Abstract
Maize is one of the most important food and feed sources at the worldwide level. Due to this importance, all the pathogens that can infect this crop can harm both food safety and security. Fungi are the most important pathogens in cultivated maize, and Fusarium spp. are one of the most important families. Reduction in yield and production of dangerous mycotoxins are the main effects of Fusarium spp. infection. Fusarium graminearum (part of the Fusarium graminearum species complex) is one the most important fungi that infect maize, and it is the causative agent of Gibberella ear rot (GER). The main characteristics of this species include its ability to infect various species and its varying infection pressures across different years. This fungus produces various harmful mycotoxins, such as deoxynivalenol, zearalenone, butanolide, and culmorin. Infection can start from silk channels or from ear wounds. In the first case, the environmental conditions are the most important factors, but in the second, a key role is played by the feeding action of lepidopteran larvae (in Europe, Ostrinia nubilalis). All these factors need to be taken into account to develop a successful management strategy, starting from cropping methods that can reduce the source of inoculum to the direct control of the fungus with fungicide, as well as insect control to reduce ear wounds. But, the most important factor that can reduce the effects of this fungus is the use of resistant hybrids. Different studies have highlighted different defensive methods developed by the plant to reduce fungal infections, like fast drying of silk and kernels, chemical compounds produced by the plant after infection, and mechanical protection from insects’ wounds. The aim of this paper is to review the scientific evidence of the most important management strategies against GER in maize and to highlight the genetic basis which is behind hybrid resistance to this disease, with a focus on genes and QTLs found in studies conducted across the world and with different types of maize from tropical cultivars to European flint.
1. Introduction
Maize (Zea mays L.) is one of the most important cultivated crops. It is the cereal that has seen the highest increase in production rate due to the high demand for maize plant products as important food resources for animals and humans, and as raw materials for use in industry and biofuels [1]. However, cultivation methods (mono-cropping) and poor gene heterogeneity in commercial hybrids have led to a serious problem of disease susceptibility [2,3]. Like all the other crops, maize has a great number of pathogens, among which fungi are some of the most critical [4]. It has been estimated that in the last decade, the average yield loss due to these pathogens ranged from 6.8% to 13.5% [5] in the USA and Canada. Fungal pathogens of maize are relevant not only for the direct damage they can cause to the plant, but also for the ability of many of these pathogens (Aspergillus spp. and Fusarium spp.) to produce mycotoxins [6] (Figure 1). The presence of mycotoxins in corn products (e.g., kernels and silage) can reach almost 100% of the examined samples, due to the large number of possibly toxicogenic fungi that can infect this species [7].

Figure 1.
Fusarium ear rot caused by Fusarium verticilloides, Gibberella ear rot caused by Fusarium graminearum, Diplodia ear rot caused by Stenocarpella maydis, and Aspergillum ear rot caused by Aspergillus flavus. (A) Fusarium ear rot; (B) Gibberella ear rot; (C) Diplodia ear rot; and (D) Aspergillum ear rot.
Several Fusarium species are known to infect maize, and among them, F. graminearum Schwabe is one of the most important pathogens. This fungus is sometimes still reported with its teleomorph name of Gibberella zeae (Schw.) Petch (Ascomycota). The complex biology of this pathogen has led researchers to define it, rather than as a single species, as the Fusarium graminearum species complex (FGSC) [8]. The Fusarium graminearum species complex is composed of 16 species: F. acaciae-mearnsii, F. aethiopicum, F. asiaticum, F. austroamericanum, F. boothii, F. brasilicum, F. cortaderiae, F. gerlachii, F. graminearum sensu stricto, F. louisianense, F. meridionale, F. mesoamericanum, F. nepalense, F. ussurianum, F. vorosii, and another one that is not yet formally described [9]. The most common species of the FGSC that affects cereals is F. graminearum, distributed at a worldwide level. Other important species in cereals are Fusarium asiaticum, the most common member of the complex on rice in Asia [10], which is now also present in rice in South America and the USA [11,12]; Fusarium meridionale, which is more prevalent in maize in South America; and Fusarium boothii, which is the most common species of this complex in South African maize [13]. The reasons for the dominance of one species over the others are not clear, but are correlated to differences in aggressiveness [14] and adaptation to different environments [15]. Other studies consider this fungus not only as part of the Fusarium graminearum species complex but also in association with F. verticillioides [16]. These two fungi have a complicated interaction, and the presence of one can reduce the effect of the other, but the symptoms in most cases are difficult to distinguish [17] unless the F. verticillioides-associated “starburst” is present, leading researchers to study these fungi together. In this review, considering the specific context of breeding resistance traits, F. graminearum will be considered as a single species associated with a single disease (Gibberella ear rot), and interaction with other species will be put into the background in order to focus only on the specific effects that this fungus causes in maize.
2. Fusarium graminearum (Schw.)
Fusarium graminearum (Ascomycota), also known as Gibberella zeae in its sexual stage, is a fungal plant pathogen diffused around the world (Figure 2).
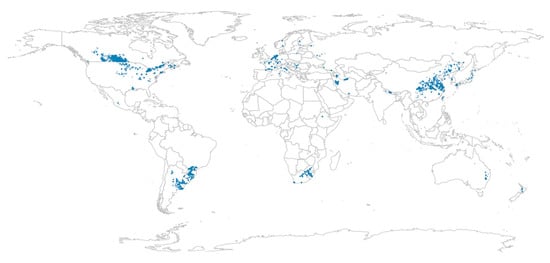
Figure 2.
Countries where Gibberella zeae has been documented (blue dots). Modified from Del Ponte [8].
Fusarium graminearum is a homothallic and self-fertile fungus. It can have both sexual and asexual life cycles. In its diploid stage, it consists of a fruiting body (perithecium) where ascospores are formed in asci and released in spring [18]. During the haploid phase, it consists of a filamentous hypha and produces mitotic spores (macroconidia). Both ascospores and macroconidia are known as sources of infection [19,20]. It can grow at temperatures between 15 and 29 °C, but when the temperature is higher than 30 °C, its development is very limited [21,22], while on the contrary, some studies have suggested that it can even grow at temperatures below 15 °C [23,24] with a limit of 8 °C for perithecia production [25]. Fusarium verticillioides, another pathogen commonly found in cereals, has a higher temperature optimum with a peak development around 27 °C, and it can produce spores even at 45 °C [26]. F. graminearum can be found in a large number of cereal grains such as wheat, barley, maize, oats, rice, and rye [27,28]. This fungus can also infect other plant genera like Pisum, Trifolium, Solanum, and Coffea [29,30]. It causes a wide range of diseases in various crops, such as head blights in wheat, tuber dry rot in potatoes, and pitch canker of Pinus species [28,29,31,32].
Fusarium graminearum is the causative agent of Gibberella ear rot (GER), which is a key maize disease in temperate regions. It appears as a reddish mold and affects the ear, starting from the tip (Figure 1). Infection can occur starting from the cob or from kernel wounds. When the fungus infects the cob, it appears as a white mycelium, and this turns into a red-pink mold. In severe cases, it can also grow on the husk leaves. In this situation, the husk, cob, and kernels become tightly bound together by the fungal mass, and they are not separable. The infection from the kernel wound has a similar development, but in this case, it seems that the fungus spreads to the top of the ear faster than to the bottom [33]. The amount of yield loss experienced in maize can significantly differ from season to season. According to Sutton’s research, there have been years in Canada where serious epidemics of GER (Gibberella ear rot) occurred, while other years saw a much lower impact of this disease [34]. A similar pattern was observed in the United States, as noted by Wetter [35]. In the case of wheat and barley, the culprit behind yield loss is fusarium head blight (FHB) [32,34], a devastating disease that has resulted in losses amounting to tens of billions of dollars over the past two decades in the United States [24,36]. Ascospores are produced from the perithecia outwinter in maize, other cereals’ residues, and also a wide range of mono and dicotyledonous weeds (Figure 3) [37,38,39] when temperatures rise to 13 °C mainly dispersed at night [40].
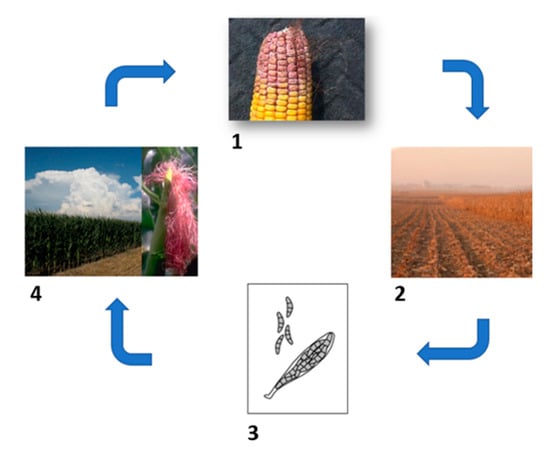
Figure 3.
Gibberella zeae life cycle. (1) F. graminearum appear as a pink or reddish ear mold. (2) Inoculum outwinters in infected crop residues like corn and wheat. (3) Fusarium graminearum can grow at temperatures between 15 and 29 °C, and it produces spores starting from 13 °C. (4) Infection occurs at flowering via silk or insect damage.
In corn, infection occurs during the silking period. Ears are more susceptible between two and six days after the emergence of the silk, and the peak of susceptibility is during their senescence [41]. The most common propagation agents of F. graminearum are rain, wind, and insects [42,43]. One of the most important insects that are correlated with more severe GER infections is the larva of the European corn borer moth (Ostrinia nubilalis Hübner) (Lepidoptera: Crambidae). Tunneling and kernel wounds during the feeding of the larvae can favor the infection by this fungus. The larvae can also spread the propagule with their movements inside the plant [44]. In temperate regions, O. nubilalis is normally bivoltine during the maize growing season, but a small number of univoltines or multivoltines can grow depending on the weather [45]. The first-generation larvae usually produce damage to leaves, while the second generation develops by feeding on the stalk or the ears. Tunnels below the ear cause breakage, while apical tunnels in the cob are linked to fungal infection due to a particular microclimatic condition that can promote fungal development [46].
3. Mycotoxins
Reduction in the yield is not the only damage caused by GER: Fusarium graminearum is also known to be a mycotoxigenic fungus. Infected maize ears can develop various types of mycotoxins, among which there are some well-established classes, such as aflatoxins, ochratoxins, trichothecenes, fumonisins, and zearalenone, and groups of minor, less-characterized, or emerging toxins for a total of over 30 different mycotoxin types [7,9,47]. The most important are deoxynivalenol and zearalenone, which cause poor livestock performance, particularly in swine. Deoxynivalenol causes feed refusal, vomiting, and decreased weight gain, while zearalenone causes reproductive problems [48,49]. Mycotoxins are secondary metabolites of fungi that have toxic properties to animals and humans [21,50], and they are produced by fungi when these organisms invade crops or their derived products [51,52] (Table 1).

Table 1.
Comparison of mycotoxins produced by F. graminearum and F. verticillioides. Fusarium nomenclature according to Nelson [31].
Due to the risks associated with the intake of mycotoxin-contaminated cereals, different countries and agencies, such as the FAO, FDA, and EFSA, have established regulations to limit their presence in both feed and food. For example, the European Union sets a maximum amount and guidance level for some mycotoxins in grain and derived products [57]. While other fungi of the same genus, such as Fusarium verticillioides (Nirenberg), produce mostly fumonisins [54], F. graminearum produces various different types of toxins, of which the most important are deoxynivalenol or vomitoxin (DON), zearalenone (ZEN), butanolide (BUT), and culmorin (CUL) [9,53].
4. Deoxynivalenol
Deoxynivalenol (DON) is a mycotoxin of the trichothecene family [58]. DON and its derivates 3-acetyldeoxynivalenol (3-ADON) and 15-acetyldeoxynivalenol (15-ADON) can be produced on many cereals like corn, wheat, barley, and rice, but also on oats, rye, and sorghum [8]. DON can be produced and accumulates both in the kernels and in the stalk of maize, depending on where the fungus infects the maize plant and, unlike in wheat, it does not seem that the toxin can be transported systemically through different plant organs [59]. Fusarium graminearum and Fusarium culmorum are the two most important species that produce this toxin. Both species possess strains capable of producing deoxynivalenol (DON) as well as other toxins as their primary metabolites [60]. Both acute and chronic toxicity are associated with DON ingestion. Acute toxicity affects mostly the intestinal mucosa. Overproduction of ROS and reduced respiratory capacities in mitochondria of the host cells and intestinal microbes are the two major causes of this toxicity [61]. The chronic effect is correlated with immune system suppression caused by the inhibition of mitophagy [62]. Other effects of DON are damage to the respiratory system that can lead to asthma [63] and alteration in the expression of MAPK (mitogen-activated protein kinase) proteins that are involved in the control of cell apoptosis, differentiation, and cell growth [64]. Recent studies linked these effects to indirect damage caused by DON to mitochondria, which will ultimately lead to cell death [65].
5. Zearalenone
Zearalenone (ZEN) is a non-steroidal estrogenic mycotoxin [66]. ZEN has a crystalline structure; it is insoluble in water, is heat stable, and has a melting point of 164–165 °C [67,68]. Like DON, Fusarium graminearum and Fusarium culmorum are the two most important zearalenone fungal producer species [53]. ZEN has been found in all the most important cultivated cereals and some legumes [69,70,71]. Zearalenone’s estrogen-like effects can cause fertility disorders both in humans and animals [72]. At high doses, ZEN induces an overproduction of ROS, thus can lead to oxidative stress. This stress can be correlated with DNA damage and mitochondrial degeneration that can lead to cell apoptosis [73,74]. At lower doses, ZEN is known for its carcinogenic activity in the liver and reproductive system [75].
6. Butenolide
Butenolide (4-acetamido-4-hydroxy-2-butenoic acid lactone or BUT) is a secondary metabolite usually co-produced with other mycotoxins (mostly deoxynivalenol) by different Fusarium species, mostly F. sporotrichioides and F. graminearum [53,76]. BUT is considered an emerging mycotoxin: this classification is used to define all the mycotoxins that are not legislatively regulated but have an important and increasing presence in feed and food [77]. This mycotoxin is associated with the cattle disease known as fescue foot [78,79]: it has been demonstrated to cause damage at the digestive system level due to significant cytotoxic effects caused by oxidative stress and oxidative damage [80,81]. In contrast, its toxicity in the long-term and at lower dosages has not yet been thoroughly studied, and more data are needed [82].
7. Culmorin
Culmorin (CUL) is a tricyclic sesquiterpene diol. Like butanolide and other compounds, it is considered an emerging mycotoxin since it is frequently observed, even in high concentrations, in grain and cereal-based products [82,83,84]. F. culmorum and F. graminearum are the two most important CUL producers [53]. A high concentration of this mycotoxin in contaminated grain correlates positively with the DON amount [85]. Taken singly, it seems that this compound does not affect animals or insects [86], but studies demonstrate that it can increase the toxicity of deoxynivalenol. CUL inhibits the glycosylation of DON, which produces less toxic compounds [86,87].
8. Management Strategies to Reduce Infection
Control methods to reduce or mitigate production and quality loss in maize caused by F. graminearum can be divided into two broad categories: direct methods that prevent the spread of fungus and infection via the use of synthetic or biological fungicides and/or insecticides, or indirect methods that include the reduction in plant stress or increasing the production of secondary metabolites to prevent the fungal infections via techniques like cropping practices and hybrid selection (Figure 4).
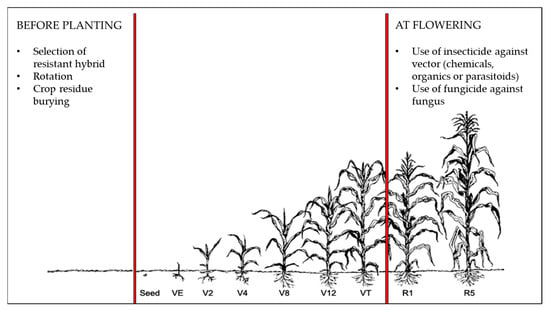
Figure 4.
Controls methods in corn cultivation to reduce the impact of Gibberella ear rot (GER). Modified from Lancashire [88].
These control methods may not always be allowed or viable in different areas, as climatic conditions may pose limitations, and different policies in different countries may prohibit or encourage the use of some methods. Fungicide and insecticide applications are not always available, mostly due to country regulations. Cropping systems that reduce the fungus inoculum, like a crop rotation [43,89], are often not employed, despite the evident advantages that this cropping method can bring [90].
9. Control with Synthetic Fungicides
Worldwide regulations on the use of fungicides on corn can differ a lot, and they can change over the years. While certain countries, such as those in the EU, do not have any registered products specifically designed to control Gibberella ear rot, other regions in the world, particularly in South and North America, have witnessed a growing trend in the use of foliar fungicides over the past two decades. Different commercial products are available for Gibberella ear rot management in corn [91,92]. The most used active ingredients for the control of GER are prothioconazole and quinone outside inhibitors (QoI). Prothioconazole is a demethylation-inhibiting (DMI) fungicide that interferes with the biosynthesis of ergosterol, a precursor of vitamin D2 and a crucial component of fungal cell walls [93,94]. Quinone outside inhibitors (QoI) are a group of compounds, such as strobilurins, which are active against the protein complex that produces ATP in the fungal cell’s membrane, leading to cell death. In particular, QoIs inhibit the transfer of electrons between cytochrome b and cytochrome c1 by the binding of the outer quinol oxidation site (Qo site) [95]. Both groups of fungicides are already used to control Fusarium head blight in wheat with different efficacies, where prothioconazole and other triazoles have a better control effect compared to strobilurins [96,97,98,99], but in corn, different studies reported that even though these compounds can control symptoms, there are contrasting results for the reduction in mycotoxin levels [98,100,101]. The biggest differences were found in the timing of application of DMI fungicides. The most efficient time of application is at flowering (VT-R2), because most of the available products for this class of fungicide are not fully systemic, and the active ingredient is not able to move from the uptake site to the newly grown tissue [101,102]. Also, DMI fungicides are more efficient in wheat compared to corn, and this could be caused by the husks covering the corn ear, preventing full penetration by the DMI, while the pathogen bypasses this protection by entering via the silks [101]. The availability of only two classes of fungicide may cause the quick development of resistance to these active ingredients in fungal strains placed under strong selective pressure in the field. Studies have already demonstrated the presence of resistance to these fungicides in species like Cercospora beticola, Mycosphaerella graminicola, Blumeria graminis, and others [94,95,102,103]
10. Insecticide against a Vector
The regulations and laws regarding insecticides can vary significantly between countries. However, unlike fungicides used against the pathogen, a range of insecticides are employed to combat Lepidoptera, such as Ostrinia nubilalis (Hübner) or O. furnacalis (Guenée), which feed on maize. These insecticides primarily belong to the following classes: pyrethroids, organophosphates, carbamates, and anthranilic diamides. Since, as previously stated, fungicide treatments can have a different degree of success regarding the accumulation of mycotoxins, it is often more effective to reduce the damage caused by these fungi by focusing on controlling the insect pests that can facilitate the infection [104]. Pyrethroids are synthetic insecticides derived from pyrethrin, a natural insecticide active against both adults and larvae [46]. Their mode of action is described as preventing the closure of voltage-sensitive sodium channels, causing inactivation of nerves and leading to complete paralysis [105]. Organophosphates inhibit the action of acetylcholinesterase and are also effective against adults and larvae. By preventing the degradation of acetylcholine, a neurotransmitter, these compounds keep synapses in a hyperexcited state, resulting in paralysis [106,107]. Carbamates are another class of compounds that are active against the enzyme acetylcholinesterase, and therefore act in a very similar manner to organophosphates [105]. The fourth group of insecticide compounds active against lepidopteran larvae are anthranilic diamides. These compounds cause paralysis of the insect via a different mechanism, affecting the calcium reserves in muscular cells by deregulation of the channels associated with the ryanodine receptor (RyR) [108,109]. The use of an insecticide active against lepidopteran larvae is one of the most important practices to reduce fungal infection and mycotoxin production, especially in countries where fungicides are not available or in a country where GMOs are not permitted [44,110]. For insects, like fungi, resistance to active substances is a reality and is promoted by incorrect insecticide management or uninterrupted usage of insecticides with the same mode of action [111].
11. Biological Control
The use of synthetic fungicides or insecticides is not the only method to control F. graminearum infection. According to reports from Reference [112], various approaches that have been successfully utilized and commercially implemented for other crops and against different fungi have been tested for controlling F. graminearum infection. They include the use of plant-associated or endophytic micro-organisms, plant growth-promoting bacteria, nontoxigenic fungal strains, and plant-derived products, which have been tested in recent decades and have proven effective in controlling both symptoms and mycotoxin production. However, no resulting commercial products are yet available on the market at the current date. Another important approach toward successful biological control is targeting O. nubilalis. In countries where GMOs that express Cry toxin-related genes from Bacillus thuringiensis Berliner (Bt) are permitted, the efficacy of control against ECB and mycotoxin level has proven to be effective [113,114]. In other countries where GMOs are forbidden, there is the possibility of using isolated BT toxins as insecticides [115]. These Cry toxins form pores in the guts of the insects, at first stopping the feeding of the insect and ultimately leading to its death, usually by septicemia. There are different Cry toxins with specificity for different insect groups but, as for other types of insecticides, resistance mechanisms can be developed against this toxin. Resistance in ECB was found to be caused by a mutation in a gut protease, preventing the conversion of the toxin crystalline form into active, monomeric molecules [116]. ECB biological control can also be achieved with the use of parasitoid insects. Trichogramma spp. (Hymenoptera), egg parasitoids, are one of the most-used parasitoids to control ECB [117]. In recent decades, different release and distribution methods have been tested, and today, with the introduction of unmanned aerial vehicles in agriculture, the efficacy and feasibility of the use of parasitoid insects have been facilitated [118]. Trichogramma spp. is also considered an important factor in the management of BT toxin-resistant insects [119].
12. Cropping Methods
Agronomic practices are fundamental to achieving the highest production in a given environment. They are also one of the most important control methods to reduce the impact of different corn diseases. The most common practices used to control diseases are tillage, crop rotation, optimization of plant density and sowing date, harvest time, and all the agronomic strategies to reduce stress during the whole life cycle of the crop, like irrigation and fertilization. Crop residue management is one of the most important methods to reduce the source of inoculum, since this fungus overwinters in maize stalks and other cereal debris [37,38]. A crop rotation with non-host species is a common strategy for the management of Fusarium graminearum in wheat [43,89,120]. In corn, it has been demonstrated that a succession of susceptible species increases the infection rate and the symptoms of this disease [28,121,122]. The positive effects of crop rotations are also related to tillage methods. Conventional tillage associated with plowing is effective in the control of this pathogen in comparison with reduced or no tillage. The burying of crop residues accelerates their decomposition, and the subsequent underground microbial activities are effective in the reduction of inoculum density [28,122]. Of course, the mineralization of debris primarily depends on various agronomic conditions and geographical locations worldwide [123]. Planting date is another important factor in the control of different fungal diseases, including GER. Late planting is associated with higher fungal presence; this brings a synchronization of flowering and ECB presence, resulting in greater insect damage and, consequently, higher infection rates [124,125]. Another factor that can affect F. graminearum infection is plant density. Higher densities are associated with higher grain contamination. Correct planting density for every cultivated area cannot be easily established because it is affected by the environment, including both persistent and seasonal factors, and different corn genetics that can be more or less suited for a high planting density [126]. In conclusion, different agronomic methods can be applied to reduce the effect of this disease, but the use of resistant hybrids is the most important method to have led to better production in terms of quantity and quality.
13. Hybrid Selection
The use of a resistant variety that does not present symptoms is considered the best practice to reduce GER infection. However, at present, the market can only offer hybrids with a varied range of tolerance, from the ones that present few symptoms to the ones that are severely infected. Even if, during the selection of new maize cultivars, the very susceptible ones are discarded, it’s not uncommon to find farmers’ fields with infection rates that are above the legal limit in terms of GER-correlated mycotoxins [127]. This is probably due to the fact that GER resistance is a complex quantitative trait, and the actual resistance is influenced by the genotype × environment interaction [128]. However, several studies have reported both dominant and additive genetic effects correlated with GER resistance [129,130,131]. Different ear defense mechanisms against fungal infection have been reported. These mechanisms are associated with silk resistance and resistance to the spread of the fungus among the kernels. It is important to note that these two mechanisms are under separate genetic control [27,132]. Kernel resistance is associated with fast drying [133], while silk resistance is associated with faster silk abscission and larger abscission zones [134]. Another type of resistance is associated with the production of defense chemical compounds like maysin and other phenolic compounds associated with antifungal activities. Maysin is a flavone glycoside active in the suppression of insects such as Helicoverpa zea (Boddie) and others in maize like Sitophilus zeamais (Motschulsky), Euschistus servus (Say), and Nezara viridula (L.) [135]. The reduction in insect damage is correlated with a reduction in fungal infection [136,137]. Phenolic compounds are produced in corn as a response to fungal infection. It seems that a more resistant variety produces more of this type of compound compared to the susceptible ones. These compounds can also oxidate to produce quinones with an even greater antifungal effect [138,139]. Other important compounds effective against fungal infection are carotenoids. In corn, zeaxanthin has been demonstrated to be effective in the inhibition of DON production due to its effect on the DON biosynthetic pathway [140]. Physical defenses are another type of resistance, and the two major characteristics in maize correlated with the reduction in GER damage are husk tightness and ear attitude. Tight husk germplasms are correlated with a higher GER susceptibility, probably because a favorable microenvironment to fungal proliferation develops inside the ear after heavy rain [141,142,143]. Another ear characteristic associated with resistance to ear rot is the attitude: a pendant ear attitude is correlated to lower susceptibility to ear rot [127]. To understand the genetic aspects that are behind the phenotypical characteristics associated with GER resistance, various studies have been conducted in recent decades (Table 2).

Table 2.
QTLs associated with GER resistance found on different corn materials.
A study conducted in Canada that evaluated 144 F2s derived from a cross between one resistant inbred line and a susceptible one found that there was no overlap in the 11 QTLs associated with silk resistance and the 18 QTLs for kernel resistance. Out of the 11 QTLs for silk resistance, 4 were located in chromosome 1, 4 QTLs were on chromosome 7, 2 QTLs were on chromosome 3, and 1 QTL was on chromosome 6. For the QTLs associated with kernel resistance, five QTLs were located on chromosome 7; three QTLs were each on chromosomes 1, 2, and 5; and one QTL was on chromosomes 3, 4, 6, and 9 [132]. In another study of the difference between QTLs associated with GER resistance in the dent and flint materials, similarities in the Manhattan plot for GER resistance and DON accumulation were found, suggesting a possible correlation with fungal resistance and DON concentration [145]. In this study, markers associated with DON resistance for the dent and flint were found in different chromosomes. For the dent materials, the two SNPs were found on chromosomes 2 and 5, and for the flint materials, the six SNPs were located on chromosomes 1, 3, 7, and 9 (Table 3).

Table 3.
SNP markers associated with DON resistance [145].
Other studies with different types of materials like European landraces [144], Chinese inbreds [146,148], Argentinian genotypes [149], and crosses between European and Brazilian inbreds [147] found various QTLs associated with GER resistance in almost all the chromosomes, with considerable difference in the position and number of markers found (Table 4).

Table 4.
Position and effect of different SNPs studied in four different studies. pG (%): additive effects and proportion of explained genotypic variance.
This can only confirm the nature of quantitative traits of GER resistance. Despite the challenges faced, a study aimed at identifying genes linked to this resistance was carried out, leading to the discovery of four genes located on chromosome 2 that showed a correlation with kernel resistance [150].
14. Conclusions
Interactions between Fusarium graminearum and corn are complex, and a great number of factors can contribute to the development of infection or resistance of the corn plant. Differences between cropping seasons seem to have a great impact on the damage caused by this disease [34,35]. Cropping methods are another important factor, but in this case, useful actions to control GER can be difficult to implement, due to economic sustainability, like a rotation, or on the contrary, environmentally sustainable methods like no tillage can increase the impact of this disease [28,89]. In the end, the use of insecticides to control the vector or fungicide to directly control the fungus may not be economically convenient and can cause the development of resistant populations [102,103,111]. The use of genetically modified organisms can be useful to control the vector, but they are not available everywhere [113,114]. Biological methods to control this fungus are still in development, and no commercial products are available [112].
In conclusion, the selection of resistant hybrids is one of the most important and viable control methods. Hybrids with greater resistance will permit a reduction in the use of pesticides and, therefore, make the development of resistant pests less likely (both fungi and insects). The use of modern breeding technologies like genome prediction and marker-assisted selection can improve the development of more resistant materials in the framework of more sustainable agriculture.
Author Contributions
Conceptualization, R.P., P.C. and A.M.; writing—original draft preparation, A.M. and A.P.; writing—review and editing, P.C., M.G. and R.P.; visualization, M.G.; supervision, R.P., P.C. and A.P.; funding acquisition, R.P. All authors have read and agreed to the published version of the manuscript.
Funding
Funded by the Agritech National Research Centre. R.P. received funding from the European Union NextGenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)–MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4–D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Acknowledgments
We wish to thank Lesley Currah for her editing and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Strom, N.; Hu, W.; Haarith, D.; Chen, S.; Bushley, K. Interactions between Soil Properties, Fungal Communities, the Soybean Cyst Nematode, and Crop Yield under Continuous Corn and Soybean Monoculture. Appl. Soil Ecol. 2020, 147, 103388. [Google Scholar] [CrossRef]
- Liu, K.; Goodman, M.; Muse, S.; Smith, J.S.; Buckler, E.; Doebley, J. Genetic Structure and Diversity Among Maize Inbred Lines as Inferred from DNA Microsatellites. Genetics 2003, 165, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bissonnette, K.M.; Bradley, C.A.; Byamukama, E.; Chilvers, M.I.; Collins, A.A.; et al. Corn Yield Loss Estimates Due to Diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 2020, 21, 238–247. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in Corn: Occurrence, Impacts, and Management. In Corn; Elsevier: Amsterdam, The Netherlands, 2019; pp. 235–287. [Google Scholar]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium graminearum Species Complex: A Bibliographic Analysis and Web-Accessible Database for Global Mapping of Species and Trichothecene Toxin Chemotypes. Phytopathology 2022, 112, 741–751. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Yang, X.; Yang, J.; Gong, A.; Zhang, J.; Chen, L.; Zhou, C. Fusarium graminearum Species Complex and Trichothecene Genotype. In Mycotoxins and Food Safety; IntechOpen: London, UK, 2020. [Google Scholar]
- Lee, S.-H.; Lee, J.-K.; Nam, Y.-J.; Lee, S.-H.; Ryu, J.-G.; Lee, T. Population Structure of Fusarium graminearum from Maize and Rice in 2009 in Korea. Plant Pathol. J. 2010, 26, 321–327. [Google Scholar] [CrossRef]
- Gale, L.R.; Harrison, S.A.; Ward, T.J.; O’Donnell, K.; Milus, E.A.; Gale, S.W.; Kistler, H.C. Nivalenol-Type Populations of Fusarium graminearum and F. asiaticum Are Prevalent on Wheat in Southern Louisiana. Phytopathology 2011, 101, 124–134. [Google Scholar] [CrossRef]
- Gomes, L.B.; Ward, T.J.; Badiale-Furlong, E.; Del Ponte, E.M. Species Composition, Toxigenic Potential and Pathogenicity of Fusarium graminearum Species Complex Isolates from Southern Brazilian Rice. Plant Pathol. 2015, 64, 980–987. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Ward, T.J.; Van Coller, G.J.; Flett, B.; Lamprecht, S.C.; O’Donnell, K.; Viljoen, A. Analysis of the Fusarium graminearum Species Complex from Wheat, Barley and Maize in South Africa Provides Evidence of Species-Specific Differences in Host Preference. Fungal Genet. Biol. 2011, 48, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.J.; Kuhnem, P.R.; Casa, R.T.; McMaster, N.; Schmale, D.G.; Vaillancourt, L.J.; Del Ponte, E.M. The Dominance of Fusarium meridionale Over F. graminearum Causing Gibberella Ear Rot in Brazil May Be Due to Increased Aggressiveness and Competitiveness. Phytopathology 2021, 111, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, D. Global Distribution of Fusarium graminearum, F. asiaticum and F. boothii from Wheat in Relation to Climate. Eur. J. Plant Pathol. 2014, 139, 161–173. [Google Scholar] [CrossRef]
- Picot, A.; Hourcade-Marcolla, D.; Barreau, C.; Pinson-Gadais, L.; Caron, D.; Richard-Forget, F.; Lannou, C. Interactions between Fusarium verticillioides and Fusarium graminearum in Maize Ears and Consequences for Fungal Development and Mycotoxin Accumulation. Plant Pathol. 2012, 61, 140–151. [Google Scholar] [CrossRef]
- Xu, X.; Nicholson, P.; Ritieni, A. Effects of Fungal Interactions among Fusarium Head Blight Pathogens on Disease Development and Mycotoxin Accumulation. Int. J. Food Microbiol. 2007, 119, 67–71. [Google Scholar] [CrossRef]
- Trail, F.; Xu, J.R.; San Miguel, P.; Halgren, R.G.; Kistler, H.C. Analysis of Expressed Sequence Tags from Gibberella zeae (Anamorph Fusarium graminearum). Fungal Genet. Biol. 2003, 38, 187–197. [Google Scholar] [CrossRef]
- Cavinder, B.; Sikhakolli, U.; Fellows, K.M.; Trail, F. Sexual Development and Ascospore Discharge in Fusarium graminearum. J. Vis. Exp. 2012, 61, e3895. [Google Scholar] [CrossRef]
- Doohan, F.M.; Brennan, J.; Cooke, B.M. Influence of Climatic Factors on Fusarium Species Pathogenic to Cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Rossi, F.; Gallo, A.; Bertuzzi, T. Emerging Mycotoxins in the Food Chain. Med. J. Nutr. Metab. 2020, 13, 7–27. [Google Scholar] [CrossRef]
- Lenc, L. Fusarium Head Blight (FHB) and Fusarium Populations in Grain of Winter Wheat Grown in Different Cultivation Systems. J. Plant Prot. Res. 2015, 55, 94–109. [Google Scholar] [CrossRef]
- Pereyra, S.A.; Dill-Macky, R.; Sims, A.L. Survival and Inoculum Production of Gibberella zeae in Wheat Residue. Plant Dis. 2004, 88, 724–730. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; de Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; van Sanford, D. A Unified Effort to Fight an Enemy of Wheat and Barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Dufault, N.S.; de Wolf, E.D.; Lipps, P.E.; Madden, L.V. Role of Temperature and Moisture in the Production and Maturation of Gibberella zeae Perithecia. Plant Dis. 2006, 90, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Scandolara, A.; Battilani, P. Effect of Environmental Conditions on Spore Production by Fusarium verticillioides, the Causal Agent of Maize Ear Rot. Eur. J. Plant Pathol. 2009, 123, 159–169. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Lemmens, M.; Reid, L.M. Breeding for Resistance to Ear Rots Caused by Fusarium Spp. in Maize—A Review. Plant Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of Environmental Conditions and Agronomic Practices on the Prevalence of Fusarium Species Associated with Ear- and Stalk Rot in Maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Agricultural Bureaux [for the] Commonwealth Mycological Institute: Oxfordshire, UK, 1971. [Google Scholar]
- Taheri, P. Cereal Diseases Caused by Fusarium graminearum: From Biology of the Pathogen to Oxidative Burst-Related Host Defense Responses. Eur. J. Plant Pathol. 2018, 152, 1–20. [Google Scholar] [CrossRef]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Penn State University Press: University Park, PA, USA, 1983. [Google Scholar]
- Valverde-Bogantes, E.; Bianchini, A.; Herr, J.R.; Rose, D.J.; Wegulo, S.N.; Hallen-Adams, H.E. Recent Population Changes of Fusarium Head Blight Pathogens: Drivers and Implications. Can. J. Plant Pathol. 2020, 42, 315–329. [Google Scholar] [CrossRef]
- Reid, L.M.; Sinha, R.C. Maize Maturity and the Development of Gibberella Ear Rot Symptoms and Deoxynivalenol after Inoculation. Eur. J. Plant Pathol. 1998, 104, 147–154. [Google Scholar] [CrossRef]
- Sutton, J.C. Epidemiology of Wheat Head Blight and Maize Ear Rot Caused by Fusarium graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Wetter, M.T. Occurrence and Distribution of Fusarium graminearum and Deoxynivalenol in Sweet Corn Ears. Food Addit. Contam. 1999, 16, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Burgess, L.; Griffin, D. The Recovery of Gibberella Zeae from Wheat Straws. Aust. J. Exp. Agric. 1968, 8, 364. [Google Scholar] [CrossRef]
- Pereyra, S.A.; Dill-Macky, R. Colonization of the Residues of Diverse Plant Species by Gibberella zeae and Their Contribution to Fusarium Head Blight Inoculum. Plant Dis. 2008, 92, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Kelpsiene, J.; Pranaitiene, S.; et al. Asymptomatic Weeds Are Frequently Colonised by Pathogenic Species of Fusarium in Cereal-based Crop Rotations. Weed Res. 2019, 59, 312–323. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Fernandes, J.M.C.; Pierobom, C.R. Factors Affecting Density of Airborne Gibberella Zeae Inoculum. Fitopatol. Bras. 2005, 30, 55–60. [Google Scholar] [CrossRef]
- Trail, F. For Blighted Waves of Grain: Fusarium graminearum in the Postgenomics Era. Plant Physiol. 2009, 149, 103–110. [Google Scholar] [CrossRef]
- Desjardins, A.; Plattner, R.; Shaner, G.; Brown, D.; Buechley, G.; Proctor, R.; Turgeon, G. Field Release of Gibberella zeae Genetically Modified to Lack Ascopores. Phytopathology 2006, 96, S28–S29. [Google Scholar]
- PARRY, D.W.; JENKINSON, P.; McLEOD, L. Fusarium Ear Blight (Scab) in Small Grain Cereals—A Review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Alma, A.; Lessio, F.; Reyneri, A.; Blandino, M. Relationships between Ostrinia Nubilalis (Lepidoptera: Crambidae) Feeding Activity, Crop Technique and Mycotoxin Contamination of Corn Kernel in Northwestern Italy. Int. J. Pest Manag. 2005, 51, 165–173. [Google Scholar] [CrossRef]
- Kozak, G.M.; Wadsworth, C.B.; Kahne, S.C.; Bogdanowicz, S.M.; Harrison, R.G.; Coates, B.S.; Dopman, E.B. Genomic Basis of Circannual Rhythm in the European Corn Borer Moth. Curr. Biol. 2019, 29, 3501–3509.e5. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Reyneri, A.; Vanara, F.; Pascale, M.; Haidukowski, M.; Saporiti, M. Effect of Sowing Date and Insecticide Application against European Corn Borer (Lepidoptera: Crambidae) on Fumonisin Contamination in Maize Kernels. Crop. Prot. 2008, 27, 1432–1436. [Google Scholar] [CrossRef]
- O’Donnell, K.; McCormick, S.P.; Busman, M.; Proctor, R.H.; Ward, T.J.; Doehring, G.; Geiser, D.M.; Alberts, J.F.; Rheeder, J.P. 1984 “Toxigenic Fusarium Species: Identity and Mycotoxicology” Revisited. Mycologia 2018, 110, 1058–1080. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and Toxicity of a Fusarium Mycotoxin, Zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Mohan, K.; Karthick Rajan, D.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, Toxicity, Interactive Effects, and Detection of Ochratoxin and Deoxynivalenol in Food: A Review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Godseill Awuchi, C.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Odilichukwu, C.; et al. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L. Some Major Mycotoxins and Their Mycotoxicoses—An Overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Thrane, U.; Samson, R.A.; Pitt, J.I. Important Mycotoxins and the Fungi Which Produce Them. Adv. Exp. Med. Biol. 2006, 571, 3–31. [Google Scholar]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium Species and Mycotoxins Associated with Maize Ear Rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Fusarium Culmorum: Infection Process, Mechanisms of Mycotoxin Production and Their Role in Pathogenesis in Wheat. Crop. Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Hera, C.; Sulyok, M.; Schäfer, K.; Capilla, J.; Guarro, J.; Di Pietro, A. The Velvet Complex Governs Mycotoxin Production and Virulence of Fusarium oxysporum on Plant and Mammalian Hosts. Mol. Microbiol. 2013, 87, 49–65. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EC). Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Larsen, J.C.; Hunt, J.; Perrin, I.; Ruckenbauer, P. Workshop on Trichothecenes with a Focus on DON: Summary Report. Toxicol. Lett. 2004, 153, 1–22. [Google Scholar] [CrossRef]
- Reed, H.; Mueller, B.; Groves, C.L.; Smith, D.L. Presence and Correlation of Fusarium graminearum and Deoxynivalenol Accumulation in Silage Corn Plant Parts. Plant Dis. 2022, 106, 87–92. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Thrane, U. Fast Methods for Screening of Trichothecenes in Fungal Cultures Using Gas Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2001, 929, 75–87. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of Deoxynivalenol Mediated Gastrointestinal Toxicity: Insights from Mitochondrial Dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef]
- Beisl, J.; Pahlke, G.; Abeln, H.; Ehling-Schulz, M.; del Favero, G.; Varga, E.; Warth, B.; Sulyok, M.; Abia, W.; Ezekiel, C.N.; et al. Combinatory Effects of Cereulide and Deoxynivalenol on in Vitro Cell Viability and Inflammation of Human Caco-2 Cells. Arch. Toxicol. 2020, 94, 833–844. [Google Scholar] [CrossRef]
- Ookawara, T.; Aihara, R.; Morimoto, A.; Iwashita, N.; Kurata, K.; Takagi, Y.; Miyasaka, A.; Kushiro, M.; Miyake, S.; Fukuyama, T. Acute and Subacute Oral Toxicity of Deoxynivalenol Exposure in a Dermatophagoides farinae -Induced Murine Asthma Model. Toxicol. Sci. 2021, 179, 229–240. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. The Toxicity Mechanisms of DON to Humans and Animals and Potential Biological Treatment Strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 790–812. [Google Scholar] [CrossRef]
- Urry, W.H.; Wehrmeister, H.L.; Hodge, E.B.; Hidy, P.H. The Structure of Zearalenone. Tetrahedron Lett. 1966, 7, 3109–3114. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, Toxicity, Production and Detection of Fusarium Mycotoxin: A Review. FoodProd. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for Animal Health Related to the Presence of Zearalenone and Its Modified Forms in Feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-L.; Feng, Y.-L.; Song, J.-L.; Zhou, X.-S. Zearalenone: A Mycotoxin with Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Sebaei, A.S.; Gomaa, A.M.; Mohamed, G.G.; Nour El-Di, F.A. Simple Validated Method for Determination of Deoxynivalenol and Zearalenone in Some Cereals Using High Performance Liquid Chromatography. Am. J. Food Technol. 2012, 7, 668–678. [Google Scholar] [CrossRef]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection Strategies of Zearalenone for Food Safety: A Review. Crit. Rev. Anal. Chem. 2022, 52, 294–313. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Sali, N.; Kőszegi, T.; Szente, L.; Peles-Lemli, B. Interactions of Zearalenone with Native and Chemically Modified Cyclodextrins and Their Potential Utilization. J. Photochem. Photobiol. B 2015, 151, 63–68. [Google Scholar] [CrossRef]
- Viera Limon, M.J.; Morlett Chavez, J.A.; Sierra Rivera, C.A.; Contreras, D.L.; Zugasti-Cruz, A. Zearalenone Induced Cytotoxicity and Oxidative Stress in Human Peripheral Blood Leukocytesevita. Toxicol. Open Access 2015, 1, 102. [Google Scholar] [CrossRef]
- Ren, Z.; Deng, H.; Deng, Y.; Liang, Z.; Deng, J.; Zuo, Z.; Hu, Y.; Shen, L.; Yu, S.; Cao, S. Combined Effects of Deoxynivalenol and Zearalenone on Oxidative Injury and Apoptosis in Porcine Splenic Lymphocytes in Vitro. Exp. Toxicol. Pathol. 2017, 69, 612–617. [Google Scholar] [CrossRef]
- Abid-Essefi, S.; Baudrimont, I.; Hassen, W.; Ouanes, Z.; Mobio, T.A.; Anane, R.; Creppy, E.E.; Bacha, H. DNA Fragmentation, Apoptosis and Cell Cycle Arrest Induced by Zearalenone in Cultured DOK, Vero and Caco-2 Cells: Prevention by Vitamin E. Toxicology 2003, 192, 237–248. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.; Hofgaard, I.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a Changing Climate: Semi-Quantitative Multi-Mycotoxin Analysis of Grain Grown in Exceptional Climatic Conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Vaclavikova, M.; Malachova, A.; Veprikova, Z.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. ‘Emerging’ Mycotoxins in Cereals Processing Chains: Changes of Enniatins during Beer and Bread Making. Food Chem. 2013, 136, 750–757. [Google Scholar] [CrossRef]
- Tookey, H.L.; Yates, S.G.; Ellis, J.J.; Grove, M.D.; Nichols, R.E. Toxic Effects of a Butenolide Mycotoxin and of Fusarium Tricinctum Cultures in Cattle. J. Am. Vet. Med. Assoc. 1972, 160, 1522–1526. [Google Scholar] [PubMed]
- Yates, S.G.; Tookey, H.L.; Ellis, J.J.; Tallent, W.H.; Wolff, I.A. Mycotoxins as a Possible Cause of Fescue Toxicity. J. Agric. Food Chem. 1969, 17, 437–442. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, J.; Chen, J.; Li, S.; Zhang, Z.; Yang, B.; Peng, S. Butenolide Induced Cytotoxicity by Disturbing the Prooxidant–Antioxidant Balance, and Antioxidants Partly Quench in Human Chondrocytes. Toxicol. Vitr. 2009, 23, 99–104. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Wang, Y.-M.; Peng, S.-Q. Metallothionein-I/II Null Cardiomyocytes Are Sensitive to Fusarium Mycotoxin Butenolide-Induced Cytotoxicity and Oxidative DNA Damage. Toxicon 2010, 55, 1291–1296. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef]
- Ashley, J.N.; Hobbs, B.C.; Raistrick, H. Studies in the Biochemistry of Micro-Organisms. Biochem. J. 1937, 31, 385–397. [Google Scholar] [CrossRef]
- Sunic, K.; Kovac, T.; Loncaric, A.; Babic, J.; Sulyok, M.; Krska, R.; Drezner, G.; Spanic, V. Fusarium Secondary Metabolite Content in Naturally Produced and Artificially Provoked Fhb Pressure in Winter Wheat. Agronomy 2021, 11, 2239. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Kamani, M.H.; Fakhri, Y.; Coppa, C.F.S.C.; de Oliveira, C.A.F.; Sant’Ana, A.S. Changes in Masked Forms of Deoxynivalenol and Their Co-Occurrence with Culmorin in Cereal-Based Products: A Systematic Review and Meta-Analysis. Food Chem. 2019, 294, 587–596. [Google Scholar] [CrossRef]
- Wipfle, R.; McCormick, S.P.; Proctor, R.H.; Teresi, J.M.; Hao, G.; Ward, T.J.; Alexander, N.J.; Vaughan, M.M. Synergistic Phytotoxic Effects of Culmorin and Trichothecene Mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Woelflingseder, L.; Warth, B.; Vierheilig, I.; Schwartz-Zimmermann, H.; Hametner, C.; Nagl, V.; Novak, B.; Šarkanj, B.; Berthiller, F.; Adam, G.; et al. The Fusarium Metabolite Culmorin Suppresses the in Vitro Glucuronidation of Deoxynivalenol. Arch. Toxicol. 2019, 93, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Lancashire, P.D.; Bleiholder, H.; Van Den Boom, T.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.-R. An Eight-Year Survey of Wheat Shows Distinctive Effects of Cropping Factors on Different Fusarium Species and Associated Mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Tanveer, A.; Ikram, R.M.; Ali, H.H. Crop Rotation: Principles and Practices. In Agronomic Crops; Springer: Singapore, 2019; pp. 1–12. [Google Scholar]
- Wise, K.; Mueller, D. Are Fungicides No Longer Just for Fungi? An Analysis of Foliar Fungicide Use in Corn. APSnet Featur. Artic. 2011, 10. [Google Scholar] [CrossRef]
- Luna, M.P.R.; Wise, K.A. Timing and Efficacy of Fungicide Applications for Diplodia Ear Rot Management in Corn. Plant Health Prog. 2015, 16, 123–131. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Lv, Q.; Yan, L.; Wang, Y.; Jiang, Y. The Fungal CYP51s: Their Functions, Structures, Related Drug Resistance, and Inhibitors. Front. Microbiol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Muellender, M.M.; Mahlein, A.; Stammler, G.; Varrelmann, M. Evidence for the Association of Target-site Resistance in Cyp51with Reduced DMI Sensitivity in European Cercospora Beticola Field Isolates. Pest Manag. Sci. 2021, 77, 1765–1774. [Google Scholar] [CrossRef]
- Fernndez-ortuo, D.; Torés, J.A.; De Vicente, A.; Prez-Garc, A. The QoI Fungicides, the Rise and Fall of a Successful Class of Agricultural Fungicides. In Fungicides; InTech: London, UK, 2010. [Google Scholar]
- Freije, A.N.; Wise, K.A. Impact of Fusarium graminearum Inoculum Availability and Fungicide Application Timing on Fusarium Head Blight in Wheat. Crop. Prot. 2015, 77, 139–147. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Bockus, W.W.; Nopsa, J.H.; de Wolf, E.D.; Eskridge, K.M.; Peiris, K.H.S.; Dowell, F.E. Effects of Integrating Cultivar Resistance and Fungicide Application on Fusarium Head Blight and Deoxynivalenol in Winter Wheat. Plant Dis. 2011, 95, 554–560. [Google Scholar] [CrossRef]
- Nakajima, T. Fungicides Application against Fusarium Head Blight in Wheat and Barley for Ensuring Food Safety. In Fungicides; InTech: London, UK, 2010. [Google Scholar]
- Feksa, H.R.; Do Couto, H.T.Z.; Garozi, R.; De Almeida, J.L.; Gardiano, C.G.; Tessmann, D.J. Pre- and Postinfection Application of Strobilurin-Triazole Premixes and Single Fungicides for Control of Fusarium Head Blight and Deoxynivalenol Mycotoxin in Wheat. Crop. Prot. 2019, 117, 128–134. [Google Scholar] [CrossRef]
- Andriolli, C.F.; Casa, R.T.; Kuhnem, P.R.; Bogo, A.; Zancan, R.L.; Reis, E.M. Timing of Fungicide Application for the Control of Gibberella Ear Rot of Maize. Trop. Plant Pathol. 2016, 41, 264–269. [Google Scholar] [CrossRef]
- Anderson, N.R.; Romero Luna, M.P.; Ravellette, J.D.; Wise, K.A. Impact of Foliar Fungicides on Gibberella Ear Rot and Deoxynivalenol Levels in Indiana Corn. Plant Health Prog. 2017, 18, 186–191. [Google Scholar] [CrossRef]
- Vincelli, P. Q(o)I (Strobilurin) Fungicides: Benefits and Risks. Plant Health Instr. 2002, 63–65. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Dufault, N.S.; Bradley, C.A.; Chilvers, M.I. Fungicides for Field Crops; Mueller, D.S., Wise, K.A., Dufault, N.S., Bradley, C.A., Chilvers, M.I., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2017; ISBN 978-0-89054-506-5. [Google Scholar]
- Li, Q.; Shi, J.; Huang, C.; Guo, J.; He, K.; Wang, Z. Asian Corn Borer (Ostrinia furnacalis) Infestation Increases Fusarium verticillioides Infection and Fumonisin Contamination in Maize and Reduces the Yield. Plant Dis. 2023, 107, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M.; Clark, J.M.; Sheets, L.P.; Mullin, L.S.; Piccirillo, V.J.; Sargent, D.; Stevens, J.T.; Weiner, M.L. Mechanisms of Pyrethroid Neurotoxicity: Implications for Cumulative Risk Assessment. Toxicology 2002, 171, 3–59. [Google Scholar] [CrossRef]
- İçen, E.; Armutçu, F.; Büyükgüzel, K.; Gürel, A. Biochemical Stress Indicators of Greater Wax Moth Exposure to Organophosphorus Insecticides. J. Econ. Entomol. 2005, 98, 358–366. [Google Scholar] [CrossRef]
- Howard, M.D.; Pope, C.N. In Vitro Effects of Chlorpyrifos, Parathion, Methyl Parathion and Their Oxons on Cardiac Muscarinic Receptor Binding in Neonatal and Adult Rats. Toxicology 2002, 170, 1–10. [Google Scholar] [CrossRef]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect Ryanodine Receptors: Molecular Targets for Novel Pest Control Chemicals. Invertebr. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M. A Retrospective Look at Anthranilic Diamide Insecticides: Discovery and Lead Optimization to Chlorantraniliprole and Cyantraniliprole. Pest Manag. Sci. 2017, 73, 658–665. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Impact of the Insecticide Application to Maize Cultivated in Different Environmental Conditions on Emerging Mycotoxins. Field Crop. Res. 2018, 217, 188–198. [Google Scholar] [CrossRef]
- Khambay, B.P.S.; Jewess, P.J. Pyrethroids. Comprehensive Molecular Insect Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–29. [Google Scholar]
- Deepa, N.; Sreenivasa, M.Y. Sustainable Approaches for Biological Control of Mycotoxigenic Fungi and Mycotoxins in Cereals. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–161. [Google Scholar]
- Abbas, H.K.; Zablotowicz, R.M.; Weaver, M.A.; Shier, W.T.; Bruns, H.A.; Bellaloui, N.; Accinelli, C.; Abel, C.A. Implications of Bt Traits on Mycotoxin Contamination in Maize: Overview and Recent Experimental Results in Southern United States. J. Agric. Food Chem. 2013, 61, 11759–11770. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Hellmich, R.L.; Rice, L.G. Comparison of Fumonisin Concentrations in Kernels of Transgenic Bt Maize Hybrids and Nontransgenic Hybrids. Plant Dis. 1999, 83, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Pardo-López, L.; Soberón, M.; Bravo, A. Bacillus thuringiensis Insecticidal Three-Domain Cry Toxins: Mode of Action, Insect Resistance and Consequences for Crop Protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Li, H.; Oppert, B.; Higgins, R.A.; Huang, F.; Buschman, L.L.; Zhu, K.Y. Susceptibility of Dipel-Resistant and -Susceptible Ostrinia nubilalis (Lepidoptera: Crambidae) to Individual Bacillus thuringiensis Protoxins. J. Econ. Entomol. 2005, 98, 1333–1340. [Google Scholar] [CrossRef]
- Smith, S.M. Biological Control with Trichogramma: Advances, Successes, and Potential of Their Use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Chen, S.; Wang, G.; Fu, J.; Lan, Y. Biological Control Technology and Application Based on Agricultural Unmanned Aerial Vehicle (UAV) Intelligent Delivery of Insect Natural Enemies (Trichogramma) Carrier. Pest Manag. Sci. 2021, 77, 3259–3272. [Google Scholar] [CrossRef]
- Zang, L.-S.; Wang, S.; Zhang, F.; Desneux, N. Biological Control with Trichogramma in China: History, Present Status, and Perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Dong, F.; Chen, X.; Lei, X.; Wu, D.; Zhang, Y.; Lee, Y.-W.; Mokoena, M.P.; Olaniran, A.O.; Li, Y.; Shen, G.; et al. Effect of Crop Rotation on Fusarium Mycotoxins and Fusarium Species in Cereals in Sichuan Province (China). Plant Dis. 2023, 107, 1060–1066. [Google Scholar] [CrossRef]
- Dill-Macky, R.; Jones, R.K. The Effect of Previous Crop Residues and Tillage on Fusarium Head Blight of Wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Tamburic-Ilincic, L.; Hooker, D.C. Effect of Previous Crop, Tillage, Field Size, Adjacent Crop, and Sampling Direction on Airborne Propagules of Gibberella zeae/Fusarium graminearum, Fusarium Head Blight Severity, and Deoxynivalenol Accumulation in Winter Wheat. Can. J. Plant Pathol. 2005, 27, 217–224. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Giordano, D.; Sulyok, M.; Krska, R.; Vanara, F.; Reyneri, A. Impact of Sowing Time, Hybrid and Environmental Conditions on the Contamination of Maize by Emerging Mycotoxins and Fungal Metabolites. Ital. J. Agron. 2017, 12, 215–224. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Bijelić, Z.; Stanković, S.; Obradović, A.; Caro Petrović, V.; Gogić, M. Influence of Sowing Time on Fusarium and Fumonisin Contamination of Maize Grains and Yield Component Traits. Agriculture 2022, 12, 1042. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Stanković, S.; Obradović, A.; Vasić, T.; Lukić, M.; Bijelić, Z. Influence of Plant Density on Toxigenic Fungal and Mycotoxin Contamination of Maize Grains. Crop. Prot. 2019, 116, 126–131. [Google Scholar] [CrossRef]
- Munkvold, G.P. Cultural and Genetic Approaches to Managing Mycotoxins in Maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Bolduan, C.; Miedaner, T.; Schipprack, W.; Dhillon, B.S.; Melchinger, A.E. Genetic Variation for Resistance to Ear Rots and Mycotoxins Contamination in Early European Maize Inbred Lines. Crop. Sci. 2009, 49, 2019–2028. [Google Scholar] [CrossRef]
- Gendloff, E.H. Components of Resistance to Fusarium Ear Rot in Field Corn. Phytopathology 1986, 76, 684. [Google Scholar] [CrossRef]
- Parlevliet, J.E. Durability of Resistance against Fungal, Bacterial and Viral Pathogens; Present Situation. Euphytica 2002, 124, 147–156. [Google Scholar] [CrossRef]
- Cullen, D. Susceptibility of Maize to Gibberella zeae Ear Rot: Relationship to Host Genotype, Pathogen Virulence, and Zearalenone Contamination. Plant Dis. 1983, 67, 89. [Google Scholar] [CrossRef]
- Ali, M.L.; Taylor, J.H.; Jie, L.; Sun, G.; William, M.; Kasha, K.J.; Reid, L.M.; Pauls, K.P. Molecular Mapping of QTLs for Resistance to Gibberella Ear Rot, in Corn, Caused by Fusarium graminearum. Genome 2005, 48, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Reid, L.M.; Zhu, X.; Parker, A.; Yan, W. Increased Resistance to Ustilago zeae and Fusarium verticilliodes in Maize Inbred Lines Bred for Fusarium graminearum Resistance. Euphytica 2009, 165, 567. [Google Scholar] [CrossRef]
- Thompson, M.E.H.; Raizada, M.N. Fungal Pathogens of Maize Gaining Free Passage along the Silk Road. Pathogens 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Krakowsky, M.D.; David Buntin, G.; Rector, B.G.; Guo, B.; Snook, M.E. Identification of Multiple Ear-Colonizing Insect and Disease Resistance in CIMMYT Maize Inbred Lines with Varying Levels of Silk Maysin. J. Econ. Entomol. 2008, 101, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P.; Krakowsky, M.D.; Scully, B.T.; Brown, R.L.; Menkir, A.; Warburton, M.L.; Windham, G.L. Identifying and Developing Maize Germplasm with Resistance to Accumulation of Aflatoxins. World Mycotoxin J. 2015, 8, 193–209. [Google Scholar] [CrossRef]
- Ni, X.; Xu, W.; Blanco, M.H.; Wilson, J.P. Evaluation of Corn Germplasm Lines for Multiple Ear-Colonizing Insect and Disease Resistance. J. Econ. Entomol. 2012, 105, 1457–1464. [Google Scholar] [CrossRef]
- Reid, L.M.; Mather, D.E.; Arnason, J.T.; Hamilton, R.I.; Bolton, A.T. Changes in Phenolic Constituents of Maize Silk Infected with Fusarium graminearum. Can. J. Bot. 1992, 70, 1697–1702. [Google Scholar] [CrossRef]
- Reid, L.M.; Bolton, A.T.; Hamilton, R.I.; Woldemariam, T.; Mather, D.E. Effect of Silk Age on Resistance of Maize to Fusarium graminearum. Can. J. Plant Pathol. 1992, 14, 293–298. [Google Scholar] [CrossRef]
- Savignac, J.-M.; Atanasova, V.; Chereau, S.; Ducos, C.; Gallegos, N.; Ortega, V.; Ponts, N.; Richard-Forget, F. Carotenoids Occurring in Maize Affect the Redox Homeostasis of Fusarium graminearum and Its Production of Type B Trichothecene Mycotoxins: New Insights Supporting Their Role in Maize Resistance to Giberella Ear Rot. J. Agric. Food Chem. 2023, 71, 3285–3296. [Google Scholar] [CrossRef]
- Warfield, C.Y. Importance of the Husk Covering on the Susceptibility of Corn Hybrids to Fusarium Ear Rot. Plant Dis. 1996, 80, 208. [Google Scholar] [CrossRef]
- Smith, D.R.; White, D.G. Diseases of Corn. In Corn and Corn Improvement; Sprague, G.F., Dudley, J.W., Eds.; American Society of Agronomy: Madison, WI, USA, 2015; Volume 18, pp. 687–766. [Google Scholar]
- Butrón, A.; Santiago, R.; Mansilla, P.; Pintos-Varela, C.; Ordás, A.; Malvar, R.A. Maize (Zea mays L.) Genetic Factors for Preventing Fumonisin Contamination. J. Agric. Food Chem. 2006, 54, 6113–6117. [Google Scholar] [CrossRef]
- Gaikpa, D.S.; Kessel, B.; Presterl, T.; Ouzunova, M.; Galiano-Carneiro, A.L.; Mayer, M.; Melchinger, A.E.; Schön, C.C.; Miedaner, T. Exploiting Genetic Diversity in Two European Maize Landraces for Improving Gibberella Ear Rot Resistance Using Genomic Tools. Theor. Appl. Genet. 2021, 134, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Miedaner, T.; Utz, H.F.; Schipprack, W.; Schrag, T.A.; Melchinger, A.E. Genomic Prediction and GWAS of Gibberella Ear Rot Resistance Traits in Dent and Flint Lines of a Public Maize Breeding Program. Euphytica 2018, 214, 6. [Google Scholar] [CrossRef]
- Zhou, G.; Li, S.; Ma, L.; Wang, F.; Jiang, F.; Sun, Y.; Ruan, X.; Cao, Y.; Wang, Q.; Zhang, Y.; et al. Mapping and Validation of a Stable Quantitative Trait Locus Conferring Maize Resistance to Gibberella Ear Rot. Plant Dis. 2021, 105, 1984–1991. [Google Scholar] [CrossRef] [PubMed]
- Galiano-Carneiro, A.L.; Kessel, B.; Presterl, T.; Gaikpa, D.S.; Kistner, M.B.; Miedaner, T. Multi-Parent QTL Mapping Reveals Stable QTL Conferring Resistance to Gibberella Ear Rot in Maize. Euphytica 2021, 217, 2. [Google Scholar] [CrossRef]
- Wen, J.; Shen, Y.; Xing, Y.; Wang, Z.; Han, S.; Li, S.; Yang, C.; Hao, D.; Zhang, Y. QTL Mapping of Resistance to Gibberella Ear Rot in Maize. Mol. Breed. 2020, 40, 94. [Google Scholar] [CrossRef]
- Giomi, G.M.; Kreff, E.D.; Iglesias, J.; Fauguel, C.M.; Fernandez, M.; Oviedo, M.S.; Presello, D.A. Quantitative Trait Loci for Fusarium and Gibberella Ear Rot Resistance in Argentinian Maize Germplasm. Euphytica 2016, 211, 287–294. [Google Scholar] [CrossRef]
- Kebede, A.Z.; Johnston, A.; Schneiderman, D.; Bosnich, W.; Harris, L.J. Transcriptome Profiling of Two Maize Inbreds with Distinct Responses to Gibberella Ear Rot Disease to Identify Candidate Resistance Genes. BMC Genom. 2018, 19, 131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).