Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico
Abstract
1. Introduction
2. Materials and Methods
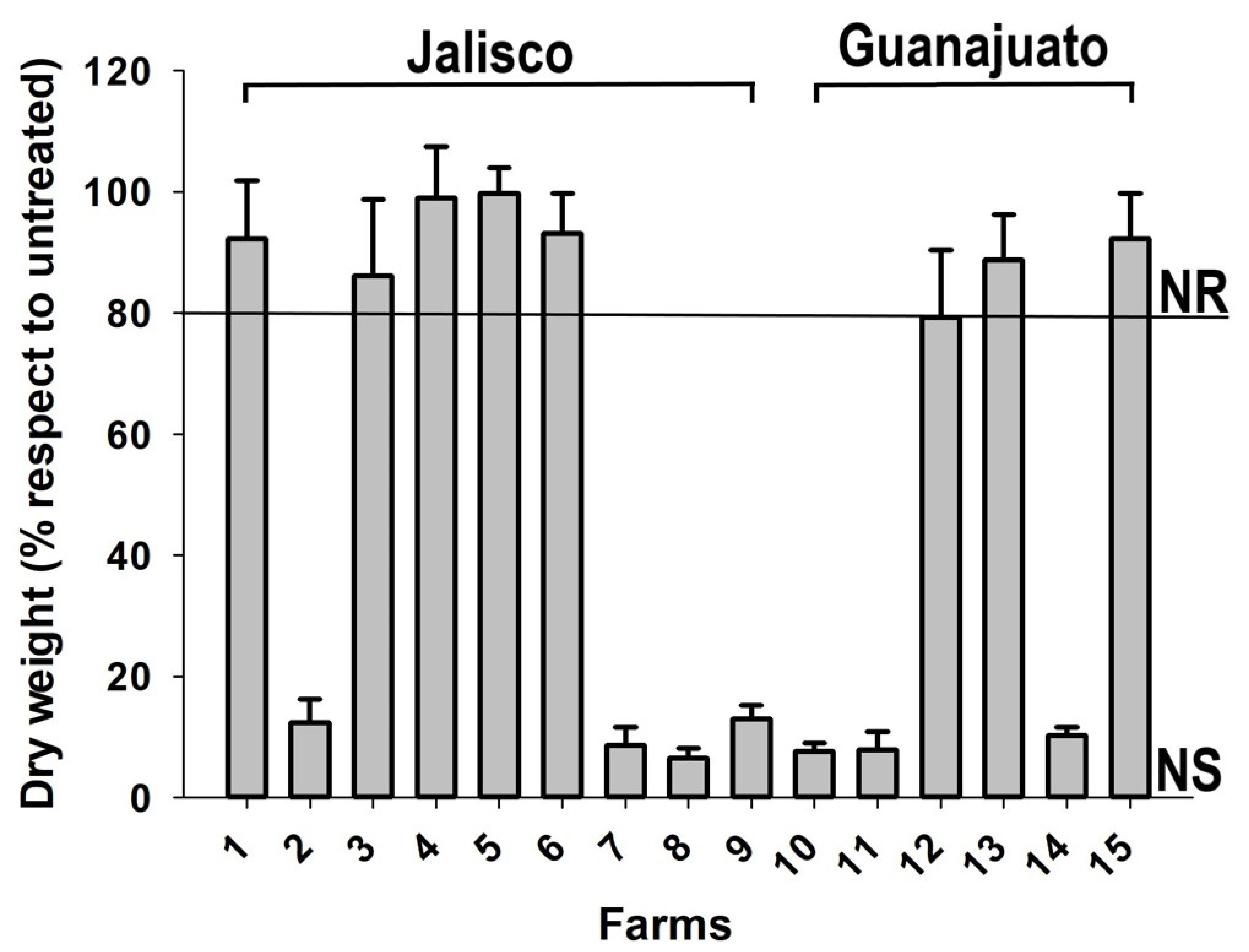
2.1. Resistance Screening in Corn Fields
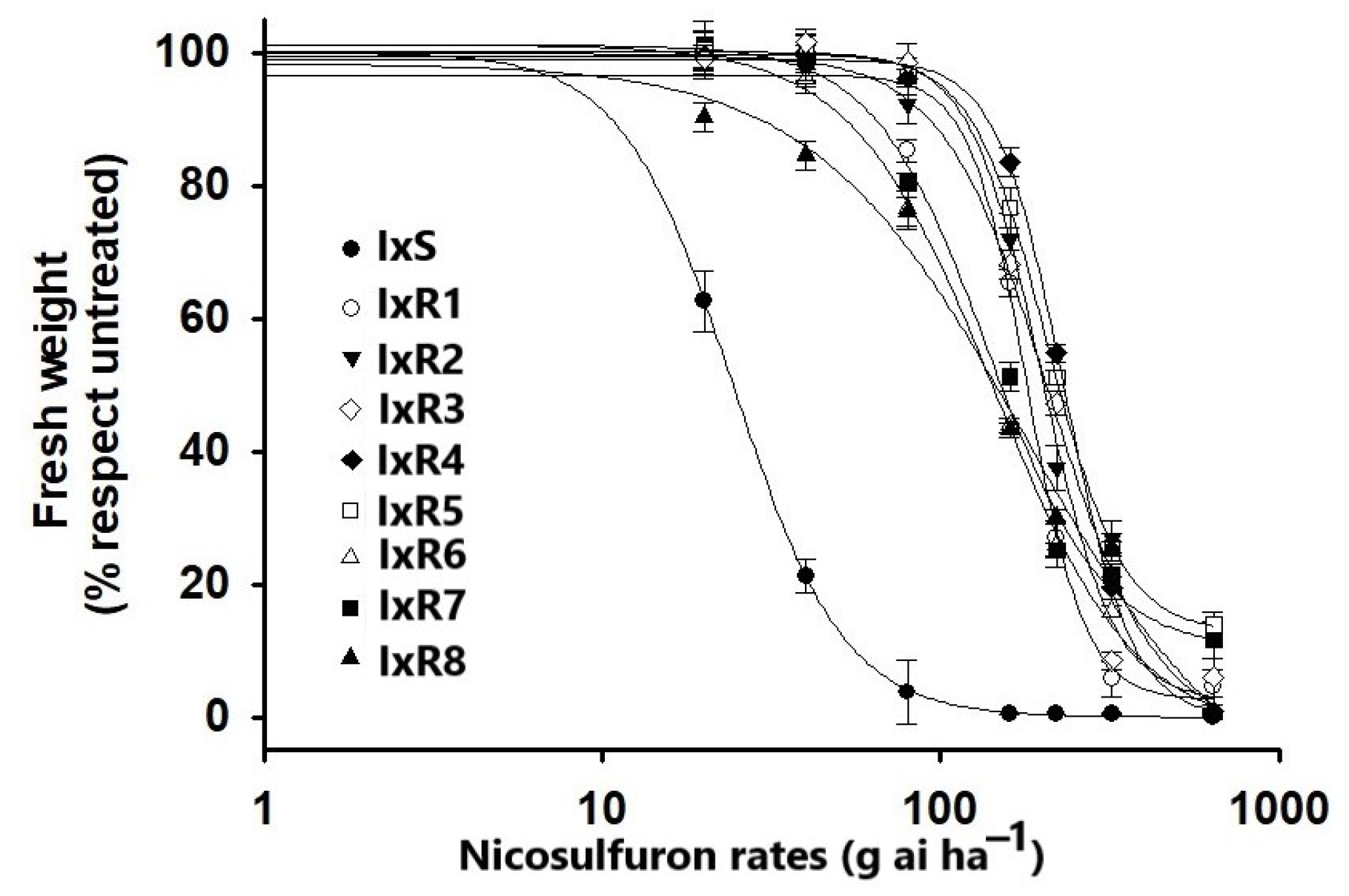
2.2. Dose–Response Curves
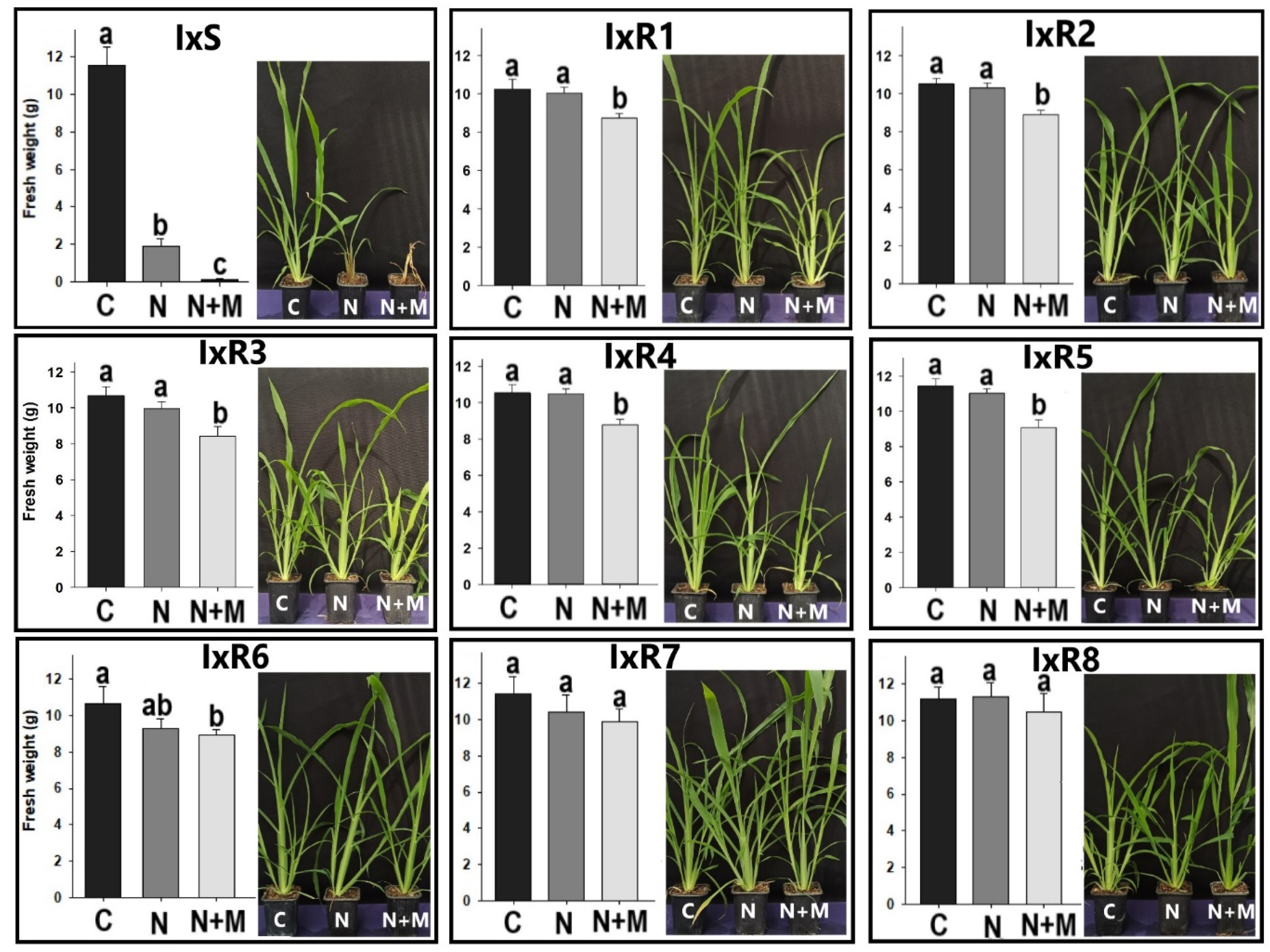
2.3. Synergism of Herbicide plus Malathion
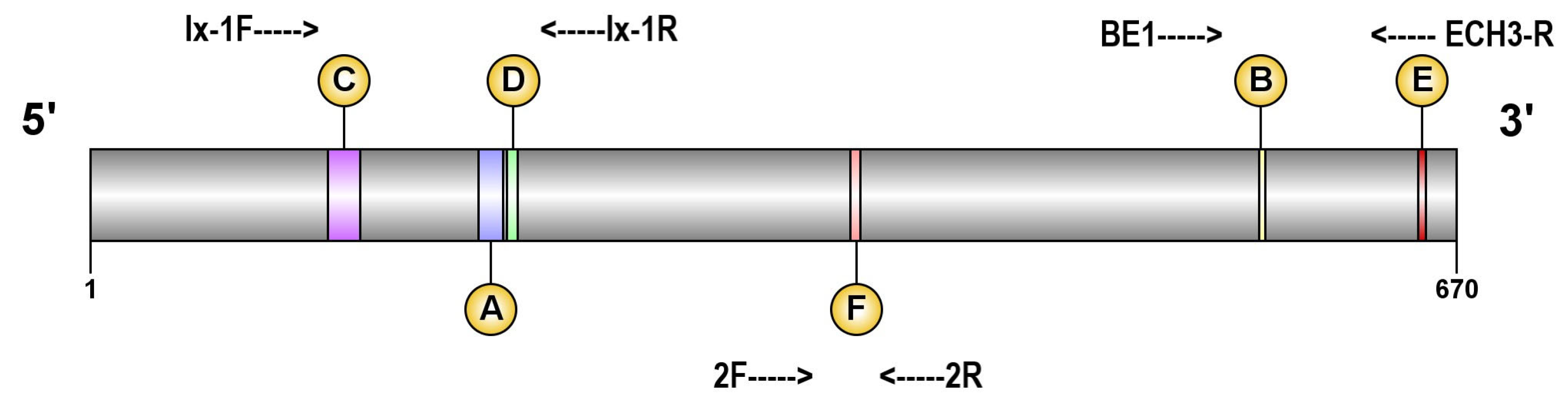
2.4. Molecular Analyses
DNA Isolation and PCR Amplification
2.5. Cross-Resistance In Vitro Assay (ALS Activity)
2.6. Cross-Resistance In Vivo Assay
2.7. Multiple-Resistance Screening
2.8. Data Analysis
2.9. Chemicals
3. Results
3.1. Herbicide-Resistance Field Screening
3.2. Dose–Response Curves
3.3. Nicosulfuron and Malathion Synergism
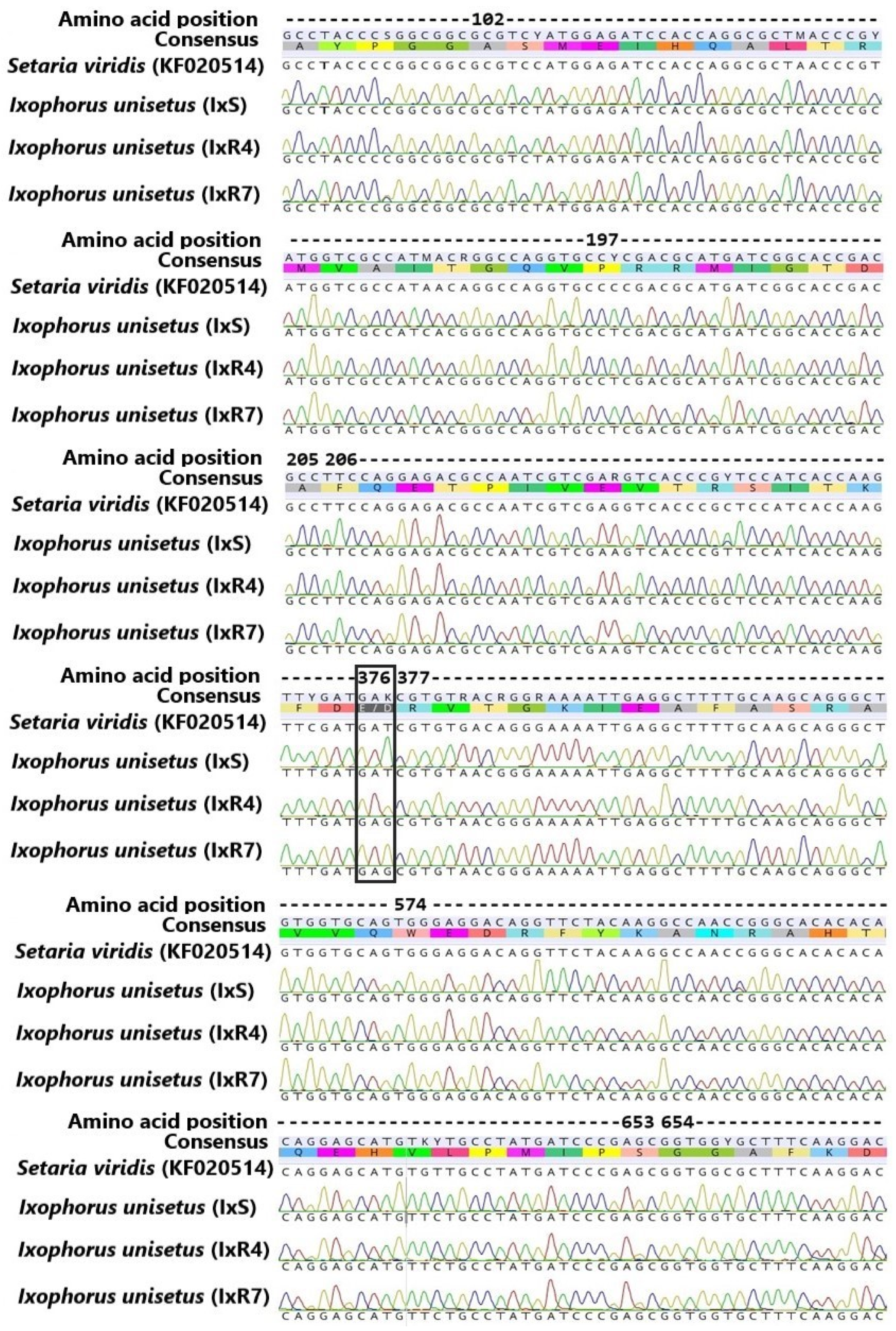
3.4. Partial ALS Gene Sequencing
3.5. Cross-Resistance In Vivo Assay
3.6. Cross-Resistance In Vitro Assay
3.7. Multiple-Resistance Screening Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vibrans, H. Malezas de México: Ixophorus unisetus (J. Presl) Schltdl. Available online: http://www.conabio.gob.mx/malezasdemexico/poaceae/ixophorus-unisetus/fichas/ficha.htm (accessed on 29 January 2023).
- Sánchez, J.G.; Zita-Padilla, G.A.; Mendoza-Cruz, M. Catálogo de las Gramíneas Malezas Nativas e Introducidas de México; CONACOFI: Montecillo, Mexico, 2012. [Google Scholar]
- Esqueda, V.A.; Tosquy, O.H. Efecto del volumen y el pH del agua en el control de Ixophorus unisetus (J. Presl) Schltdl. con glifosato. Rev. Mex. De Cienc. Agrícolas 2015, 6, 97–109. [Google Scholar] [CrossRef]
- Cano, O.; López, E. Control preemergente y postemergente de malezas en frijol de humedad residual en Veracruz, Mexico. Agron. Mesoam. 1996, 7, 42–49. [Google Scholar] [CrossRef]
- Herrera, F. Efecto de rastrojos de malezas y herbicidas pre-emergentes en el control de malezas en frijol. Agron. Mesoam. 2000, 11, 63–71. [Google Scholar] [CrossRef]
- Portillo, H.; Pitre, H.N.; Meckenstock, D.H.; Andrews, K.L. Oviposition preference of Spodoptera latifascia (Lepidoptera:Noctuidae) for sorghum, maize and non-crop vegetation. Fla. Entomol. 1996, 79, 552–562. [Google Scholar] [CrossRef]
- Pleasant, J.M.T.; Burt, J.; Frisch, J.C. Integrating mechanical and chemical weed management in corn (Zea mays). Weed Technol. 1994, 8, 217–223. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: http://weedscience.org (accessed on 25 January 2023).
- HRAC. Herbicide Resistance Action Comitte: Herbicide Resistance. Available online: https://hracglobal.com/herbicide-resistance/overview (accessed on 23 January 2023).
- Preston, C.; Powles, S.B. Evolution of herbicide resistance in weeds: Initial frequency of target site-based resistance to acetolactate synthase-inhibiting herbicides in Lolium rigidum. Heredity 2002, 88, 8–13. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef]
- Panozzo, S.; Mascanzoni, E.; Scarabel, L.; Milani, A.; Dalazen, G.; Merotto, A.J.; Tranel, P.J.; Sattin, M. Target-site mutations and expression of ALS gene copies vary according to Echinochloa species. Genes 2021, 12, 1841. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-García, J.G.; De Portugal, J.; Torra, J.; Osuna, M.D.; Palma-Bautista, C.; Cruz-Hipolito, H.E.; De Prado, R. Comparison between the mechanisms of Clearfield® wheat and Lolium rigidum multiple resistant to acetyl CoA carboxylase and acetolactate synthase inhibitors. Environ. Pollut. 2022, 306, 119438. [Google Scholar] [CrossRef]
- Fang, J.; Yang, D.; Zhao, Z.; Chen, J.; Dong, L. A novel Phe-206-Leu mutation in acetolactate synthase confers resistance to penoxsulam in barnyardgrass (Echinochloa crus-galli (L.) P. Beauv). Pest Manag. Sci. 2022, 78, 2560–2570. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, Y.; Liu, W.; Wang, J. Cytochrome P450 CYP709C56 metabolizing mesosulfuron-methyl confers herbicide resistance in Alopecurus aequalis. Cell. Mol. Life Sci. 2022, 75, 205. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Laplante, J.; Rajcan, I.; Tardif, F.J. Multiple allelic forms of acetohydroxyacid synthase are responsible for herbicide resistance in Setaria viridis. Theor. Appl. Genet. 2009, 119, 577–585. [Google Scholar] [CrossRef]
- Amaro-Blanco, I.; Romano, Y.; Palmerin, J.A.; Gordo, R.; Palma-Bautista, C.; De Prado, R.; Osuna, M.D. Different mutations providing target site resistance to ALS- ACCase-inhibiting herbicides in Echinochloa spp. from rice fields. Agriculture 2021, 11, 382. [Google Scholar] [CrossRef]
- Panozzo, S.; Scarabel, L.; Tranel, P.J.; Sattin, M. Target-site resistance to ALS inhibitors in polyploid species Echinochloa crus-galli. Pest. Biochem. Physiol. 2013, 105, 93–101. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; De Prado, R. Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response análisis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; da Silva Amaral, G.; Ferreira Mendes, K.; Rojano-Delgado, A.M.; De Prado, R.; das Graças Fernandes da Silva, M.F. Absorption, translocation, and metabolism studies of herbicides in weeds and crops. In Radioisotopes Weed Research; Ferreira Mendes, K., Ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 127–154. [Google Scholar]
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An ecological future for weed science to sustain crop production and the environment. A review. Agron. Sustain. Dev. 2020, 40, 24. [Google Scholar] [CrossRef]
- Ramiz, Z.; Malone, J.; Preston, C.; Gurjeet, G. Genetic control of seed dormancy in Lolium rigidum and its association with GA20oX and ABA1 expression. Crop Pasture Sci. 2022, 73, 1406–1415. [Google Scholar] [CrossRef]
- Christopher, J.T.; Preston, C.; Powles, S.B. Malathion antagonizes metabolism-based chlorsulfuron resistance in Lolium rigidum. Pest. Biochem. Physiol. 1994, 49, 172–182. [Google Scholar] [CrossRef]
- Mei, Y.; Si, C.; Liu, M.; Qiu, L.; Zheng, M. Investigation of resistance levels and mechanisms to nicosulfuron conferred by non-target-site mechanisms in large crabgrass (Digitaria sanguinalis L.) from China. Pest. Biochem. Physiol. 2017, 141, 84–89. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, Z.; Huang, H.; Li, W.; Cao, Y.; Wei, S. Target site mutations and cytochrome P450s-involved metabolism confer resistance to nicosulfuron in green foxtail (Setaria viridis). Pest. Biochem. Physiol. 2021, 179, 104956. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, Y.; Zhang, Y.; Dong, L.; Li, J. Mechanisms of resistance to pyroxsulam and ACCase inhibitors in Japanase foxtail (Alopecurus japonicus). Weed Sci. 2016, 64, 695–704. [Google Scholar] [CrossRef]
- Menegat, A.; Bailly, G.C.; Aponte, R.; Heinrich, G.M.T.; Sievernich, B.; Gerhards, R. Acetohydroxyacid synthase (AHAS) amino acid substitution Asp376Glu in Lolium perenne: Effect on herbicide efficacy and plant growth. J. Plant Dis. Prot. 2016, 123, 145–153. [Google Scholar] [CrossRef]
- Panozzo, S.; Milani, A.; Scarabel, L.; Balogh, A.; Dancza, I.; Sattin, M. Ocurrence of different resistance mechanisms to acetolactate synthase inhibitors in European Sorghum halepense. J. Agric. Food Chem. 2017, 65, 7320–7327. [Google Scholar] [CrossRef]
- McCourt, J.A.; Pang, S.S.; King-Scott, J.; Guddat, L.W.; Duggleby, R.G. Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc. Natl. Acad. Sci. USA 2006, 103, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shuang, L.; Hao, L.; Li, X.; Zheng, M. Tribenuron-methyl-resistant Descurainia sophia L. exhibits negative cross-resistance to imazethapyr conferred by a Pro197Ser mutation in acetolactate synthase and reduced metabolism. Pest Manag. Sci. 2021, 78, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G.; Carbonari, C.A.; Duke, S.O. The potential influence of hormesis on evolution of resistance to herbicides. Curr. Opin. Environ. Sci. Health 2022, 27, 100360. [Google Scholar] [CrossRef]


| Tradename | Active Ingredient | MoA WSSA/HRAC a | Timing b | Field Dose (g ai ha−1) |
|---|---|---|---|---|
| Herbicide-resistance field screening and dose–response curves | ||||
| SANSON® 4 SC | Nicosulfuron | 2 | Post | 40 |
| Herbicide and Cyt-P450-inhibitor synergism | ||||
| SANSON® 4 SC | Nicosulfuron | 2 | Post | 40 |
| INMAR 50 | Malathion | - | - | 1000 |
| Cross-resistance in vivo assay | ||||
| Everest 70 | Flucarbazone | 2 | Post | 25 |
| Pulsar® | Imazamox | 2 | Post | 40 |
| Nomine® | Bispyribac-Na | 2 | Post | 40 |
| Viper | Penoxsulam | 2 | Post | 20 |
| Multiple-resistance screening | ||||
| Asgard® | Petoxamida | 15 | Pre | 1800 |
| Anthem-Maxx 518 | Piroxasulfone + Fluthiacet | 15 + 14 | Pre | 376.7 + 9.8 |
| Roundup | Glyphosate | 9 | Post | 720 |
| Leopard 5% | Quizalofop | 1 | Post | 100 |
| Laudis 20% | Tembotrione | 27 | Post | 120 |
| Callisto 100 | Mesotrione | 27 | Post | 150 |
| Convey® | Topramezone | 27 | Post | 26.9 |
| Raker PRO® | Tolpyralate | 27 | Post | 40 |
| Elumis | Nicosulfuron + Mesotrione | 2 + 27 | Post | 60 + 150 |
| Population Code | Place | b | d | GR50 | 95% CI | RI a | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| IxS | Jalisco | 2.6 | 99.9 | 24.3 | 22.2 | 26.4 | -- |
| IxR1 | Jalisco | 3.98 | 97.2 | 186.6 | 176.5 | 196.7 | 7.6 |
| IxR2 | Jalisco | 2.9 | 98.9 | 214.4 | 201.9 | 226.8 | 8.8 |
| IxR3 | Jalisco | 2.7 | 100.7 | 237.2 | 223.6 | 250.8 | 9.7 |
| IxR4 | Jalisco | 4.6 | 98.3 | 245.5 | 235.0 | 255.8 | 10.1 |
| IxR5 | Jalisco | 3.61 | 100.1 | 209.5 | 198.6 | 220.3 | 8.6 |
| IxR6 | Guanajuato | 2.4 | 99.9 | 140.3 | 128.4 | 152.1 | 5.7 |
| IxR7 | Guanajuato | 2.0 | 102.5 | 155.0 | 141.8 | 168.1 | 6.3 |
| IxR8 | Guanajuato | 1.7 | 96.1 | 151.9 | 135.1 | 168.5 | 6.2 |
| Treatment | Plant Mortality (%) a | |||||
|---|---|---|---|---|---|---|
| IxS | IxR4 | IxR7 | ||||
| Petoxamide | 100 ± 0 | a | 100 ± 0 | b | 100 ± 0 | a |
| Pyroxasulfone + Fluthiacet | 100 ± 0 | a | 90 ± 0 | b | 95 ± 7 | ab |
| Glyphosate | 100 ± 0 | a | 100 ± 0 | a | 100 | a |
| Quizalofop | 100 ± 0 | a | 100 ± 0 | a | 100 ± 0 | a |
| Tembotrione | 85 ± 7 | bc | 85 ± 0 | b | 75 ± 7 | c |
| Mesotrione | 85 ± 7 | bc | 90 ± 0 | b | 85 ± 7 | bc |
| Topramezone | 100 ± 0 | a | 90 ± 14 | ab | 100 ± 0 | a |
| Tolpyralate | 100 ± 0 | a | 95 ± 7 | ab | 100 ± 0 | a |
| Nicosulfuron + Mesotrione | 100 ± 0 | a | 100 ± 0 | a | 100 ± 0 | a |
| Species a | Herbicide Family Abbreviations b,c | ||||
|---|---|---|---|---|---|
| SU | IMI | SCT | PTB | TP | |
| Ixophorus unisetus (studied here) (SW) | R | r | R | r | R |
| Sorghum halepense (SW) | R | S | R | R | ND |
| Alopecurus japonicus (WW) | R | R | R | r | R |
| Setaria viridis (SW) | R | R | ND | ND | ND |
| Lolium perenne (WW) | R | r | R | ND | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Valenzuela, J.A.; Vázquez-García, J.G.; Castro, P.; Palma-Bautista, C.; Cruz-Hipólito, H.E.; Rey, M.-D.; De Prado, R.; Portugal, J. Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico. Agronomy 2023, 13, 1682. https://doi.org/10.3390/agronomy13071682
Domínguez-Valenzuela JA, Vázquez-García JG, Castro P, Palma-Bautista C, Cruz-Hipólito HE, Rey M-D, De Prado R, Portugal J. Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico. Agronomy. 2023; 13(7):1682. https://doi.org/10.3390/agronomy13071682
Chicago/Turabian StyleDomínguez-Valenzuela, José Alfredo, José G. Vázquez-García, Patricia Castro, Candelario Palma-Bautista, Hugo E. Cruz-Hipólito, Maria-Dolores Rey, Rafael De Prado, and João Portugal. 2023. "Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico" Agronomy 13, no. 7: 1682. https://doi.org/10.3390/agronomy13071682
APA StyleDomínguez-Valenzuela, J. A., Vázquez-García, J. G., Castro, P., Palma-Bautista, C., Cruz-Hipólito, H. E., Rey, M.-D., De Prado, R., & Portugal, J. (2023). Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl. from the Bajio, Mexico. Agronomy, 13(7), 1682. https://doi.org/10.3390/agronomy13071682











