Chemical and Biological Control of Wet Bubble Disease (Hypomyces perniciosus) in Mushroom Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungicides and Biological Control Agents
2.2. Trial Conditions
2.3. Preparation of the Inoculum of Hypomyces perniciosus
2.4. Data Analysis
3. Results
3.1. Yield
3.2. Biological Efficiency
3.3. Incidence of Wet Bubble Disease
3.4. Effectiveness of the Different Biological and Chemical Products
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fletcher, J.T.; Gaze, R.H. Mushroom. In Pest and Disease Control; Manson Publishing: London, UK, 2008; 192p. [Google Scholar]
- Du, Y.X.; Shi, N.N.; Ruan, H.C.; Chen, F.R. Three Mycogone species, including a new species, cause wet bubble disease of Agaricus bisporus in China. Plant Dis. 2021, 105, 3967–3977. [Google Scholar] [CrossRef]
- Shi, N.-N.; Ruan, H.-C.; Chen, W.-L.; Chen, Q.-H.; Chen, F.-R.; Dun, Y.-X. Development of species-specific PCR detection for three Mycogone species causing wet bubble disease in white button mushroom. Crop Prot. 2023, 164, 106141. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J. Mushroom Diseases and Control. In Edible and Medicinal Mushrooms: Technology and Application; Cunha, Z.D., Pardo-Giménez, A., Eds.; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 239–259. [Google Scholar]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Carrasco, J. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: A Review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Chen, B.Z.; Wang, S.; Li, C.H.; Wen, Z.Q. Analysis of genetic diversity and development of SCAR markers in a Mycogone perniciosa population. Curr. Microbiol. 2016, 73, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, D.; Chen, L.; Li, Y. Genetic diversity analysis of Mycogone perniciosa causing wet bubble disease of Agaricus bisporus in China using SRAP. J. Phytopathol. 2016, 164, 271–275. [Google Scholar] [CrossRef]
- Li, D.; Sossah, F.L.; Sun, L.; Fu, Y.; Li, Y. Genome analysis of Hypomyces perniciosus, the causal agent of wet bubble disease of button mushroom (Agaricus bisporus). Genes 2019, 10, 417. [Google Scholar] [CrossRef]
- Shi, N.; Ruan, H.; Jie, Y.; Chen, F.; Du, Y. Sensitivity and efficacy of fungicides against wet bubble disease of Agaricus bisporus caused by Mycogone perniciosa. Eur. J. Plant Pathol. 2020, 157, 873–885. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Efficacy and effects on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Navarro, M.J.; Carrasco, J.; Gea-Alegría, F.J. The role of water content in the casing layer for mushroom crop production and the occurrence of fungal diseases. Agronomy 2021, 11, 2063. [Google Scholar] [CrossRef]
- Carrasco, J.; Tello, M.L.; de Toro, M.; Tkacz, A.; Poole, P.; Pérez-Clavijo, M. Casing microbiome dynamics during button mushroom cultivation: Implications for dry and wet bubble diseases. Microbiology 2019, 165, 611–624. [Google Scholar] [CrossRef]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Gea, F.J. Effect of five fungicides with different modes of action on mushroom cobweb disease (Cladobotryum mycophilum) and mushroom yield. Ann. Appl. Biol. 2017, 171, 62–69. [Google Scholar] [CrossRef]
- American Mushroom, Grower Resources, Integrated Pest Management, Pesticides, Fungicides. American Mushroom. 2023. Available online: https://www.americanmushroom.org/integrated-pest-management/fungicides/ (accessed on 22 February 2023).
- APVMA (Australian Pesticides and Veterinary Medicines Authority). 2023. Available online: https://portal.apvma.gov.au/es/permits (accessed on 22 February 2023).
- Soković, M.; Van Griensven, L.J. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Tanović, B.; Milijasevic, S.; Obradovic, A. In vitro effect of plant essential oils on growth of some soil-borne pathogens. Acta Hortic. 2007, 729, 483–487. [Google Scholar] [CrossRef]
- Tanović, B.; Potočnik, I.; Delibašić, G.; Ristić, M.; Kostić, M.; Marković, M. In vitro effect of essential oils from aromatic and medicinal plants on mushroom pathogens: Verticillium fungicola var. fungicola, Mycogone perniciosa, and Cladobotryum sp. Arch. Biol. Sci. 2009, 61, 231–237. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S. In vitro and in vivo screening of essential oils for the control of wet bubble disease of Agaricus bisporus. S. Afr. J. Bot. 2010, 76, 681–685. [Google Scholar] [CrossRef]
- Gea, F.J.; Navarro, M.J.; Santos, M.; Diánez, F.; Herraiz-Peñalver, D. Screening and evaluation of essential oils from mediterranean aromatic plants against the mushroom cobweb disease, Cladobotryum mycophilum. Agronomy 2019, 9, 656. [Google Scholar] [CrossRef]
- Beyer, D.M.; Pecchia, J.A.; Paley, K. Evaluation of bio-fungicides for the control of fungal diseases on Agaricus bisporus. In Proceedings of the 19th International Society for Mushroom Science (ISMS) Conference, Amsterdam, The Netherlands, 29 May–2 June 2016; Baars, J.J.P., Sonnenberg, A.S.M., Eds.; pp. 86–90. [Google Scholar]
- Preston, G.M.; Carrasco, J.; Gea, F.J.; Navarro, M.J. Biological control of microbial pathogens in edible mushrooms. In Biology of Macrofungi. Fungal Biology; Singh, B., Lallawmsanga, Passari, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Marín, F.; Santos, M.; Diánez, F.; Carretero, F.; Gea, F.J.; Yau, J.; Navarro, M.J. Characters of compost teas from different sources and their suppressive effect on fungal phytopathogens. World J. Microbiol. Biotechnol. 2013, 29, 1371–1382. [Google Scholar] [CrossRef]
- Gea, F.J.; Carrasco, J.; Santos, M.; Diánez, F.; Navarro, M.J. Control of dry bubble disease (Lecanicillium fungicola) in button mushroom (Agaricus bisporus) by spent mushroom substrate tea. Eur. J. Plant Pathol. 2014, 138, 711–720. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Ann. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Kalkhove, S.I.; Lugones, L.G.; Wösten, H.A.; Bakker, P.A. Germination of Lecanicillium fungicola in the mycosphere of Agaricus bisporus. Environ. Microbiol. Rep. 2012, 4, 227–233. [Google Scholar] [CrossRef]
- Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Duduk, B.; Stepanović, J.; Rekanović, E.; Potočnik, I. Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. Eur. J. Plant Pathol. 2017, 148, 509–519. [Google Scholar] [CrossRef]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Diánez, F.; Gea, F.J. Incidence, identification and pathogenicity of Cladobotryum mycophilum, causal agent of cobweb disease on Agaricus bisporus mushroom crops in Spain. Ann. Appl. Biol. 2016, 168, 214–224. [Google Scholar] [CrossRef]
- Vieira, F.R.; Pecchia, J.A. An exploration into the bacterial community under different pasteurization conditions during substrate preparation (composting-Phase II) for Agaricus bisporus cultivation. Microb. Ecol. 2018, 75, 318–330. [Google Scholar] [CrossRef]
- Carrasco, J.; Preston, G. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biochem. 2018, 278, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Kosanovic, D.; Dyas, M.; Grogan, H.; Kavanagh, K. Differential proteomic response of Agaricus bisporus and Trichoderma aggressivum f. europaeum to Bacillus velezensis supernatant. Eur. J. Plant Pathol. 2021, 160, 397–409. [Google Scholar] [CrossRef]
- Clarke, J.; Grogan, H.; Fitzpatrick, D.; Kavanagh, K. Analysis of the effect of Bacillus velezensis culture filtrate on the growth and proteome of Cladobotryum mycophilum. Fungal Biol. 2022, 126, 11–19. [Google Scholar] [CrossRef]
- Borriss, R. Bacillus, a plant-beneficial bacterium. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2015; pp. 379–391. [Google Scholar] [CrossRef]
- Nagy, A.; Manczinger, L.; Tombácz, D.; Hatvani, L.; Gyõrfi, J.; Antal, Z.; Sajben, E.; Vágvõllgyi, C.; Kredics, L. Biological control of oyster mushroom green mould disease by antagonistic Bacillus species. Biol. Control Fungal Bact. Plant Pathog. IOBC-WPRS Bull. 2012, 78, 289–293. [Google Scholar]
- Kim, G.; Weon, H.Y.; Seok, S.J.; Lee, K.H. In vitro antagonistic characteristics of bacilli isolates against Trichoderma spp. and three species of mushrooms. Mycobiology 2008, 36, 266–269. [Google Scholar] [CrossRef]
- Liu, C.; Sheng, J.; Chen, L.; Zheng, Y.; Lee, D.Y.W.; Yang, Y.; Xu, M.; Shen, L. Biocontrol activity of Bacillus subtilis isolated from Agaricus bisporus mushroom compost against pathogenic fungi. J. Agric. Food Chem. 2015, 63, 6009–6018. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Grogan, H.; Fitzpatrick, D.; Kavanagh, K. Characterising the proteomic response of mushroom pathogen Lecanicillium fungicola to Bacillus velezensis QST713 and Kos biocontrol agents. Eur. J. Plant Pathol. 2022, 163, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Potocnik, I.; Todorovic, B.; Rekanovic, E.; Lukovic, J.; Paunovic, D.; Milijasevic-Marcic, S. Impact of Bacillus subtilis QST713 mushroom grain spawn treatment on yield and green mould control. Pestic. Phytomed. 2018, 33, 205–211. [Google Scholar] [CrossRef]
- Kosanović, D.; Potočnik, I.; Duduk, B.; Vukojević, J.; Stajić, M.; Rekanović, E.; Milijašević-Marčić, S. Trichoderma species on Agaricus bisporus farms in Serbia and their biocontrol. Ann. Appl. Biol. 2013, 163, 218–230. [Google Scholar] [CrossRef]
 ), second (
), second ( ), and third (
), and third ( ) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; Ibs: inoculated and Bacillus subtilis treatment.
) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; Ibs: inoculated and Bacillus subtilis treatment.
 ), second (
), second ( ), and third (
), and third ( ) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; Ibs: inoculated and Bacillus subtilis treatment.
) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; Ibs: inoculated and Bacillus subtilis treatment.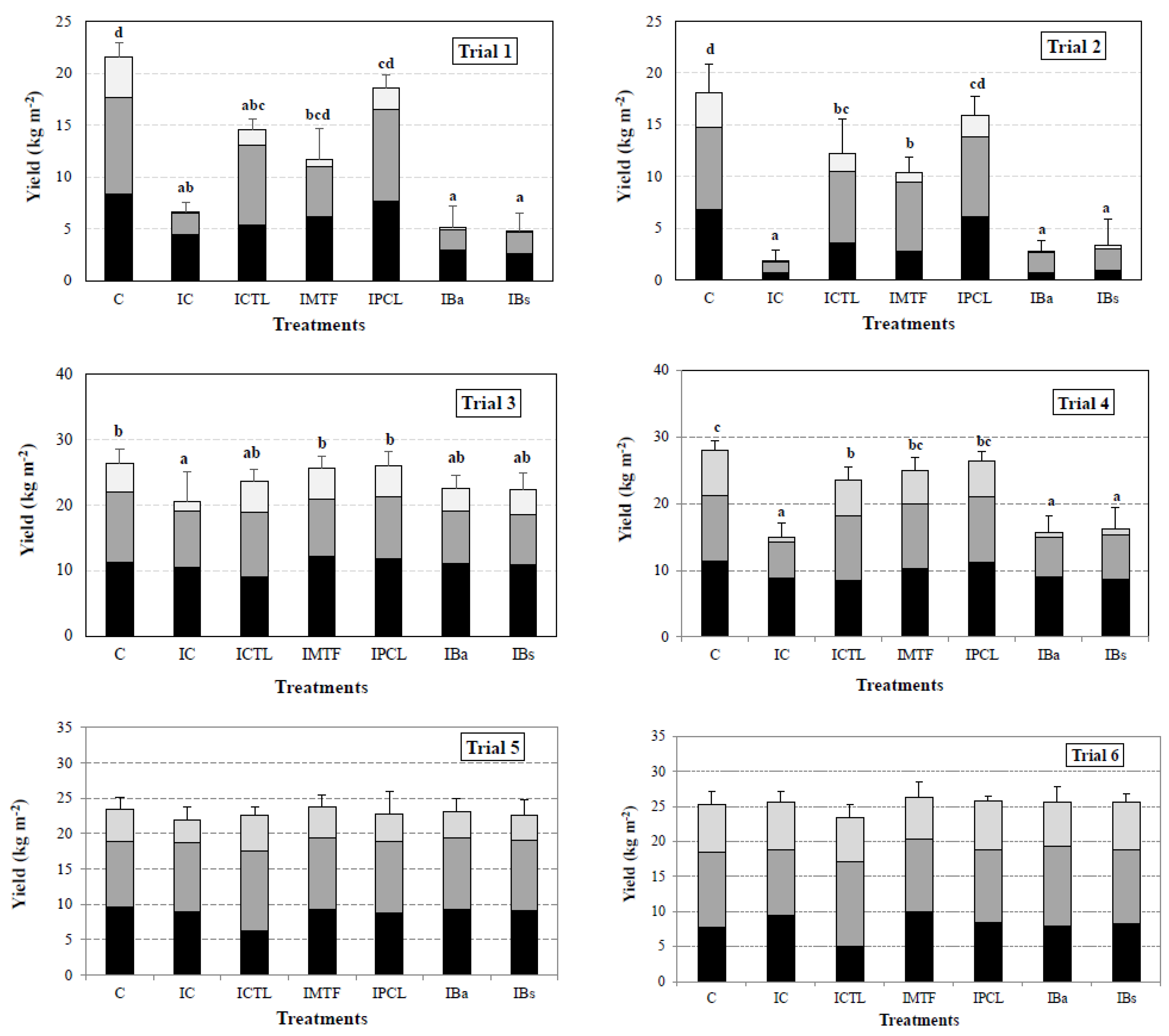
 ), second (
), second ( ), and third (
), and third ( ) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; IBs: inoculated and Bacillus subtilis treatment.
) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; IBs: inoculated and Bacillus subtilis treatment.
 ), second (
), second ( ), and third (
), and third ( ) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; IBs: inoculated and Bacillus subtilis treatment.
) flushes. In each trial, different letters indicate statistical differences between treatments, at 95% of significance (p < 0.01). Figures without statistical analysis markers denote no statistical differences between the treatments. Inoculation rates: trials 1 and 2 (106 CFU m−2); trials 3 and 4 (105 CFU m−2); trials 5 and 6 (103 CFU m−2). C: non-inoculated control; IC: inoculated control; ICTL: inoculated and chlorothalonil treatment; IMTF: inoculated and metrafenone treatment; IPCL: inoculated and prochloraz-Mn treatment; IBa: inoculated and Bacillus amyloliquefaciens treatment; IBs: inoculated and Bacillus subtilis treatment.
 C
C  IC
IC  ICTL
ICTL  IMTF
IMTF  IPCL
IPCL  IBa
IBa  IBs.
IBs.
 C
C  IC
IC  ICTL
ICTL  IMTF
IMTF  IPCL
IPCL  IBa
IBa  IBs.
IBs.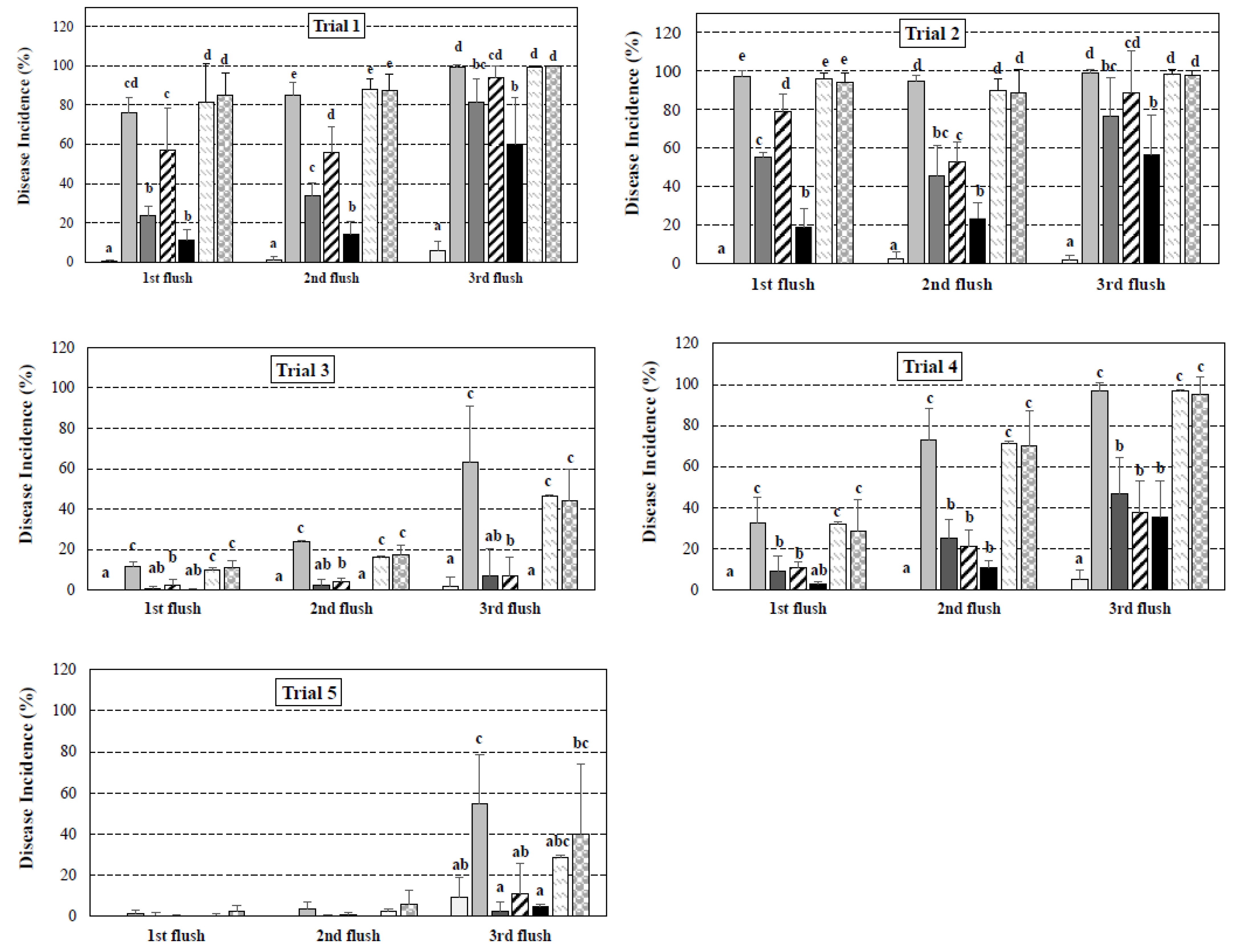
| Commercial Mark | Formulation | Code | Dose |
|---|---|---|---|
| Daconil® | Chlorothalonil 50% CS | (CTL) | 3 mL L−1 m−2 |
| Vivando® | Metrafenone 50% CS | (MTF) | 1 mL L−1 m−2 |
| Sporgon® | Prochloraz-Mn 46% WP | (PCL) | 0.5 g m−2 |
| Serenade® | B. subtilis 1.34% CS strain QST 713 | (Bs) | 3 g m−2 |
| Amylo-X® | B. amyloliquefaciens subsp. plantarum 25% WG strain D747 (Ba) | (Ba) | 3 g m−2 |
| Treatment | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 |
|---|---|---|---|---|---|
| C * | 86 ± 4 a | 70 ± 11 a | 104 ± 8 a | 121 ± 6 a | 108 ± 7 a |
| IC | 102 ± 5 bc | 74 ± 5 a | 104 ± 23 a | 120 ± 8 a | 104 ± 10 a |
| ICTL | 87 ± 5 a | 77 ± 15 a | 96 ± 8 a | 115 ± 5 a | 103 ± 6 a |
| IMTF | 92 ± 8 ab | 82 ± 7 a | 104 ± 4 a | 118 ± 6 a | 109 ± 8 a |
| IPCL | 88 ± 5 a | 77 ± 4 a | 103 ± 8 a | 120 ± 4 a | 103 ± 15 a |
| IBa | 108 ± 8 c | 74 ± 6 a | 103 ± 5 a | 125 ± 6 a | 108 ± 6 a |
| IBs | 102 ± 5 bc | 76 ± 7 a | 102 ± 10 a | 125 ± 12 a | 109 ± 6 a |
| F | 12.24 | 1.08 | 0.35 | 1.38 | 0.66 |
| P | 0.0000 | 0.3912 | 0.9039 | 0.2499 | 0.6811 |
| LSD | 11.30 | 15.44 | 10.31 | 13.58 | 15.78 |
| SED | 2.56 | 3.49 | 0.23 | 3.07 | 3.57 |
| Inoculum | Treatment | F1 | F2 | F3 | F2,35 | p | LSD | SED |
|---|---|---|---|---|---|---|---|---|
| 106 CFU m−2 | CTL * | 56.1 cB ** | 55.9 cB | 20.4 bA | 22.38 | 0.0000 | 9.94 | 2.86 |
| MTF | 23.8 bB | 39.2 bB | 8.3 aA | 14.17 | 0.0000 | 12.10 | 3.49 | |
| PCL | 83.1 dB | 79.2 dB | 41.4 cA | 27.96 | 0.0000 | 9.74 | 2.80 | |
| Ba | 4.6 aA | 3.2 aA | 0.9 aA | 0.76 | 0.4746 | 8.33 | 2.40 | |
| Bs | 2.8 aA | 5.6 aA | 0.9 aA | 1.07 | 0.3546 | 8.57 | 2.47 | |
| F4,59 | 79.30 | 100.25 | 26.36 | |||||
| p | 0.0000 | 0.0000 | 0.0000 | |||||
| LSD | 11.79 | 9.89 | 12.09 | |||||
| SED | 2.96 | 2.48 | 3.03 | |||||
| 105 CFU m−2 | CTL | 83.0 bcA | 77.8 bA | 70.1 bA | 0.84 | 0.4389 | 18.14 | 5.23 |
| MTF | 71.9 bA | 77.0 bA | 74.8 bA | 0.05 | 0.9511 | 13.56 | 3.91 | |
| PCL | 95.0 cA | 92.2 bA | 82.0 bA | 1.17 | 0.3233 | 14.75 | 4.25 | |
| Ba | 14.8 aA | 19.0 aA | 15.4 aA | 0.23 | 0.7952 | 18.62 | 5.36 | |
| Bs | 20.7 aA | 18.5 aA | 17.0 aA | 0.09 | 0.9106 | 119.83 | 5.71 | |
| F4,59 | 35.99 | 38.52 | 23.09 | |||||
| p | 0.0000 | 0.0000 | 0.0000 | |||||
| LSD | 19.50 | 17.25 | 22.07 | |||||
| SED | 4.89 | 4.32 | 5.53 | |||||
| Inoculum | CTL * | MTF | PCL | Ba | Bs |
|---|---|---|---|---|---|
| 105 CFU m−2 | 75.9 ± 20.4 b ** | 77.9 ± 9.9 b | 90.5 ± 10.2 b | 14.5 ± 18.2 b | 18.2 ± 17.0 b |
| 106 CFU m−2 | 43.4 ± 11.6 a | 25.6 ± 12.3 a | 71.9 ± 9.9 a | 1.9 ± 2.5 a | 2.9 ± 5.1 a |
| F1,23 | 20.97 | 108.26 | 19.21 | 5.91 | 8.91 |
| p | 0.0001 | 0.0000 | 0.0002 | 0.0237 | 0.0068 |
| LSD | 9.97 | 6.51 | 8.63 | 10.33 | 10.73 |
| SED | 3.40 | 2.22 | 2.94 | 3.52 | 3.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, M.J.; Santos, M.; Diánez, F.; Gea, F.J. Chemical and Biological Control of Wet Bubble Disease (Hypomyces perniciosus) in Mushroom Crops. Agronomy 2023, 13, 1672. https://doi.org/10.3390/agronomy13071672
Navarro MJ, Santos M, Diánez F, Gea FJ. Chemical and Biological Control of Wet Bubble Disease (Hypomyces perniciosus) in Mushroom Crops. Agronomy. 2023; 13(7):1672. https://doi.org/10.3390/agronomy13071672
Chicago/Turabian StyleNavarro, María Jesús, Mila Santos, Fernando Diánez, and Francisco José Gea. 2023. "Chemical and Biological Control of Wet Bubble Disease (Hypomyces perniciosus) in Mushroom Crops" Agronomy 13, no. 7: 1672. https://doi.org/10.3390/agronomy13071672
APA StyleNavarro, M. J., Santos, M., Diánez, F., & Gea, F. J. (2023). Chemical and Biological Control of Wet Bubble Disease (Hypomyces perniciosus) in Mushroom Crops. Agronomy, 13(7), 1672. https://doi.org/10.3390/agronomy13071672







