Metabolome and Transcriptome Analyses Reveal the Differences in the Molecular Mechanisms of Oat Leaves Responding to Salt and Alkali Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment Conditions
2.2. Transcriptome Sequencing and Analyses
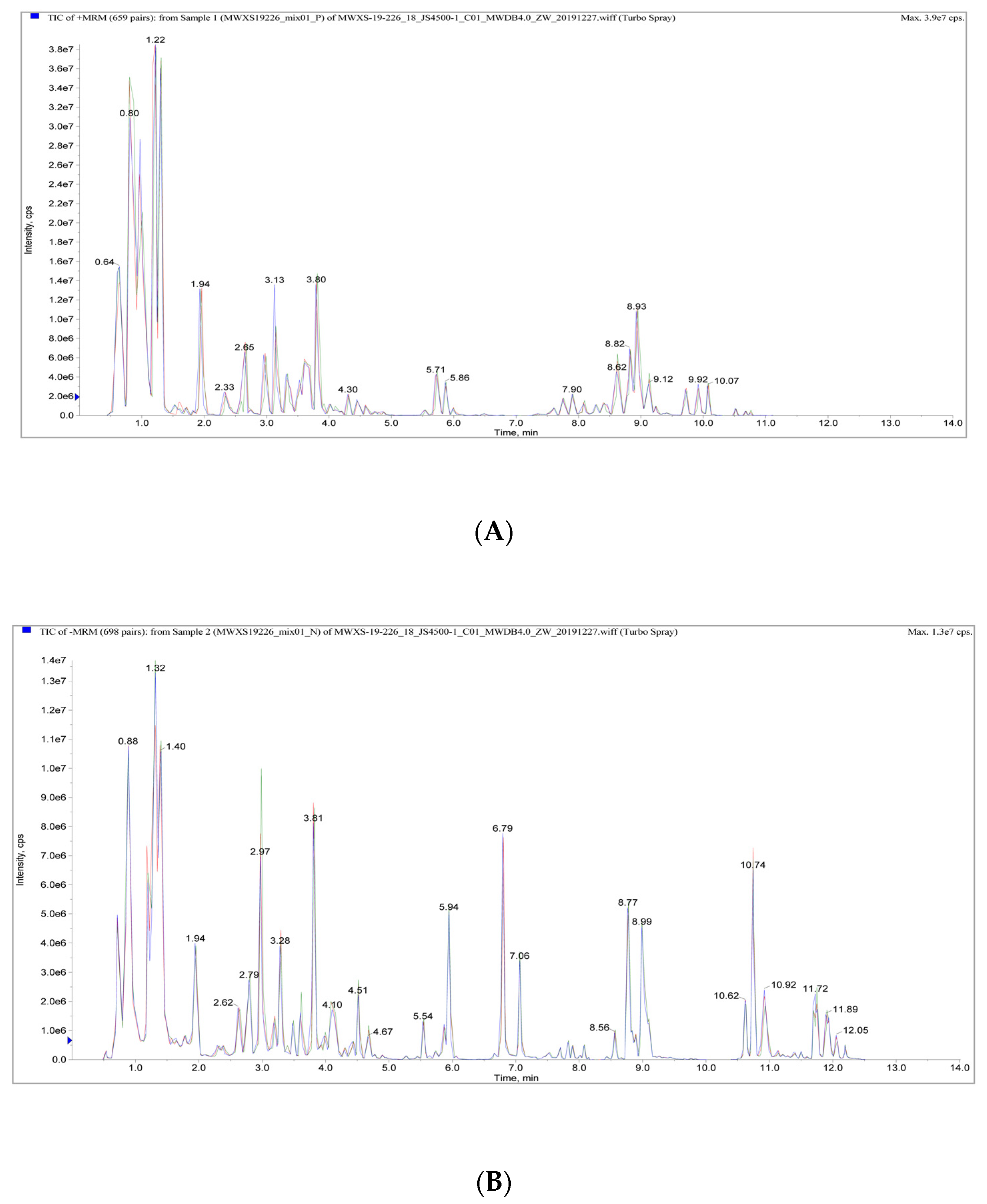
2.3. Metabolite Profiling
2.4. RT-qPCR Analysis
2.5. Chlorophyll Fluorescence Analysis
2.6. Sodium (Na+), Potassium (K+), and Calcium (Ca2+) Levels
2.7. Statistical Analysis
3. Results
3.1. Analysis of Transcriptomic Data
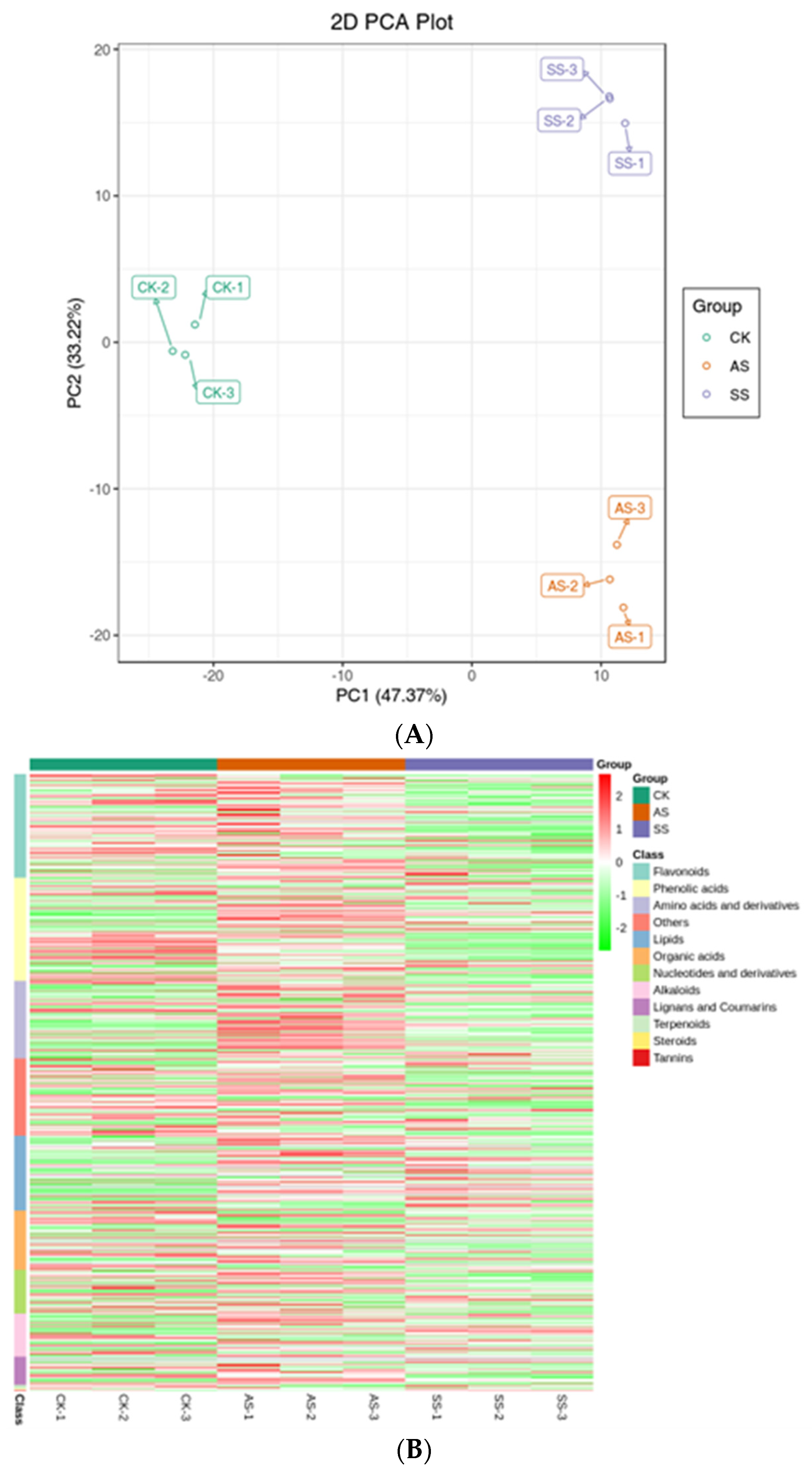
3.2. Analysis of Metabolic Data
4. Discussion
4.1. Genes Related to Growth Inhibition, Chlorophyll Content Decrease and Antioxidation Due to the Two Stress Conditions
4.2. Metabolites Related to Growth Inhibition and Chlorophyll Content Decrease Due to the Two Stress Conditions
4.3. Metabolic Pathways Related to the Improvement of Salt and Alkali Tolerance
4.3.1. Metabolites Related to the Improvement of the Chlorophyll Content under Salt and Alkali Stress Conditions
4.3.2. Metabolites Related to the Elimination of ROS under Salt and Alkali Stress Conditions
4.3.3. Metabolites Related to Inorganic Ion Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, M.C.; Liao, P.M.; Kuo, W.W.; Lin, T.P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chai, J.; Zhang, Y.; Zhang, C.; Lei, Y.; Li, Q.; Yao, T. Halotolerant rhizobacteria mitigate the effects of salinity stress on maize growth by secreting exopolysaccharides. Environ. Exp. Bot. 2022, 204, 105098. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, J.; Guo, L.; Fang, H.; Liu, X.; Liang, K.; Niu, W.; Liu, F.; Siddique, K.H.M. Effects of Two Biochar Types on Mitigating Drought and Salt Stress in Tomato Seedlings. Agronomy 2023, 13, 1039. [Google Scholar] [CrossRef]
- Li, H.; Tang, X.; Yang, X.; Zhang, H. Comprehensive transcriptome and metabolome profiling reveal metabolic mechanisms of Nitraria sibirica Pall. to salt stress. Sci. Rep. 2021, 11, 12878. [Google Scholar] [CrossRef] [PubMed]
- Niron, H.; Barlas, N.; Salih, B.; Türet, M. Comparative Transcriptome, Metabolome, and Ionome Analysis of Two Contrasting Common Bean Genotypes in Saline Conditions. Front. Plant Sci. 2020, 11, 599501. [Google Scholar] [CrossRef]
- Song, T.; Xu, H.; Sun, N.; Jiang, L.; Tian, P.; Yong, Y.; Yang, W.; Cai, H.; Cui, G. Metabolomic Analysis of Alfalfa ( Medicago sativa L.) Root-Symbiotic Rhizobia Responses under Alkali Stress. Front. Plant Sci. 2017, 8, 1208. [Google Scholar] [CrossRef]
- Bai, J.; Yan, W.; Wang, Y.; Yin, Q.; Liu, J.; Wight, C.; Ma, B. Screening Oat Genotypes for Tolerance to Salinity and Alkalinity. Front. Plant Sci. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Faizan, S.; Gulzar, B. Salt stress, its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biochem. Biotechnol. 2020, 8, 81–91. [Google Scholar]
- Wen, W.; Li, K.; Alseekh, S.; Omranian, N.; Zhao, L.; Zhou, Y.; Xiao, Y.; Jin, M.; Yang, N.; Liu, H.; et al. Genetic Determinants of the Network of Primary Metabolism and Their Relationships to Plant Performance in a Maize Recombinant Inbred Line Population. Plant Cell 2015, 27, 1839–1856. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol. 2011, 29, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S. Advances in detection of stress tolerance in plants through metabolomics approaches. Plant Omics 2017, 10, 153–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X.; et al. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef]
- Wu, D.; Cui, M.; Hao, Y.; Liu, L.; Zhou, Y.; Wang, W.; Xue, A.; Chingin, K.; Luo, L. In Situ Study of Metabolic Response of Arabidopsis thaliana Leaves to Salt Stress by Neutral Desorption-Extractive Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 12945–12952. [Google Scholar] [CrossRef]
- Ma, S.; Lv, L.; Meng, C.; Zhang, C.; Li, Y. Integrative Analysis of the Metabolome and Transcriptome of Sorghum bicolor Reveals Dynamic Changes in Flavonoids Accumulation under Saline-Alkali Stress. J. Agric. Food Chem. 2020, 68, 14781–14789. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yang, C.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H. Comparison of Ionomic and Metabolites Response under Alkali Stress in Old and Young Leaves of Cotton (Gossypium hirsutum L.) Seedlings. Front. Plant Sci. 2016, 7, 1785. [Google Scholar] [CrossRef]
- Song, T.; Sun, N.; Dong, L.; Cai, H. Enhanced alkali tolerance of rhizobia-inoculated alfalfa correlates with altered proteins and metabolic processes as well as decreased oxidative damage. Plant Physiol. Biochem. 2021, 159, 301–311. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, J.; Wang, Y.; Liu, J.; Hu, Y.; Dong, Z.; Ji, L. Comparative effects of salt and alkali stress on photosynthesis and root physiology of oat at anthesis. Arch. Biol. Sci. 2017, 70, 50. [Google Scholar] [CrossRef]
- Jia, X.; Wang, H.; Svetla, S.; Zhu, Y.; Hu, Y.; Cheng, L.; Zhao, T.; Wang, Y. Comparative physiological responses and adaptive strategies of apple Malus halliana to salt, alkali and saline-alkali stress. Sci. Hortic. 2019, 245, 154–162. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite Profiles of Maize Leaves in Drought, Heat, and Combined Stress Field Trials Reveal the Relationship between Metabolism and Grain Yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Bai, J.; Jin, K.; Qin, W.; Wang, Y.; Yin, Q. Proteomic Responses to Alkali Stress in Oats and the Alleviatory Effects of Exogenous Spermine Application. Front. Plant Sci. 2021, 12, 627129. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant. 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Deng, B.; Tian, S.; Guo, M.; Liu, H.; Zhao, X. Metabolic and transcriptomic analyses reveal different metabolite biosynthesis profiles between leaf buds and mature leaves in Ziziphus jujuba mill. Food Chem. 2021, 347, 129005. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Zhang, H.; Lai, B.; Liu, L.; Pan, X.; Ma, Z.; Wang, Y.; Xie, J.; Shi, S.; Wei, Y. Integrative Analysis of the Coloring Mechanism of Red Longan Pericarp through Metabolome and Transcriptome Analyses. J. Agric. Food Chem. 2021, 69, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-H.; Liu, J.-H.; Zhang, N.; Yang, J.-H.; Sa, R.-L.; Wu, L. Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat. J. Integr. Agric. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−11 CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crops Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Sa, R.L.; Liu, J.H.; Liu, W.; Jiao, W.H.; Bai, J.H.; Wang, Z.H. Effects of salt stress and alkali stress on the content of Na+, K+ and yield of oat seedings. Acta Agric. Boreali-Occident Sin. 2014, 23, 50–54. [Google Scholar]
- Hannie, S.; Kieft, H.; Emons, A.; Ketelaar, T. Arabidopsis VILLIN2 and VILLIN3 Are Required for the Generation of Thick Actin Filament Bundles and for Directional Organ Growth. Plant Physiol. 2012, 158, 1426–1438. [Google Scholar]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Li, Q.; Yu, B.; Gao, Y.; Dai, A.-H.; Bai, J.-G. Cinnamic acid pretreatment mitigates chilling stress of cucumber leaves through altering antioxidant enzyme activity. J. Plant Physiol. 2011, 168, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Kuhlmann, M.; Rutten, T.; Mette, M.F.; Scharfenberg, S.; Petridis, A.; Berreth, D.C.; Mock, H.P. Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant J. 2018, 93, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Xuan, T.D.; Khang, D.T. Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence. Molecules 2018, 23, 620. [Google Scholar] [CrossRef]
- Hossain, A.K.M.Z.; Ohno, T.; Koyama, H.; Hara, T. Effect of Enhanced Calcium Supply on Aluminum Toxicity in Relation to Cell Wall Properties in the Root Apex of Two Wheat Cultivars Differing in Aluminum Resistance. Plant Soil 2005, 276, 193–204. [Google Scholar] [CrossRef]
- Mohamadi, N.; Sharififar, F.; Pournamdari, M.; Ansari, M. A Review on Biosynthesis, Analytical Techniques, and Pharmacological Activities of Trigonelline as a Plant Alkaloid. J. Diet. Suppl. 2018, 15, 207–222. [Google Scholar] [CrossRef]
- Nagata, N.; Suzuki, M.; Yoshida, S.; Muranaka, T. Mevalonic acid partially restores chloroplast and etioplast development in Arabidopsis lacking the non-mevalonate pathway. Planta 2002, 216, 345–350. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Hong, C.; Jiang, D.; Zheng, B. Exogenous malic acid alleviates cadmium toxicity in Miscanthus sacchariflorus through enhancing photosynthetic capacity and restraining ROS accumulation. Ecotoxicol. Environ. Saf. 2017, 141, 119–128. [Google Scholar] [CrossRef]
- Darandeh, N.; Hadavi, E. Effect of Pre-Harvest Foliar Application of Citric Acid and Malic Acid on Chlorophyll Content and Post-Harvest Vase Life of Lilium cv. Brunello. Front. Plant Sci. 2011, 2, 106. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Qin, Y.; Liu, J.; Wang, Y.; Sa, R.; Zhang, N.; Jia, R. Proteomic response of oat leaves to long-term salinity stress. Environ. Sci. Pollut. Res. Int. 2017, 24, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Gong, B.; Chen, Y.; Wang, J.; Wei, M.; Shi, Q. Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci. Hortic. 2018, 235, 413–423. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235. [Google Scholar] [CrossRef] [PubMed]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Karahan, H.; Kucukoduk, M.; Turkan, I. Ferulic acid confers tolerance against excess boron by regulating ROS levels and inducing antioxidant system in wheat leaves (Triticum aestivum). Environ. Exp. Bot. 2019, 161, 193–202. [Google Scholar] [CrossRef]
- Wan, Y.-Y.; Chen, S.-Y.; Huang, Y.-W.; Li, X.; Zhang, Y.; Wang, X.-J.; Bai, J.-G. Caffeic acid pretreatment enhances dehydration tolerance in cucumber seedlings by increasing antioxidant enzyme activity and proline and soluble sugar contents. Sci. Hortic. 2014, 173, 54–64. [Google Scholar] [CrossRef]
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and gentisic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; Luis, J.C.; Suarez, E.; Hernández, M.; Valdés, F.; Borges, A.A. Lettuce plants treated with L-pyroglutamic acid increase yield under water deficit stress. Environ. Exp. Bot. 2019, 158, 215–222. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Z.; Xu, C.; Li, L.; Yang, C. Multiomics analysis provides insights into alkali stress tolerance of sunflower (Helianthus annuus L.). Plant Physiol. Biochem. 2021, 166, 66–77. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and Seed Application of Amino Acids Affects the Antioxidant Metabolism of the Soybean Crop. Front. Plant Sci. 2017, 8, 327. [Google Scholar] [CrossRef]
- Wang, W.; Pang, J.; Zhang, F.; Sun, L.; Yang, L.; Zhao, Y.; Yang, Y.; Wang, Y.; Siddique, K.H.M. Integrated transcriptomics and metabolomics analysis to characterize alkali stress responses in canola (Brassica napus L.). Plant Physiol. Biochem. 2021, 166, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Imran, M.; Ashraf, M.; Mahmood, K. Perspectives of Using L-Tryptophan for Improving Productivity of Agricultural Crops: A Review. Pedosphere 2018, 28, 16–34. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019, 42, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Shah, M.K.; Naveed, M.; Akhter, M.J. Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vigna radiata L. under salt stress conditions. J. Microbiol. Biotechnol. 2010, 20, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, F.; Yaghoobi, M.M.; Kalantari, K.M. Effect of exogenous arginine on alleviation of oxidative damage in tomato plant underwater stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef]
- Sabetta, W.; Vannini, C.; Sgobba, A.; Marsoni, M.; Paradiso, A.; Ortolani, F.; Bracale, M.; Viggiano, L.; Blanco, E.; de Pinto, M.C. Cyclic AMP deficiency negatively affects cell growth and enhances stress-related responses in tobacco Bright Yellow-2 cells. Plant Mol. Biol. 2016, 90, 467–483. [Google Scholar] [CrossRef]
- Paradiso, A.; Domingo, G.; Blanco, E.; Buscaglia, A.; Fortunato, S.; Marsoni, M.; Scarcia, P.; Caretto, S.; Vannini, C.; de Pinto, M.C. Cyclic AMP mediates heat stress response by the control of redox homeostasis and ubiquitin-proteasome system. Plant Cell Environ. 2020, 43, 2727–2742. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef]
- Chen, H.N.; Tao, L.Y.; Shi, J.M.; Han, X.R.; Cheng, X.G. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021, 188, 104498. [Google Scholar]



| ID | Function | Metabolite | Class | Response Ratio | VIP | Regulated | |||
|---|---|---|---|---|---|---|---|---|---|
| AS/CK | SS/CK | AS/CK | SS/CK | AS/CK | SS/CK | ||||
| mws2213 | Antioxidation | Cinnamic acid | Phenolic acids | 0.40 | 1 | 1.14 | - | down | No |
| mws1077 | Scopolin | Lignans and Coumarins | 0.17 | 1 | 1.15 | - | down | No | |
| Hmfn00531 | L-ascorbic acid | Vitamin/othets | 0.46 | 0.41 | 1.21 | 1.16 | down | down | |
| mws0281 | Citric acid | Organic acid | 2.09 | 2.4 | 1.20 | 1.17 | up | up | |
| pme2024 | Serotonin | Alkaloid | 8.76 | 5.72 | 1.21 | 1.16 | up | up | |
| mws0263 | Pyroglutamic acid | Amino acids(derivatives) | 893.00 | 1 | 1.21 | - | up | No | |
| mws0282 | L-tryptophan | Amino acids (derivatives) | 4.87 | 3.26 | 1.21 | 1.17 | up | up | |
| mws0260 | Arginine | Amino acids(derivatives) | 9.03 | 4.87 | 1.09 | 1.13 | up | up | |
| pme0008 | L-Citrulline | Amino acids(derivatives) | 3.83 | 2.21 | 1.19 | 1.10 | up | up | |
| mws0014 | Ferulic acid | Phenolic acid | 2.50 | 2.46 | 1.19 | 1.14 | up | up | |
| pma6561 | caffeic acid | Phenolic acid | 2.42 | 3.23 | 1.20 | 1.26 | up | up | |
| mws0183 | Chlorophyll accumulation | Protocatechuic acid | Flavanols | 0.00009 | 0.00009 | 1.21 | 1.17 | down | down |
| mws0275 | Malic acid | Organic acid | 2.98 | 1 | 1.21 | - | up | No | |
| pmb3142 | Salicylic acid | Phenolic acid | 126,582 | 84,066 | 1.21 | 1.17 | up | up | |
| pme3154 | Mevalonic acid | Organic acid | 54,302 | 10,372 | 1.21 | 1.17 | Up | Up | |
| pme1439 | Growth | O-coumaric acid | Phenolic acid | 2.37 | 1 | 1.18 | - | up | No |
| mws0884 | Inorganic ion regulation | Cyclic AMP | Amino acids(derivatives) | 5.36 | 4.59 | 1.21 | 1.17 | up | up |
| pme0006 | Osmoregulation | Proline | Amino acids(derivatives) | 12.1 | 10.65 | 1.21 | 1.17 | up | up |
| pme2268 | Trigonelline | Alkaloid | 0.41 | 1 | 1.16 | - | No | Down | |
| mws0191 | Betaine | Alkaloid | 2.82 | 4.14 | 1.17 | 1.56 | up | up | |
| pmb0069 | Unknown function | Benzamide | Others | 206.87 | 456.62 | 1.21 | 1.17 | up | up |
| mws0491 | Phenethylamine | Others | 212.01 | 446.58 | 1.21 | 1.17 | up | up | |
| mws1433 | N-Feruloyltyramine | Alkaloid | 1156.76 | 1592.89 | 1.21 | 1.17 | up | up | |
| pmb0629 | Chrysoeriol 6-C-hexoside | Flavonoid | 3320.44 | 2262.04 | 1.21 | 1.17 | up | up | |
| pmn001318 | 1,3-O-Di-p-Coumaroyl glycerol | Phenolic acid | 38,091.85 | 128,392.59 | 1.21 | 1.17 | up | up | |
| mws1715 | Cordycepin | Nucleotides(derivatives) | 1356.00 | 1352.74 | 1.21 | 1.17 | up | up | |
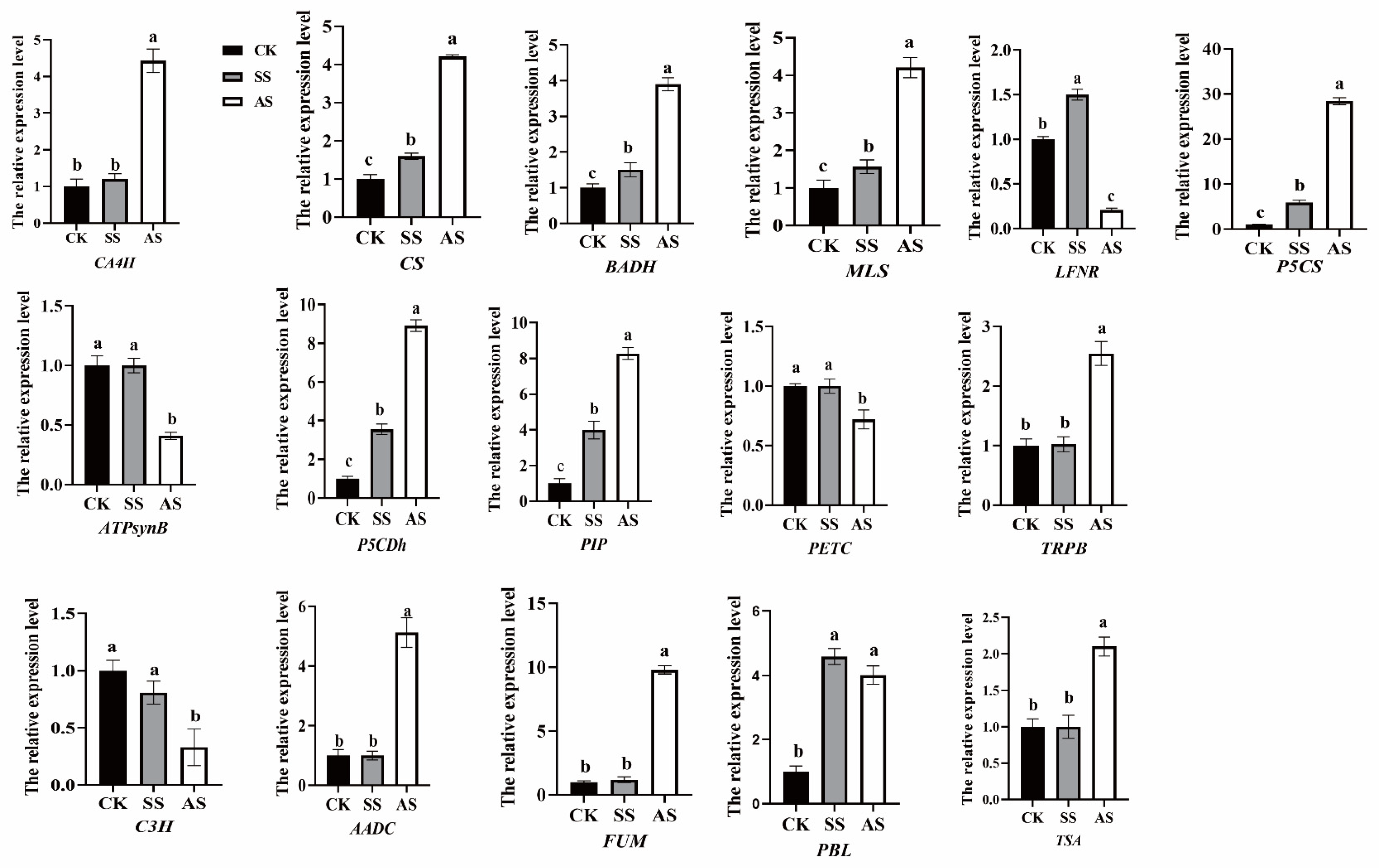
| Function | Gene ID | Gene Name | Description | Fold Change | Log2Fold Change | Regulated | |||
|---|---|---|---|---|---|---|---|---|---|
| SS/CK | AS/CK | SS/CK | AS/CK | SS/CK | AS/CK | ||||
| O-coumaric acid synthesis | TRINITY_DN35234_c0_g1_i2.path1 | CA4H | Trans-cinnamate 4-monooxygenase | 2.040 | 40.203 | 1.028 | 5.329 | up | up |
| Serotonin synthesis | TRINITY_DN28969_c2_g1_i1.path1 | AADC | Aromatic-L-amino-acid decarboxylase | 4.003 | 36.063 | 2.001 | 5.172 | up | up |
| Caffeic acid | TRINITY_DN35234_c0_g1_i2.path1 | CA4H | Trans-cinnamate 4-monooxygenase | 2.040 | 40.203 | 1.028 | 5.329 | up | up |
| TRINITY_DN49630_c0_g1_i2.path2 | C3H | p-coumarate 3-hydroxylase | 0.747 | 0.426 | −0.421 | −1.231 | No significant | down | |
| Citric acid | TRINITY_DN2130_c0_g1_i1.path1 | CS | Citrate synthase | 2.874 | 32.258 | 1.523 | 5.012 | up | up |
| Proline | TRINITY_DN22005_c0_g1_i2.path3 | PIP | Proline iminopeptidase | 2.046 | 8.934 | 1.033 | 3.159 | up | up |
| TRINITY_DN17408_c0_g1_i3.path1 | P5CS | Delta-1-pyrroline-5-carboxylate synthase | 3.563 | 40.663 | 1.833 | 5.346 | up | up | |
| TRINITY_DN1782_c0_g1_i2.path1 | P5CDh | Delta-1-pyrroline-5-carboxylate dehydrogenase | 2.362 | 10.674 | 1.240 | 3.416 | up | up | |
| Betaine | TRINITY_DN33834_c0_g1_i1.path2 | BADH | Betaine aldehyde dehydrogenase | 2.138 | 9.135 | 1.096 | 3.191 | up | up |
| Salicylic acid | TRINITY_DN5328_c0_g1_i4.path1 | EDS | Enhanced disease susceptibility | 2.029 | 3.640 | 1.021 | 1.864 | up | up |
| TRINITY_DN28752_c0_g1_i1.path2 | PBL | avrPphB Susceptible | 3.201 | 2.809 | 1.679 | 1.490 | up | up | |
| Malic acid | TRINITY_DN12835_c0_g1_i1.path1 | MLS | Malate synthase | 48.034 | 1137.412 | 5.586 | 10.152 | up | up |
| TRINITY_DN4496_c0_g1_i2.path1 | FUM | Fumarase | 1.945 | 14.678 | 0.96 | 3.876 | No significant | up | |
| L-tryptophan. | TRINITY_DN7542_c0_g1_i2.path1 | TSA | Tryptophan synthase alpha chain | 1.414 | 3.577 | 0.5 | 1.839 | No significant | up |
| TRINITY_DN13657_c0_g1_i2.path2 | TRPB | Tryptophan synthase beta chain | 0.97 | 3.287 | −0.044 | 1.717 | No significant | up | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Lu, P.; Li, F.; Li, L.; Yin, Q. Metabolome and Transcriptome Analyses Reveal the Differences in the Molecular Mechanisms of Oat Leaves Responding to Salt and Alkali Stress Conditions. Agronomy 2023, 13, 1441. https://doi.org/10.3390/agronomy13061441
Bai J, Lu P, Li F, Li L, Yin Q. Metabolome and Transcriptome Analyses Reveal the Differences in the Molecular Mechanisms of Oat Leaves Responding to Salt and Alkali Stress Conditions. Agronomy. 2023; 13(6):1441. https://doi.org/10.3390/agronomy13061441
Chicago/Turabian StyleBai, Jianhui, Peina Lu, Feng Li, Lijun Li, and Qiang Yin. 2023. "Metabolome and Transcriptome Analyses Reveal the Differences in the Molecular Mechanisms of Oat Leaves Responding to Salt and Alkali Stress Conditions" Agronomy 13, no. 6: 1441. https://doi.org/10.3390/agronomy13061441
APA StyleBai, J., Lu, P., Li, F., Li, L., & Yin, Q. (2023). Metabolome and Transcriptome Analyses Reveal the Differences in the Molecular Mechanisms of Oat Leaves Responding to Salt and Alkali Stress Conditions. Agronomy, 13(6), 1441. https://doi.org/10.3390/agronomy13061441





