Morphophysiological and Biochemical Responses of Zea mays L. under Cadmium and Drought Stresses Integrated with Fungal and Bacterial Inoculation
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Analysis
2.2. Microbial Inoculation
2.3. Experimental Design and Growth Condition
- microorganism [without microbial inoculation (uninoculated), bacterial inoculation, and fungal inoculation].
2.4. Sample Collection and Parameter Measurement
2.4.1. Morphological Parameters
2.4.2. Electrolyte Leakage (EL)
2.4.3. Relative Water Content (RWC)
2.4.4. Malondialdehyde and Total Protein
2.4.5. Enzyme Extraction
2.4.6. Superoxide Dismutase Activity
2.4.7. Catalase Activity
2.4.8. Peroxidase Activity
2.4.9. Ascorbate Peroxidase Activity
2.4.10. Translocation Factor (TF) and Bioconcentration Factor (BF)
2.5. Statistical Analysis
3. Results
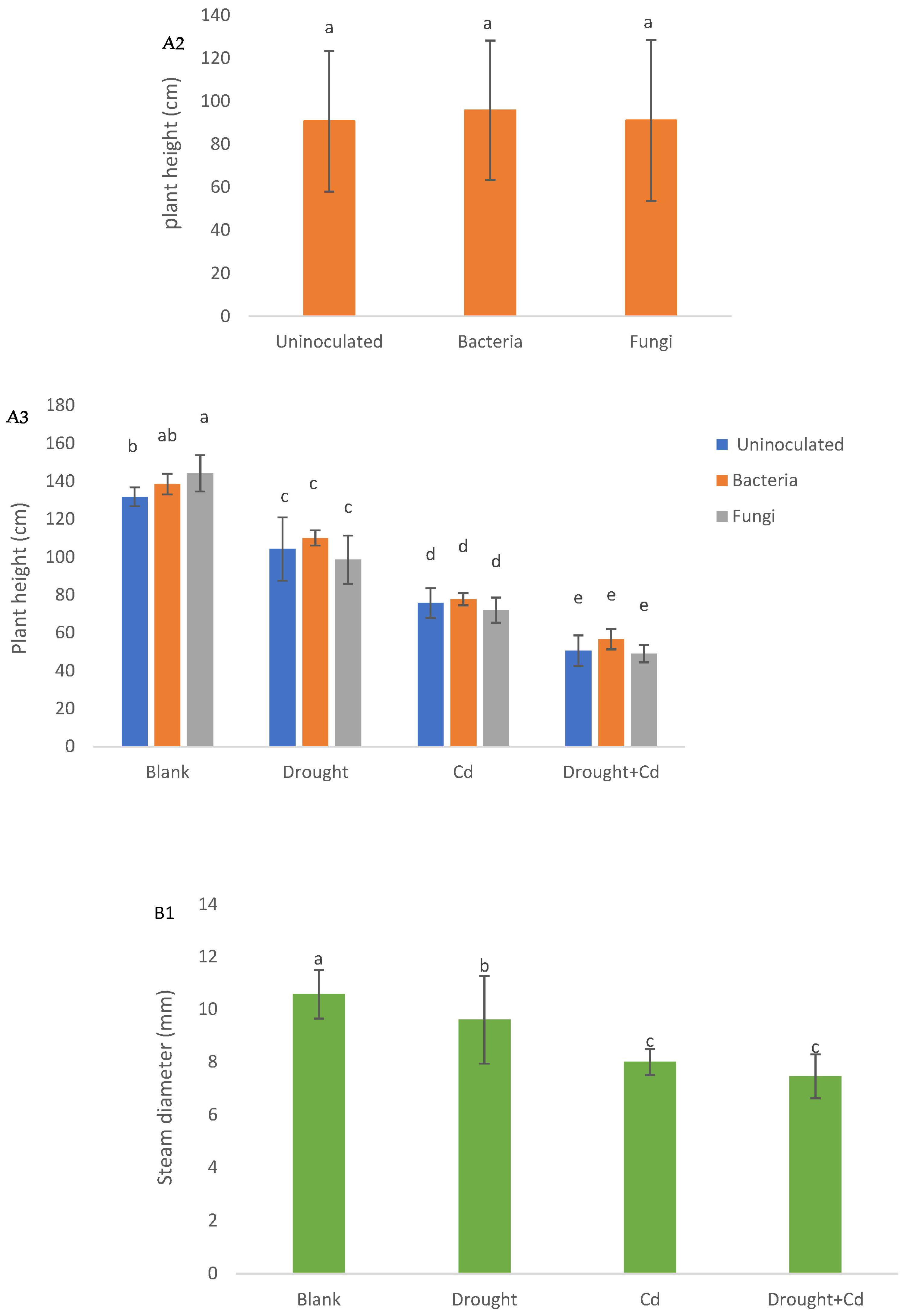
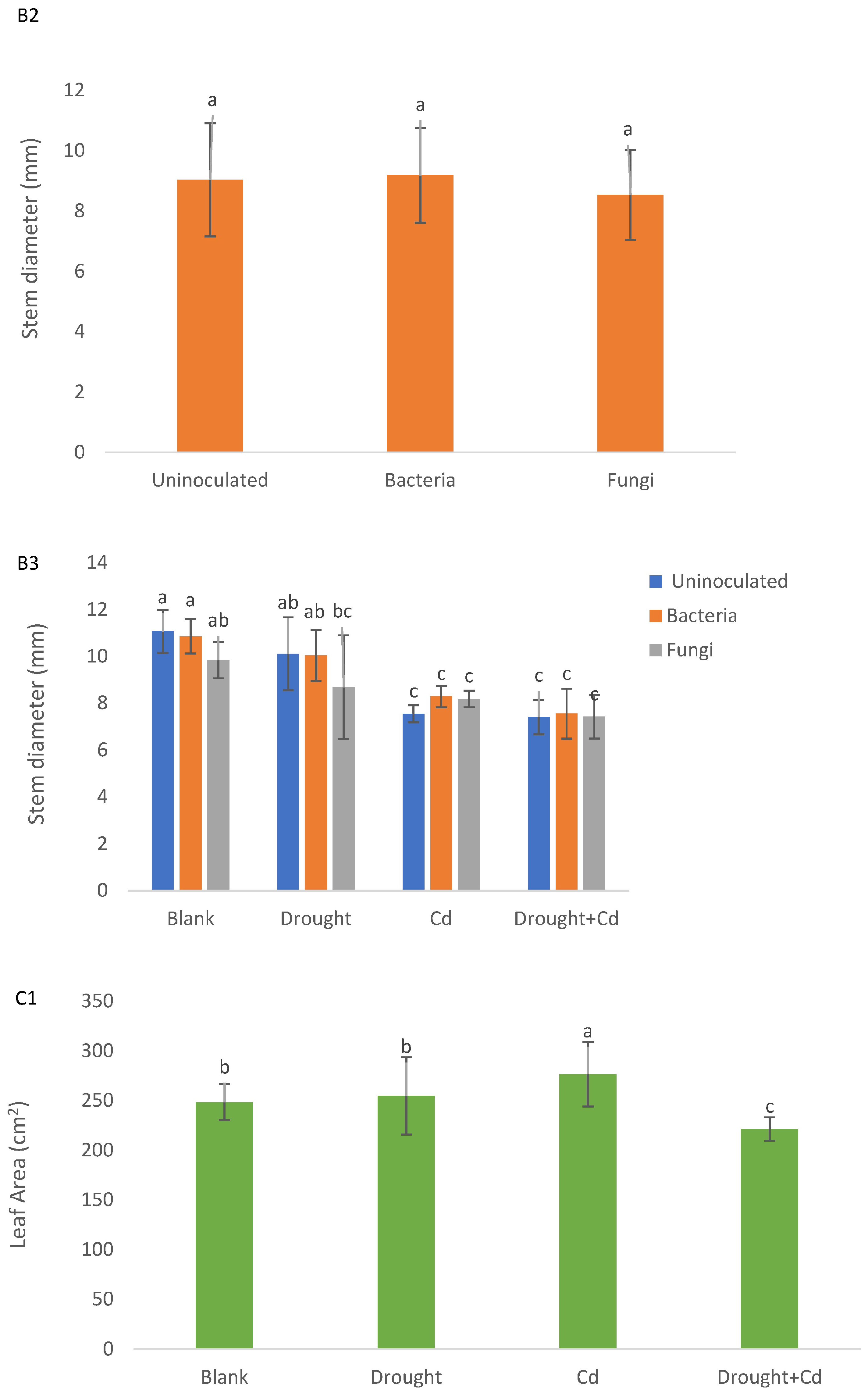
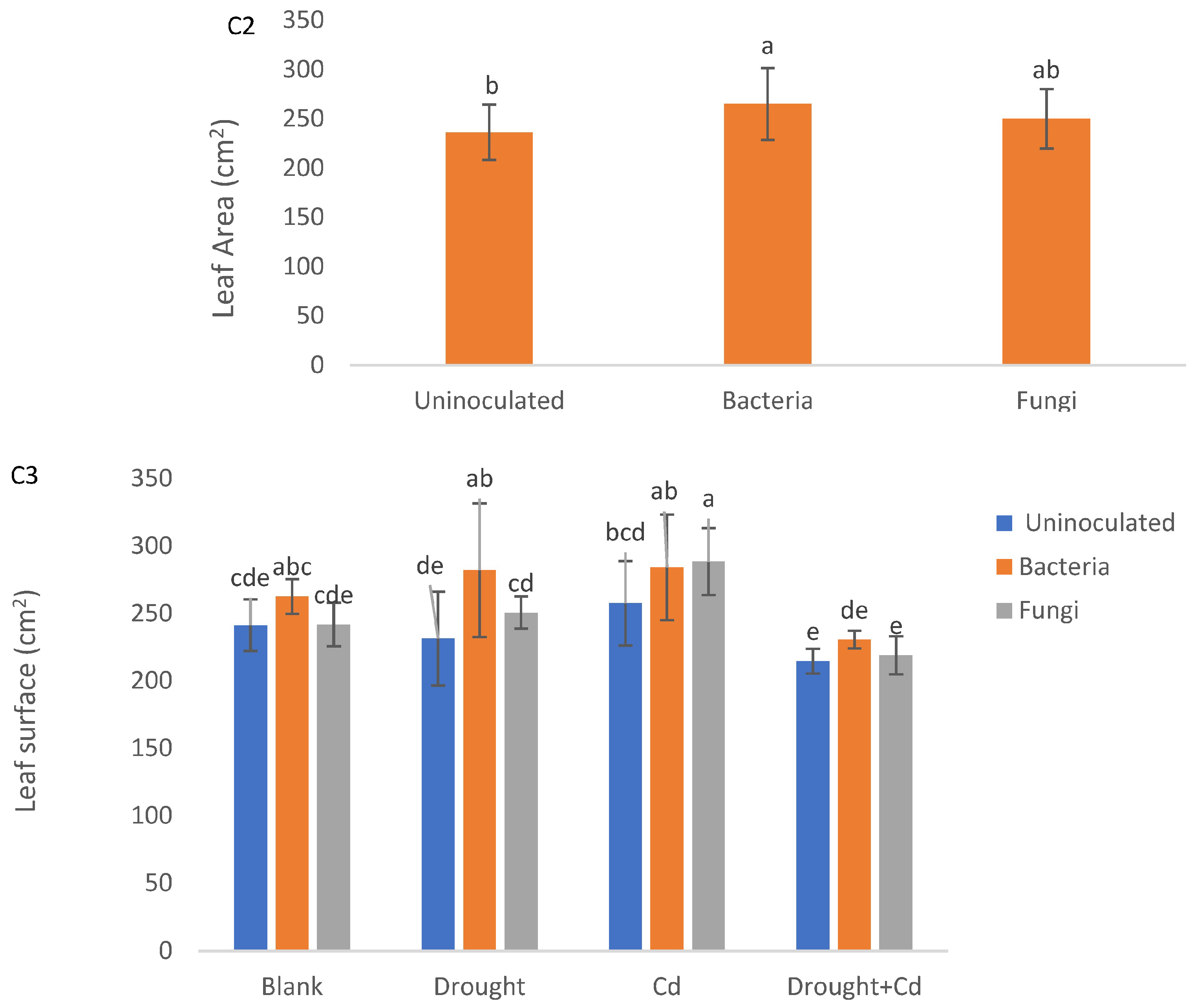
3.1. Plant Height, Stem Diameter, and Leaf Area
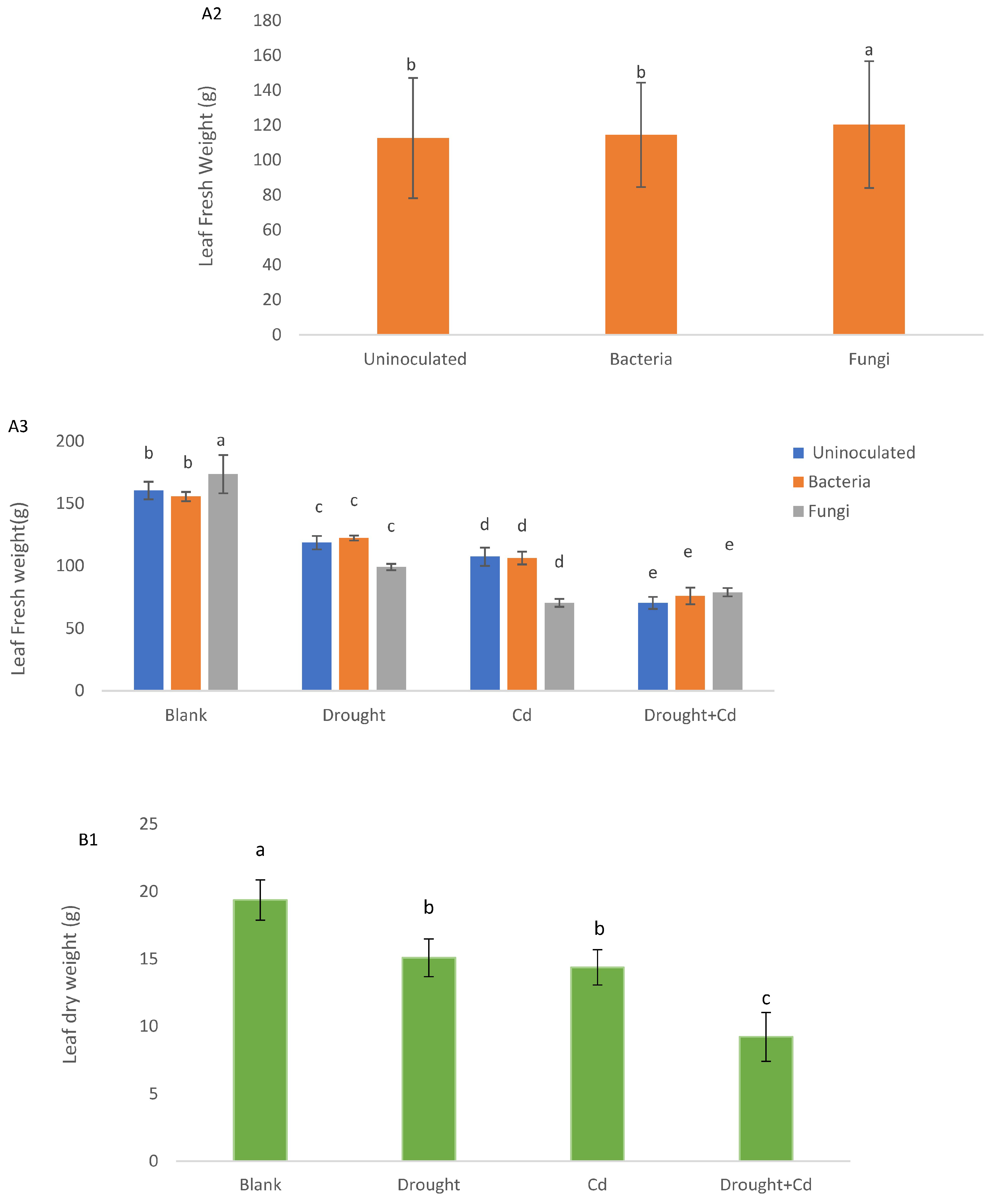
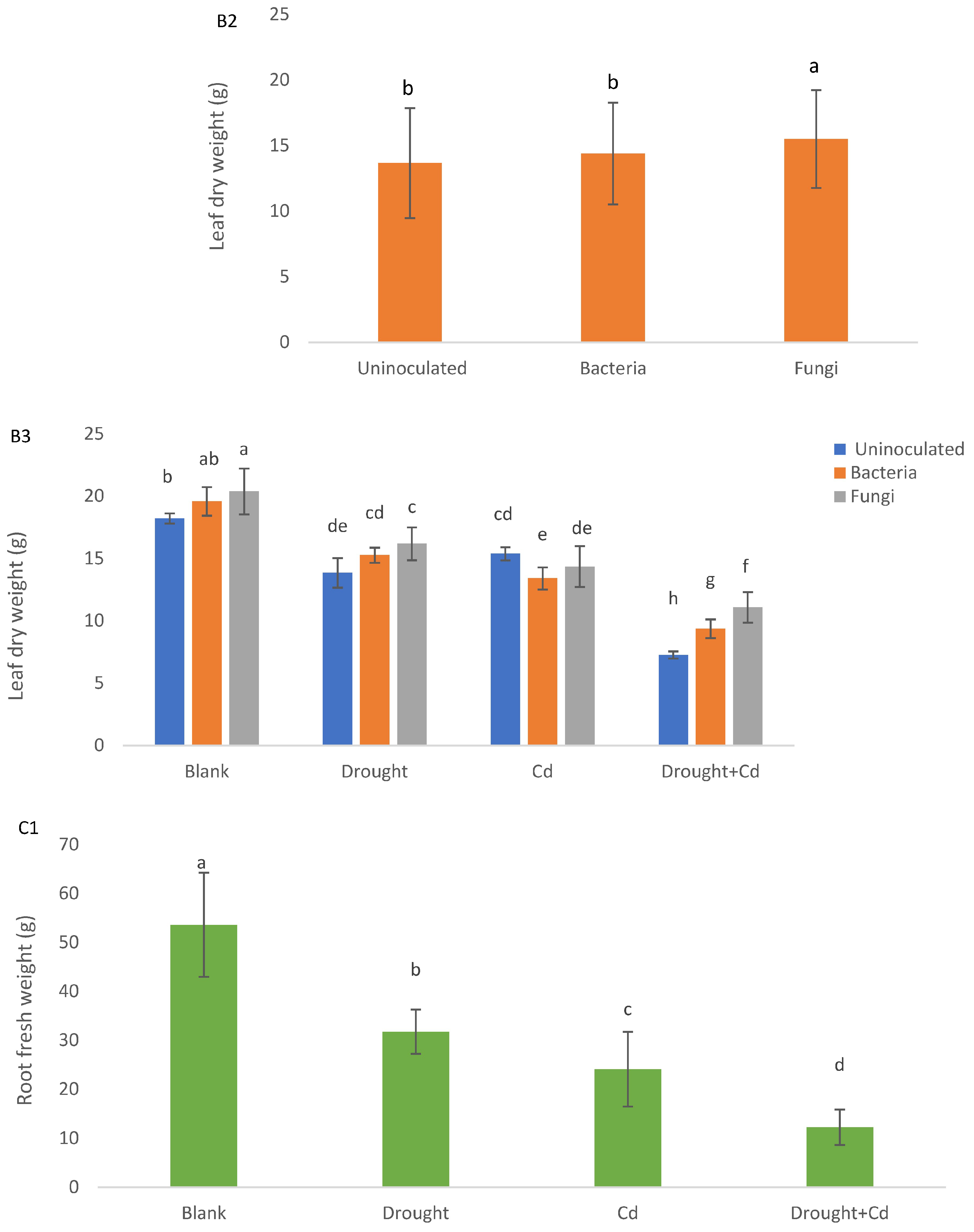
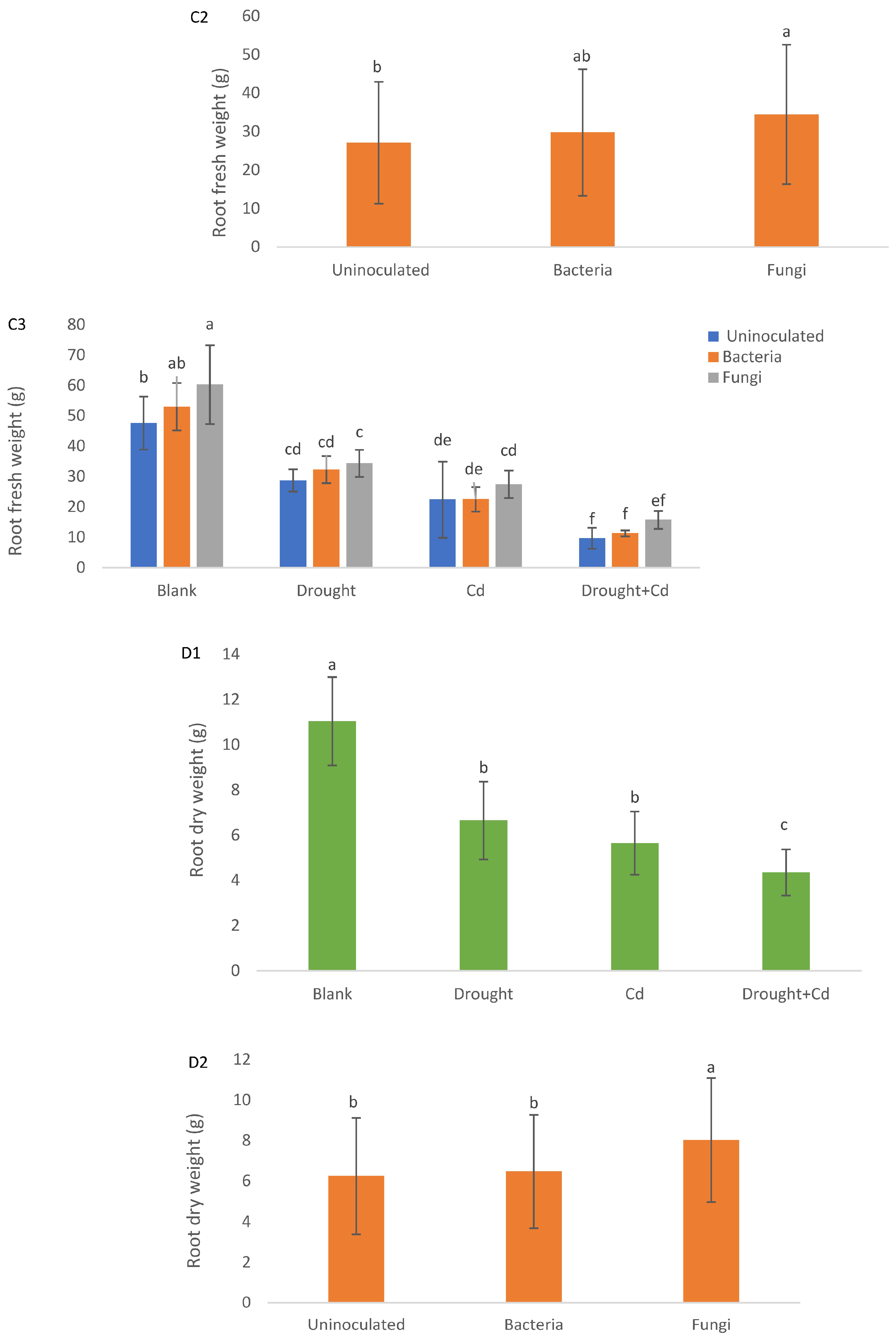
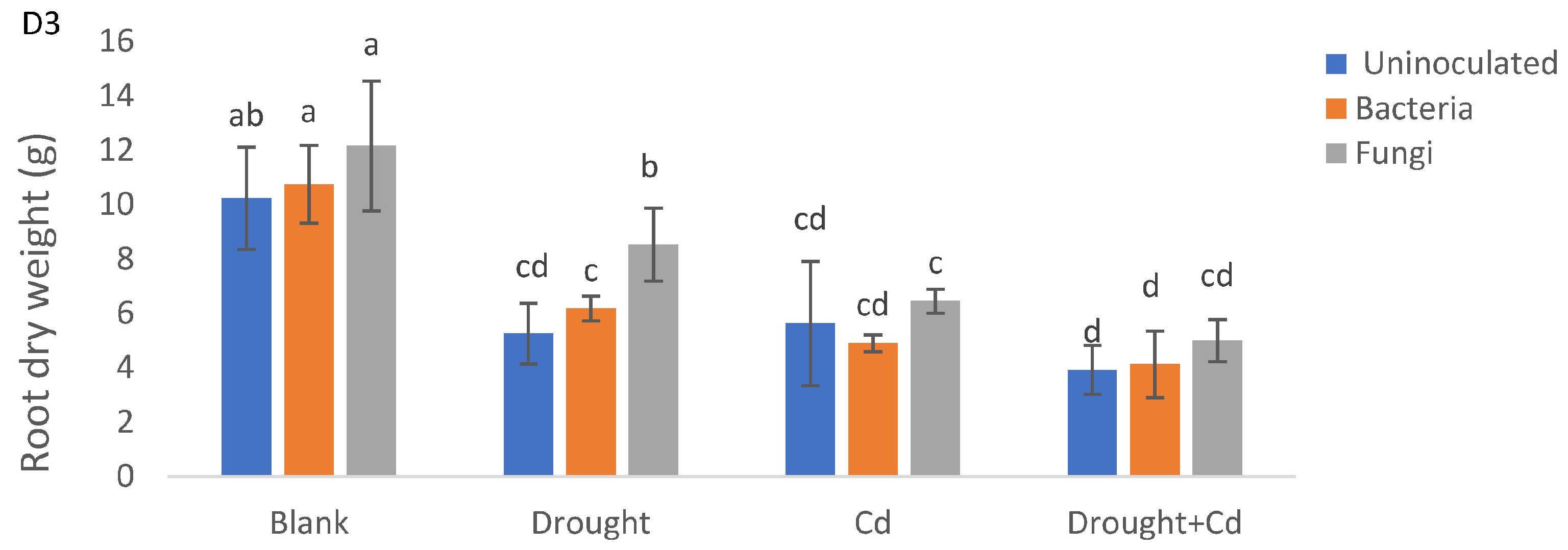
3.2. Leaf and Root Biomass
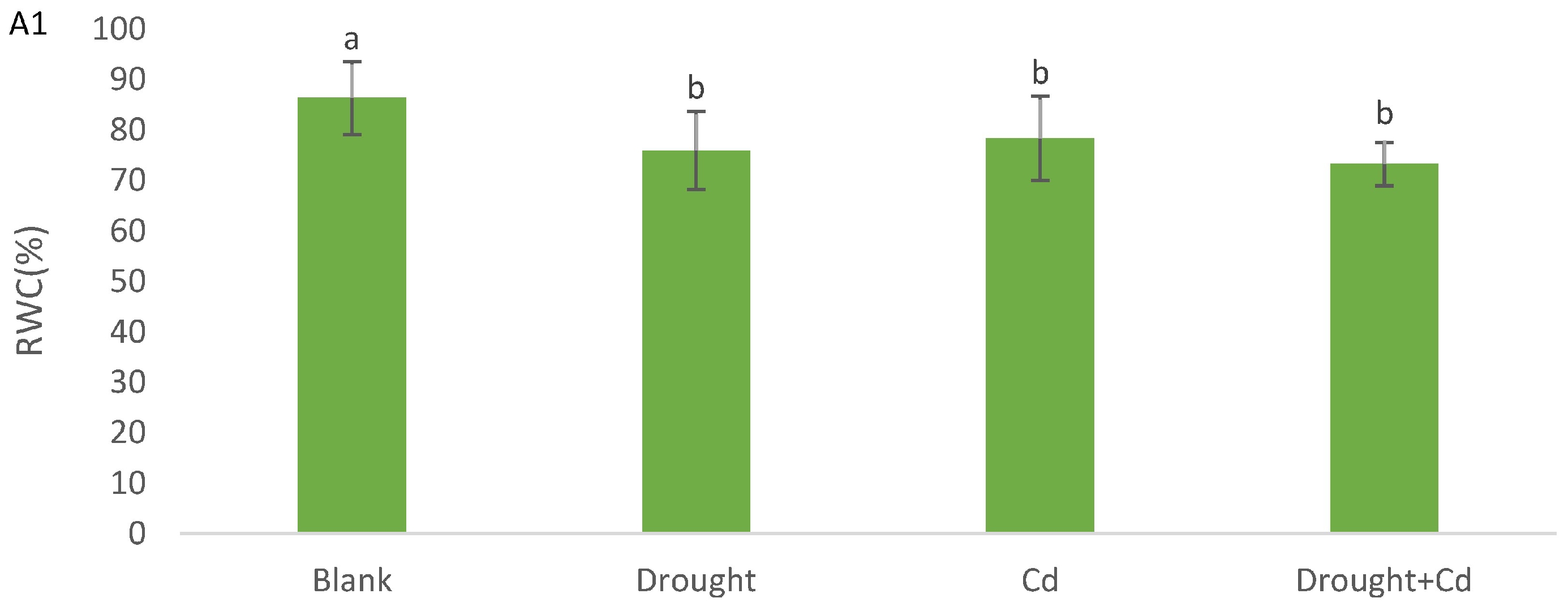
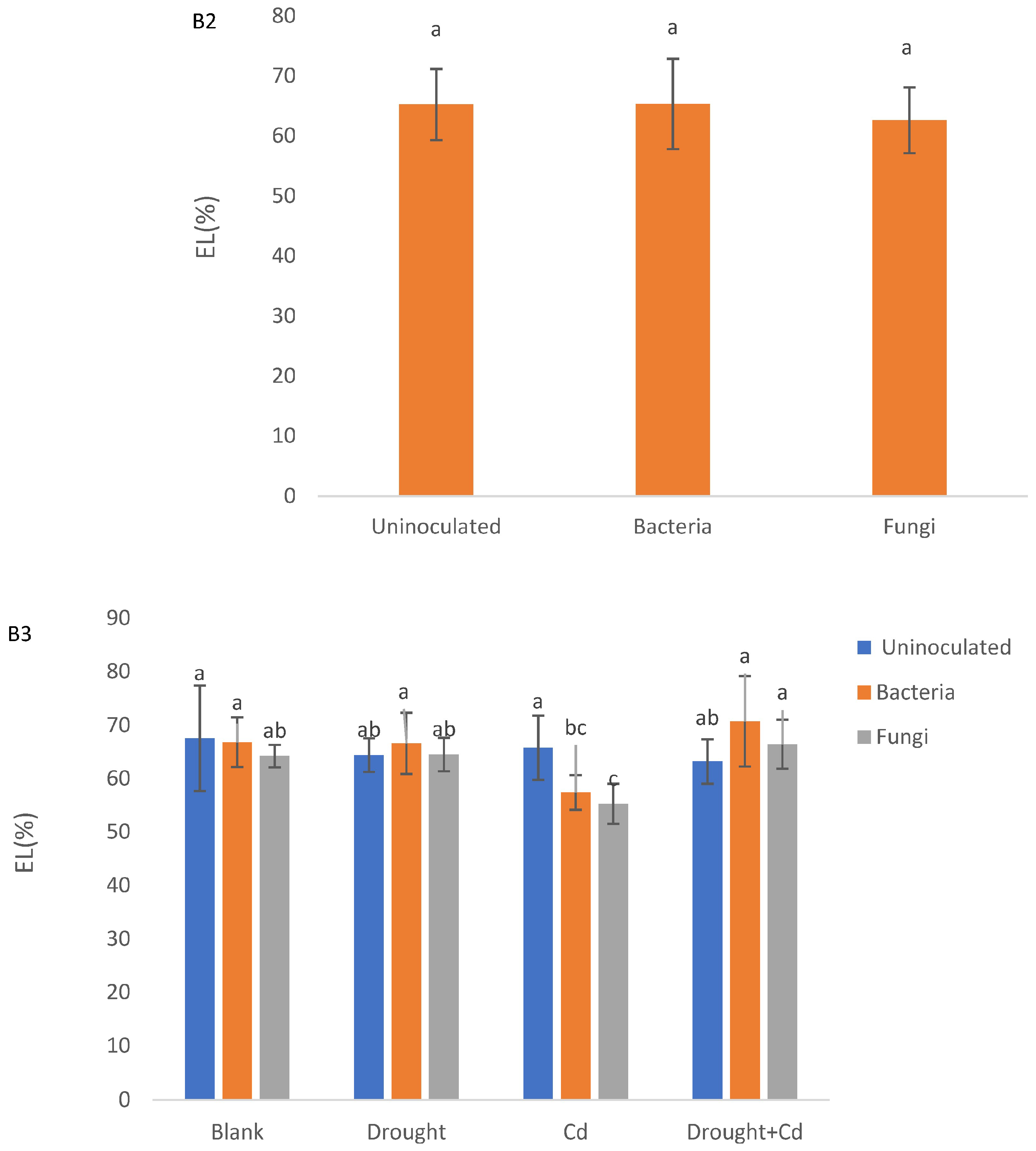
3.3. Relative Water Content (RWC) and Electrolyte Leakage (EL)
3.4. SPAD
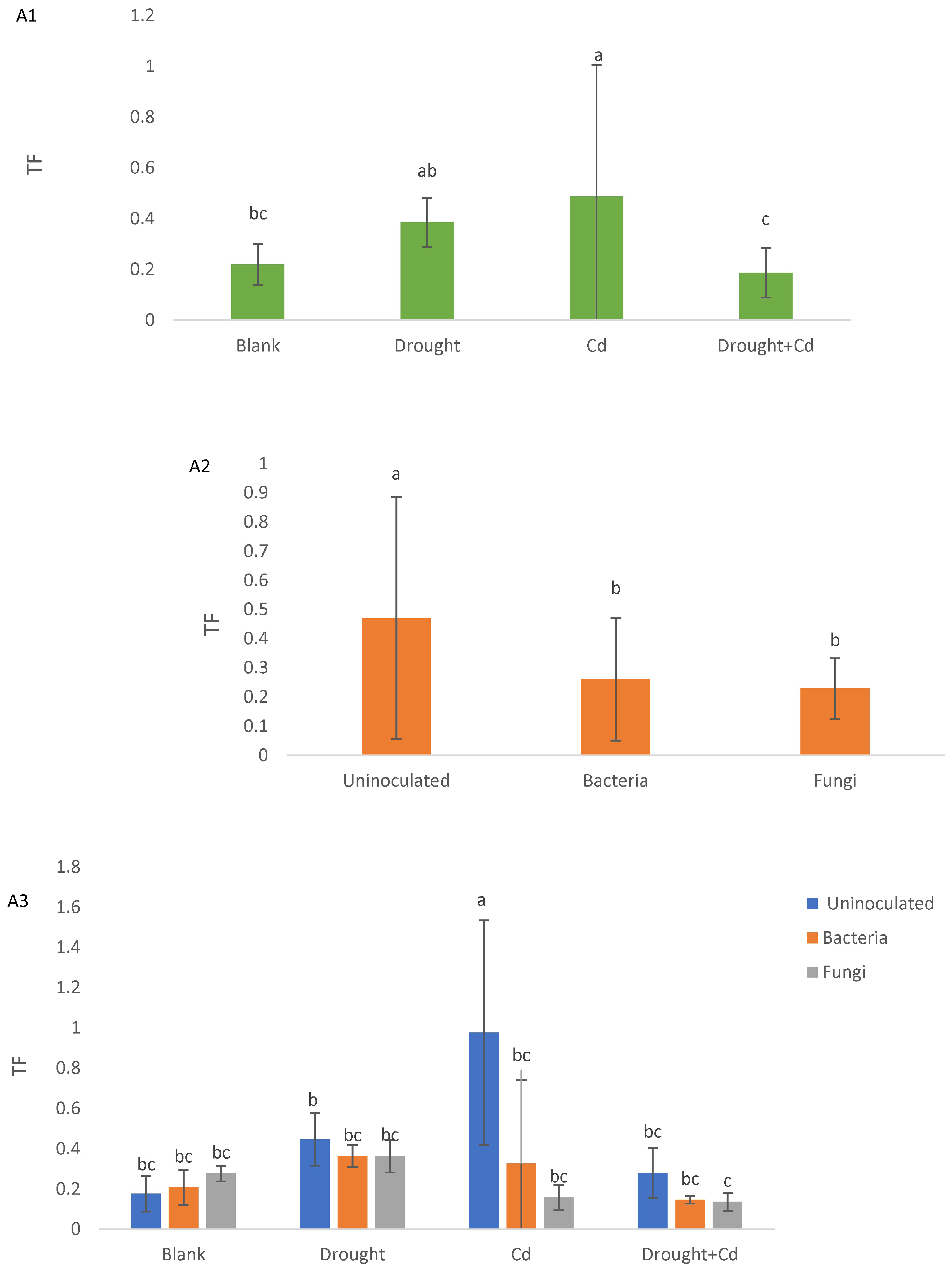
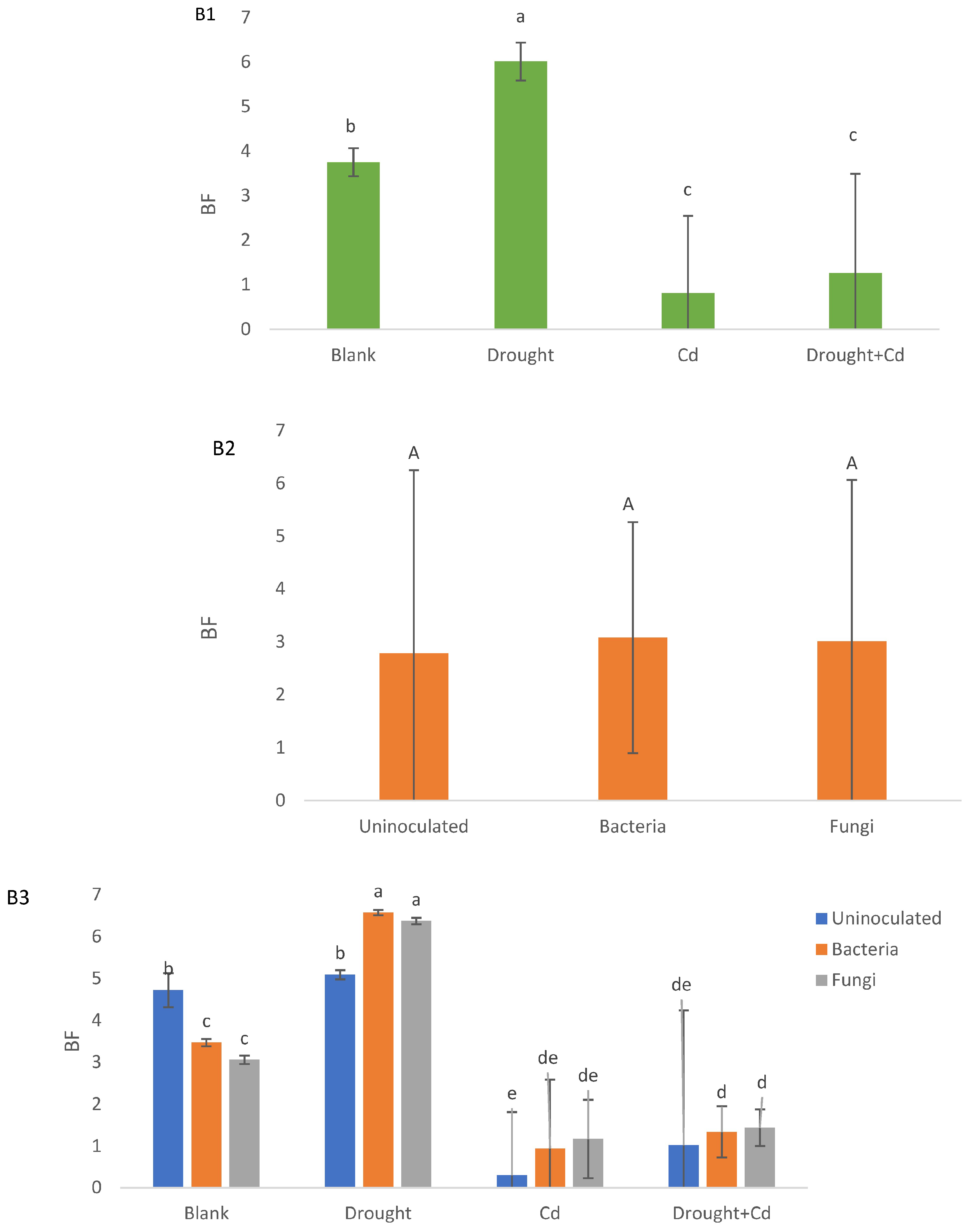
3.5. Translocation Factor (TF) and Bioconcentration Factor (BF)
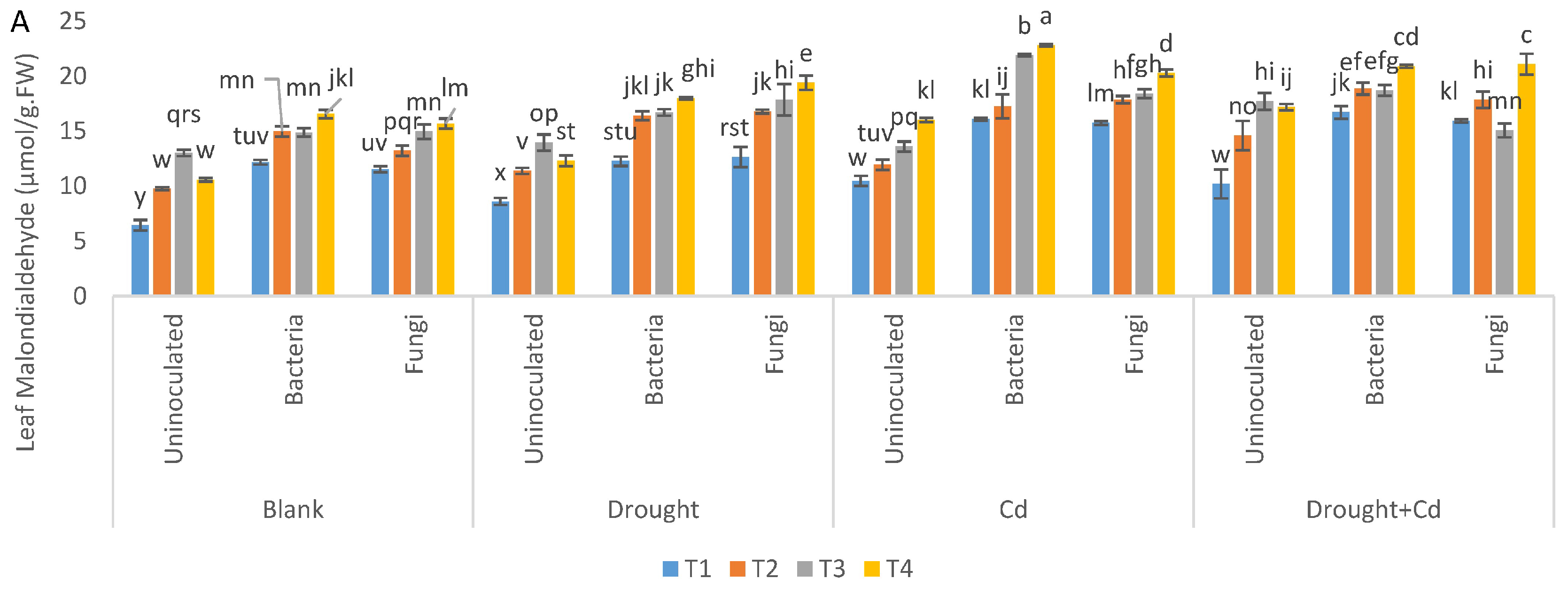
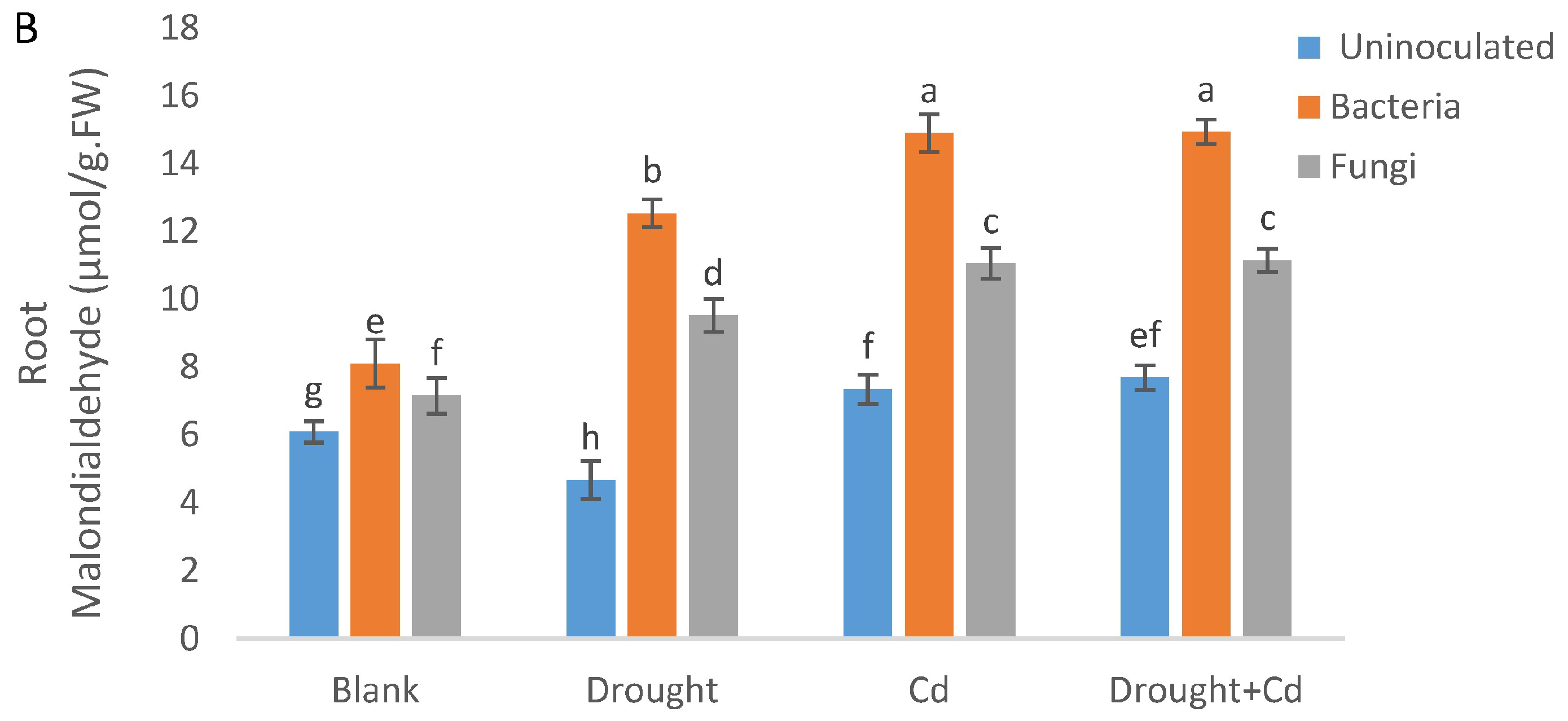
3.6. Leaf and Root MDA
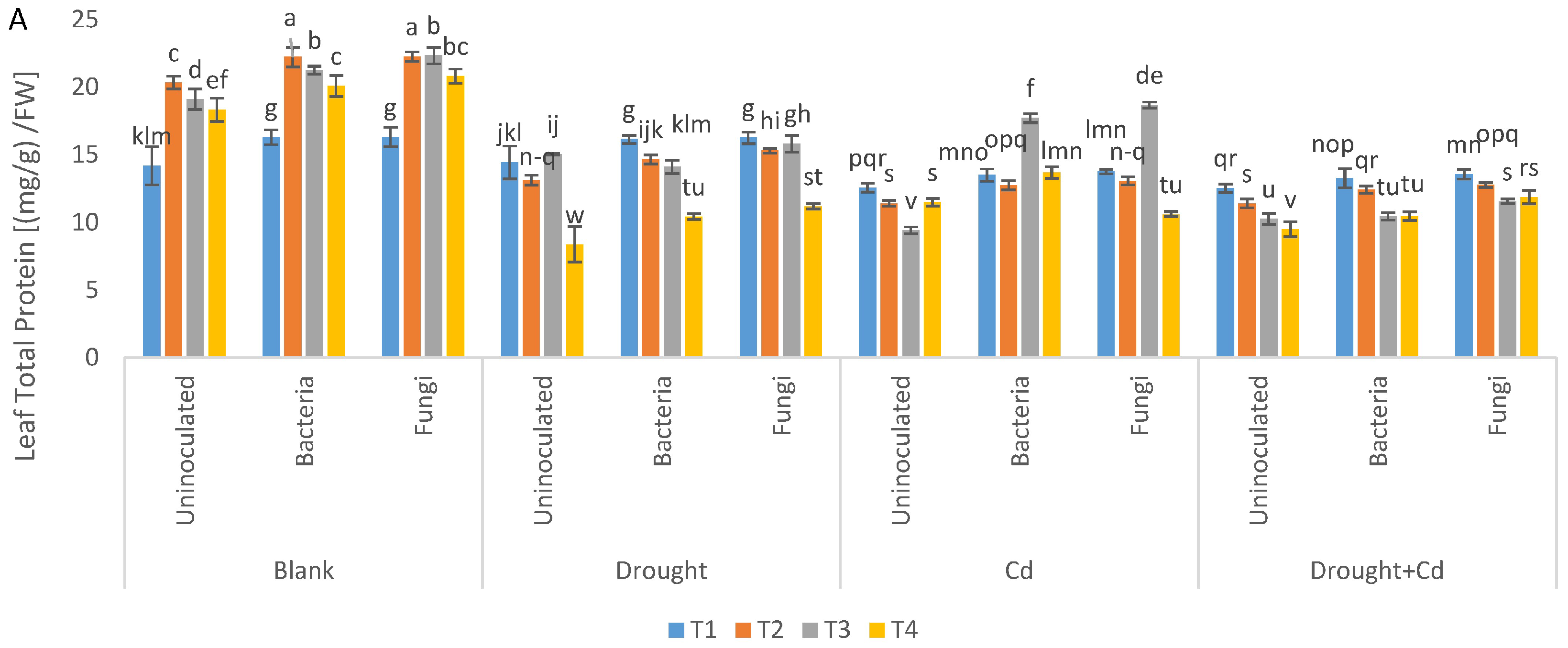
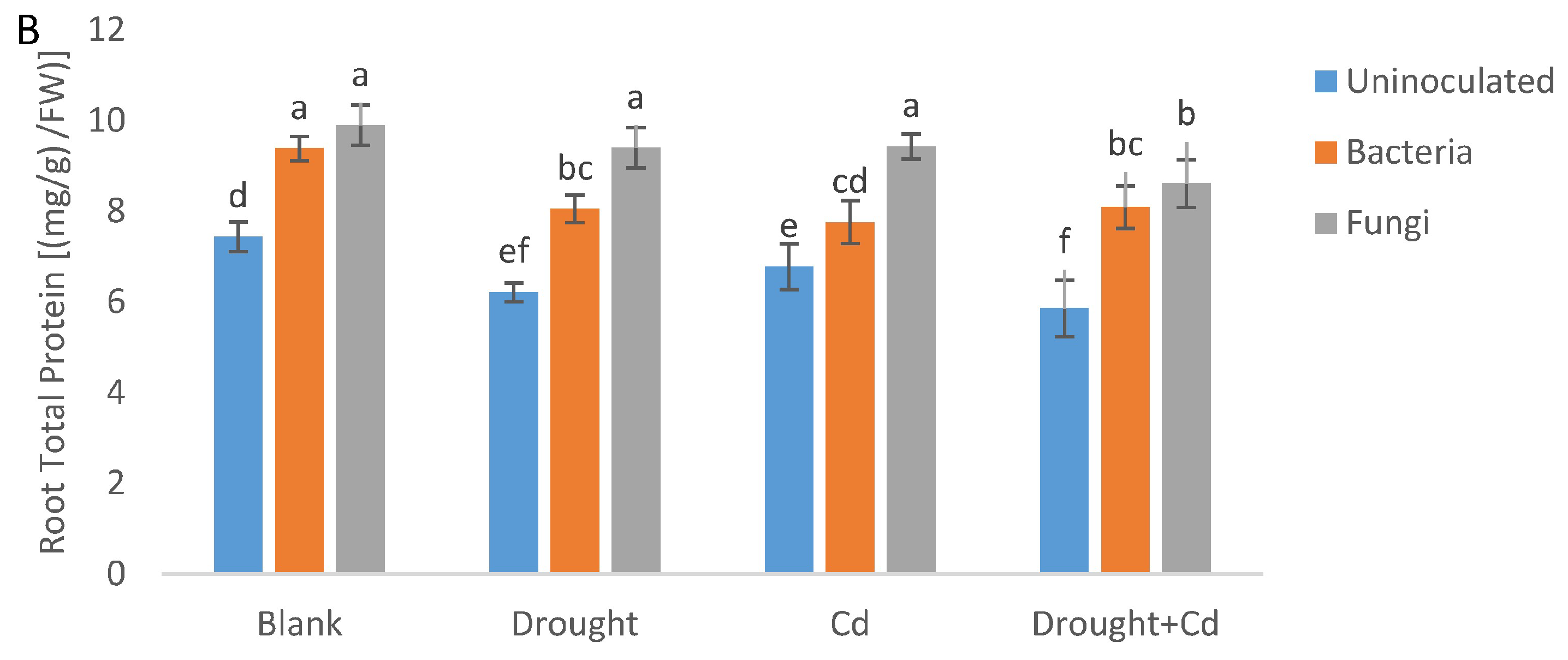
3.7. Leaf and Root Protein
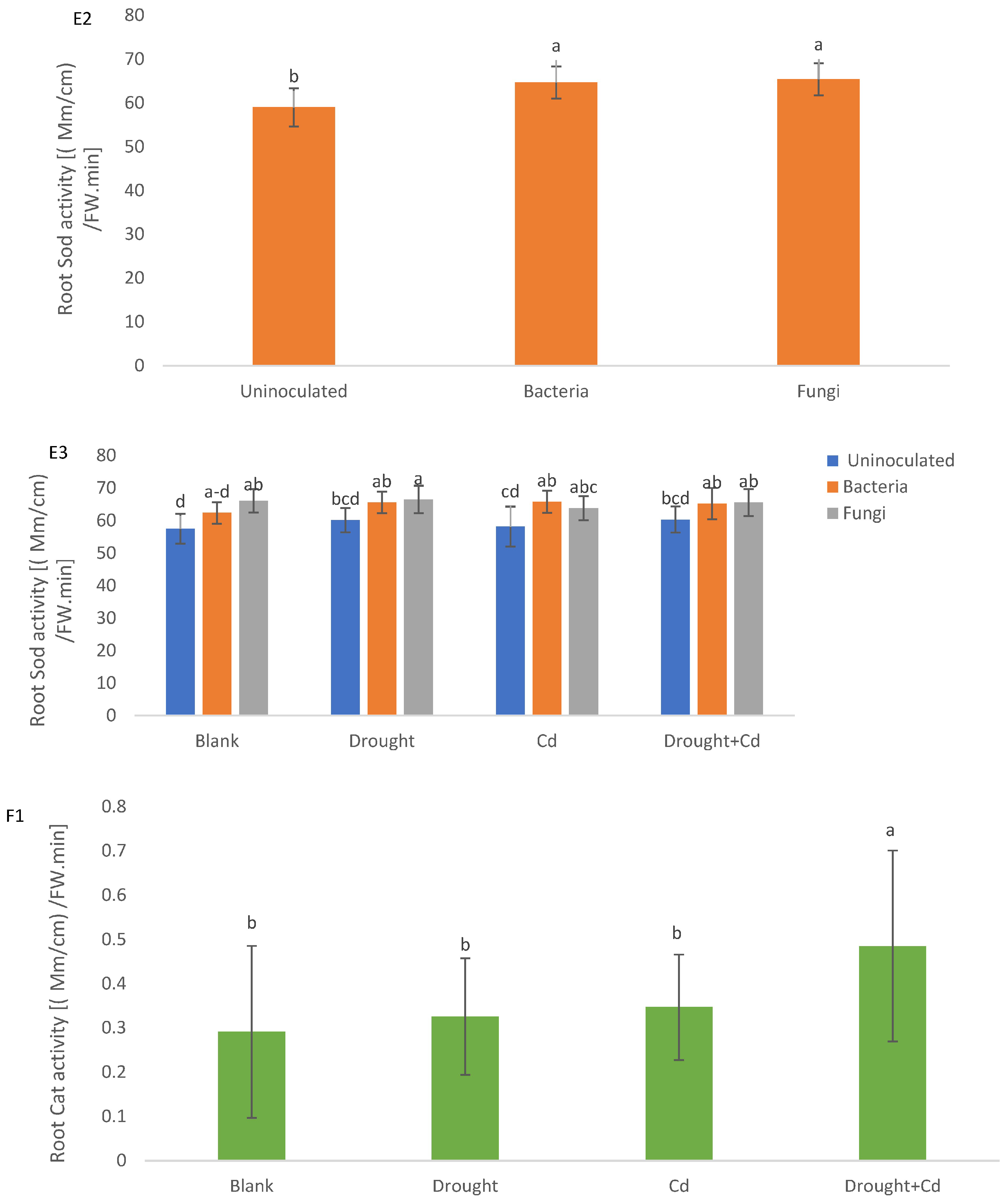
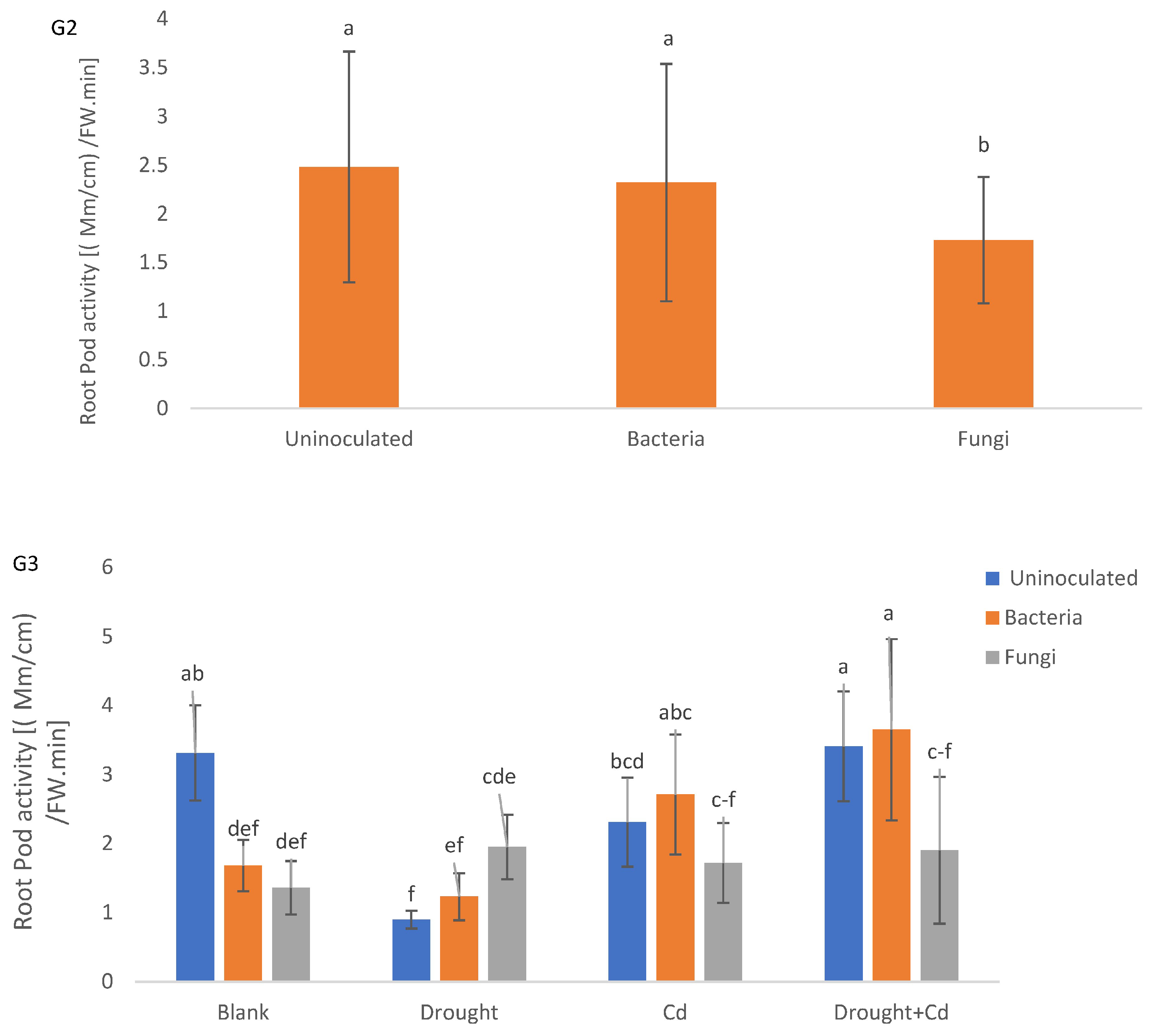
3.8. Aerial Part and Root Enzymatic Activity
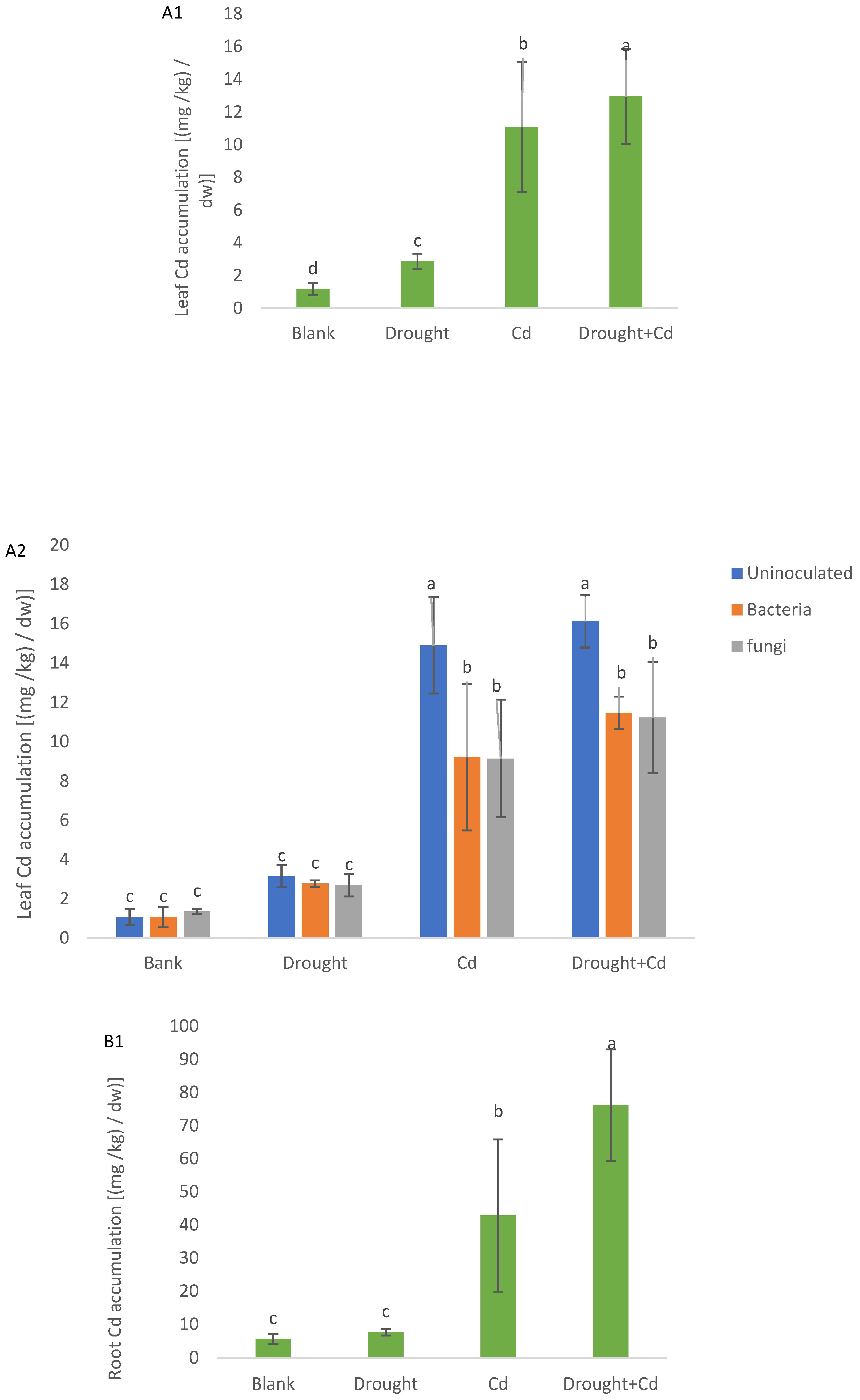
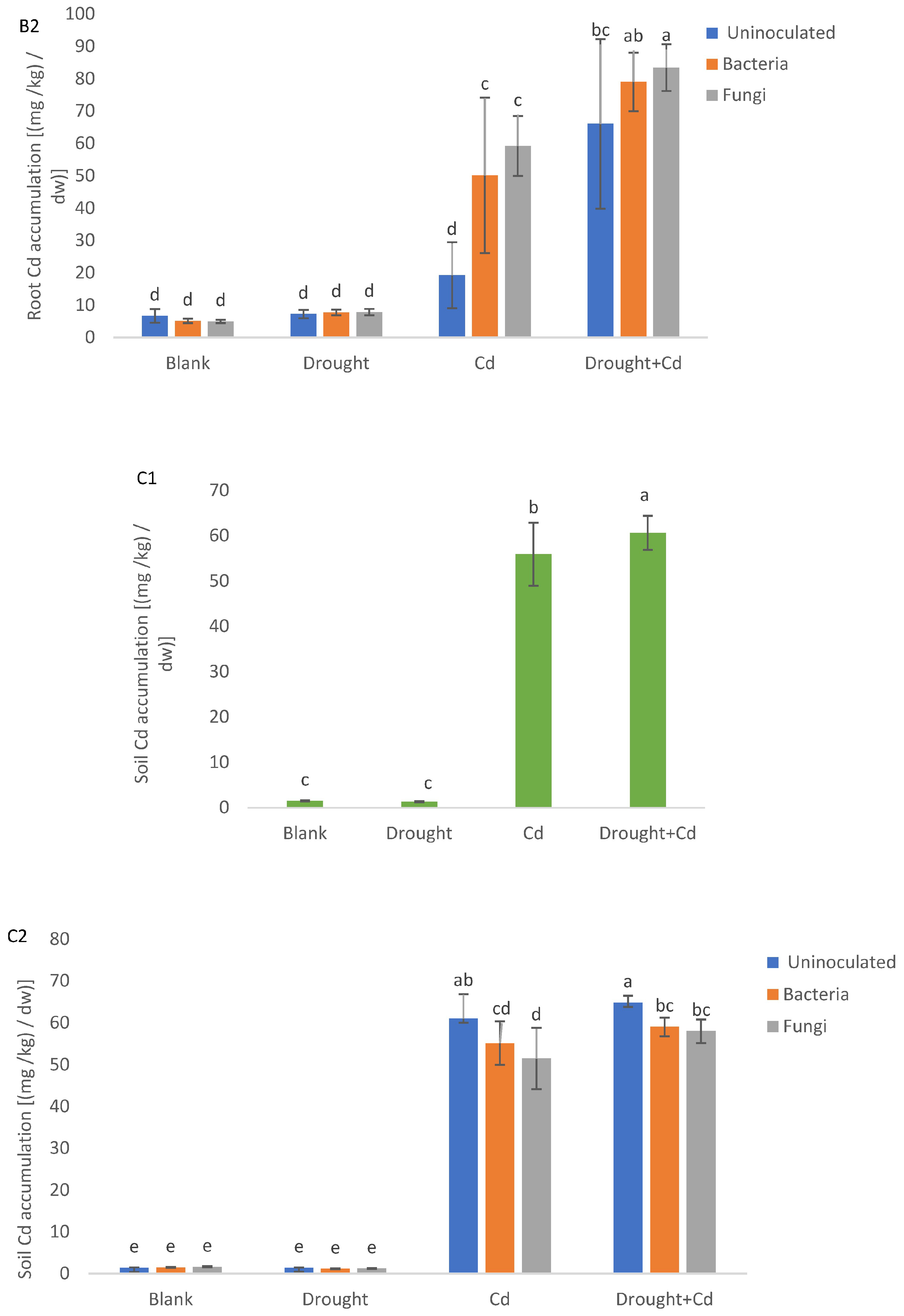
3.9. Cd Accumulation in the Plant Material and Soil
3.10. Principle Component Analysis (PCA) of the Studied Traits of Z. mays under Various Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wild, A. Soils, Land and Food: Managing the Land during the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Yousefi, Z.; Babanejad, E.; Mohammadpour, R.; Esbokolaee, H.N. Evaluation of Cd phytoremediation by Portulaca oleracea irrigated by contaminated water. Environ. Health Eng. Manag. J. 2023, 15, 286. [Google Scholar] [CrossRef]
- Pacheco, D.D.R.; Santana, B.C.G.; Pirovani, C.P.; de Almeida, A.-A.F. Zinc/Iron-Regulated Transporter-Like Protein (ZIP) Gene Family in Theobroma cacao L: Characteristics, evolution, function and 3D structure analysis. Front. Plant Sci. 2023, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 232, pp. 1–44. [Google Scholar]
- Kohli, S.K.; Handa, N.; Gautam, V.; Bali, S.; Sharma, A.; Khanna, K.; Arora, S.; Thukral, A.K.; Ohri, P.; Karpets, Y.V. ROS signaling in plants under heavy metal stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017; pp. 185–214. [Google Scholar]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. ROS Generation in Plant Cells Orchestrated by Stress. In Reactive Oxygen Species in Plants: The Right Balance; Springer: Berlin/Heidelberg, Germany, 2023; pp. 23–43. [Google Scholar]
- Islam, M.; Sandhi, A. Heavy Metal and Drought Stress in Plants: The Role of Microbes—A Review. Gesunde Pflanz. 2022. [Google Scholar] [CrossRef]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Open Biol. 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Fahadi Hoveizeh, N.; Kadkhodaei, S.; Vaculík, M. Physiological and Biochemical Responses of Commercial Strawberry Cultivars under Optimal and Drought Stress Conditions. Plants 2023, 12, 496. [Google Scholar] [CrossRef]
- Chugh, V.; Kaur, N.; Gupta, A.K. Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought. Indian J. Biochem. Biophys. 2011, 48, 47–53. [Google Scholar]
- More, S.J.; Bardhan, K.; Ravi, V.; Pasala, R.; Chaturvedi, A.K.; Lal, M.K.; Siddique, K.H.M. Morphophysiological responses and tolerance mechanisms in cassava (Manihot esculenta Crantz) under drought stress. J. Soil Sci. Plant Nutr. 2023, 23, 71–91. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in agriculture: A sustainable approach to increasing climate change resilience. Front. Sustain. Food 2021, 5, 667546. [Google Scholar] [CrossRef]
- Pal, A.; Bhattacharjee, S.; Saha, J.; Sarkar, M.; Mandal, P. Bacterial survival strategies and responses under heavy metal stress: A comprehensive overview. Crit. Rev. Microbiol. 2022, 48, 327–355. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Nguyen, P.-T.; Nguyen, T.-T.; Bui, D.-C.; Hong, P.-T.; Hoang, Q.-K.; Nguyen, H.-T. Exopolysaccharide production by lactic acid bacteria: The manipulation of environmental stresses for industrial applications. AIMS Microbiol. 2020, 6, 451. [Google Scholar] [CrossRef]
- Looijesteijn, P.J.; Trapet, L.; de Vries, E.; Abee, T.; Hugenholtz, J. Physiological function of exopolysaccharides produced by Lactococcus lactis. Int. J. Food Microbiol. 2001, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, S.; Petrillo, C.; Donadio, G.; Piaz, F.D.; Cimmino, A.; Masi, M.; Evidente, A.; Isticato, R. Plant growth promotion function of Bacillus sp. strains isolated from salt-pan rhizosphere and their biocontrol potential against Macrophomina phaseolina. Int. J. Mol. Sci. 2021, 22, 3324. [Google Scholar] [CrossRef]
- Liu, J.; Fimognari, L.; de Almeida, J.; Jensen, C.N.G.; Compant, S.; Oliveira, T.; Baelum, J.; Pastar, M.; Sessitsch, A.; Moelbak, L. Effect of Bacillus paralicheniformis on soybean (Glycine max) roots colonization, nutrient uptake and water use efficiency under drought stress. J. Agron. Crop Sci. 2023. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P. Plant responses to Trichoderma spp. and their tolerance to abiotic stresses: A review. J. Pharmacogn. Phytochem. 2018, 7, 758–766. [Google Scholar]
- Doni, F.; Isahak, A.; Che Mohd Zain, C.R.; Wan Yusoff, W.M. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Express 2014, 4, 45. [Google Scholar] [CrossRef]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubana, B.; De Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum inoculation as a tool for attenuating drought stress in sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef] [PubMed]
- Gee, G.W.; Or, D. 2.4 Particle-size analysis. Methods Soil Anal. Part 4 Phys. Methods 2002, 5, 255–293. [Google Scholar]
- Nelson, D.A.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]
- Rhoades, J. Salinity: Electrical conductivity and total dissolved solids. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 417–435. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1201–1229. [Google Scholar]
- Watanabe, F.; Olsen, S. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 1085–1121. [Google Scholar]
- Knudsen, D.; Peterson, G.; Pratt, P.; Page, A. Methods of Soil Analysis Chemical and Microbiological Properties. Am. Soc. Agron. Madison Wis. USA 1982, 225–246. [Google Scholar]
- Zarei, M.; Saleh-Rastin, N.; Jouzani, G.S.; Savaghebi, G.; Buscot, F. Arbuscular mycorrhizal abundance in contaminated soils around a zinc and lead deposit. Eur. J. Soil Biol. 2008, 44, 381–391. [Google Scholar] [CrossRef]
- Goodarzian Ghahfarokhi, M.; Mansurifar, S.; Taghizadeh-Mehrjardi, R.; Saeidi, M.; Jamshidi, A.M.; Ghasemi, E. Effects of drought stress and rewatering on antioxidant systems and relative water content in different growth stages of maize (Zea mays L.) hybrids. Arch. Agron. Soil Sci. 2015, 61, 493–506. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Zia-ur-Rehman, M.; Abbas, Z.; Hannan, F. Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: A critical review. Environ. Geochem. Health 2017, 39, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Volk, T.A.; Quackenbush, L.J.; Stehman, S.V. Estimation of shrub willow leaf chlorophyll concentration across different growth stages using a hand-held chlorophyll meter to monitor plant health and production. Biomass Bioenergy 2021, 150, 106132. [Google Scholar] [CrossRef]
- Lamacque, L.; Charrier, G.; Farnese, F.d.S.; Lemaire, B.; Améglio, T.; Herbette, S. Drought-induced mortality: Branch diameter variation reveals a point of no recovery in lavender species. Plant Physiol. 2020, 183, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Thalhammer, A.; Pagter, M.; Hincha, D.K.; Zuther, E. Measuring freezing tolerance of leaves and rosettes: Electrolyte leakage and chlorophyll fluorescence assays. In Plant Cold Acclimation: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 9–21. [Google Scholar]
- Badr, A.; Brüggemann, W. Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescence measurements and leaf relative water content. Photosynthetica 2020, 58, 38–645. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Jouyban, A. Challenges on determination of malondialdehyde in plant samples. Arch. Crop Sci. 2020, 4, 64–66. [Google Scholar]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and assessment of nano-encapsulated bioherbicides based on biopolymers and essential oil. Ind. Crop. Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in bioactive compounds, antioxidant activity, and nutritional quality of blood orange cultivars at different storage temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef]
- Bose, S.; Jain, A.; Rai, V.; Ramanathan, A. Chemical fractionation and translocation of heavy metals in Canna indica L. grown on industrial waste amended soil. J. Hazard. Mater. 2008, 160, 187–193. [Google Scholar]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Vishnupradeep, R.; Bruno, L.B.; Taj, Z.; Karthik, C.; Challabathula, D.; Kumar, A.; Freitas, H.; Rajkumar, M. Plant growth promoting bacteria improve growth and phytostabilization potential of Zea mays under chromium and drought stress by altering photosynthetic and antioxidant responses. Environ. Technol. Innov. 2022, 25, 102154. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Natarajan, D.; Ma, Y. Plant growth-promoting bacteria in metal-contaminated soil: Current perspectives on remediation mechanisms. Front. Microbiol. 2022, 13, 966226. [Google Scholar] [CrossRef]
- Saleem, M.H.; Parveen, A.; Khan, S.U.; Hussain, I.; Wang, X.; Alshaya, H.; El-Sheikh, M.A.; Ali, S. Silicon fertigation regimes attenuates cadmium toxicity and phytoremediation potential in two maize (Zea mays L.) cultivars by minimizing its uptake and oxidative stress. Sustain. Sci. 2022, 14, 1462. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Moreno, A.; Zhang, C.; Freitas, H. Serpentine endophytic bacterium Pseudomonas azotoformans ASS1 accelerates phytoremediation of soil metals under drought stress. Chemosphere 2017, 185, 75–85. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Woo, O.-G.; Kim, H.; Kim, J.-S.; Keum, H.L.; Lee, K.-C.; Sul, W.J.; Lee, J.-H. Bacillus subtilis strain GOT9 confers enhanced tolerance to drought and salt stresses in Arabidopsis thaliana and Brassica campestris. Plant Physiol. Biochem. 2020, 148, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J. Hazard. Mater. 2019, 377, 391–398. [Google Scholar] [CrossRef] [PubMed]
- BÖLÜKbaŞI, E. Methylation Modelling and Epigenetic Analysis of Sunflower (Helianthus annuus L.) Seedlings Exposed to Cadmium Heavy Metal Stress. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Doğa Derg. 2022, 25, 467–475. [Google Scholar] [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Baranov, O.; Belbahri, L. Plant Growth-Promoting Rhizobacteria (PGPR): A Rampart against the Adverse Effects of Drought Stress. Water 2023, 15, 418. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Karami, A.; Maggi, F. Photosynthesis and chlorophyll fluorescence of Iranian licorice (Glycyrrhiza glabra L.) accessions under salinity stress. Front. Plant Sci. 2022, 13, 984944. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S. Oxidative stress and antioxidative defense system in plants growing under abiotic stresses. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 93–136. [Google Scholar]
- Singh, R.P.; Mishra, S.; Jha, P.; Raghuvanshi, S.; Jha, P.N. Effect of inoculation of zinc-resistant bacterium Enterobacter ludwigii CDP-14 on growth, biochemical parameters and zinc uptake in wheat (Triticum aestivum L.) plant. Ecol. Eng. 2018, 116, 163–173. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S. Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. 2021, 41, 549–569. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, X.; Saleem, M.H.; Hafeez, A.; Afridi, M.S.; Khan, S.; Ullah, I.; Amaral Júnior, A.T.d.; Alatawi, A.; Ali, S. PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Valentine, A.; Cornejo, P. Role of Curtobacterium herbarum strain CAH5 on aluminum bioaccumulation and enhancement of Lactuca sativa growth under aluminum and drought stresses. Ecotoxicol. Environ. Saf. 2019, 183, 109573. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, J.; Mishra, A.; Kumari, S.; Singh, S.; Agarwal, H.; Pudake, R.N.; Varma, A.; Joshi, N.C. Deploying a microbial consortium of Serendipita indica, Rhizophagus intraradices, and Azotobacter chroococcum to boost drought tolerance in maize. Environ. Exp. Bot. 2023, 206, 105142. [Google Scholar] [CrossRef]
- Moreira, H.; Marques, A.P.; Franco, A.R.; Rangel, A.O.; Castro, P.M. Phytomanagement of Cd-contaminated soils using maize (Zea mays L.) assisted by plant growth-promoting rhizobacteria. Environ. Sci. Pollut. Res. 2014, 21, 9742–9753. [Google Scholar] [CrossRef]
- Liu, Y.; Tie, B.; Li, Y.; Lei, M.; Wei, X.; Liu, X.; Du, H. Inoculation of soil with cadmium-resistant bacterium Delftia sp. B9 reduces cadmium accumulation in rice (Oryza sativa L.) grains. Ecotoxicol. Environ. Saf. 2018, 163, 223–229. [Google Scholar] [CrossRef]
- Lima e Silva, A.A.d.; Carvalho, M.A.; de Souza, S.A.; Dias, P.M.T.; Silva Filho, R.G.d.; Saramago, C.S.; Bento, C.A.; Hofer, E. Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz. J. Microbiol. 2012, 43, 1620–1631. [Google Scholar] [CrossRef]
- Thakur, M.; Praveen, S.; Divte, P.R.; Mitra, R.; Kumar, M.; Gupta, C.K.; Kalidindi, U.; Bansal, R.; Roy, S.; Anand, A. Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere 2022, 287, 131957. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Singh, S.; Kumar, P. Reconditioning of plant metabolism by arbuscular mycorrhizal networks in cadmium contaminated soils: Recent perspectives. Microbiol. Res. 2022, 268, 127293. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, B.; Xu, J.; Li, Z.; Tang, Z.; Wu, X. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ. Sci. Pollut. Res. 2022, 29, 32988–33001. [Google Scholar] [CrossRef] [PubMed]
- Amanullah, F.; Khan, W.-u.-D. Trichoderma asperellum L. Coupled the Effects of Biochar to Enhance the Growth and Physiology of Contrasting Maize Cultivars under Copper and Nickel Stresses. Plants 2023, 12, 958. [Google Scholar] [PubMed]
- Singh, A.; Mehta, S.; Yadav, S.; Nagar, G.; Ghosh, R.; Roy, A.; Chakraborty, A.; Singh, I.K. How to cope with the challenges of environmental stresses in the era of global climate change: An update on ROS stave off in plants. Int. J. Mol. Sci. 2022, 23, 1995. [Google Scholar] [CrossRef] [PubMed]
- Mirzai, M.; Moeini, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cultivars. J. Agr. Sci. Tech. 2013, 15, 593–602. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Alharby, H.F.; Al-Zahrani, H.S.; Alsamadany, H.; Alghamdi, K.M.; Ahmed, N.; Nahar, K. Foliar Application of Ascorbic Acid and Tocopherol in Conferring Salt Tolerance in Rapeseed by Enhancing K+/Na+ Homeostasis, Osmoregulation, Antioxidant Defense, and Glyoxalase System. Agronomy 2023, 13, 361. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, C.; Liu, H.; Jiao, Q.; Li, G.; Zhang, J.; Zhang, B.; Jin, W.; Lin, D.; Chen, G. Exogenous ascorbic acid application alleviates cadmium toxicity in seedlings of two wheat (Triticum aestivum L.) varieties by reducing cadmium uptake and enhancing antioxidative capacity. Environ. Sci. Pollut. Res. 2022, 29, 21739–21750. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian tomato cultivars under drought stress show different content of bioactives in pulp and peel of fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of Proline in Plants under Contaminated Soils. Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Pacheco, A.O.; García Díaz, J.; Morris-Quevedo, H.J.; Ferrer Dubois, A.; Aleman, E.I. Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef]
- Song, L.; Niu, X.; Zhou, B.; Xiao, Y.; Zou, H. Application of biochar-immobilized Bacillus sp. KSB7 to enhance the phytoremediation of PAHs and heavy metals in a coking plant. Chemosphere 2022, 307, 136084. [Google Scholar]
- Azeem, M.; Haider, M.Z.; Javed, S.; Saleem, M.H.; Alatawi, A. Drought stress amelioration in maize (Zea mays L.) by inoculation of Bacillus spp. strains under sterile soil conditions. Agriculture 2022, 12, 50. [Google Scholar] [CrossRef]








| Feature | The Amount |
|---|---|
| Sand | 32% |
| Silt | 37% |
| Clay | 31% |
| Texture class | Clay loam |
| pH saturated dough | 7.8 |
| Electrical conductivity of the saturated extract | 0.9 dS/m |
| Cation exchange capacity | 15 cmol+/kg |
| CaCO3 equivalent | 2.25% |
| Organic C | 0.81% |
| Organic matter | 1.04% |
| Total N | 0.09% |
| P extractable by NaHCO3 | 25 mg/kg |
| K extractable by C2H7NO2 | 386 mg/kg |
| Extractable Fe with DTPA | 1.43 mg/kg |
| Extractable Cu with DTPA | 21.2 mg/kg |
| Extractable Mn with DTPA | 47.3 mg/kg |
| Extractable Zn with DTPA | 1.02 mg/kg |
| Extractable Cd with DTPA | 0.8 mg/kg |
| Treatments |
|---|
| Blank without microbial inoculation |
| Blank with bacterial inoculation |
| Blank with fungal inoculation |
| Drought without microbial inoculation |
| Drought with bacterial inoculation |
| Drought with fungal inoculation |
| Cadmium without microbial inoculation |
| Cadmium with bacterial inoculation |
| Cadmium with fungal inoculation |
| Drought + Cadmium without microbial inoculation |
| Drought + Cadmium with bacterial inoculation |
| Drought + Cadmium with fungal inoculation |
| PH (cm) | SD (mm) | LA (cm2) | LFW (g) | LDW (g) | RFW (g) | RDW (g) | RWC (%) | EL (%) | TF | BF | SVT1 | SVT2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| M | ns | ns | * | ** | ** | * | ** | ** | ns | ** | ns | ** | ** |
| S × M | ns | ns | ns | ns | ** | ns | ns | ns | ns | ** | ** | ** | ** |
| RMDA (µmol/g FW) | RTP (mg/gFW) | RSOD (Unit/mg FW) | RCAT [(Mm/cm)/FW.min] | RAPX [(Mm/cm)/FW.min] | RPOD [(Mm cm)/FW.min] | LCA [(mg kg)/DW] | RCA [(mg kg)/DW] | SCA [(mg kg)/DW] | EC (ds/m) | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | ** | ** | ns | ** | ns | ** | ** | ** | ** | ** | ** |
| M | ** | ** | ** | ** | ns | * | ** | ** | ** | ns | ** |
| S × M | ** | ** | ns | ns | ns | ** | ** | * | ns | ns | ** |
| LMDA [(µmol/g)/FW] | LTP [(mg/g)/FW)] | LSOD (Unit/mg FW) | LCAT [(Mm/cm)/FW.min] | LAPX [(Mm/cm)/FW.min] | LPOD [(Mm/cm)/FW.min] | |
|---|---|---|---|---|---|---|
| S | ** | ** | ** | ** | ** | ** |
| M | ** | ** | ** | ** | ** | ** |
| Time | ** | ** | ** | ** | ** | ** |
| S × M × T | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavian, S.; Zarei, M.; Niazi, A.; Ghasemi-Fasaei, R.; Shahriari, A.G.; Janda, T. Morphophysiological and Biochemical Responses of Zea mays L. under Cadmium and Drought Stresses Integrated with Fungal and Bacterial Inoculation. Agronomy 2023, 13, 1675. https://doi.org/10.3390/agronomy13071675
Kavian S, Zarei M, Niazi A, Ghasemi-Fasaei R, Shahriari AG, Janda T. Morphophysiological and Biochemical Responses of Zea mays L. under Cadmium and Drought Stresses Integrated with Fungal and Bacterial Inoculation. Agronomy. 2023; 13(7):1675. https://doi.org/10.3390/agronomy13071675
Chicago/Turabian StyleKavian, Saba, Mehdi Zarei, Ali Niazi, Reza Ghasemi-Fasaei, Amir Ghaffar Shahriari, and Tibor Janda. 2023. "Morphophysiological and Biochemical Responses of Zea mays L. under Cadmium and Drought Stresses Integrated with Fungal and Bacterial Inoculation" Agronomy 13, no. 7: 1675. https://doi.org/10.3390/agronomy13071675
APA StyleKavian, S., Zarei, M., Niazi, A., Ghasemi-Fasaei, R., Shahriari, A. G., & Janda, T. (2023). Morphophysiological and Biochemical Responses of Zea mays L. under Cadmium and Drought Stresses Integrated with Fungal and Bacterial Inoculation. Agronomy, 13(7), 1675. https://doi.org/10.3390/agronomy13071675








