Constitution of a Camelina sativa L. Synthetic Population and Agronomic Comparison between Spring and Winter Cultivation in North Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Constitution of Synthetic Population
2.3. Field Experimentation
2.4. Agronomic Parameters
2.5. Bromatological Analysis
2.6. Glucosinolates Quantification
2.7. Informatic Tools
3. Results
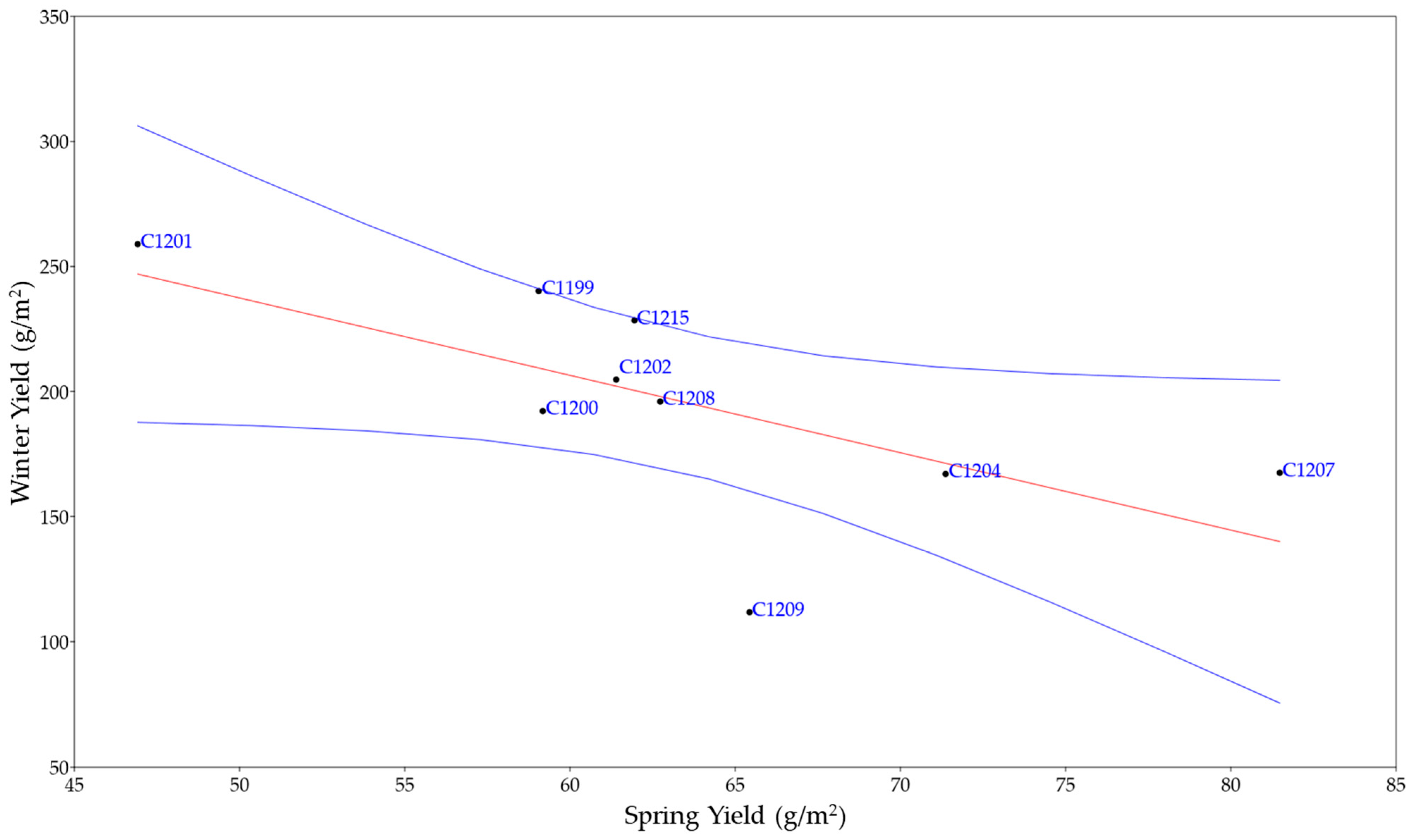
3.1. Comparison among Varieties Cultivated at Two Different Sowing Times
3.2. Dissection of Agronomic Parameters Involved in Yield
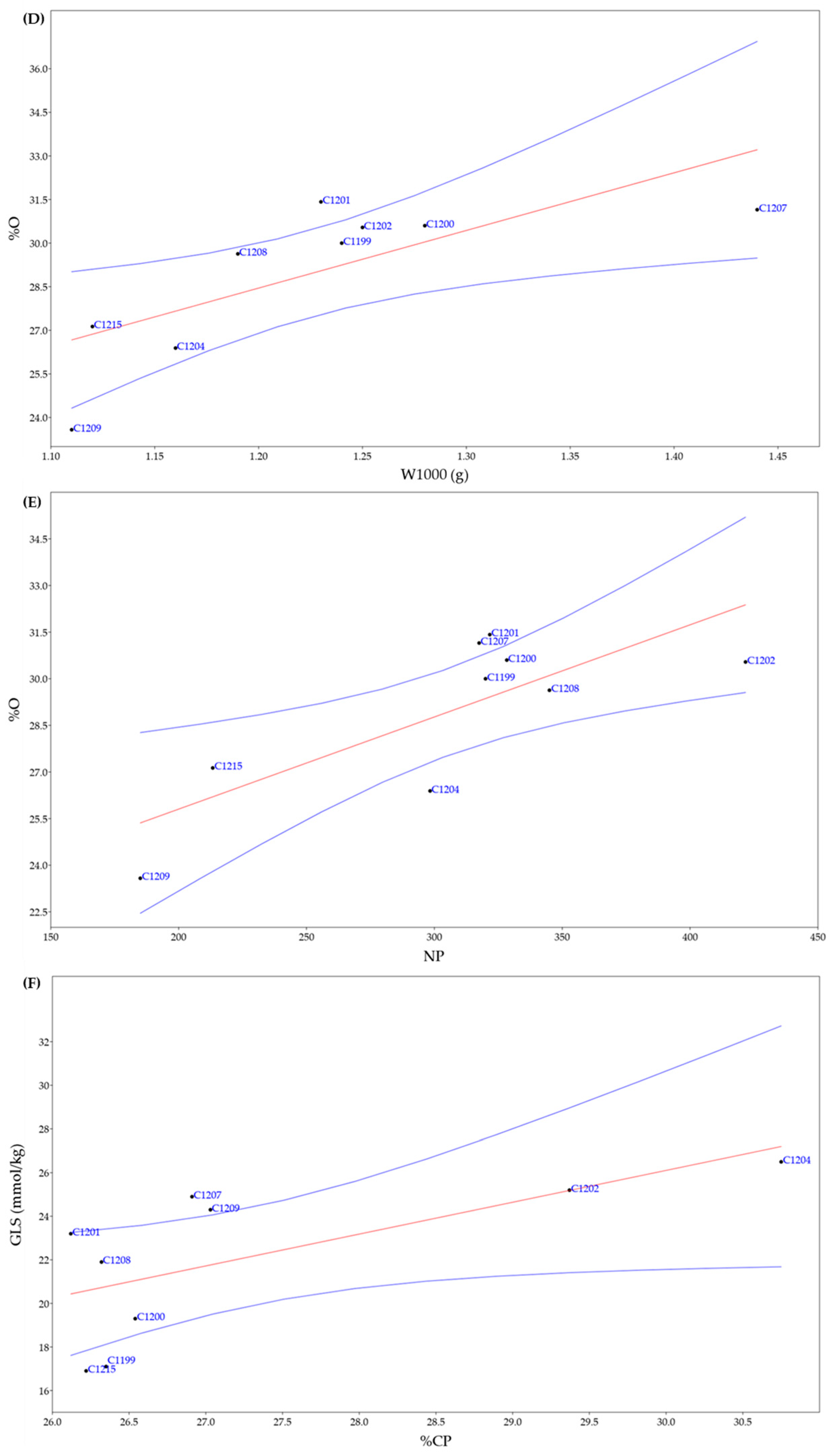
3.3. Bromatological Analyses and Glucosinolates Content
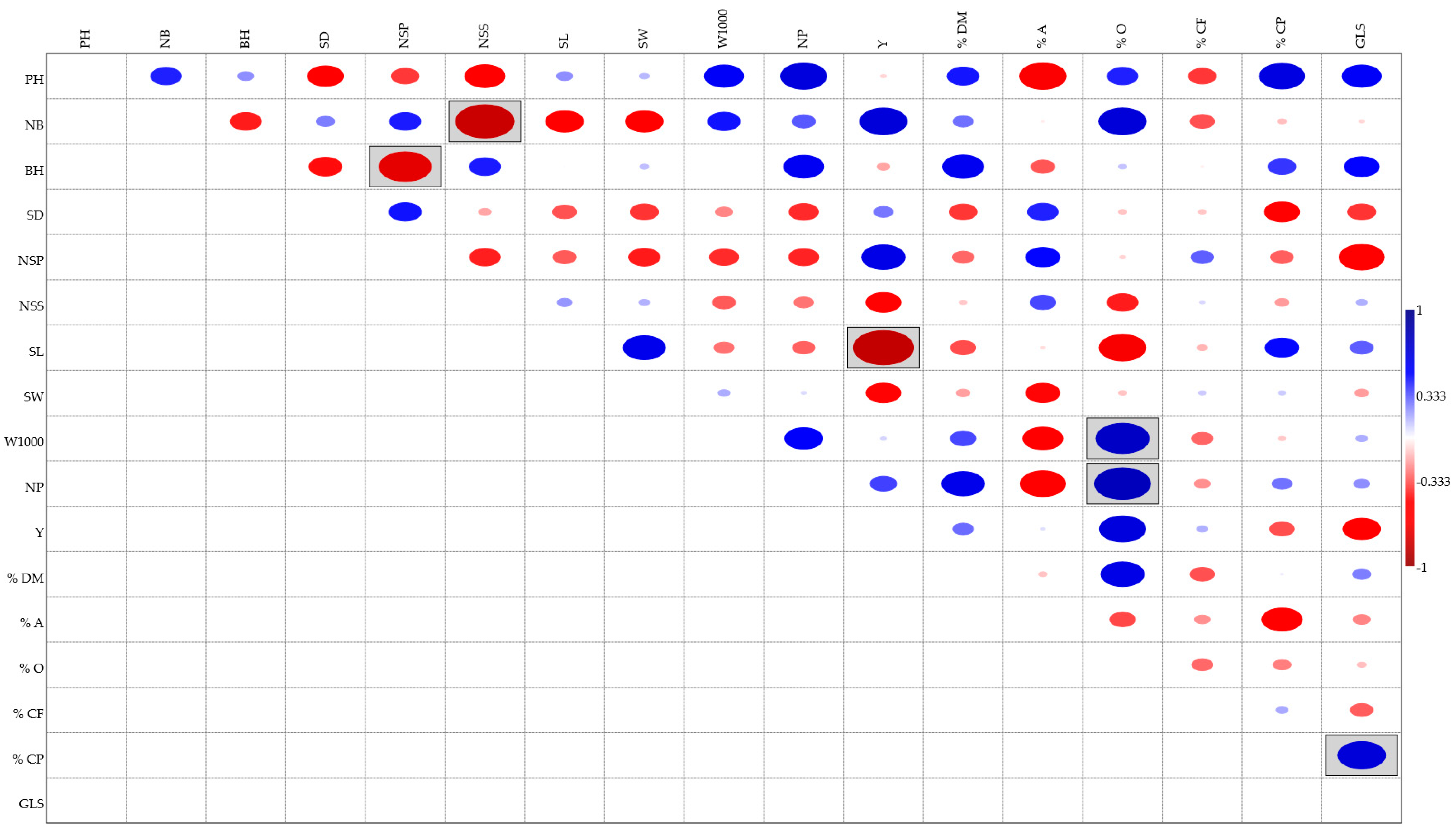
3.4. Correlation among Agronomic and Chemical Traits
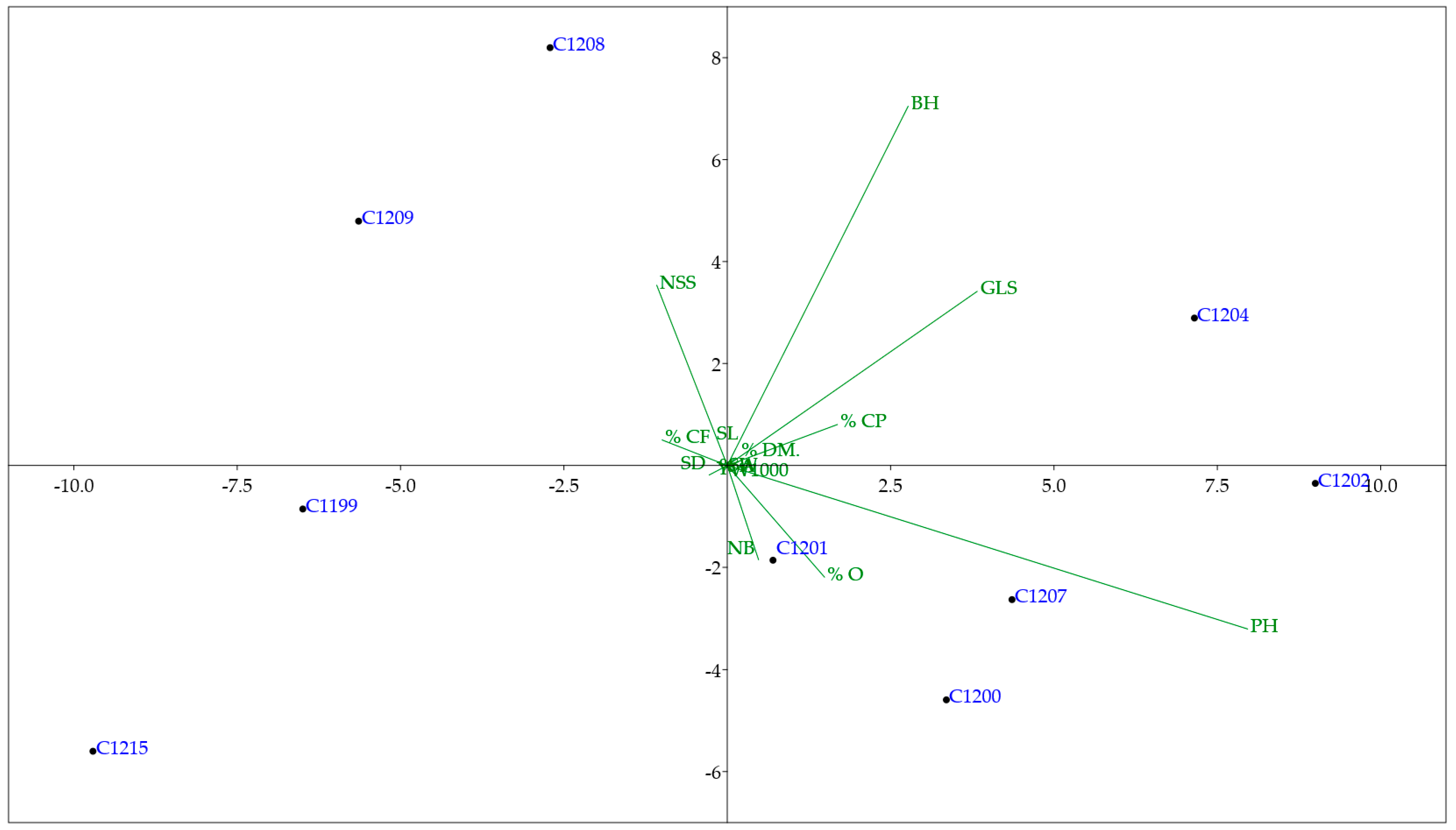
3.5. Multivariate Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic Evaluation of Camelina Genotypes Selected for Seed Quality Characteristics. Ind. Crops Prod. 2007, 26, 270–277. [Google Scholar] [CrossRef]
- Falk, K.C. Camelina (Camelina sativa). In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 369–391. [Google Scholar]
- Gore, M.; Kurt, O. Evaluation of Camelina Genotypes Grown in Winter at Different Sowing Times in Northern Turkey Ecological Conditions in Terms of Yield and Oil Ratio. Agrotech. Ind. Crops 2022, 1, 154–159. [Google Scholar]
- Government of Canada; Canadian Food Inspection Agency. The Biology of Camelina sativa (L.) Crantz (Camelina). Available online: https://inspection.canada.ca/plant-varieties/plants-with-novel-traits/applicants/directive-94-08/biology-documents/camelina-sativa-l-/eng/1330971423348/1330971509470 (accessed on 18 November 2022).
- Ghidoli, M.; Ponzoni, E.; Araniti, F.; Miglio, D.; Pilu, R. Genetic Improvement of Camelina sativa (L.) Crantz: Opportunities and Challenges. Plants 2023, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Zubr, J. Oil-Seed Crop: Camelina sativa. Ind. Crops Prod. 1997, 6, 113–119. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.F.; Moreno-Pérez, A.J.; Makni, S.; Troncoso-Ponce, M.A.; Acket, S.; Thomasset, B.; Sánchez, R.; Venegas-Calerón, M.; Garcés, R.; Martínez-Force, E.; et al. Lipid Profiling and Oil Properties of Camelina sativa Seeds Engineered to Enhance the Production of Saturated and Omega-7 Fatty Acids. Ind. Crops Prod. 2021, 170, 113765. [Google Scholar] [CrossRef]
- Matthäus, B.; Zubr, J. Variability of Specific Components in Camelina sativa Oilseed Cakes. Ind. Crops Prod. 2000, 12, 9–18. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Graczyk, M.; Stuper-Szablewska, K. Winter Camelina Seeds as a Raw Material for the Production of Erucic Acid-Free Oil. Food Chem. 2020, 330, 127265. [Google Scholar] [CrossRef]
- Russo, R.; Reggiani, R. Antinutritive Compounds in Twelve Camelina sativa Genotypes. Am. J. Plant Sci. 2012, 3, 1408–1412. [Google Scholar] [CrossRef]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina Uses, Genetics, Genomics, Production, and Management. Ind. Crops Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Landoni, M.; Scapin, A.; Cassani, E.; Borlini, G.; Follador, A.; Giupponi, L.; Ghidoli, M.; Hejna, M.; Rossi, L.; Pilu, R. Comparison among Four Maize Varieties in Conventional and Low Input Cultivation. Maydica 2021, 65, 1–13. [Google Scholar]
- Angelini, L.G.; Abou Chehade, L.; Foschi, L.; Tavarini, S. Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy 2020, 10, 1929. [Google Scholar] [CrossRef]
- Berti, M.; Wilckens, R.; Fischer, S.; Solis, A.; Johnson, B. Seeding Date Influence on Camelina Seed Yield, Yield Components, and Oil Content in Chile. Ind. Crops Prod. 2011, 34, 1358–1365. [Google Scholar] [CrossRef]
- Pavlista, A.D.; Isbell, T.A.; Baltensperger, D.D.; Hergert, G.W. Planting Date and Development of Spring-Seeded Irrigated Canola, Brown Mustard and Camelina. Ind. Crops Prod. 2011, 33, 451–456. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Caldwell, C.D. Glucosinolate Content of Camelina Genotypes as Affected by Applied Nitrogen and Sulphur. Crop Sci. 2016, 56, 3250–3262. [Google Scholar] [CrossRef]
- Perera, S.P.; McIntosh, T.; Coutu, C.; Tyler, R.T.; Hegedus, D.D.; Wanasundara, J.P.D. Profiling and Characterization of Camelina sativa (L.) Crantz Meal Proteins. J. Am. Oil Chem. Soc. 2022, 99, 873–889. [Google Scholar] [CrossRef]
- Brock, J.R.; Scott, T.; Lee, A.Y.; Mosyakin, S.L.; Olsen, K.M. Interactions between Genetics and Environment Shape Camelina Seed Oil Composition. BMC Plant Biol. 2020, 20, 423. [Google Scholar] [CrossRef]
- Russo, R.; Galasso, I.; Reggiani, R. Variability in Glucosinolate Content among Camelina Species. Am. J. Plant Sci. 2014, 5, 42529. [Google Scholar] [CrossRef]
- Biofumigation Potential of Brassicas|SpringerLink. Available online: https://link.springer.com/article/10.1023/A:1004364713152 (accessed on 29 May 2023).
- Castro, A.; Aires, A.; Rosa, E.; Bloem, E.; Stulen, I.; De Kok, L. Distribution of Glucosinolates in Brassica Oleracea Cultivars. Phyton Ann. Rei Bot. 2004, 44, 133–143. [Google Scholar]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The Effect of Sulfur Nutrition on Plant Glucosinolate Content: Physiology and Molecular Mechanisms. Plant Biol. Stuttg. Ger. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica Species for Glucosinolate Content. J. Environ. Sci. Health B 2009, 44, 311–316. [Google Scholar] [CrossRef]
- Amiri-Darban, N.; Nourmohammadi, G.; Shirani Rad, A.H.; Mirhadi, S.; Heravan, I. Potassium Sulfate and Ammonium Sulfate Affect Quality and Quantity of Camelina Oil Grown with Different Irrigation Regimes. Ind. Crops Prod. 2020, 148, 112308. [Google Scholar] [CrossRef]
- Li, H.; Hu, X.; Lovell, J.T.; Grabowski, P.P.; Mamidi, S.; Chen, C.; Amirebrahimi, M.; Kahanda, I.; Mumey, B.; Barry, K.; et al. Genetic Dissection of Natural Variation in Oilseed Traits of Camelina by Whole-Genome Resequencing and QTL Mapping. Plant Genome 2021, 14, e20110. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Critchley, C.; Pun, S.; Nottingham, S.; O’Hare, T.J. Epithiospecifier Protein Activity in Broccoli: The Link between Terminal Alkenyl Glucosinolates and Sulphoraphane Nitrile. Phytochemistry 2008, 69, 2765–2773. [Google Scholar] [CrossRef]
- Becker, H.C.; Svensk, H.; Engqvist, G. Chances and Limitations for the use of Heterosis in Synthetic Cultivars of Rapeseed. Groupe Consult. Int. Rech. Colza (GCIRC) Bull. 1998, 15, 51–57. [Google Scholar]


| Name Varieties | Genetic Constitution | Code |
|---|---|---|
| Calena | Pure line | C1201 |
| Omich | Pure line | C1204 |
| Madalina | Pure line | C1202 |
| Experimental material | Pure line | C1199 |
| Experimental material | Pure line | C1200 |
| Experimental material | Pure line | C1207 |
| Experimental material | Pure line | C1208 |
| Experimental material | Pure line | C1209 |
| Experimental material | Synthetic population (C1199 × C1204) | C1215 |
| Parameters | Values |
|---|---|
| pH (H2O) | 6.5 ± 0.01 |
| Organic matter | 14.2 ± 0.12 g/kg |
| Sand | 56.2 ± 0.37% |
| Coarse Silt | 14.6 ± 0.52% |
| Fine Silt | 22.6 ± 1.53% |
| Clay | 6.6 ± 0.63% |
| P available (Bray/Kurtz) | 136 ± 1.44 mg/kg |
| Cation Exchange Capacity | 10.6 ± 0.24 cmol/kg |
| K exchangeable | 432 ± 8.16 mg/kg |
| Mg exchangeable | 229 ± 9.40 mg/kg |
| Ca exchangeable | 1331 ± 22.6 mg/kg |
| Estimated water holding capacity | 24.3 ± 1.59% |
| Year | Month | Monthly Rainfall (mm) | Mean Monthly Temperature (°C) |
|---|---|---|---|
| 2021 | April | 70.6 | 13.22 |
| May | 60.8 | 18.67 | |
| June | 15.8 | 26.03 | |
| 2021 | October | 52 | 14.12 |
| November | 165.6 | 9.49 | |
| December | 46 | 3.11 | |
| 2022 | January | 28 | 3.18 |
| February | 16 | 7.59 | |
| March | 9.8 | 9.13 | |
| April | 25 | 14.44 | |
| May | 62 | 22.47 |
| ID Code | Cultivation Period | Trait | |
|---|---|---|---|
| Yield (g/m2) | Estimated Yield (kg/ha) | ||
| C1199 | S | 59.68 ± 15.01 | 596.83 ± 150 |
| W | 240.18 ± 36.46 * | 2401.83 ± 365 * | |
| C1200 | S | 56.39 ± 20.14 | 563.92 ± 201 |
| W | 192.18 ± 21.20 * | 1921.83 ± 212 * | |
| C1201 | S | 45.64 ± 10.06 | 456.38 ± 101 |
| W | 258.93 ± 59.93 * | 2589.33 ± 599 * | |
| C1202 | S | 60.47 ± 20.01 | 604.66 ± 200 |
| W | 204.77 ± 69.43 * | 2047.67 ± 694 * | |
| C1204 | S | 70.46 ± 20.02 | 704.55 ± 200 |
| W | 167.02 ± 13.19 * | 1670.17 ± 132 * | |
| C1207 | S | 81.48 ± 18.25 | 814.80 ± 183 |
| W | 167.48 ± 24.40 * | 1674.83 ± 244 * | |
| C1208 | S | 62.73 ± 19.81 | 627.30 ± 198 |
| W | 195.98 ± 20.18 * | 1959.83 ± 202 * | |
| C1209 | S | 65.43 ± 23.42 | 654.32 ± 234 |
| W | 111.77 ± 21.80 * | 1117.67 ± 218 * | |
| C1215 | S | 63.98 ± 15.10 | 639.83 ± 151 |
| W | 228.42 ± 9.03 * | 2284.20 ± 90.3 * | |
| Total | S | 62.17 ± 16.36 | 621.66 ± 164 |
| W | 196.26 ± 58.57 * | 1962.57 ± 586 * | |
| Traits | ID Code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1199 | C1200 | C1201 | C1202 | C1204 | C1207 | C1208 | C1209 | C1215 | |
| PH (cm) | 70.85 ± 12.66 | 81.00 ± 5.50 | 75.83 ± 6.37 | 83.55 ± 9.28 | 81.25 ± 5.82 | 80.18 ± 12.64 | 71.10 ± 20.44 | 69.78 ± 9.31 | 70.14 ± 7.45 |
| NB | 4.08 ± 2.14 ab | 4.63 ± 2.07 ab | 6.17 ± 4.36 a | 4.64 ± 2.06 ab | 3.75 ± 1.60 ab | 4.80 ± 1.99 ab | 2.67 ± 1.32 ab | 2.56 ± 1.81 b | 4.57 ± 1.90 ab |
| BH (cm) | 39.25 ± 9.76 a | 38.75 ± 5.39 a | 38.83 ± 9.06 a | 42.00 ± 10.73 a | 42.92 ± 10.15 a | 37.60 ± 12.84 a | 46.67 ± 6.71 a | 38.88 ± 6.10 a | 31.71 ± 10.93 b |
| SD (mm) | 2.74 ± 0.41 | 2.25 ± 0.63 | 2.76 ± 0.82 | 2.08 ± 0.62 | 1.97 ± 0.73 | 2.05 ± 0.47 | 1.90 ± 0.45 | 2.66 ± 1.02 | 2.36 ± 0.59 |
| NSP | 45.17 ± 6.85 | 37.08 ± 4.09 | 52.64 ± 12.18 | 29.80 ± 10.10 | 37.52 ± 2.96 | 26.68 ± 3.75 | 26.82 ± 3.75 | 30.84 ± 3.67 | 41.57 ± 20.96 |
| NSS | 13.05 ± 3.34 b | 12.10 ± 3.11 b | 12.40 ± 3.20 b | 13.00 ± 2.62 b | 12.90 ± 3.73 b | 13.70 ± 2.45 ab | 17.80 ± 2.20 a | 16.78 ± 2.78 a | 13.21 ± 3.05 b |
| SL (mm) | 5.60 ± 1.01 b | 6.42 ± 0.81 a | 5.40 ± 0.70 b | 6.30 ± 0.95 ab | 6.40 ± 0.52 ab | 6.00 ± 0.29 ab | 5.90 ± 0.74 ab | 6.70 ± 0.81 a | 6.11 ± 0.89 ab |
| SW (mm) | 3.05 ± 0.22 b | 3.30 ± 0.46 a | 2.30 ± 0.48 c | 3.00 ± 0.11 ab | 2.90 ± 0.35 b | 3.00 ± 0.11 ab | 3.00 ± 0.15 ab | 3.00 ± 0.14 b | 2.85 ± 0.47 b |
| W1000 (g) | 1.24 ± 0.08 bc | 1.28 ± 0.03 c | 1.23 ± 0.03 bc | 1.25 ± 0.03 bc | 1.16 ± 0.05 bd | 1.44 ± 0.04 a | 1.19 ± 0.03 cd | 1.11 ± 0.02 d | 1.24 ± 0.04 bc |
| NP | 320 ± 77.62 ab | 328.33 ± 35.11 ab | 321.67 ± 79.43 ab | 421.67 ± 85.20 a | 298.33 ± 46.46 ab | 317.50 ± 29.30 ab | 345.00 ± 50.00 ab | 185 ± 56.35 b | 213.33 ± 65.26 b |
| Y (g/m2) | 240.18 ± 36.46 a | 192.18 ± 21.20 ab | 258.93 ± 59.93 a | 204.77 ± 69.43 ab | 167.02 ± 13.19 ab | 167.48 ± 25.83 ab | 195.98 ± 27.41 ab | 111.77 ± 13.30 b | 228.42 ± 9.03 a |
| Y (kg/ha) | 2401.83 ± 365 a | 1921.83 ± 212 ab | 2589.33 ± 599 a | 2047.67 ± 694 ab | 1670.17 ± 132 ab | 1674.83 ± 258 ab | 1959.83 ± 274 ab | 1117.67 ± 133 b | 2284.20 ± 903 a |
| ID Code | %DM | %A | %CF | %CP | %O |
|---|---|---|---|---|---|
| C1199 | 95.21 ± 0.95 | 4.27 ± 0.02 cd | 24.49 ± 1.31 a | 26.35 ± 1.47 b | 29.99 ± 1.00 ab |
| C1200 | 95.56 ± 0.22 | 4.37 ± 0.02 ad | 20.01 ± 0.52 b | 26.54 ± 1.06 b | 30.61 ± 0.58 a |
| C1201 | 95.61 ± 0.31 | 4.55 ± 0.04 a | 20.22 ± 0.77 b | 26.12 ± 1.54 b | 31.43 ± 0.78 a |
| C1202 | 95.31 ± 0.30 | 4.25 ± 0.05 d | 21.05 ± 1.33 ab | 29.37 ± 0.26 ab | 30.54 ± 1.37 a |
| C1204 | 95.48 ± 0.20 | 4.28 ± 0.06 bd | 24.04 ± 1.70 a | 30.75 ± 1.26 a | 26.39 ± 0.78 cd |
| C1207 | 95.39 ± 0.29 | 4.28 ± 0.12 bd | 21.79 ± 0.19 ab | 26.91 ± 1.16 b | 31.15 ± 0.30 a |
| C1208 | 95.62 ± 0.27 | 4.46 ± 0.07 abc | 22.74 ± 1.82 ab | 26.32 ± 0.21 b | 29.63 ± 1.49 ab |
| C1209 | 95.05 ± 0.85 | 4.54 ± 0.03 a | 21.16 ± 0.47 ab | 27.03 ± 1.87 b | 23.58 ± 1.70 d |
| C1215 | 95.08 ± 0.35 | 4.55 ± 0.12 a | 23.91 ± 1.75 a | 26.22 ± 1.55 b | 27.13 ± 0.88 bc |
| ID Code | GLS (mmol/kg) |
|---|---|
| C1199 | 17.10 ± 0.03 de |
| C1200 | 19.25 ± 0.07 d |
| C1201 | 23.21 ± 0.19 bc |
| C1202 | 25.15 ± 1.22 ab |
| C1204 | 26.52 ± 0.35 a |
| C1207 | 24.88 ± 1.50 ab |
| C1208 | 21.90 ± 0.40 c |
| C1209 | 24.29 ± 0.11 ab |
| C1215 | 16.92 ± 1.22 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidoli, M.; Frazzini, S.; De Benedetti, S.; Sangiorgio, S.; Landoni, M.; Scarafoni, A.; Rossi, L.; Pilu, R. Constitution of a Camelina sativa L. Synthetic Population and Agronomic Comparison between Spring and Winter Cultivation in North Italy. Agronomy 2023, 13, 1562. https://doi.org/10.3390/agronomy13061562
Ghidoli M, Frazzini S, De Benedetti S, Sangiorgio S, Landoni M, Scarafoni A, Rossi L, Pilu R. Constitution of a Camelina sativa L. Synthetic Population and Agronomic Comparison between Spring and Winter Cultivation in North Italy. Agronomy. 2023; 13(6):1562. https://doi.org/10.3390/agronomy13061562
Chicago/Turabian StyleGhidoli, Martina, Sara Frazzini, Stefano De Benedetti, Stefano Sangiorgio, Michela Landoni, Alessio Scarafoni, Luciana Rossi, and Roberto Pilu. 2023. "Constitution of a Camelina sativa L. Synthetic Population and Agronomic Comparison between Spring and Winter Cultivation in North Italy" Agronomy 13, no. 6: 1562. https://doi.org/10.3390/agronomy13061562
APA StyleGhidoli, M., Frazzini, S., De Benedetti, S., Sangiorgio, S., Landoni, M., Scarafoni, A., Rossi, L., & Pilu, R. (2023). Constitution of a Camelina sativa L. Synthetic Population and Agronomic Comparison between Spring and Winter Cultivation in North Italy. Agronomy, 13(6), 1562. https://doi.org/10.3390/agronomy13061562











