Monitoring Patch Expansion Amends to Evaluate the Effects of Non-Chemical Control on the Creeping Perennial Cirsium arvense (L.) Scop. in a Spring Wheat Crop
Abstract
1. Introduction
2. Materials and Methods
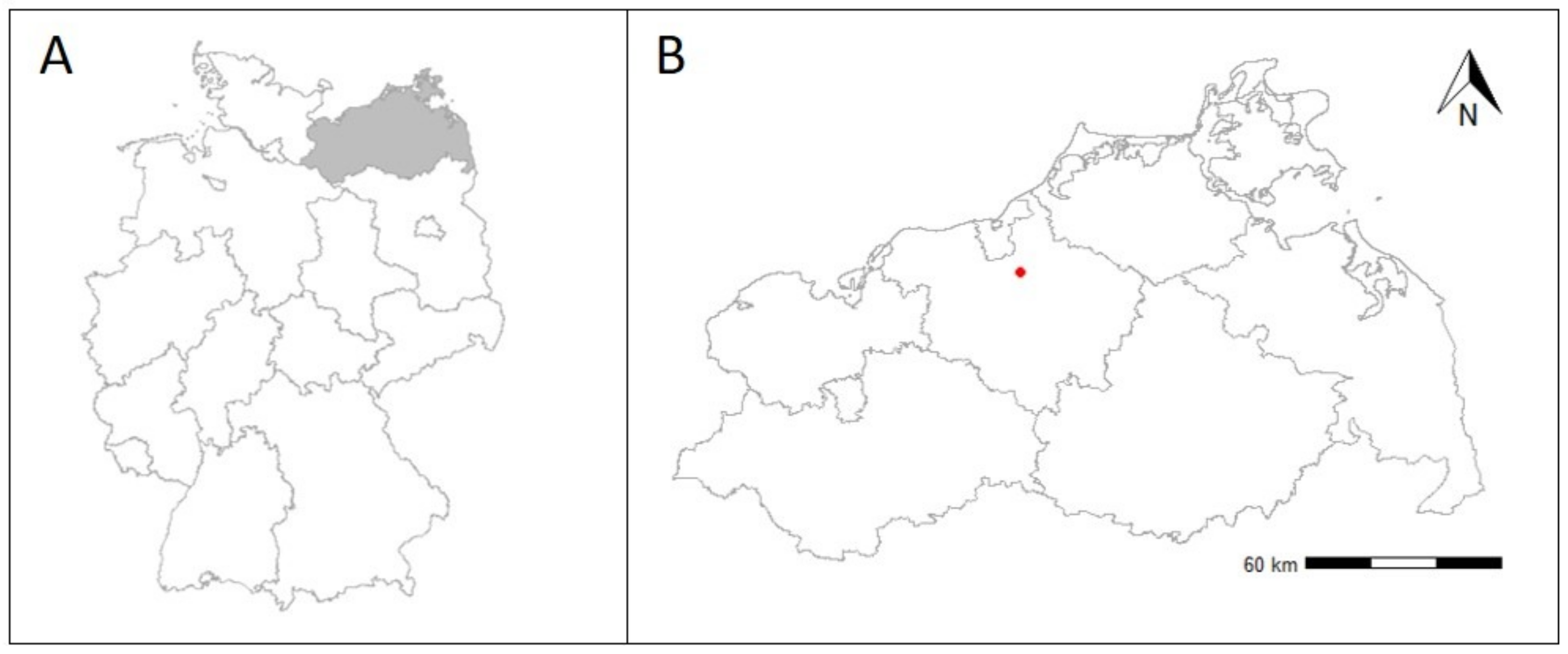
2.1. Experimental Site
2.2. Experimental Set Up
- -
- Untreated control (UC)
- -
- Cover crop (CC)—Sowing white mustard in September at a seeding rate of 25 kg ha−1
- -
- Root cutter (RC)—Root cutting in autumn (10 cm depth) and spring (20–25 cm depth)
- -
- Plough (PL)—Mouldboard ploughing in spring (20–25 cm depth)
- -
- Root cutter + Cover crop = (RC + CC)
- -
- Plough + Cover crop = (PL + CC)
- -
- Plough + Root cutter = (PL + RC)
- -
- Plough + Root cutter + Cover crop = (PL + RC + CC)
2.3. Weed Assessments
- “Shoot density”—counted shoots m−2, 10 subplots of 1 m2 per plot
- “Shoot height”—measured shoot height in cm, 10 randomly chosen shoots per plot
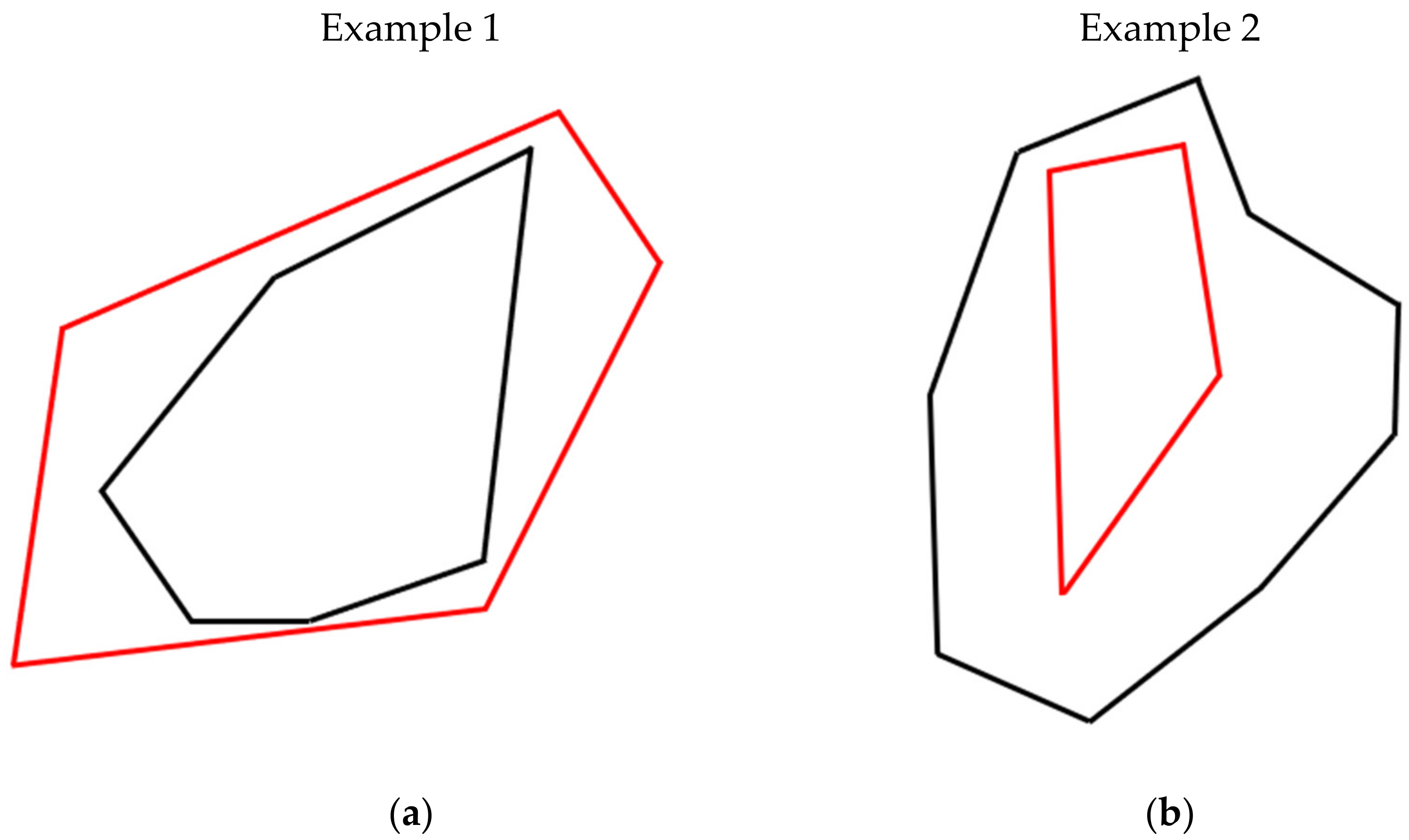
- “ExpansionGPS”—measured patch expansion (m2) monitored with GPS
- “ExpansionUAV”—patch expansion (m2) camera monitored with UAV
- “CoverageUAV”—estimated % of cover by thistle, plots divided into subplots of one m2 assessed in photographs taken by UAV.
2.4. Statistical Analyses
3. Results and Discussion
3.1. Measuring Thistle Response
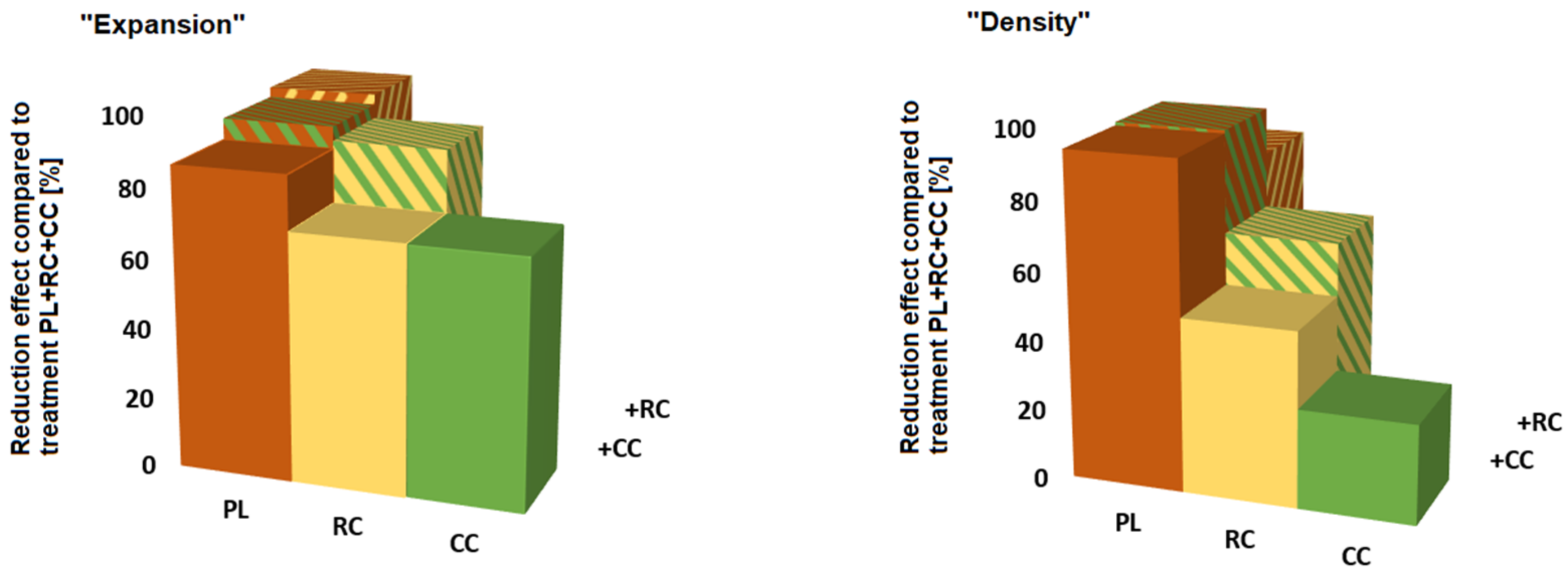
3.2. Treatment Effects
3.3. UAV Monitored Thistle Response Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| “Expansion” | “Density” | “Coverage” | “Height” | |
|---|---|---|---|---|
| Factor | p-Value | |||
| PL | <0.001 | <0.001 | 0.039 | 0.001 |
| RC | 0.005 | 0.002 | 0.515 | 0.431 |
| CC | 0.008 | 0.011 | 0.959 | 0.638 |
| PL:RC | 0.047 | <0.001 | 0.851 | 0.372 |
| PL:CC | 0.033 | 0.271 | 0.952 | 0.036 |
| RC:CC | 0.122 | 0.848 | 0.241 | 0.111 |
| PL:RC:CC | 0.145 | 0.192 | 0.886 | 0.081 |
References
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Heisenberger, J.P. The World’s Worst Weeds: Distribution and Biology. Q. Rev. Biol. 1978, 53, 319–320. [Google Scholar] [CrossRef]
- Leth, V.; Netland, J.; Andreasen, C. Phomopsis cirsii: A potential biocontrol agent of Cirsium arvense. Weed Res. 2008, 48, 533–541. [Google Scholar] [CrossRef]
- Eber, S.; Brandl, R. Regional pathch dynamics of Cirsium arvense and possible implications for plant-animal interactions. J. Veg. Sci. 2003, 14, 259–266. [Google Scholar] [CrossRef]
- Tiley, G.E.D. Biological Flora of the British Isles: Cirsium arvense (L.) Scop. J. Ecol. 2010, 98, 938–983. [Google Scholar] [CrossRef]
- Hamouz, P.; Hamouzová, K.; Tyšer, L.; Holec, J. Effect of site-specific weed management in winter crops on yield and weed populations. Plant Soil Environ. 2014, 60, 27–35. [Google Scholar] [CrossRef]
- Nadeau, L.B.; Vanden Born, W.H. The root system of canada thistle. Can. J. Plant Sci. 1989, 69, 1199–1206. [Google Scholar] [CrossRef]
- Heimann, B.; Cussans, G.W. The importance of seeds and sexual reproduction in the population biology of Cirsium arvense—A literature review. Weed Res. 1996, 36, 493–503. [Google Scholar] [CrossRef]
- Solé, M.; Durka, W.; Eber, S.; Brandl, R. Genotypic and Genetic Diversity of the Common Weed Cirsium arvense (Asteraceae). Int. J. Plant Sci. 2004, 165, 437–444. [Google Scholar] [CrossRef]
- Hettwer, U.; Gerowitt, B. An investigation of genetic variation in Cirsium arvense field patches. Weed Res. 2004, 44, 289–297. [Google Scholar] [CrossRef]
- Blumenthal, D.; Jordan, N. Weeds in field margins: A spatially explicit simulation analysis of Canada thistle population dynamics. Weed Sci. 2001, 49, 509–519. [Google Scholar] [CrossRef]
- Rasmussen, J.; Nielsen, J. A novel approach to estimating the competitive ability of Cirsium arvense in cereals using unmanned aerial vehicle imagery. Weed Res. 2020, 60, 150–160. [Google Scholar] [CrossRef]
- Graglia, E.; Melander, B.; Jensen, R.K. Mechanical and cultural strategies to control Cirsium arvense in organic arable cropping systems. Weed Res. 2006, 46, 304–312. [Google Scholar] [CrossRef]
- Thomsen, M.G.; Mangerud, K.; Riley, H.; Brandsæter, L.O. Method, timing and duration of bare fallow for the control of Cirsium arvense and other creeping perennials. Crop. Prot. 2015, 77, 31–37. [Google Scholar] [CrossRef]
- Bicksler, A.J.; Masiunas, J.B. Canada Thistle (Cirsium arvense) Suppression with Buckwheat or Sudangrass Cover Crops and Mowing. Weed Technol. 2009, 23, 556–563. [Google Scholar] [CrossRef]
- Gruber, S.; Claupein, W. Effect of tillage intensity on weed infestation in organic farming. Soil Tillage Res. 2009, 105, 104–111. [Google Scholar] [CrossRef]
- Hoeft, E.V.; Jordan, N.; Zhang, J.; Wyse, D.L. Integrated cultural and biological control of Canada thistle in conservation tillage soybean. Weed Sci. 2001, 49, 642–646. [Google Scholar] [CrossRef]
- Brandsæter, L.O.; Mangerud, K.; Helgheim, M.; Berge, T.W. Control of perennial weeds in spring cereals through stubble cultivation and mouldboard ploughing during autumn or spring. Crop. Prot. 2017, 98, 16–23. [Google Scholar] [CrossRef]
- Wilson, R.G. Canada thistle—The problem, distribution and economics. In Proceedings of the North Central Weed Conference, Moines, IO, USA, 8–10 December 1981. [Google Scholar]
- Schroeder, D.; Mueller-Schaerer, H.; Stinson, C.S.A. A European weed survey in 10 major crop systems to identify targets for biological control. Weed Res. 1993, 33, 449–458. [Google Scholar] [CrossRef]
- Riesinger, P.; Hyvönen, T. Impact of Management on Weed Species Composition in Organically Cropped Spring Cereals. Biol. Agric. Hortic. 2006, 24, 257–274. [Google Scholar] [CrossRef]
- Glemnitz, M.; Radics, L.; Mackensen, K. Weed management in organic farming in the new EU member states and the acceding countries—Status quo and main limitations. In Proceedings of the 3rd QLIF Congress, Hohenheim, Germany, 20–23 March 2007. [Google Scholar]
- Håkansson, S. Weeds and Weed Management on Arable Land: An Ecological Approach; CABI Pub: Cambridge, UK, 2003; pp. 81–193. [Google Scholar]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. Current and future scenarios of glyphosate use in Europe: Are there alternatives? Adv. Agron. 2020, 163, 219–278. [Google Scholar] [CrossRef]
- Zhang, H.; Andert, S.; Brandsæter, L.O.; Rasmussen, J.; Robin, M.-H.; Salonen, J.; Tørresen, K.S.; Valantin-Morison, M.; Gerowitt, B. Future management of arable perennials—An introduction to the project AC/DC-weeds. Jul. Kühn-Inst. 2020, 464, 280–285. [Google Scholar] [CrossRef]
- Aronsson, H.; Ringselle, B.; Andersson, L.; Bergkvist, G. Combining mechanical control of couch grass (Elymus repens L.) with reduced tillage in early autumn and cover crops to decrease nitrogen and phosphorus leaching. Nutr. Cycl. Agroecosyst. 2015, 102, 383–396. [Google Scholar] [CrossRef]
- Bakker, D. A comparative life-history study of Cirsium arvense (L.). In The Biology of Weeds; Harper, J.L., Ed.; Blackwell Scientific Publications Ltd.: Oxford, UK, 1960. [Google Scholar]
- Edwards, G.R.; Bourdot, G.W.; Crawley, M.J. Influence of herbivory, competition and soil fertility on the abundance of Cirsium arvense in acid grassland. J. Appl. Ecol. 2000, 37, 321–334. [Google Scholar] [CrossRef]
- Boström, U.; Fogelfors, H. Long-term effects of herbicide-application strategies on weeds and yield in spring-sown cereals. Weed Sci. 2002, 50, 196–203. [Google Scholar] [CrossRef]
- Weigel, M.; Gerowitt, B. Mechanical disturbance of Cirsium arvense—Results from a multi-year field study. Jul. Kühn-Inst. 2022, 468, 79–85. [Google Scholar] [CrossRef]
- Tørresen, K.S.; Gerowitt, B. Late-Autumn Ramet Sprouting of Three Arable Creeping Perennial Weed Species. Agronomy 2022, 12, 2175. [Google Scholar] [CrossRef]
- Hakansson, I.; Steneberg, M.; Rydberg, T. Long-term experiments with different depths of mouldboard ploughing in Sweden. Soil Tillage Res. 1998, 46, 209–223. [Google Scholar] [CrossRef]
- Thomsen, M.G.; Brandsæter, L.O.; Fykse, H. Sensitivity of Cirsium arvense to simulated tillage and competition. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 693–700. [Google Scholar] [CrossRef]
- Melander, B.; Munier-Jolain, N.; Charles, R.; Wirth, J.; Schwarz, J.; van der Weide, R.; Bonin, L.; Jensen, P.K.; Kudsk, P. European Perspectives on the Adoption of Nonchemical Weed Management in Reduced-Tillage Systems for Arable Crops. Weed Technol. 2013, 27, 231–240. [Google Scholar] [CrossRef]
- Rasmussen, J.; Nielsen, J.; Streibig, J.C.; Jensen, J.E.; Pedersen, K.S.; Olsen, S.I. Pre-harvest weed mapping of Cirsium arvense in wheat and barley with off-the-shelf UAVs. Precis. Agric. 2019, 20, 983–999. [Google Scholar] [CrossRef]
- Rodene, E.; Xu, G.; Palali Delen, S.; Zhao, X.; Smith, C.; Ge, Y.; Schnable, J.; Yang, J. A UAV-based high-throughput phenotyping approach to assess time-series nitrogen responses and identify trait-associated genetic components in maize. Plant Phenome J. 2022, 5, e20030. [Google Scholar] [CrossRef]
- Marino, S.; Alvino, A. Detection of Spatial and Temporal Variability of Wheat Cultivars by High-Resolution Vegetation Indices. Agronomy 2019, 9, 226. [Google Scholar] [CrossRef]
- Duddu, H.S.N.; Johnson, E.N.; Willenborg, C.J.; Shirtliffe, S.J. High-Throughput UAV Image-Based Method Is More Precise Than Manual Rating of Herbicide Tolerance. Plant Phenom. 2019, 2019, 6036453. [Google Scholar] [CrossRef]
- Mink, R.; Dutta, A.; Peteinatos, G.; Sökefeld, M.; Engels, J.; Hahn, M.; Gerhards, R. Multi-Temporal Site-Specific Weed Control of Cirsium arvense (L.) Scop. and Rumex crispus L. in Maize and Sugar Beet Using Unmanned Aerial Vehicle Based Mapping. Agriculture 2018, 8, 65. [Google Scholar] [CrossRef]
- Ølsen, S.I.; Nielsen, J.; Rasmussen, J. Image Analysis. In Thistle Detection; Sharma, P., Bianchi, F.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 413–425. [Google Scholar]
- R Studio Team (Ed.) R Studio: Integrated Development for R; PBC: Boston, MA, USA, 2022. [Google Scholar]
- Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research, R package version 1.4.0; The R Project for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Usinglme4. J. Stat. Softw 2015, 67, 48. [Google Scholar] [CrossRef]
- Amor, R.L.; Harris, R.V. Seedling establishment and vegetative spread of Cirsium arvense (L.) Scop, in Victoria, Australia. Weed Res. 1975, 15, 407–411. [Google Scholar] [CrossRef]
- Melander, B.; Holst, N.; Rasmussen, I.A.; Hansen, P.K. Direct control of perennial weeds between crops—Implications for organic farming. Crop. Prot. 2012, 40, 36–42. [Google Scholar] [CrossRef]
- Tworkoski, T. Developmental and Environmental Effects on Assimilate Partitioning in Canada Thistle (Cirsium arvense). Weed Sci. 1992, 40, 79–85. [Google Scholar] [CrossRef]
- Burdon, J.J.; Jarosz, A.M.; Kirby, G.C. Pattern and Patchiness in Plant-Pathogen Interactions—Causes and Consequences. Annu. Rev. Ecol. Syst. 1989, 20, 119–136. [Google Scholar] [CrossRef]
- Yin, C.; Hulbert, S.H.; Schroeder, K.L.; Mavrodi, O.; Mavrodi, D.; Dhingra, A.; Schillinger, W.F.; Paulitz, T.C. Role of bacterial communities in the natural suppression of Rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.). Appl. Environ. Microbiol. 2013, 79, 7428–7438. [Google Scholar] [CrossRef]
- Tavaziva, V.J.; Lundkvist, A.; Verwijst, T. Effects of selective cutting and timing of herbicide application on growth and development of Cirsium arvense in spring barley. Weed Res. 2019, 59, 349–356. [Google Scholar] [CrossRef]
- Wedryk, S.; Cardina, J. Smother Crop Mixtures for Canada Thistle (Cirsium arvense) Suppression in Organic Transition. Weed Sci. 2012, 60, 618–623. [Google Scholar] [CrossRef]
- Gruntman, M.; Groß, D.; Májeková, M.; Tielbörger, K. Decision-making in plants under competition. Nat. Commun. 2017, 8, 2235. [Google Scholar] [CrossRef] [PubMed]
- Brandsæter, L.O.; Goul Thomsen, M.; Wærnhus, K.; Fykse, H. Effects of repeated clover undersowing in spring cereals and stubble treatments in autumn on Elymus repens, Sonchus arvensis and Cirsium arvense. Crop. Prot. 2012, 32, 104–110. [Google Scholar] [CrossRef]
- Dock Gustavsson, A.M. Growth and regenerative capacity of plants of Cirsium arvense. Weed Res. 1997, 37, 229–236. [Google Scholar] [CrossRef]
- Forero, M.G.; Herrera-Rivera, S.; Ávila-Navarro, J.; Franco, C.A.; Rasmussen, J.; Nielsen, J. Color Classification Methods for Perennial Weed Detection in Cereal Crops. In Progress in Pattern Recognition, Image Analysis, Computer Vision, and Applications; Vera-Rodriguez, R., Fierrez, J., Morales, A., Eds.; Springer: Cham, Germany, 2019; Volume 11401, pp. 117–123. [Google Scholar] [CrossRef]
- Sørensen, R.A.; Rasmussen, J.; Nielsen, J.; Jørgensen, R.N. Thistle detection using convolutional neural networks. In Proceedings of the EFITA WCCA 2017 Conference, Montpellier, France, 2–6 July 2017; p. 75. [Google Scholar]
- Suir, G.; Saltus, C.; Sasser, C.; Harris, J.; Reif, M.; Diaz, R.; Giffin, G. Evaluating Drone Truthing as an Alternative to Ground Truthing: An Example with Wetland Plant Identification, ERDC/TN APCRP-MI-9; Engineer Research and Development Center: Vicksburg, MI, USA, 2021. [Google Scholar] [CrossRef]

| Field Operations | 2019 | 2020 | 2021 |
|---|---|---|---|
| Root cutting (spring, 20 cm) | - | 27-January | 31-March |
| Ploughing (spring, 20 cm) | - | 28-January | 31-March |
| Sowing spring wheat | - | 18-March | 31-March |
| Grain harvest | - | 20-August | 31-August |
| Field cultivator (5 cm), disc harrow (7 cm) | 24-August | 22-August | - |
| Field cultivator (10 cm) | 13-March | 30-March | |
| Sowing cover crop | 5-September | 15-September | - |
| Root cutting (autumn, 10 cm) | 5-September | 15-September | - |
| Estimate | ||||
|---|---|---|---|---|
| Fixed Treatment Effects | “Expansion” | “Density” | “Coverage” | “Height” |
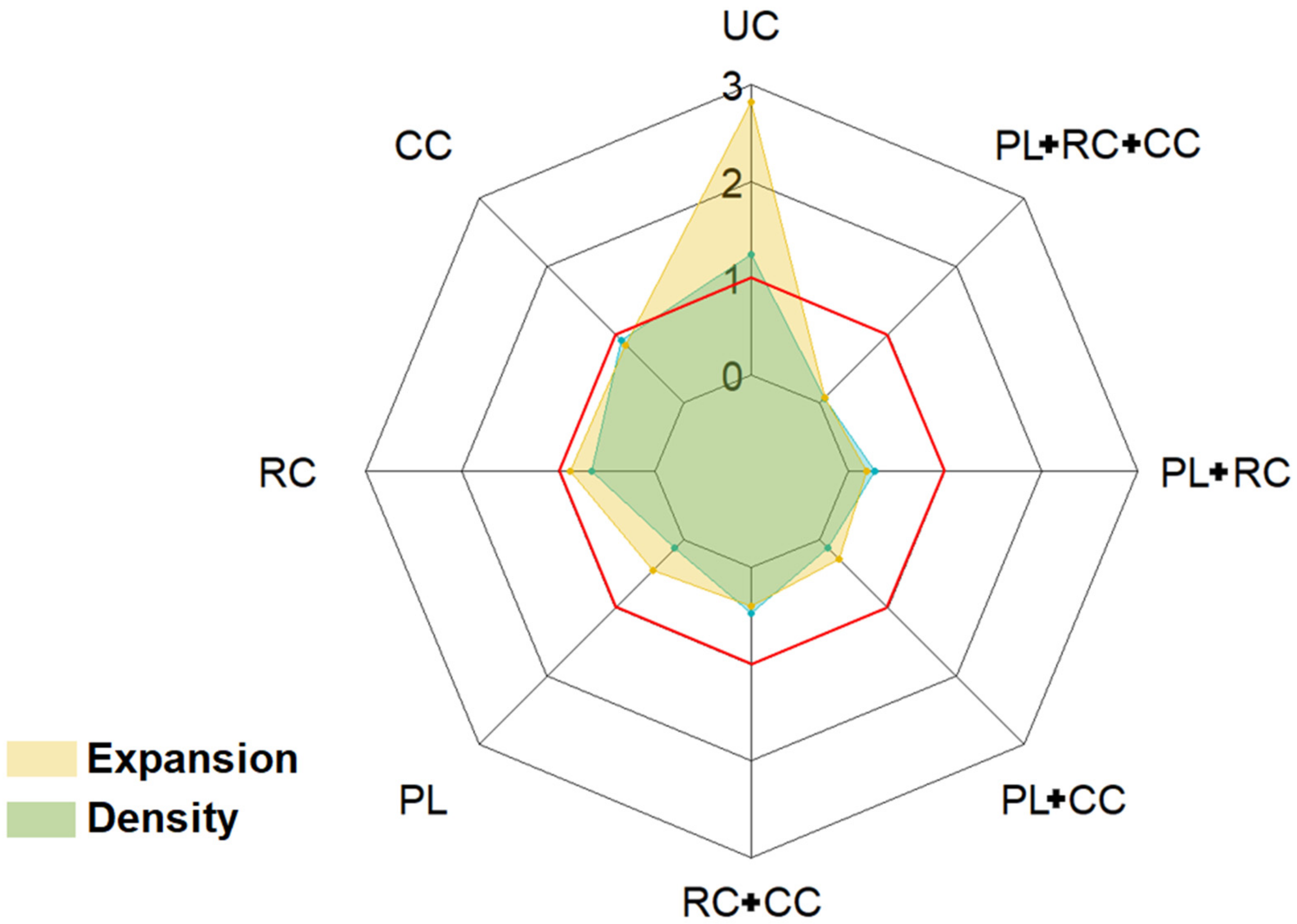
| Intercept: UC | 2.83 *** | 1.25 *** | 0.99 ** | 0.93 *** |
| CC | −1.95 *** | −0.34 * | +0.19 | −0.23 |
| RC | −1.95 *** | −0.6 *** | +0.04 | −0.15 |
| PL | −2.38 *** | −1.12 *** | −0.51 | −0.69 *** |
| RC + CC | −2.44 *** | −0.78 *** | −0.19 | −0.35 * |
| PL + CC | −2.55 *** | −1.13 *** | −0.24 | −0.28. |
| PL + RC | −2.63 *** | −0.97 *** | −0.34 | −0.40 * |
| PL + RC + CC | −2.76 *** | −1.19 *** | −0.60 | −0.54 ** |
| Estimate | ||||
|---|---|---|---|---|
| y | x | Intercept | Slope | r2 |
| ExpansionGPS | ExpansionUAV | 1.34 | 0.96 *** | 0.92 |
| Shoot density | CoverageUAV | 10.33 ** | 0.37 *** | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigel, M.M.; Andert, S.; Gerowitt, B. Monitoring Patch Expansion Amends to Evaluate the Effects of Non-Chemical Control on the Creeping Perennial Cirsium arvense (L.) Scop. in a Spring Wheat Crop. Agronomy 2023, 13, 1474. https://doi.org/10.3390/agronomy13061474
Weigel MM, Andert S, Gerowitt B. Monitoring Patch Expansion Amends to Evaluate the Effects of Non-Chemical Control on the Creeping Perennial Cirsium arvense (L.) Scop. in a Spring Wheat Crop. Agronomy. 2023; 13(6):1474. https://doi.org/10.3390/agronomy13061474
Chicago/Turabian StyleWeigel, Marian Malte, Sabine Andert, and Bärbel Gerowitt. 2023. "Monitoring Patch Expansion Amends to Evaluate the Effects of Non-Chemical Control on the Creeping Perennial Cirsium arvense (L.) Scop. in a Spring Wheat Crop" Agronomy 13, no. 6: 1474. https://doi.org/10.3390/agronomy13061474
APA StyleWeigel, M. M., Andert, S., & Gerowitt, B. (2023). Monitoring Patch Expansion Amends to Evaluate the Effects of Non-Chemical Control on the Creeping Perennial Cirsium arvense (L.) Scop. in a Spring Wheat Crop. Agronomy, 13(6), 1474. https://doi.org/10.3390/agronomy13061474






