Maize and Wheat Responses to the Legacies of Different Cover Crops under Warm Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Soil and Growing Conditions
2.3. Sampling and Analysis
2.4. Statistical Analysis
3. Results
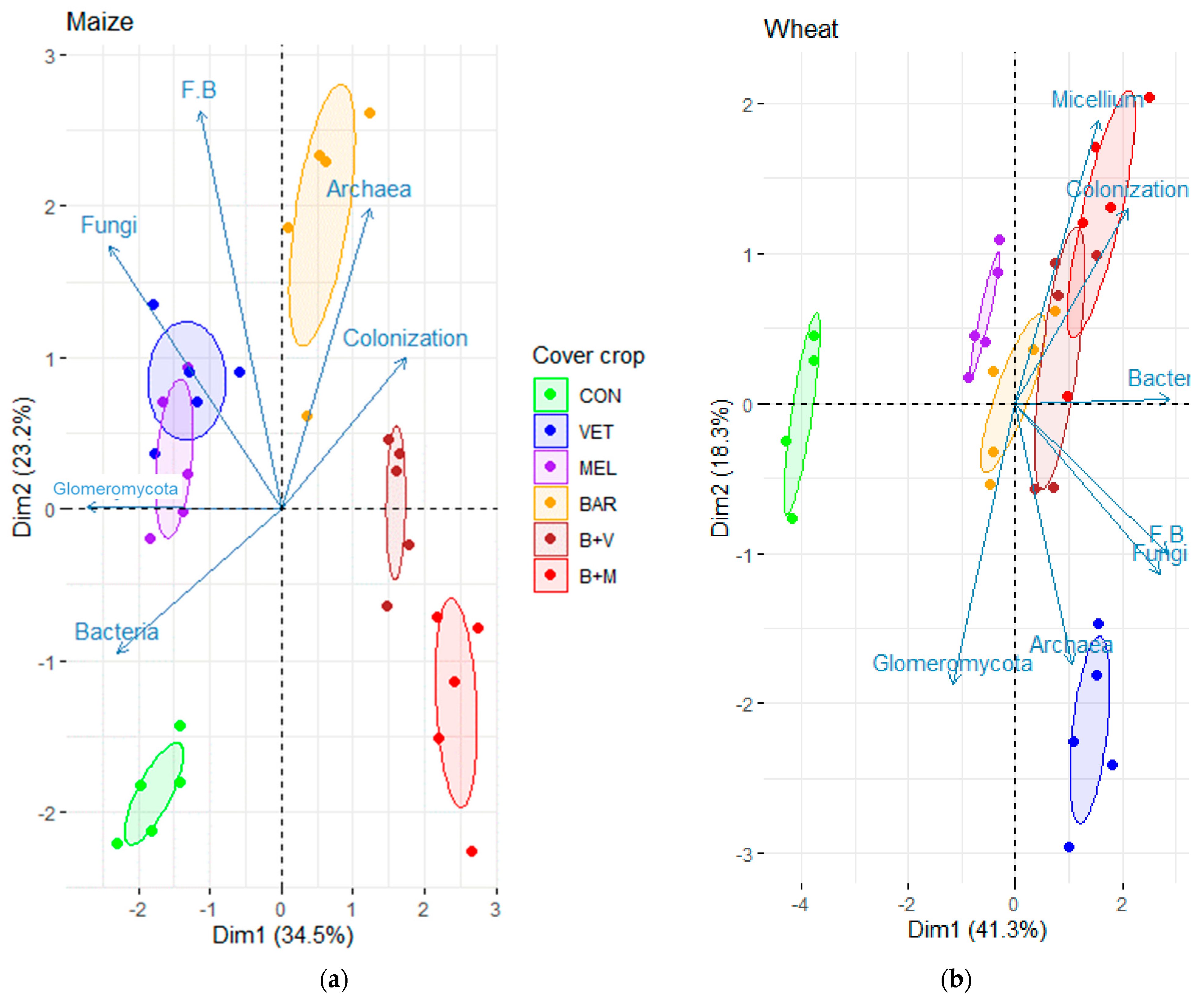
3.1. Microbial Variables in Maize after Cover Crops
3.2. Microbial Variables in Wheat after Cover Crops
3.3. Effects of Soil Properties on Crop Biomass
3.4. Maize Biomass and Shoot Nutrient Contents
3.5. Wheat Biomass and Shoot Nutrient Contents
4. Discussion
4.1. Crops Shape Soil Microbiomes
4.2. Cover Crop Mixtures
4.3. Benefits of Cover Crop Residues on Biomass of Cash Crops
4.4. Relationship between Soil Microbiology and Nitrogen Content in Maize
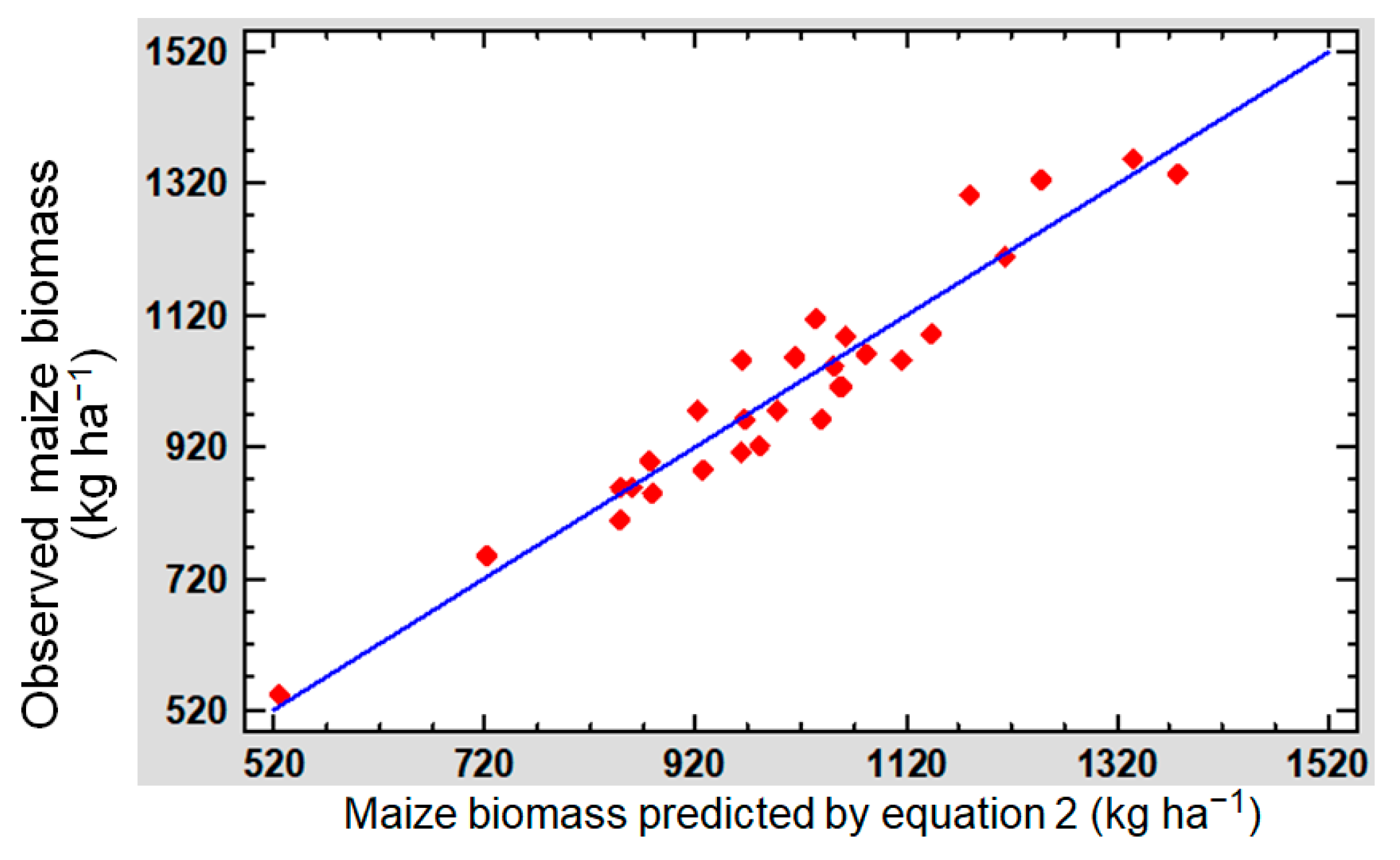
4.5. Relationship between Maize Biomass and Soil Variables
- (i)
- Firstly, increased penetration resistance is detrimental to maize biomass production because it impairs seed emergence and water/oxygen accessibility [67]. In our experiment, penetration resistance was not only negatively correlated with maize biomass production but also with biomass production in previous CCs (r = −0.58 ***, r = −0.48 **, respectively). Thus, penetration resistance informs efficiently on the potential of the soil for biomass production [68].
- (ii)
- Secondly, we recall that Fe availability in carbonate soils is limited due to the formation of insoluble compounds such as Fe hydroxides and carbonates [69]. Thus, increasing Fe availability in these soils may improve maize biomass production, as indicated by Equation (1). However, none of the treatments studied was able to significantly increase soil Fe concentration.
- (iii)
- As soil S increased, maize biomass increased. In addition, a positive correlation (p < 0.01) was found between soil S and previous CC biomass production, which reinforces the idea that S played a key role in biomass production. There are several reasons why S has this relevance. In our calcareous soil, a significant amount of sulphates (the assimilable form of S) is co-precipitated with CaCO3 [70]. On the other hand, maize demands significant supplies of S due to its high requirements and sensitivity to S deficiency. Finally, and in the case of Europe, S fertilization needs have increased as inputs through atmospheric deposition or fertilizers have decreased substantially [71]. S concentrations in the maize soils of the CC mixtures were classified as very low or low (Table 4a) [72], but we did not find a satisfactory explanation for this last phenomenon.
- (iv)
- Nitrogen is often the most limiting nutrient for maize development [73]. In our case, we applied low-nitrogen fertilization to all treatments. Therefore, maize biomass production was able to respond to small variations in available nitrogen, such as from the mineralization of the previous CC. Although legume-based CC (such as vetch and melilotus) are well known for their N fixation capacity [74], in the maize rotation, BAR was the treatment with the highest shoot N content (Table 5a). This could be due to the fact that barley and other grasses tend to be more efficient than legumes at capturing and replenishing aboveground residual N from the soil [10].
- (v)
- Finally, as expected, soil pH affected the availability of most nutrients. In moderately alkaline soils, such as ours, the availability of most micronutrients (except for Mo) is seriously impaired [10,75]. Under these conditions, maize biomass increased with decreasing pH, as shown in Equation (1). It was also observed that the pH in maize soil (Table 3a) varied significantly depending on the treatment. According to Vives-Peris et al. [76], root exudates and respiration can change the pH of rhizospheric soil in order to solubilize nutrients and thus increase their availability. In addition, certain rhizosphere-associated soil microorganisms can also modify the pH of the surrounding soil [77].
4.6. Relationship between Maize Biomass and Plant Variables
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Du, X.; Jian, J.; Du, C.; Stewart, R.D. Conservation management decreases surface runoff and soil erosion. Int. Soil Water Conserv. Res. 2022, 10, 188–196. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Ren, W.; Coyne, M.; Jacinthe, P.; Tao, B.; Hui, D.; Yang, J.; Matocha, C. Responses of soil carbon sequestration to climate-smart agriculture practices: A meta-analysis. Glob. Change Biol. 2019, 25, 2591–2606. [Google Scholar] [CrossRef] [PubMed]
- Smit, B.; Janssens, B.; Haagsma, W.; Hennen, W.; Adrados, J.L.; Kathage, J.; Domínguez, I.P. Adoption of Cover Crops for Climate Change Mitigation in the EU; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar] [CrossRef]
- Nivelle, E.; Verzeaux, J.; Habbib, H.; Kuzyakov, Y.; Decocq, G.; Roger, D.; Lacoux, J.; Duclercq, J.; Spicher, F.; Nava-Saucedo, J. Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Appl. Soil Ecol. 2016, 108, 147–155. [Google Scholar] [CrossRef]
- Valkama, E.; Kunypiyaeva, G.; Zhapayev, R.; Karabayev, M.; Zhusupbekov, E.; Perego, A.; Schillaci, C.; Sacco, D.; Moretti, B.; Grignani, C. Can conservation agriculture increase soil carbon sequestration? A modelling approach. Geoderma 2020, 369, 114298. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. Cover crop impacts on soil physical properties: A review. Soil Sci. Soc. Am. J. 2020, 84, 1527–1576. [Google Scholar] [CrossRef]
- Qin, Z.; Guan, K.; Zhou, W.; Peng, B.; Tang, J.; Jin, Z.; Grant, R.; Hu, T.; Villamil, M.B.; DeLucia, E. Assessing long-term impacts of cover crops on soil organic carbon in the central US Midwestern agroecosystems. Glob. Change Biol. 2023, 29, 2572–2590. [Google Scholar] [CrossRef]
- White, C.M.; DuPont, S.T.; Hautau, M.; Hartman, D.; Finney, D.M.; Bradley, B.; LaChance, J.C.; Kaye, J.P. Managing the trade off between nitrogen supply and retention with cover crop mixtures. Agric. Ecosyst. Environ. 2017, 237, 121–133. [Google Scholar] [CrossRef]
- Lapierre, J.; Machado, P.V.F.; Debruyn, Z.; Brown, S.E.; Jordan, S.; Berg, A.; Biswas, A.; Henry, H.A.; Wagner-Riddle, C. Cover crop mixtures: A powerful strategy to reduce post-harvest surplus of soil nitrate and leaching. Agric. Ecosyst. Environ. 2022, 325, 107750. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Cheng, K.; Yue, Q.; Chadwick, D.; Espenberg, M.; Truu, J.; Rees, R.M.; Smith, P. A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob. Change Biol. 2019, 25, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Hättenschwiler, S.; Fromin, N. Litter fingerprint on microbial biomass, activity, and community structure in the underlying soil. Plant Soil 2014, 379, 79–91. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Brunetti, P.; Campiglia, E. Soil quality, microbial functions and tomato yield under cover crop mulching in the Mediterranean environment. Soil Tillage Res. 2015, 145, 20–28. [Google Scholar] [CrossRef]
- Ulcuango, K.; Navas, M.; Centurión, N.; Ibañez, M.Á.; Hontoria, C.; Mariscal-Sancho, I. Interaction of Inherited Microbiota from Cover Crops with Cash Crops. Agronomy 2021, 11, 2199. [Google Scholar] [CrossRef]
- Mahama, G.Y.; Prasad, P.; Roozeboom, K.L.; Nippert, J.B.; Rice, C.W. Reduction of nitrogen fertilizer requirements and nitrous oxide emissions using legume cover crops in a no-tillage sorghum production system. Sustainability 2020, 12, 4403. [Google Scholar] [CrossRef]
- Lavergne, S.; Vanasse, A.; Thivierge, M.; Halde, C. Using fall-seeded cover crop mixtures to enhance agroecosystem services: A review. J. Geosci. Environ. Prot. 2021, 4, e20161. [Google Scholar] [CrossRef]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.R.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 48. [Google Scholar] [CrossRef]
- Finney, D.M.; Murrell, E.G.; White, C.M.; Baraibar, B.; Barbercheck, M.E.; Bradley, B.A.; Cornelisse, S.; Hunter, M.C.; Kaye, J.P.; Mortensen, D.A. Ecosystem services and disservices are bundled in simple and diverse cover cropping systems. Agric. Environ. Lett. 2017, 2, 170033. [Google Scholar] [CrossRef]
- Blesh, J.; VanDusen, B.M.; Brainard, D.C. Managing ecosystem services with cover crop mixtures on organic farms. Agron. J. 2019, 111, 826–840. [Google Scholar] [CrossRef]
- Garba, I.I.; Bell, L.W.; Williams, A. Cover crop legacy impacts on soil water and nitrogen dynamics, and on subsequent crop yields in drylands: A meta-analysis. Agron. Sustain. Dev. 2022, 42, 34. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Alletto, L. Ecosystem services of cover crops: A research roadmap. Trends Plant Sci. 2022, 27, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the global number and distribution of maize and wheat farms. Glob. Food Sec. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Casler, M.D. Fundamentals of experimental design: Guidelines for designing successful experiments. Agron. J. 2015, 107, 692–705. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Villalobos, F.J.; Delgado, A.; López-Bernal, Á.; Quemada, M. FertiliCalc: A decision support system for fertilizer management. J. Plant Prod. Sci. 2020, 14, 299–308. [Google Scholar] [CrossRef]
- Enz, M.; Dachler, C. Compendio Para la Identificación de Los Estadios Fenológicos de Especies Mono-y Dicotiledóneas Cultivadas Escala BBCH Extendida; BBA: Limburgerhof, Germany, 1998. [Google Scholar]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- García-González, I.; Quemada, M.; Gabriel, J.L.; Hontoria, C. Arbuscular mycorrhizal fungal activity responses to winter cover crops in a sunflower and maize cropping system. Appl. Soil Ecol. 2016, 102, 10–18. [Google Scholar] [CrossRef]
- Jakobsen, I.; Abbott, L.K.; Robson, A.D. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in applied soil microbiology and biochemistry. In Enzyme Activities; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 311–373. [Google Scholar]
- Yakovchenko, V.P.; Sikora, L.J. Modified dichromate method for determining low concentrations of extractable organic carbon in soil. Commun. Soil Sci. Plant Anal. 1998, 29, 421–433. [Google Scholar] [CrossRef]
- Kodešová, R.; Kodeš, V.; Mraz, A. Comparison of two sensors ECH2O EC-5 and SM200 for measuring soil water content. Soil Water Res. 2011, 6, 102–110. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P.W. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef] [PubMed]
- López-Gutiérrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreiter, T.; Selezi, D.; Quaiser, A.; Bonch-Osmolovskaya, L.; Schleper, C. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ. Microbiol. 2003, 5, 787–797. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R. RStudio; PBC: Boston, MA, USA, 2022; Available online: http://www.r-project.org/ (accessed on 2 January 2022).
- Kassambara, A.; Mundt, F. Package ‘factoextra’. Extr. Vis. Results Multivar. Data Anal. 2017, 76, 1–74. [Google Scholar]
- Tosi, M.; Drummelsmith, J.; Obregón, D.; Chahal, I.; Van Eerd, L.L.; Dunfield, K.E. Cover crop-driven shifts in soil microbial communities could modulate early tomato biomass via plant-soil feedbacks. Sci. Rep. 2022, 12, 9140. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-associated microbiomes of wheat under the combined effect of plant development and nitrogen fertilization. Microbiome 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, J.; Choi, M.M.; Chen, Y.; Chen, H.; Mohammad, R.; Zhuang, R.; Chen, H.; Wang, F.; Maskow, T. A combination method to study microbial communities and activities in zinc contaminated soil. J. Hazard. Mater. 2009, 169, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Florence, A.M.; McGuire, A.M. Do diverse cover crop mixtures perform better than monocultures? A systematic review. Agron. J. 2020, 112, 3513–3534. [Google Scholar] [CrossRef]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; De Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models: A review. In Sustainable Agriculture; Springer: Cham, Switzerland, 2009; pp. 329–353. [Google Scholar] [CrossRef]
- De Tombeur, F.; Lemoine, T.; Violle, C.; Fréville, H.; Thorne, S.J.; Hartley, S.E.; Lambers, H.; Fort, F. Nitrogen availability and plant–plant interactions drive leaf silicon concentration in wheat genotypes. Funct. Ecol. 2022, 36, 2833–2844. [Google Scholar] [CrossRef]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, A.H.; Asadi, G.A.; Ghorbani, R. Allelopathic Potential of Lavender’s Extract and Coumarin Applied as Pre-Plant Incorporated Into Soil Under Agronomic Conditions. Planta Daninha 2018, 36. [Google Scholar] [CrossRef]
- Li, F.; Sorensen, P.; Li, X.; Olesen, J.E. Carbon and nitrogen mineralization differ between incorporated shoots and roots of legume versus non-legume based cover crops. Plant Soil 2020, 446, 243–257. [Google Scholar] [CrossRef]
- Udom, B.E.; Omovbude, S. Soil physical properties and carbon/nitrogen relationships in stable aggregates under legume and grass fallow. Acta Ecol. Sin. 2019, 39, 56–62. [Google Scholar] [CrossRef]
- Sievers, T.; Cook, R.L. Aboveground and root decomposition of cereal rye and hairy vetch cover crops. Soil Sci. Soc. Am. J. 2018, 82, 147–155. [Google Scholar] [CrossRef]
- Singh, G.; Williard, K.W.; Schoonover, J.E. Cover crops and tillage influence on nitrogen dynamics in plant-soil-water pools. Soil Sci. Soc. Am. J. 2018, 82, 1572–1582. [Google Scholar] [CrossRef]
- Perdigão, A.; Pereira, J.L.; Moreira, N.; Trindade, H.; Coutinho, J. Assessment of Mineralized Nitrogen During Maize Growth Succeeding Different Winter Cover Crops in the Mediterranean Environment. Open Agric. J. 2022, 16, e187433152208150. [Google Scholar] [CrossRef]
- Roldán, A.; Caravaca, F.; Hernández, M.T.; Garcıa, C.; Sánchez-Brito, C.; Velásquez, M.; Tiscareno, M. No-tillage, crop residue additions, and legume cover cropping effects on soil quality characteristics under maize in Patzcuaro watershed (Mexico). Soil Tillage Res. 2003, 72, 65–73. [Google Scholar] [CrossRef]
- Nielsen, D.C.; Lyon, D.J.; Higgins, R.K.; Hergert, G.W.; Holman, J.D.; Vigil, M.F. Cover crop effect on subsequent wheat yield in the central Great Plains. Agron. J. 2016, 108, 243–256. [Google Scholar] [CrossRef]
- Clay, D.C.; Carlson, C.G.; Dalsted, K. iGrow wheat: Best management practices for wheat production. In Agronomy, Horticulture, and Plant Science Books; South Dakota State University: Brookings, SD, USA, 2012; Volume 1. [Google Scholar]
- Skoracka, A.; Rector, B.; Kuczyński, L.; Szydło, W.; Hein, G.; French, R. Global spread of wheat curl mite by its most polyphagous and pestiferous lineages. Ann. Appl. Biol. 2014, 165, 222–235. [Google Scholar] [CrossRef]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.; Fierer, N. Temporal variability in soil microbial communities across land-use types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef]
- Myrold, D.D.; Posavatz, N.R. Potential importance of bacteria and fungi in nitrate assimilation in soil. Soil Biol. Biochem. 2007, 39, 1737–1743. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. Reclamation of arid and semi-arid soils: The role of plant growth-promoting archaea and bacteria. Curr. Plant Biol. 2021, 25, 100173. [Google Scholar] [CrossRef]
- Jung, J.; Kim, J.; Taffner, J.; Berg, G.; Ryu, C. Archaea, tiny helpers of land plants. Comput. Struct. Biotechnol. J. 2020, 18, 2494–2500. [Google Scholar] [CrossRef]
- Natheer, S.E.; Muthukkaruppan, S. Assessing the in vitro zinc solubilization potential and improving sugarcane growth by inoculating Gluconacetobacter diazotrophicus. Ann. Microbiol. 2012, 62, 435–441. [Google Scholar] [CrossRef]
- Colombi, T.; Torres, L.C.; Walter, A.; Keller, T. Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth–A vicious circle. Sci. Total Environ. 2018, 626, 1026–1035. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, J.; Ji, W.; Sun, J.; Huo, D.; Liu, Y.; Chen, H. Effects of crop residue managements and tillage practices on variations of soil penetration resistance in sloping farmland of Mollisols. Int. J. Agric. Biol. 2022, 15, 164–171. [Google Scholar] [CrossRef]
- Priyadarshini, P.; Chitdeshwari, T.; Sudhalakshmi, C. A Iron Availability in Calcareous and Non Calcareous Soils as Influenced by Various Sources and Levels of Iron. Madras Agric. J. 2019, 106, 301–315. [Google Scholar] [CrossRef]
- Wilhelm Scherer, H. Sulfur in soils. J. Soil Sci. Plant Nutr. 2009, 172, 326–335. [Google Scholar] [CrossRef]
- Sutar, R.K.; Pujar, A.M.; Kumar, B.A.; Hebsur, N.S. Sulphur nutrition in maize-a critical review. Int. J. Pure App Biosci. 2017, 5, 1582–1596. [Google Scholar] [CrossRef]
- Zbíral, J.; Smatanová, M.; Němec, P. Sulphur status in agricultural soils determined using the Mehlich 3 method. Plant Soil Environ. 2018, 64, 255–259. [Google Scholar] [CrossRef]
- Ten Berge, H.F.; Hijbeek, R.; Van Loon, M.P.; Rurinda, J.; Tesfaye, K.; Zingore, S.; Craufurd, P.; van Heerwaarden, J.; Brentrup, F.; Schröder, J.J. Maize crop nutrient input requirements for food security in sub-Saharan Africa. Glob. Food Sec. 2019, 23, 9–21. [Google Scholar] [CrossRef]
- De Notaris, C.; Olesen, J.E.; Sorensen, P.; Rasmussen, J. Input and mineralization of carbon and nitrogen in soil from legume-based cover crops. Nutr. Cycl. Agroecosyst. 2020, 116, 1–18. [Google Scholar] [CrossRef]
- Shuman, L.M. Nutrient use in crop production. In Micronutrient Fertilizers; CRC Press: Boca Ratón, FL, USA, 2017; pp. 165–195. [Google Scholar]
- Vives-Peris, V.; De Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Gondal, A.H.; Hussain, I.; Ijaz, A.B.; Zafar, A.; Ch, B.I.; Zafar, H.; Sohail, M.D.; Niazi, H.; Touseef, M.; Khan, A.A. Influence of soil pH and microbes on mineral solubility and plant nutrition: A review. Int. J. Agric. Biol. Sci. 2021, 5, 71–81. [Google Scholar]
- Zhu, X.; Song, F.; Liu, F. Arbuscular mycorrhizal fungi and tolerance of temperature stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer Nature: Singapore, 2017; pp. 163–194. [Google Scholar]
- Toom, M.; Talgre, L.; Pechter, P.; Narits, L.; Tamm, S.; Lauringson, E. The effect of sowing date on cover crop biomass and nitrogen accumulation. Agron. Res. 2019, 17, 4. [Google Scholar] [CrossRef]
- Dos Santos Cordeiro, L.F.; dos Santos Cordeiro, C.F.; Ferrari, S. Cotton yield and boron dynamics affected by cover crops and boron fertilization in a tropical sandy soil. Field Crop. Res. 2022, 284, 108575. [Google Scholar] [CrossRef]
- Ahmad, W.; Zia, M.H.; Malhi, S.S.; Niaz, A.; Ullah, S. Boron Deficiency in soils and crops: A review. Crop. plant. 2012, 2012, 65–97. [Google Scholar] [CrossRef]
| March | April | May | June | July | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Covered Crops sowing (15 March) and development for 11 weeks. | ||||||||||
| Mean aerial temperature during the CC: 18.5 °C | ||||||||||
| Sampling of cover crops and their termination (30 May) | ||||||||||
| Wheat sowing (3 June) and development for 8 weeks | ||||||||||
| Mean aerial temperature between CC termination and main sampling: 22.5 °C | ||||||||||
| Maize sowing (24 June) and development for 5 weeks | ||||||||||
| Fertilization with NH4NO3 91/0/0 (11 July) | ||||||||||
| Irrigation: 112.5 ml per pot, 3 times per week (≈ 40 mm·month−1 of rain) | ||||||||||
| Main Sampling and termination of the main crops (29 July) | ||||||||||
| Previous | AMF Colonization | Mycelium Length | Bacteria | Fungi | F:B | Glomeromycetes | Archaea | SIR * | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | (%) | (cm g−1) | (Log Copies 16S g−1) | (Log Copies ITS g−1) | (Log (ITS:16S)) | (Log Copies g−1) | (Log Copies g−1) | (mg C-CO2 kg−1h−1) | ||||||||||||||||
| (a) Under maize | ||||||||||||||||||||||||
| CON | 13 | ±2.4 | a | 5.93 | ±2.54 | a | 9.033 | ±0.018 | c | 6.705 | ±0.038 | c | −2.33 | ±0.03 | ab | 5.67 | ±0.03 | f | 6.59 | ±0.03 | a | 2.21 | ±1.37 | a |
| VET | 22 | ±5.0 | bc | 6.01 | ±2.41 | a | 9.038 | ±0.032 | c | 6.946 | ±0.054 | e | −2.09 | ±0.08 | c | 5.43 | ±0.04 | e | 7.05 | ±0.02 | c | 2.12 | ±0.96 | a |
| MEL | 20 | ±5.2 | b | 6.59 | ±1.46 | a | 8.942 | ±0.053 | b | 6.938 | ±0.035 | e | −2.00 | ±0.06 | cd | 5.34 | ±0.04 | d | 6.67 | ±0.04 | a | 1.93 | ±1.08 | a |
| BAR | 26 | ±4.5 | c | 7.51 | ±2.02 | a | 8.791 | ±0.128 | a | 6.836 | ±0.028 | d | −1.95 | ±0.14 | d | 4.99 | ±0.04 | b | 7.10 | ±0.04 | c | 2.02 | ±1.03 | a |
| B+V | 34 | ±6.7 | d | 6.97 | ±2.29 | a | 8.77 | ±0.097 | a | 6.531 | ±0.039 | b | −2.24 | ±0.09 | b | 5.07 | ±0.03 | c | 6.88 | ±0.03 | b | 1.86 | ±0.86 | a |
| B+M | 22 | ±6.3 | bc | 6.56 | ±2.25 | a | 8.801 | ±0.051 | a | 6.38 | ±0.055 | a | −2.42 | ±0.10 | a | 4.39 | ±0.04 | a | 6.91 | ±0.13 | b | 1.97 | ±0.59 | a |
| (b) Under wheat | ||||||||||||||||||||||||
| CON | 8.8 | ±4.3 | a | 4.33 | ±1.97 | a | 8.424 | ±0.025 | a | 6.302 | ±0.178 | a | −2.201 | ±0.030 | a | 4.407 | ±0.041 | a | 7.1 | ±0.052 | a | 2.14 | ±1.22 | a |
| VET | 14.6 | ±3.6 | bc | 5.91 | ±1.26 | ab | 8.852 | ±0.025 | d | 7.337 | ±0.027 | f | −1.515 | ±0.033 | e | 4.524 | ±0.035 | b | 7.39 | ±0.041 | c | 1.73 | ±1.18 | a |
| MEL | 16.8 | ±2.2 | bc | 6.41 | ±1.05 | ab | 8.664 | ±0.017 | C | 6.838 | ±0.030 | b | −1.826 | ±0.047 | b | 5.789 | ±0.047 | d | 7.24 | ±0.038 | b | 1.78 | ±0.97 | a |
| BAR | 13.8 | ±3.9 | ab | 7.23 | ±1.60 | bc | 8.619 | ±0.027 | B | 6.952 | ±0.044 | c | −1.667 | ±0.060 | d | 6.003 | ±0.041 | e | 7.37 | ±0.030 | c | 1.95 | ±1.12 | a |
| B+V | 19.4 | ±4.4 | c | 6.31 | ±1.93 | ab | 8.822 | ±0.040 | D | 7.071 | ±0.019 | d | −1.751 | ±0.053 | c | 6.032 | ±0.032 | e | 7.36 | ±0.043 | c | 2.33 | ±1.19 | a |
| B+M | 19.6 | ±5.0 | c | 8.87 | ±2.45 | c | 8.933 | ±0.014 | E | 7.250 | ±0.017 | e | −1.684 | ±0.028 | d | 5.599 | ±0.036 | c | 7.06 | ±0.020 | a | 2.04 | ±0.89 | a |
| Previous | Soil Moisture | Soil Temperature | pH | EC | DOC | Penetration Resistance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | (%v) | (°C) | (µS cm−1) | (mg kg−1) | (kg cm−2) | |||||||||||||
| (a) Under maize | ||||||||||||||||||
| CON | 9.26 | ±1.67 | bc | 25.24 | ±0.73 | a | 7.68 | ±0.51 | a | 493 | ±263 | a | 30.5 | ±2.0 | a | 2.15 | ±0.19 | ab |
| VET | 8.3 | ±0.42 | ab | 25.25 | ±0.40 | a | 7.88 | ±0.42 | ab | 345 | ±183 | a | 30.3 | ±8.4 | a | 2.34 | ±0.78 | b |
| MEL | 7.41 | ±0.83 | a | 25.71 | ±0.82 | a | 8.29 | ±0.33 | c | 381 | ±83 | a | 32.3 | ±2.7 | a | 2.25 | ±0.87 | ab |
| BAR | 10.09 | ±1.01 | c | 25.22 | ±0.79 | a | 8.15 | ±0.11 | bc | 348 | ±143 | a | 34.6 | ±9.9 | a | 1.51 | ±0.82 | ab |
| B+V | 7.62 | ±0.53 | a | 25.47 | ±0.46 | a | 7.92 | ±0.12 | abc | 433 | ±121 | a | 31.2 | ±1.7 | a | 2.15 | ±0.60 | ab |
| B+M | 9.32 | ±1.60 | bc | 25.56 | ±0.53 | a | 7.93 | ±0.50 | abc | 341 | ±164 | a | 28.4 | ±4.0 | a | 1.46 | ±0.85 | a |
| (b) Under wheat | ||||||||||||||||||
| CON | 7.76 | ±0.72 | b | 25.24 | ±0.79 | ab | 8.1 | ±0.25 | a | 386 | ±167 | a | 30.6 | ±6.7 | a | 3.83 | ±0.45 | ab |
| VET | 7.52 | ±0.51 | b | 25.29 | ±0.78 | ab | 8.02 | ±0.27 | a | 457 | ±255 | a | 39.1 | ±10.3 | a | 4.9 | ±0.94 | b |
| MEL | 7.21 | ±0.79 | ab | 25.45 | ±0.64 | b | 8.09 | ±0.12 | a | 437 | ±153 | a | 33 | ±6.8 | a | 3.65 | ±0.79 | ab |
| BAR | 8.08 | ±0.83 | b | 24.82 | ±0.61 | a | 8.08 | ±0.17 | a | 348 | ±140 | a | 34.5 | ±11.9 | a | 3.87 | ±1.25 | ab |
| B+V | 7.49 | ±0.51 | b | 25.76 | ±0.50 | b | 8.03 | ±0.21 | a | 396 | ±140 | a | 53.2 | ±10.0 | b | 3.8 | ±1.22 | ab |
| B+M | 6.52 | ±0.45 | a | 25.23 | ±1.10 | ab | 8.44 | ±0.22 | b | 268 | ±152 | a | 32.1 | ±5.1 | a | 3.53 | ±0.73 | a |
| (a) Under maize | ||||||||||||||||||
| Previous | Ca | K | Mg | N* | P | S | ||||||||||||
| CC | (mg kg−1) | |||||||||||||||||
| CON | 887 | ±72 | a | 100.5 | ±5.6 | a | 159.7 | ±11.4 | b | 381 | ±199 | a | 32.9 | ±2.4 | b | 19.4 | ±10.3 | c |
| VET | 834 | ±61 | a | 103 | ±3.1 | a | 155.1 | ±7.6 | ab | 525 | ±158 | abc | 33.1 | ±4.2 | b | 12.9 | ±3.0 | abc |
| MEL | 862 | ±85 | a | 99.3 | ±1.8 | a | 145.8 | ±6.6 | a | 611 | ±294 | abc | 30.9 | ±1.5 | b | 10.8 | ±3.1 | ab |
| BAR | 894 | ±127 | a | 111.9 | ±7.7 | b | 155.3 | ±13.7 | ab | 693 | ±207 | bc | 27.0 | ±1.3 | a | 15.7 | ±4.2 | bc |
| B+V | 847 | ±88 | a | 103.4 | ±6.3 | a | 159.8 | ±10.5 | b | 715 | ±99 | c | 31.5 | ±1.7 | b | 9.6 | ±4.2 | a |
| B+M | 868 | ±67 | a | 95.8 | ±8.4 | a | 148.9 | ±11.5 | ab | 418 | ±191 | ab | 27.6 | ±1.9 | a | 9.1 | ±2.8 | a |
| Previous | Co | Fe | Mn | Na | Ni | Zn | ||||||||||||
| CC | (mg kg−1) | |||||||||||||||||
| CON | 0.889 | ±0.041 | c | 58.5 | ±9.3 | a | 51.3 | ±4.6 | a | 58.6 | ±15.2 | b | 0.626 | ±0.058 | a | 2.51 | ±0.85 | ab |
| VET | 0.841 | ±0.059 | abc | 63.5 | ±9.9 | a | 50.1 | ±2.8 | a | 45.5 | ±14.0 | ab | 0.625 | ±0.035 | a | 2.86 | ±0.35 | b |
| MEL | 0.816 | ±0.045 | ab | 60.4 | ±7.1 | a | 48.1 | ±2.4 | a | 51.9 | ±4.9 | ab | 0.607 | ±0.024 | a | 2.89 | ±0.70 | b |
| BAR | 0.807 | ±0.056 | ab | 66.5 | ±9.4 | a | 50.3 | ±5.6 | a | 54.5 | ±13.4 | ab | 0.614 | ±0.048 | a | 2.19 | ±0.23 | a |
| B+V | 0.861 | ±0.039 | bc | 65.0 | ±5.5 | a | 50.7 | ±2.0 | a | 50.3 | ±21.5 | ab | 0.625 | ±0.031 | a | 2.5 | ±0.42 | ab |
| B+M | 0.800 | ±0.026 | a | 62.3 | ±7.7 | a | 47.5 | ±1.5 | a | 40.8 | ±9.6 | a | 0.589 | ±0.032 | a | 2.46 | ±0.22 | ab |
| (b) Under wheat | ||||||||||||||||||
| Previous | Ca | K | Mg | N* | P | S | ||||||||||||
| CC | (mg kg−1) | |||||||||||||||||
| CON | 840 | ±55 | ab | 96.8 | ±9.7 | a | 148.7 | ±10.3 | a | 416 | ±329 | a | 31.9 | ±3.4 | ab | 11.8 | ±5.3 | a |
| VET | 792 | ±97 | a | 95.5 | ±9.0 | a | 141.6 | ±18.3 | a | 552 | ±217 | a | 33.0 | ±3.3 | b | 14.1 | ±7.6 | a |
| MEL | 838 | ±62 | ab | 97.3 | ±20.5 | a | 146.9 | ±14.6 | a | 559 | ±103 | a | 29.6 | ±2.2 | a | 16.5 | ±10.2 | ab |
| BAR | 947 | ±91 | c | 97.4 | ±10.1 | a | 163.7 | ±8.2 | c | 624 | ±239 | a | 30.5 | ±2.8 | ab | 21.3 | ±3.6 | b |
| B+V | 844 | ±85 | ab | 94.6 | ±3.8 | a | 151.5 | ±13.4 | ab | 573 | ±171 | a | 29.8 | ±1.8 | ab | 16.5 | ±5.0 | ab |
| B+M | 904 | ±47 | bc | 104.5 | ±10.2 | a | 160.1 | ±6.2 | bc | 586 | ±249 | a | 30.7 | ±2.1 | ab | 24.9 | ±13.0 | b |
| Previous | Co | Fe | Mn | Na | Ni | Zn | ||||||||||||
| CC | (mg kg−1) | |||||||||||||||||
| CON | 0.818 | ±0.058 | ab | 57.7 | ±6.9 | a | 47.6 | ±3.5 | ab | 44.2 | ±16.6 | ab | 0.58 | ±0.032 | ab | 2.24 | ±0.53 | a |
| VET | 0.774 | ±0.089 | a | 57.3 | ±6.3 | a | 45.7 | ±5.2 | a | 37.3 | ±21.7 | a | 0.558 | ±0.044 | a | 2.95 | ±0.89 | bc |
| MEL | 0.817 | ±0.080 | ab | 56.1 | ±13.7 | a | 48.4 | ±5.3 | ab | 42.1 | ±22.3 | a | 0.595 | ±0.066 | abc | 2.58 | ±0.66 | ab |
| BAR | 0.864 | ±0.090 | b | 54.7 | ±8.8 | a | 50.7 | ±4.5 | b | 63.1 | ±13.0 | b | 0.610 | ±0.030 | bc | 2.65 | ±0.74 | ab |
| B+V | 0.800 | ±0.079 | ab | 56.2 | ±4.1 | a | 47.6 | ±4.2 | ab | 55.5 | ±7.6 | ab | 0.584 | ±0.035 | ab | 2.54 | ±0.48 | ab |
| B+M | 0.870 | ±0.054 | b | 62.7 | ±9.7 | a | 51.2 | ±3.2 | b | 52.8 | ±9.0 | ab | 0.633 | ±0.038 | c | 3.23 | ±0.72 | c |
| (a) Under maize | |||||||||||||||||||||
| Previous | Dry aerial biomass | Ca | K | Mg | N* | P | S | ||||||||||||||
| CC | (kg ha−1) | (mg microcosm−1) | |||||||||||||||||||
| CON | 950 | ±75 | ab | 10.7 | ±1.1 | ab | 57.1 | ±14.0 | ab | 5.64 | ±2.17 | a | 74.8 | ±4.8 | a | 5.57 | ±0.81 | a | 5.58 | ±1.40 | A |
| VET | 1000 | ±220 | ab | 10.5 | ±1.7 | a | 45.7 | ±14.6 | a | 6.71 | ±3.03 | ab | 81.5 | ±13.2 | ab | 5.86 | ±1.06 | a | 5.76 | ±1.64 | a |
| MEL | 869 | ±206 | a | 9.7 | ±2.3 | a | 46.6 | ±17.8 | a | 6.2 | ±3.05 | ab | 74.6 | ±15.4 | a | 5.59 | ±1.46 | a | 5.26 | ±1.88 | a |
| BAR | 1141 | ±149 | b | 13 | ±1.7 | b | 61.7 | ±20.4 | b | 7.27 | ±1.99 | b | 91.7 | ±8.8 | b | 6.84 | ±1.84 | a | 5.9 | ±1.27 | a |
| B+V | 1015 | ±100 | ab | 10.4 | ±2.6 | a | 46.6 | ±13.2 | a | 6.19 | ±2.47 | ab | 85.3 | ±5.8 | ab | 6.22 | ±1.22 | a | 5.53 | ±1.77 | a |
| B+M | 1096 | ±213 | ab | 11.4 | ±1.6 | ab | 53.1 | ±16.4 | ab | 7.12 | ±2.72 | ab | 88.1 | ±13.9 | ab | 6.8 | ±1.45 | a | 6.68 | ±2.03 | a |
| Previous | Root biomass | B | Cu | Fe | Mn | Na | Zn | ||||||||||||||
| CC | (kg ha−1) | (µg microcosm−1) | |||||||||||||||||||
| CON | 741 | ±272 | a | 43.9 | ±5.4 | ab | 18.4 | ±1.5 | a | 278 | ±107 | a | 232 | ±35 | a | 315 | ±166 | a | 76.3 | ±7.8 | a |
| VET | 843 | ±219 | a | 39.8 | ±10.1 | a | 19.5 | ±2.7 | a | 302 | ±137 | a | 262 | ±58 | ab | 629 | ±497 | abc | 96.1 | ±19.5 | ab |
| MEL | 831 | ±103 | a | 39.6 | ±10.6 | a | 18 | ±5.0 | a | 294 | ±150 | a | 234 | ±71 | a | 349 | ±282 | a | 87.3 | ±21.6 | ab |
| BAR | 1281 | ±323 | b | 51.8 | ±6.4 | b | 20.7 | ±2.7 | a | 426 | ±247 | bc | 306 | ±27 | b | 435 | ±140 | ab | 111.5 | ±16.9 | b |
| B+V | 898 | ±229 | ab | 38.7 | ±6.7 | a | 19 | ±4.3 | a | 322 | ±139 | ab | 250 | ±54 | ab | 713 | ±593 | bc | 102.7 | ±15.4 | b |
| B+M | 1049 | ±439 | ab | 48.6 | ±7.8 | ab | 20.8 | ±5.5 | a | 470 | ±217 | c | 282 | ±56 | ab | 817 | ±463 | c | 110.1 | ±17.7 | b |
| (b) Under wheat | |||||||||||||||||||||
| Previous | Dry aerial biomass | Ca | K | Mg | N* | P | S | ||||||||||||||
| CC | (kg ha−1) | (mg microcosm−1) | |||||||||||||||||||
| CON | 1144 | ±283 | c | 12.3 | ±3.36 | b | 79.3 | ±32.5 | c | 5.29 | ±2.05 | a | 111.8 | ±26.0 | bc | 6.38 | ±1.49 | bc | 7.42 | ±3.24 | bc |
| VET | 1125 | ±104 | bc | 11.1 | ±2.41 | ab | 62.1 | ±15.5 | b | 5.93 | ±2.88 | a | 107.6 | ±22.8 | bc | 7.05 | ±1.42 | bc | 6.87 | ±2.20 | bc |
| MEL | 1078 | ±266 | bc | 11.3 | ±3.01 | ab | 62.9 | ±25.3 | b | 6.01 | ±2.75 | a | 113.3 | ±28.4 | c | 7.44 | ±2.05 | c | 7.52 | ±2.97 | c |
| BAR | 718 | ±157 | a | 8.1 | ±1.86 | a | 35.1 | ±13.5 | a | 4.39 | ±2.00 | a | 66.1 | ±11.9 | a | 4.59 | ±0.49 | a | 4.44 | ±1.44 | a |
| B+V | 848 | ±163 | a | 9.3 | ±2.2 | ab | 47.4 | ±19.6 | b | 4.74 | ±2.01 | a | 88.7 | ±15.1 | ab | 5.71 | ±1.44 | ab | 5.74 | ±2.13 | b |
| B+M | 910 | ±162 | ab | 10.1 | ±1.84 | ab | 52.8 | ±16.9 | b | 5.54 | ±1.98 | a | 96.4 | ±17.0 | bc | 6.79 | ±1.61 | bc | 6.64 | ±2.40 | bc |
| Previous | Root biomass | B | Cu | Fe | Mn | Na | Zn | ||||||||||||||
| CC | (kg ha−1) | (µg microcosm−1) | |||||||||||||||||||
| CON | 1236 | ±230 | ab | 50.2 | ±12.4 | c | 22.2 | ±6.8 | bc | 466 | ±292 | bc | 261 | ±58 | b | 508 | ±362 | ab | 88.5 | ±24.3 | ab |
| VET | 1328 | ±180 | b | 47.2 | ±12.5 | bc | 22.3 | ±5.2 | bc | 332 | ±146 | b | 265 | ±57 | b | 685 | ±534 | abc | 123.2 | ±30.9 | c |
| MEL | 1156 | ±324 | ab | 45.6 | ±12.2 | abc | 24.0 | ±6.9 | c | 498 | ±305 | c | 278 | ±65 | b | 927 | ±758 | bcd | 125.3 | ±41.1 | c |
| BAR | 1066 | ±131 | ab | 32 | ±7.3 | a | 15.4 | ±4.0 | a | 214 | ±85 | a | 173 | ±38 | a | 441 | ±324 | a | 73.3 | ±16.6 | a |
| B+V | 991 | ±183 | a | 35.5 | ±7.7 | ab | 18.1 | ±5.4 | ab | 318 | ±184 | ab | 210 | ±43 | ab | 1058 | ±805 | cd | 100.7 | ±33.5 | abc |
| B+M | 1002 | ±164 | a | 37.3 | ±7.2 | ab | 21 | ±5.1 | bc | 440 | ±261 | bc | 240 | ±38 | ab | 1222 | ±.931 | d | 114.1 | ±34.1 | bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariscal-Sancho, I.; Hontoria, C.; Centurión, N.; Navas, M.; Moliner, A.; Peregrina, F.; Ulcuango, K. Maize and Wheat Responses to the Legacies of Different Cover Crops under Warm Conditions. Agronomy 2023, 13, 1721. https://doi.org/10.3390/agronomy13071721
Mariscal-Sancho I, Hontoria C, Centurión N, Navas M, Moliner A, Peregrina F, Ulcuango K. Maize and Wheat Responses to the Legacies of Different Cover Crops under Warm Conditions. Agronomy. 2023; 13(7):1721. https://doi.org/10.3390/agronomy13071721
Chicago/Turabian StyleMariscal-Sancho, Ignacio, Chiquinquirá Hontoria, Nelly Centurión, Mariela Navas, Ana Moliner, Fernando Peregrina, and Kelly Ulcuango. 2023. "Maize and Wheat Responses to the Legacies of Different Cover Crops under Warm Conditions" Agronomy 13, no. 7: 1721. https://doi.org/10.3390/agronomy13071721
APA StyleMariscal-Sancho, I., Hontoria, C., Centurión, N., Navas, M., Moliner, A., Peregrina, F., & Ulcuango, K. (2023). Maize and Wheat Responses to the Legacies of Different Cover Crops under Warm Conditions. Agronomy, 13(7), 1721. https://doi.org/10.3390/agronomy13071721







