and Iron(III)-Modified Smectites and Their Use in Nanoformulations. Pest Manag. Sci. 2016, 72, 1285–1294. [Google Scholar] [CrossRef]
- Cal-IPC (California Invasive Plant Council). Cal-IPC Plant Assessment Form for Brassica Nigra; Cal-IPC: Berkeley, CA, USA, 2004. [Google Scholar]
- Sánchez-Verdejo, T.; Undabeytia, T.; Nir, S.; Villaverde, J.; Maqueda, C.; Morillo, E. Environmentally Friendly Formulations of Alachlor and Atrazine: Preparation, Characterization, and Reduced Leaching. J. Agric. Food Chem. 2008, 56, 10192–10199. [Google Scholar] [CrossRef]
- Wall, D.A.; Friesen, G.H. Effect of Duration of Green Foxtail (Setaria viridis) Competition on Potato (Solanum tuberosum) Yield Effect of Duration of Green Foxtail (Setaria viridis) Competition on Potato (Solanum tuberosum) Yield. Weed Technol. 1990, 4, 539–542. [Google Scholar] [CrossRef]
- Li, J.; Yao, J.; Li, Y.; Shao, Y. Controlled Release and Retarded Leaching of Pesticides by Encapsulating in Carboxymethyl Chitosan/Bentonite Composite Gel. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2012, 47, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Mishael, Y.G.; Undabeytia, T.; Rabinovitz, O.; Rubin, B.; Nir, S. Sulfosulfuron Incorporated in Micelles Adsorbed on Montmorillonite for Slow Release Formulations. J. Agric. Food Chem. 2003, 51, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Galán-Jiménez, M.D.C.; Mishael, Y.-G.; Nir, S.; Morillo, E.; Undabeytia, T. Factors Affecting the Design of Slow Release Formulations of Herbicides Based on Clay-Surfactant Systems. A Methodological Approach. PLoS ONE 2013, 8, e59060. [Google Scholar] [CrossRef] [PubMed]
- Zait, Y.; Segev, D.; Schweitzer, A.; Goldwasser, Y.; Rubin, B.; Mishael, Y.G. Development and Employment of Slow-Release Pendimethalin Formulations for the Reduction of Root Penetration into Subsurface Drippers. J. Agric. Food Chem. 2015, 63, 1682–1688. [Google Scholar] [CrossRef]
- Goldreich, O.; Goldwasser, Y.; Mishael, Y.G. Effect of Soil Wetting and Drying Cycles on Metolachlor Fate in Soil Applied as a Commercial or Controlled-Release Formulation. J. Agric. Food Chem. 2011, 59, 645–653. [Google Scholar] [CrossRef]
- Hermosin, M.C.; Calderón, M.J.; Aguer, J.P.; Cornejo, J. Organoclays for Controlled Release of the Herbicide Fenuron. Pest Manag. Sci. 2001, 57, 803–809. [Google Scholar] [CrossRef]
- Mishael, Y.G.; Undabeytia, T.; Rabinovitz, O.; Rubin, B.; Nir, S. Slow-Release Formulations of Sulfometuron Incorporated in Micelles Adsorbed on Montmorillonite. J. Agric. Food Chem. 2002, 50, 2864–2869. [Google Scholar] [CrossRef]
- Galán-Pérez, J.A.; Gámiz, B.; Celis, R. Granulated Organoclay as a Sorbent to Protect the Allelochemical Scopoletin from Rapid Biodegradation in Soil. Environ. Technol. Innov. 2022, 28, 102707. [Google Scholar] [CrossRef]
- del Carmen Galán-Jiménez, M.; Morillo, E.; Bonnemoy, F.; Mallet, C.; Undabeytia, T. A Sepiolite-Based Formulation for Slow Release of the Herbicide Mesotrione. Appl. Clay Sci. 2020, 189, 105503. [Google Scholar] [CrossRef]
- Doane, W.M.; Shasha, B.S.; Russell, C.R. Encapsulation of Pesticides within a Starch Matrix. Control. Release Pestic. 1977, 53, 74–83. [Google Scholar]
- Schreiber, M.M.; White, M.D. Effect of Formulation Technology on the Release of Starch Encapsulated EPTC. Proc. Br. Crop Prot. Conf. Weeds 1980, 15, 225–229. [Google Scholar]
- Schreiber, M.M.; White, M.D. Granule Structure and Rate of Release with Starch-Encapsulated Thiocarbamates. Weed Sci. 1980, 28, 685–690. [Google Scholar] [CrossRef]
- Shasha, B.S.; Trimnell, D.; Otey, F.H. Encapsulation of Pesticides in a Starch-Calcium Adduct. J. Polym. Sci. Part A Polym. Chem. 1981, 19, 1891–1899. [Google Scholar] [CrossRef]
- Trimnell, D.; Shasha, B.S.; Doane, W.M. Release of Trifluralin from Starch Xanthide Encapsulated Formulations. J. Agric. Food Chem. 1981, 29, 637–640. [Google Scholar] [CrossRef]
- Trimnell, D.; Shasha, B.S.; Otey, F.H. The Effect of α-Amylases upon the Release of Trifluralin Encapsulated in Starch. J. Control. Release 1985, 1, 183–190. [Google Scholar] [CrossRef]
- Wing, R.E.; Doane, W.M. The Role of Retrogradation on Starch Encapsulation and Release of Active Agents. Polym. Prepr. 1987, 28, 108–109. [Google Scholar]
- Wing, R.E.; Maiti, S.; Doane, W.M. Effectiveness of Jet-cooked Pearl Cornstarch as a Controlled Release Matrix. Starch-Stäke 1987, 39, 422–425. [Google Scholar] [CrossRef]
- White, M.D.; Schreiber, M.M. Herbicidal Activity of Starch Encapsulated Trifluralin. Weed Sci. 1984, 32, 387–394. [Google Scholar] [CrossRef]
- Trimnell, D.; Shasha, B.S. Autoencapsulation: A New Method for Entrapping Pesticides within Starch. J. Control. Release 1988, 7, 25–31. [Google Scholar] [CrossRef]
- Grover, R.; Wolt, J.; Cessna, A.; Schiefer, B. Environmental Fate of Trifluralin. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 1997; pp. 1–64. [Google Scholar]
- European Union European Union-Final Regulatory Action. Available online: http://archive.pic.int/view_displayFRA.php?id=1186&back=viewB_FRAchems.php?sort=chemical (accessed on 7 June 2023).
- Schreiber, M.M.; Hickman, M.V.; Vail, G.D. Starch-encapsulated Atrazine: Efficacy and Transport. J. Environ. Qual. 1993, 22, 443–453. [Google Scholar] [CrossRef]
- Vail, G.D.; Hickman, M.V.; Schreiber, M.M. Atrazine Dissipation and Carryover from Commercial and Starch-Encapsulated Atrazine Formulations. Weed Sci. 1997, 45, 842–847. [Google Scholar] [CrossRef]
- Fleming, G.F.; Wax, L.M.; Simmons, F.W. Leachability and Efficacy of Starch-Encapsulated Atrazine. Weed Technol. 1992, 6, 297–302. [Google Scholar] [CrossRef]
- Buhler, D.D.; Schreiber, M.M.; Koskinen, W.C. Weed Control with Starch-Encapsulated Alachlor, Metolachlor, and Atrazine. Weed Technol. 1994, 8, 277–284. [Google Scholar] [CrossRef]
- Williams, C.F.; Nelson, S.D.; Gish, T.J. Release Rate and Leaching of Starch-Encapsulated Atrazine in a Calcareous Soil. Soil Sci. Soc. Am. J. 1999, 63, 425–432. [Google Scholar] [CrossRef]
- Gish, T.G.; Shirmohammadi, A.; Wienhold, B.J. Field-Scale Mobility and Persistence of Commercial and Starch-Encapsulated Atrazine and Alachlor. J. Environ. Qual. 1994, 23, 355–359. [Google Scholar] [CrossRef]
- Wienhold, B.J.; Sadeghi, A.M.; Gish, T.J. Effect of Starch Encapsulation and Temperature on Volatilization of Atrazine and Alachlor. J. Environ. Qual. 1993, 22, 162–166. [Google Scholar] [CrossRef]
- Wienhold, B.J.; Gish, T.J. Effect of Formulation and Tillage Practice on Volatilization of Atrazine and Alachlor. J. Environ. Qual. 1994, 23, 292–298. [Google Scholar] [CrossRef]
- Wing, R.E.; Maiti, S.; Doane, M. Amylose Content of Starch Control the Release of Encapsulated Bioactive Agents. J. Control. Release 1988, 7, 33–37. [Google Scholar] [CrossRef]
- Trimnell, D.; Wing, R.E.; Carr, M.E.; Doane, W.M. Encapsulation of EPTC in Starch by Twin-screw Extrusion. Ind. Crops Prod. 1991, 43, 146–151. [Google Scholar] [CrossRef]
- Wing, R.E.; Carr, M.E.; Trimnell, D.; Doane, W.M. Comparison of Steam Injection Cooking versus Twin-Screw Extrusion of Pearl Cornstarch for Encapsulation of Chloroacetanilide Herbicides. J. Control. Release 1991, 16, 267–277. [Google Scholar] [CrossRef]
- Carr, M.E.; Wing, R.E.; Doane, W.M. Encapsulation of Atrazine within a Starch Matrix by Extrusion Processing. Cereal Chem. 1991, 68, 262–266. [Google Scholar]
- Fleming, G.F.; Wax, L.M.; Simmons, F.W.; Felsot, A.S. Movement of Alachlor and Metribuzin from Controlled Release Formulations in a Sandy Soil. Weed Sci. 1992, 40, 606–613. [Google Scholar] [CrossRef]
- Reed, J.P.; Hall, F.R.; Trimnell, D. Effect of Encapsulating Thiocarbamate Herbicides within Starch for Overcoming Enhanced Degradation in Soils. Starch-Stärke 1989, 41, 184–186. [Google Scholar] [CrossRef]
- Giroto, A.S.; de Campos, A.; Pereira, E.I.; Cruz, C.C.T.; Marconcini, J.M.; Ribeiro, C. Study of a Nanocomposite Starch-Clay for Slow-Release of Herbicides: Evidence of Synergistic Effects between the Biodegradable Matrix and Exfoliated Clay on Herbicide Release Control. J. Appl. Polym. Sci. 2014, 131, 41188. [Google Scholar] [CrossRef]
- Riyajan, S.A. Physical Property Testing of a Novel Hybrid Natural Rubber-Graft-Cassava Starch/Sodium Alginate Bead for Encapsulating Herbicide. Polym. Test. 2017, 58, 300–307. [Google Scholar] [CrossRef]
- Riyajan, S.A. A Novel Hybrid 2,4-Dichlorophenoxy Acetate Bead from Modified Cassava Starch and Sodium Alginate with Modified Natural Rubber Coating. J. Polym. Environ. 2018, 26, 1950–1961. [Google Scholar] [CrossRef]
- Alipour, M.; Saharkhiz, M.J.; Niakousari, M.; Seidi Damyeh, M. Phytotoxicity of Encapsulated Essential Oil of Rosemary on Germination and Morphophysiological Features of Amaranth and Radish Seedlings. Sci. Hortic. 2019, 243, 131–139. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Naderi, R. A Natural Post-Emergence Herbicide Based on Essential Oil Encapsulation by Cross-Linked Biopolymers: Characterization and Herbicidal Activity. Environ. Sci. Pollut. Res. 2020, 27, 45844–45858. [Google Scholar] [CrossRef]
- Dong, H.; Li, F.; Li, J.; Li, Y. Characterizations of Blend Gels of Carboxymethylated Polysaccharides and Their Use for the Controlled Release of Herbicide. J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 235–241. [Google Scholar] [CrossRef]
- Li, J.; Jiang, M.; Wu, H.; Li, Y. Addition of Modified Bentonites in Polymer Gel Formulation of 2,4-D for Its Controlled Release in Water and Soil. J. Agric. Food Chem. 2009, 57, 2868–2874. [Google Scholar] [CrossRef] [PubMed]
- Flores-Céspedes, F.; Daza-Fernández, I.; Villafranca-Sánchez, M.; Fernández-Pérez, M. Use of Ethylcellulose To Control Chlorsulfuron Leaching in a Calcareous Soil. J. Agric. Food Chem. 2009, 57, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, M.; Villafranca-Sánchez, M.; Flores-Céspedes, F.; Daza-Fernández, I. Ethylcellulose and Lignin as Bearer Polymers in Controlled Release Formulations of Chloridazon. Carbohydr. Polym. 2011, 83, 1672–1679. [Google Scholar] [CrossRef]
- Fernández-Pérez, M.; Villafranca-Sánchez, M.; Flores-Céspedes, F.; Daza-Fernández, I. Lignin-Polyethylene Glycol Matrices and Ethylcellulose to Encapsulate Highly Soluble Herbicides. J. Appl. Polym. Sci. 2014, 132, 41422. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Kangwansupamonkon, W.; Kiatkamjornwong, S. Lignin-Based Nanogels for the Release of Payloads in Alkaline Conditions. Eur. Polym. J. 2021, 145, 110241. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Xuan, G. Preparation and Characterization of β-Cyclodextrin Nanosponges and Study on Enhancing the Solubility of Insoluble Nicosulfuron. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012108. [Google Scholar] [CrossRef]
- Gámiz, B.; López-Cabeza, R.; Velarde, P.; Spokas, K.A.; Cox, L. Biochar Changes the Bioavailability and Bioefficacy of the Allelochemical Coumarin in Agricultural Soils. Pest Manag. Sci. 2021, 77, 834–843. [Google Scholar] [CrossRef]
- Luo, J.; Gao, Y.; Liu, Y.; Huang, X.; Zhang, D.; Cao, H.; Jing, T.; Liu, F.; Li, B. Self-Assembled Degradable Nanogels Provide Foliar Affinity and Pinning for Pesticide Delivery by Flexibility and Adhesiveness Adjustment. ACS Nano 2021, 15, 14598–14609. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.C.; Glotfelty, D.E.; Taylor, A.W.; Watson, D.R. Volatilization of Microencapsulated and Conventionally Applied Chlorpropham in the Field. Agron. J. 1978, 70, 933–937. [Google Scholar] [CrossRef]
- Petersen, B.B.; Shea, P.J. Microencapsulated Alachlor and Its Behavior on Wheat (Triticum aestivum) Straw. Weed Sci. 1989, 37, 719–723. [Google Scholar] [CrossRef]
- Chang, Y.N.; Mueller, R.E.; Iannotti, E.L. Use of Low MW Polylactic Acid and Lactide to Stimulate Growth and Yield of Soybeans. Plant Growth Regul. 1996, 19, 223–232. [Google Scholar] [CrossRef]
- Stloukal, P.; Kucharczyk, P.; Sedlarik, V.; Bazant, P.; Koutny, M. Low Molecular Weight Poly(Lactic Acid) Microparticles for Controlled Release of the Herbicide Metazachlor: Preparation, Morphology, and Release Kinetics. J. Agric. Food Chem. 2012, 60, 4111–4119. [Google Scholar] [CrossRef]
- Boehm, A.L.; Martinon, I.; Zerrouk, R.; Rump, E.; Fessi, H. Nanoprecipitation Technique for the Encapsulation of Agrochemical Active Ingredients. J. Microencapsul. 2003, 20, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.K.; Oh, S.B.; Rhim, J.W.; Lee, J.M. Microencapsulation of Water-Soluble Herbicide by Interfacial Reaction. I. Characterization of Microencapsulation. J. Appl. Polym. Sci. 2000, 78, 1645–1655. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J. Encapsulation of the Herbicide Picloram by Using Polyelectrolyte Biopolymers as Layer-by-Layer Materials. J. Agric. Food Chem. 2013, 61, 3789–3796. [Google Scholar] [CrossRef]
- Quiñones, J.P.; García, Y.C.; Curiel, H.; Covas, C.P. Microspheres of Chitosan for Controlled Delivery of Brassinosteroids with Biological Activity as Agrochemicals. Carbohydr. Polym. 2010, 80, 915–921. [Google Scholar] [CrossRef]
- Yeom, C.K.; Kim, Y.H.; Lee, J.M. Microencapsulation of Water-Soluble Herbicide by Interfacial Reaction. II. Release Properties of Microcapsules. J. Appl. Polym. Sci. 2002, 84, 1025–1034. [Google Scholar] [CrossRef]
- Grillo, R.; de Melo, N.F.S.; de Lima, R.; Lourenço, R.W.; Rosa, A.H.; Fraceto, L.F. Characterization of Atrazine-Loaded Biodegradable Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Microspheres. J. Polym. Environ. 2010, 18, 26–32. [Google Scholar] [CrossRef]
- Lobo, F.A.; De Aguirre, C.L.; Silva, M.S.; Grillo, R.; De Melo, N.F.S.; De Oliveira, L.K.; De Morais, L.C.; Campos, V.; Rosa, A.H.; Fraceto, L.F. Poly(Hydroxybutyrate-Co-Hydroxyvalerate) Microspheres Loaded with Atrazine Herbicide: Screening of Conditions for Preparation, Physico-Chemical Characterization, and in Vitro Release Studies. Polym. Bull. 2011, 67, 479–495. [Google Scholar] [CrossRef]
- Pérez-Martínez, J.I.; Morillo, E.; Maqueda, C.; Ginés, J.M. Ethyl Cellulose Polymer Microspheres for Controlled Release of Norfluazon. Pest Manag. Sci. 2001, 57, 688–694. [Google Scholar] [CrossRef]
- Dailey, O.D.J. Volatilization of Alachlor from Polymeric Formulations. J. Agric. Food Chem. 2004, 52, 6742–6746. [Google Scholar] [CrossRef] [PubMed]
- Dowler, C.C.; Dailey, O.D.; Mullinix, B.G. Polymeric Microcapsules of Alachlor and Metolachlor: Preparation and Evaluation of Controlled-Release Properties. J. Agric. Food Chem. 1999, 47, 2908–2913. [Google Scholar] [CrossRef] [PubMed]
- Elbahri, Z.; Taverdet, J.L. Optimization of an Herbicide Release from Ethylcellulose Microspheres. Polym. Bull. 2005, 54, 353–363. [Google Scholar] [CrossRef]
- Sopeña, F.; Cabrera, A.; Maqueda, C.; Morillo, E. Controlled Release of the Herbicide Norflurazon into Water from Ethylcellulose Formulations. J. Agric. Food Chem. 2005, 53, 3540–3547. [Google Scholar] [CrossRef]
- Cho, J.; Seo, Y.; Yim, T.; Lee, H. Effect of Nanoencapsulated Vitamin B1 Derivative on Inhibition of Both Mycelial Growth and Spore Germination of Fusarium oxysporum f. sp. Raphani. Int. J. Mol. Sci. 2013, 14, 4283–4297. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Grillo, R.; Jonsson, M.; Santos, N.Z.P.; Feitosa, L.O.; Lima, R.; Fraceto, L.F. Ecotoxicological Evaluation of Poly(ε-Caprolactone) Nanocapsules Containing Triazine Herbicides. J. Nanosci. Nanotechnol. 2014, 14, 4911–4917. [Google Scholar] [CrossRef]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Poly(ε-Caprolactone) Nanocapsules Carrying the Herbicide Atrazine: Effect of Chitosan-Coating Agent on Physico-Chemical Stability and Herbicide Release Profile. Int. J. Environ. Sci. Technol. 2014, 11, 1691–1700. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of Poly(Epsilon-Caprolactone) Nanoparticles Containing Atrazine Herbicide as an Alternative Technique to Control Weeds and Reduce Damage to the Environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef]
- Diyanat, M.; Saeidian, H. The Metribuzin Herbicide in Polycaprolactone Nanocapsules Shows Less Plant Chromosome Aberration than Non-Encapsulated Metribuzin. Environ. Chem. Lett. 2019, 17, 1881–1888. [Google Scholar] [CrossRef]
- Preisler, A.C.; Pereira, A.E.S.; Campos, E.V.R.; Dalazen, G.; Fraceto, L.F.; Oliveira, H.C. Atrazine Nanoencapsulation Improves Pre-Emergence Herbicidal Activity against Bidens Pilosa without Enhancing Long-Term Residual Effect on Glycine Max. Pest Manag. Sci. 2020, 76, 141–149. [Google Scholar] [CrossRef]
- Sousa, B.T.; Santo Pereira, A.D.E.; Fraceto, L.F.; de Oliveira, H.C.; Dalazen, G. Effectiveness of Nanoatrazine in Post-Emergent Control of the Tolerant Weed Digitaria Insularis. J. Plant Prot. Res. 2020, 60, 185–192. [Google Scholar] [CrossRef]
- Babaei, S.; Kahrizi, D.; Nosratti, I.; Karimi, N.; Arkan, E.; Tahir, M.B. Preparation and Characterization of Chloridazon-Loaded Alginate/Chitosan Nanocapsules. Cell. Mol. Biol. 2022, 68, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Artusio, F.; Casà, D.; Granetto, M.; Tosco, T.; Pisano, R. Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes 2021, 9, 1641. [Google Scholar] [CrossRef]
- Faria, D.M.; Dourado Júnior, S.M.; Nascimento, J.P.L.D.; Nunes, E.D.S.; Marques, R.P.; Rossino, L.S.; Moreto, J.A. Development and Evaluation of a Controlled Release System of TBH Herbicide Using Alginate Microparticles. Mater. Res. 2016, 20, 225–235. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sarita; Sidhu, M.C.; Dilbaghi, N. Herbicide Loaded Carboxymethyl Cellulose Nanocapsules as Potential Carrier in Agrinanotechnology. Sci. Adv. Mater. 2015, 7, 1143–1148. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Nanoparticles Based on Chitosan as Carriers for the Combined Herbicides Imazapic and Imazapyr. Sci. Rep. 2016, 6, 19768–19781. [Google Scholar] [CrossRef]
- Gerardo, R.; de Lima, I.P. Monitoring Duckweeds (Lemna minor) in Small Rivers Using Sentinel-2 Satellite Imagery: Application of Vegetation and Water Indices to the Lis River (Portugal). Water 2022, 14, 2284. [Google Scholar] [CrossRef]
- Torbati, S.; Mahmoudian, M.; Alimirzaei, N. Nanocapsulation of Herbicide Haloxyfop-R-Methyl in Poly(Methyl Methacrylate): Phytotoxicological Effects of Pure Herbicide and Its Nanocapsulated Form on Duckweed as a Model Macrophyte. Turk. J. Chem. 2018, 42, 132–145. [Google Scholar] [CrossRef]
- Torbati, S.; Mahmoudian, M.; Alimirzaei, N. Toxicological Effects of a Post Emergent Herbicide on Spirodela Polyrhiza as a Model Macrophyte: A Comparison of the Effects of Pure and Nano-Capsulated Form of the Herbicide. Iran. J. Toxicol. 2018, 12, 45–54. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Torbati, S.; AliMirzayi, N.; Nozad, E.; Kochameshki, M.G.; Shokri, A. Preparation and Investigation of Poly(methylmethacrylate) Nano-Capsules Containing Haloxyfop-R-Methyl and Their Release Behavior. J. Environ. Sci. Health Part B 2019, 55, 301–309. [Google Scholar] [CrossRef]
- Falsini, S.; Clemente, I.; Papini, A.; Tani, C.; Schiff, S.; Salvatici, M.C.; Petruccelli, R.; Benelli, C.; Giordano, C.; Gonnelli, C.; et al. When Sustainable Nanochemistry Meets Agriculture: Lignin Nanocapsules for Bioactive Compound Delivery to Plantlets. ACS Sustain. Chem. Eng. 2019, 7, 19935–19942. [Google Scholar] [CrossRef]
- Synowiec, A.; Lenart-Boroń, A.; Bocianowski, J.; Lepiarczyk, A.; Kalemba, D. How Soil-Applied Maltodextrin with Caraway (Carum carvi L.) Oil Affects Weed and Soil Microbiological Activity in Maize (Zea mays L.) Stands. Pol. J. Environ. Stud. 2019, 29, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and Assessment of Nano-Encapsulated Bioherbicides Based on Biopolymers and Essential Oil. Ind. Crops Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Wing, R.E.; Maiti, S.; Doane, M. Factors Affecting Release of Butylate from Calcium Ion-Modified Starch-Borate Matrices. J. Control. Release 1987, 5, 79–89. [Google Scholar] [CrossRef]
- Shasha, B.S.; Trimnell, D.; Otey, F.H. Starch–Borate Complexes for EPTC Encapsulation. J. Appl. Polym. Sci. 1984, 29, 67–73. [Google Scholar] [CrossRef]
- Mejías, F.J.R.; Trasobares, S.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Macías, F.A. One-Step Encapsulation of Ortho-Disulfides in Functionalized Zinc MOF. Enabling Metal–Organic Frameworks in Agriculture. ACS Appl. Mater. Interfaces 2021, 13, 7997–8005. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.; Natarajan, S. Investigation of Pesticidal and Anti-Biofilm Potential of Calotropis Gigantea Latex Encapsulated Zeolitic Imidazole Nanoframeworks. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2771–2780. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Y.; Yang, X.; Ni, B.; Lü, S. MOF Hybrid for Long-Term Pest Management and Micronutrient Supply Triggered with Protease. ACS Appl. Mater. Interfaces 2022, 14, 17783–17793. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, S.; Yang, X.; Li, Y.; Lü, S. Facile Synthesis of the Dual Pesticide-Loaded Metal–Organic Framework Hybrid for PH-Responsive Release. ACS Agric. Sci. Technol. 2022, 2, 1267–1275. [Google Scholar] [CrossRef]
- Shan, Y.; Xu, C.; Zhang, H.; Chen, H.; Bilal, M.; Niu, S.; Cao, L.; Huang, Q. Polydopamine-Modified Metal–Organic Frameworks, NH2-Fe-MIL-101, as PH-Sensitive Nanocarriers for Controlled Pesticide Release. Nanomaterials 2020, 10, 2000. [Google Scholar] [CrossRef]
- Dong, J.; Chen, W.; Feng, J.; Liu, X.; Xu, Y.; Wang, C.; Yang, W.; Du, X. Facile, Smart, and Degradable Metal–Organic Framework Nanopesticides Gated with Fe III-Tannic Acid Networks in Response to Seven Biological and Environmental Stimuli. ACS Appl. Mater. Interfaces 2021, 13, 19507–19520. [Google Scholar] [CrossRef]
- Lunn, R.D.J.; Tocher, D.A.; Sidebottom, P.J.; Montgomery, M.G.; Keates, A.C.; Carmalt, C.J. Encapsulation of Aromatic Compounds and a Non-Aromatic Herbicide into a Gadolinium-Based Metal–Organic Framework via the Crystalline Sponge Method. Cryst. Growth Des. 2020, 20, 7238–7245. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, S.; Yang, Y.; Tong, Z.; Shen, T.; Yu, Z.; Xie, J.; Yao, Y.; Gao, B.; Li, Y.C.; et al. Development of Multifunctional Copper Alginate and Bio-Polyurethane Bilayer Coated Fertilizer: Controlled-Release, Selenium Supply and Antifungal. Int. J. Biol. Macromol. 2023, 224, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Song, J.; Dong, H.; Huo, Z.; Gao, Y.; Zhou, Z.; Tian, Y.; Li, Y.; Cao, Y. Fabrication of PH-Responsive Nanoparticles for High Efficiency Pyraclostrobin Delivery and Reducing Environmental Impact. Sci. Total Environ. 2021, 787, 147422. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, B.; Bindra, P.; Panneerselvam, P.; Dwivedi, N.; Senapati, A.; Adholeya, A.; Shanmugam, V. Triple-Smart Eco-Friendly Chili Anthracnose Control Agro-Nanocarrier. ACS Appl. Mater. Interfaces 2021, 13, 9143–9155. [Google Scholar] [CrossRef] [PubMed]
- Karthick Raja Namasivayam, S.; Aruna, A. Gokila Evaluation of Silver Nanoparticles-Chitosan Encapsulated Synthetic Herbicide Paraquate (AgNp-CS-PQ) Preparation for the Controlled Release and Improved Herbicidal Activity against Eichhornia crassipes. Res. J. Biotechnol. 2014, 9, 19–27. [Google Scholar]
- Dendrinou-Samara, C.; Alevizopoulou, L.; Iordanidis, L.; Samaras, E.; Kessissoglou, D.P. 15-MC-5 Manganese Metallacrowns Hosting Herbicide Complexes. Structure and Bioactivity. J. Inorg. Biochem. 2002, 89, 89–96. [Google Scholar] [CrossRef]
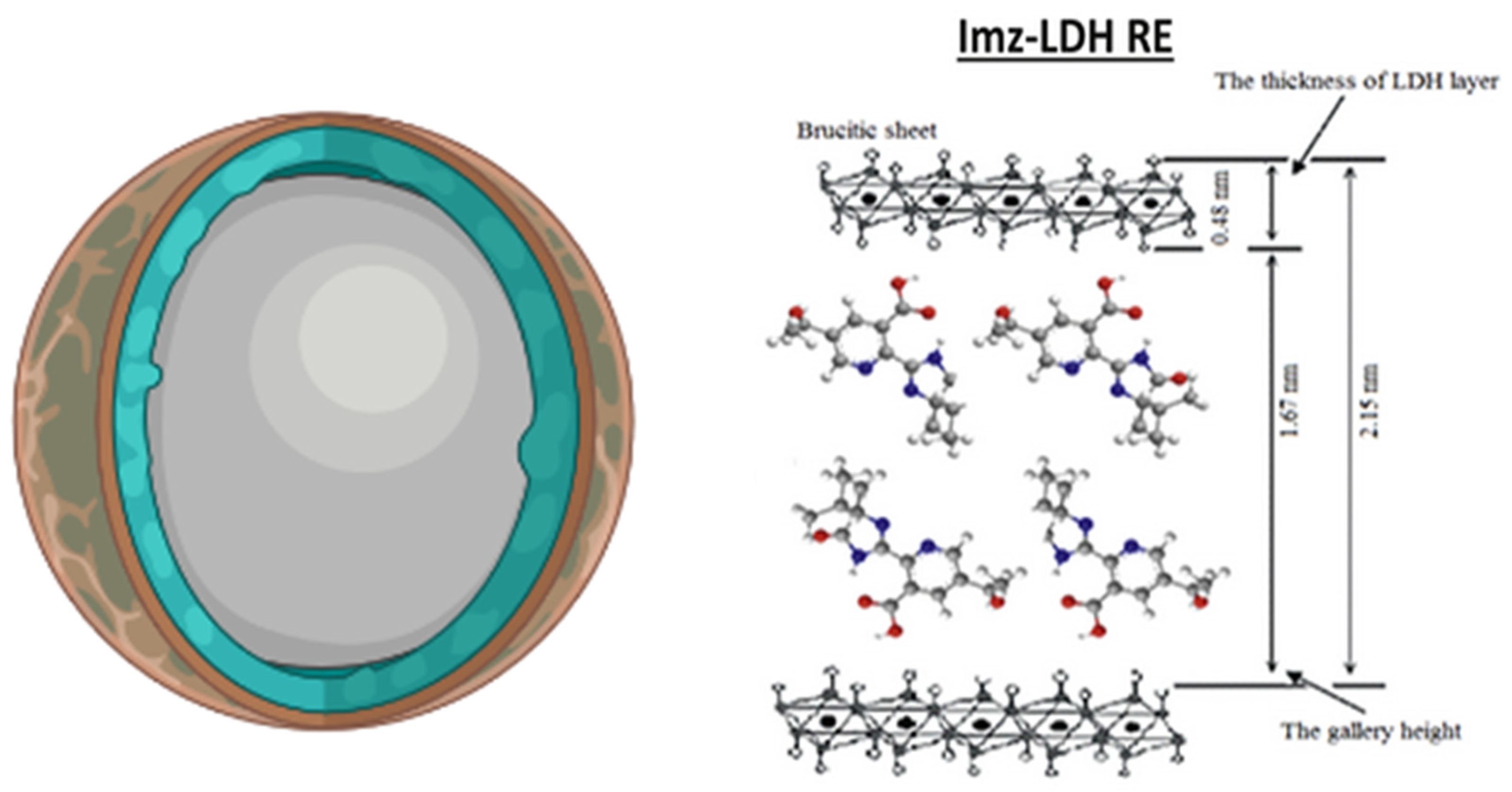
- Hussein, M.Z.; Hashim, N.; Yahaya, A.H.; Zainal, Z. Synthesis of an Herbicides-Inorganic Nanohybrid Compound by Ion Exchange-Intercalation of 3(2-Chlorophenoxy)Propionate into Layered Double Hydroxide. J. Exp. Nanosci. 2010, 5, 548–558. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y. Fabrication of Smart Stimuli-Responsive Mesoporous Organosilica Nano-Vehicles for Targeted Pesticide Delivery. J. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef]
- Sarlak, N.; Taherifar, A.; Salehi, F. Synthesis of Nanopesticides by Encapsulating Pesticide Nanoparticles Using Functionalized Carbon Nanotubes and Application of New Nanocomposite for Plant Disease Treatment. J. Agric. Food Chem. 2014, 62, 4833–4838. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of Carbon Nanotubes: A Review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Mejías, F.J.R.; Trasobares, S.; López-Haro, M.; Varela, R.M.; Molinillo, J.M.G.; Calvino, J.J.; Macías, F.A. In Situ Eco Encapsulation of Bioactive Agrochemicals within Fully Organic Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 41925–41934. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Paul, A.; Banerjee, R.; Bera, M.; Ikbal, M.; Dhara, D.; Singh, N.P. Photoresponsive Polymers Based on a Coumarin Moiety for the Controlled Release of Pesticide 2,4-D. RSC Adv. 2015, 5, 99968–99975. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Kavoosi, G. Development of Pre-Emergence Herbicide Based on Arabic Gum-Gelatin, Apple Pectin and Savory Essential Oil Nano-Particles: A Potential Green Alternative to Metribuzin. Int. J. Biol. Macromol. 2021, 167, 756–765. [Google Scholar] [CrossRef]
- Chariou, P.L.; Dogan, A.B.; Welsh, A.G.; Saidel, G.M.; Baskaran, H.; Steinmetz, N.F. Soil Mobility of Synthetic and Virus-Based Model Nanopesticides. Nat. Nanotechnol. 2019, 14, 712–718. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Mejías, F.J.; Scavo, A.; Chinchilla, N.; Molinillo, J.M.G.; Schwaiger, S.; Mauromicale, G.; Macías, F.A. Perspectives and Advances in Organic Formulations for Agriculture: Encapsulation of Herbicides for Weed Control. Agronomy 2023, 13, 1898. https://doi.org/10.3390/agronomy13071898
Rodríguez-Mejías FJ, Scavo A, Chinchilla N, Molinillo JMG, Schwaiger S, Mauromicale G, Macías FA. Perspectives and Advances in Organic Formulations for Agriculture: Encapsulation of Herbicides for Weed Control. Agronomy. 2023; 13(7):1898. https://doi.org/10.3390/agronomy13071898
Chicago/Turabian StyleRodríguez-Mejías, Francisco J., Aurelio Scavo, Nuria Chinchilla, José M. G. Molinillo, Stefan Schwaiger, Giovanni Mauromicale, and Francisco A. Macías. 2023. "Perspectives and Advances in Organic Formulations for Agriculture: Encapsulation of Herbicides for Weed Control" Agronomy 13, no. 7: 1898. https://doi.org/10.3390/agronomy13071898
APA StyleRodríguez-Mejías, F. J., Scavo, A., Chinchilla, N., Molinillo, J. M. G., Schwaiger, S., Mauromicale, G., & Macías, F. A. (2023). Perspectives and Advances in Organic Formulations for Agriculture: Encapsulation of Herbicides for Weed Control. Agronomy, 13(7), 1898. https://doi.org/10.3390/agronomy13071898