Bioponics—An Organic Closed-Loop Soilless Cultivation System: Yields and Characteristics Compared to Hydroponics and Soil Cultivation
Abstract
1. Introduction
2. Materials and Methods
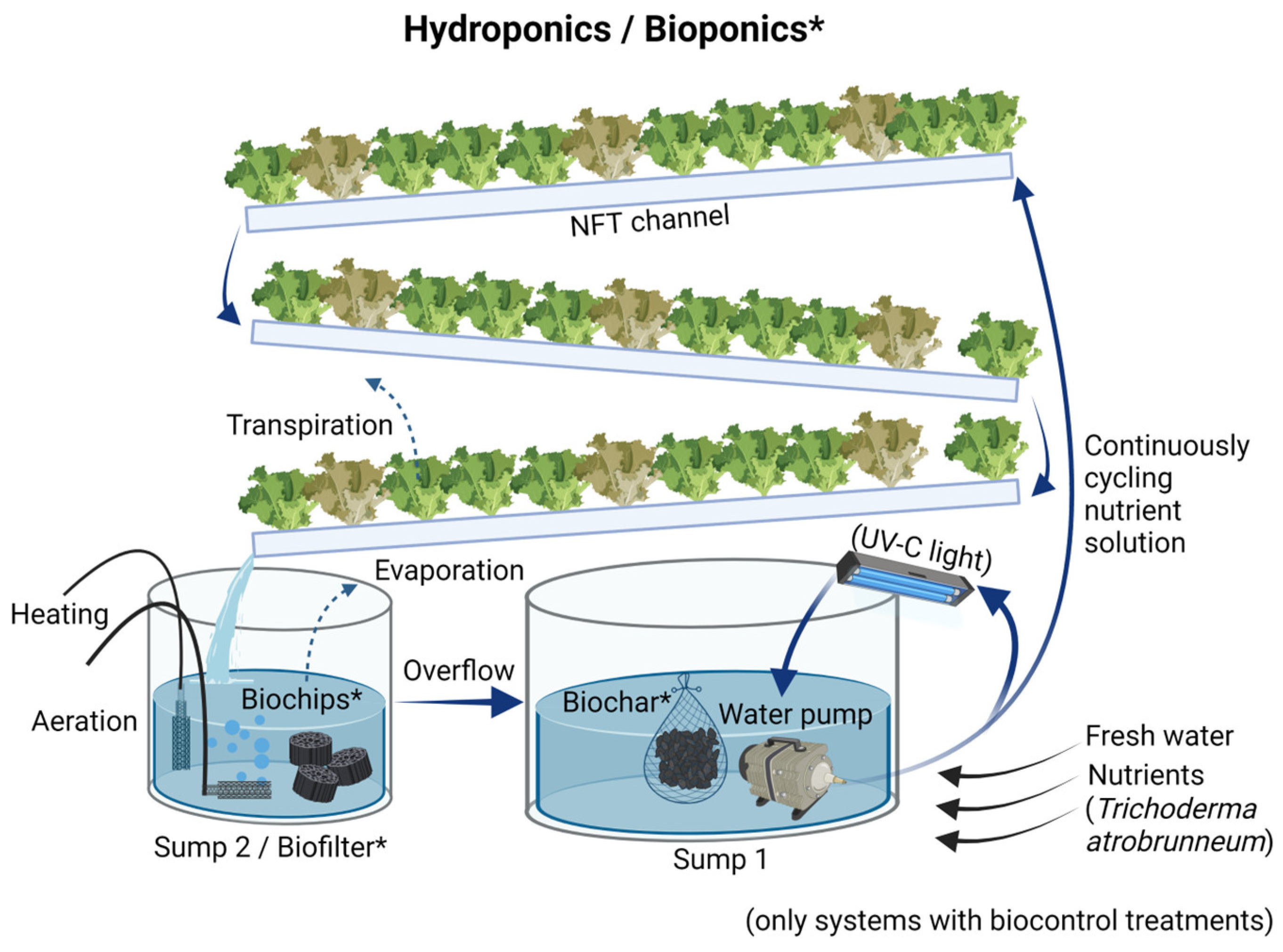
2.1. Soilless Cultivation System Setup
2.2. Soil Cultivation System
2.3. Seedling Preparation
2.4. Fertilizer Management
2.4.1. Bioponics (BP)
2.4.2. Hydroponics (HPs)
2.4.3. Soil Cultivation (SO)
2.5. Nutrient Solution Analyses
2.6. Plant Growth and Quality
2.6.1. Plant Growth
2.6.2. Fresh and Dry Weight Determination
2.6.3. Shelf-Life and Consumer Test
2.6.4. Plant Nutrient Analyses
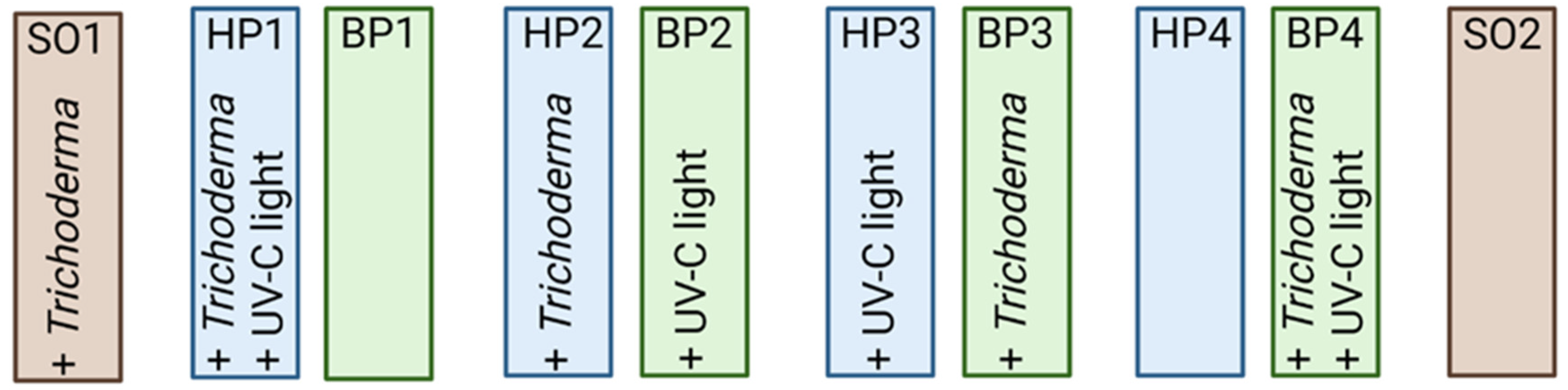
2.7. Biocontrol
2.7.1. UV-C Treatment
2.7.2. Trichoderma atrobrunneum Strain T720 Application
2.8. Data Analyses
3. Results
3.1. Nutrient Solution Characteristics
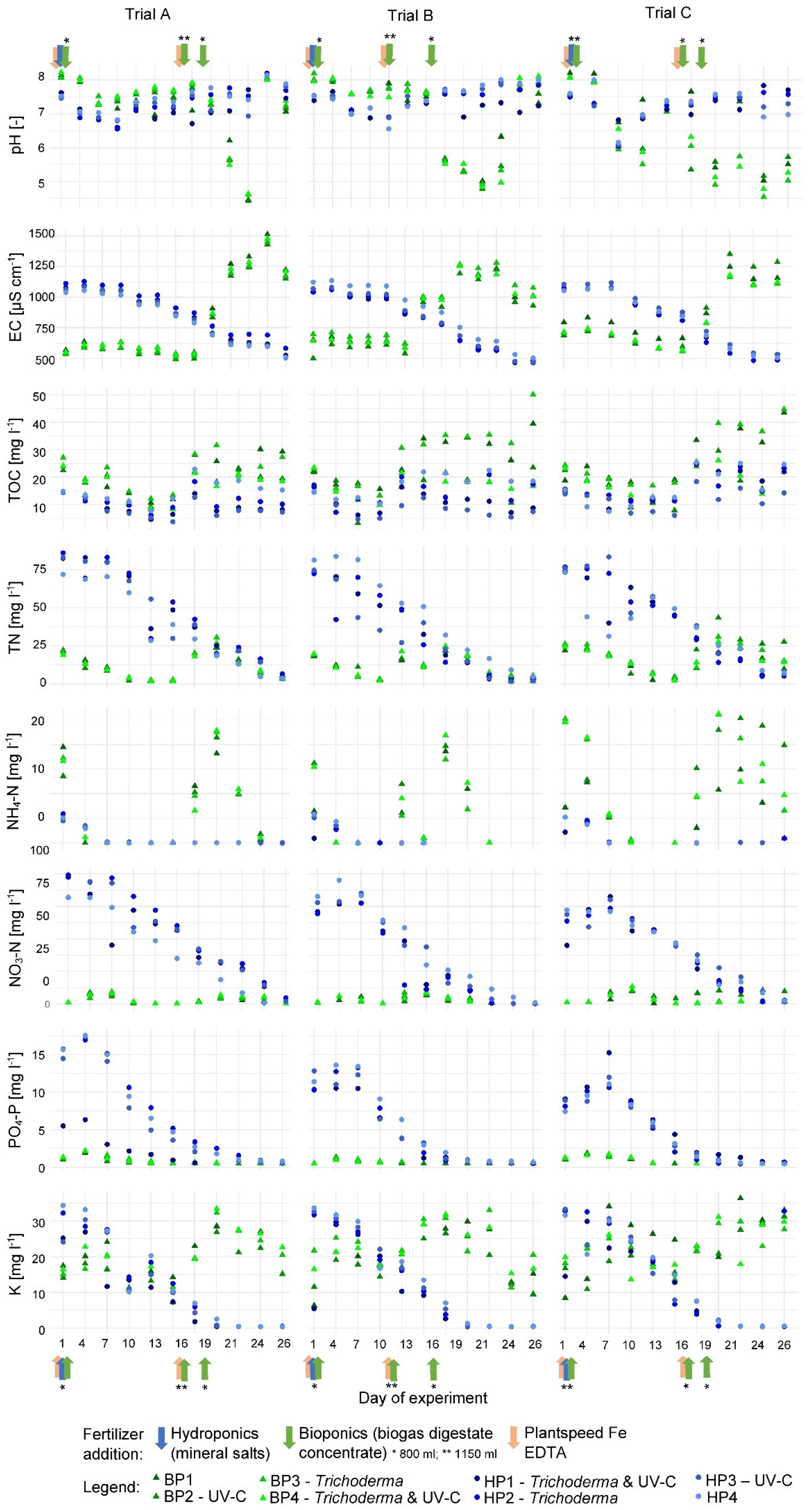
3.1.1. Physiochemical Parameters
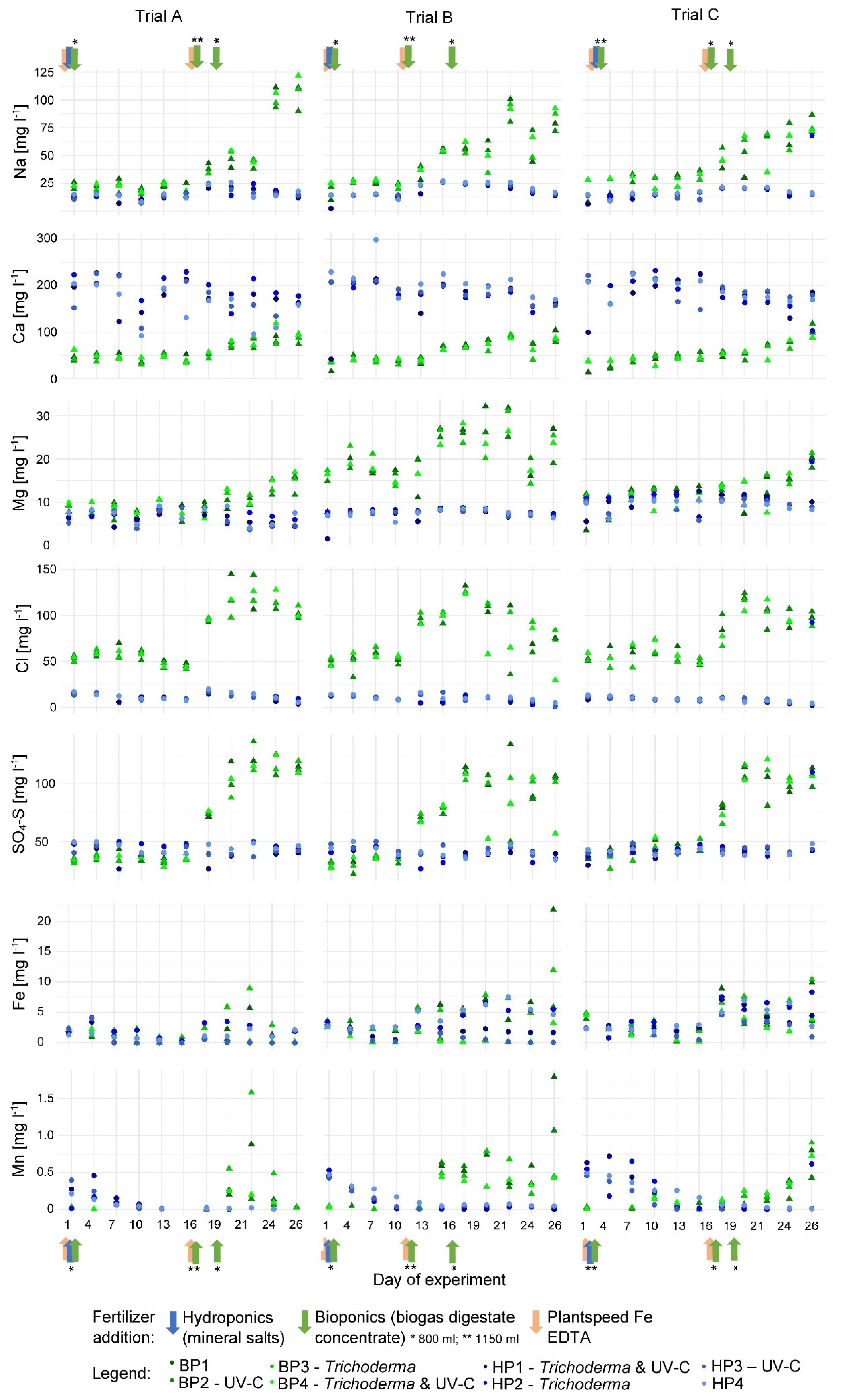
3.1.2. Nutrient Concentrations in the Nutrient Solution
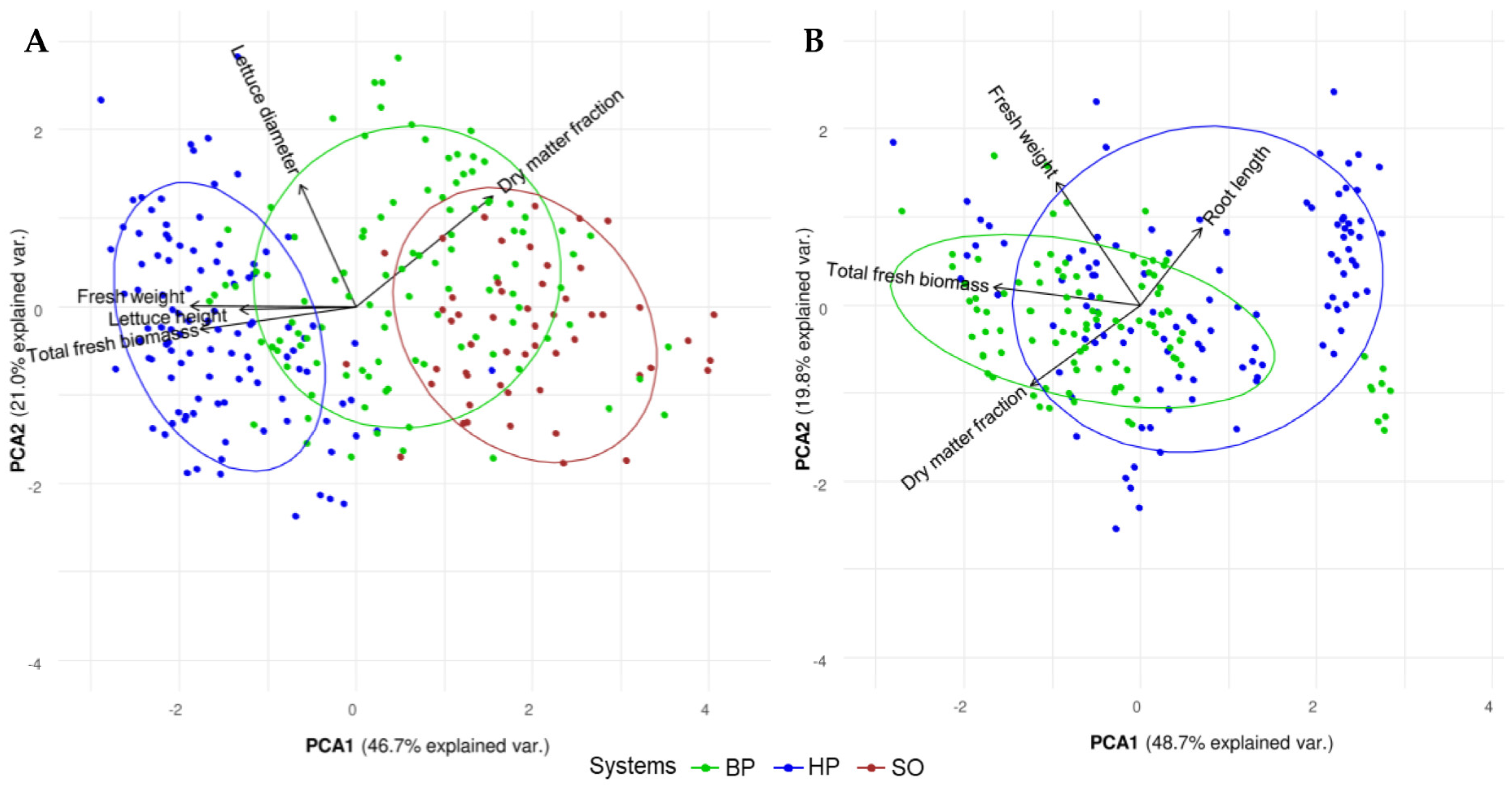
3.2. Plant Growth and Quality
3.2.1. Plant Growth
3.2.2. Fresh and Dry Weight
3.2.3. Shelf-Life and Consumer Test
3.2.4. Plant Nutrients
3.3. Food Safety
3.3.1. Leaf Nitrate
3.3.2. Heavy Metals
3.4. Biocontrol
3.4.1. UV-C Treatment
3.4.2. Application of Trichoderma atrobrunneum as Biocontrol Organism
4. Discussion
4.1. Nutrient Solution Characteristics
4.1.1. Physiochemical Parameters
4.1.2. Nutrient Concentrations in the Nutrient Solution
4.2. Plant Growth and Quality
4.3. Food Safety
4.3.1. Leaf Nitrate
4.3.2. Heavy Metals
4.3.3. Other Harmful Substances
4.4. Biocontrol
4.4.1. UV-C Treatment
4.4.2. Trichoderma atrobrunneum Strain T720 Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States. The 17 Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 14 June 2021).
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781482239263. [Google Scholar]
- Sánchez, H.J.A. Lactuca Sativa Production in An Anthroponics System. 2015. Available online: https://www.hemmaodlat.se/research/lactuca%20sativa%20production%20in%20an%20anthroponics%20system.pdf (accessed on 5 January 2021).
- Nicola, S.; Ferrante, A.; Cocetta, G.; Bulgari, R.; Nicoletto, C.; Sambo, P.; Ertani, A. Food supply and urban gardening in the time of covid-19. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2020, 77, 141. [Google Scholar] [CrossRef]
- Bulgari, R.; Petrini, A.; Cocetta, G.; Nicoletto, C.; Ertani, A.; Sambo, P.; Ferrante, A.; Nicola, S. The impact of Covid-19 on horticulture: Critical issues and opportunities derived from an unexpected occurrence. Horticulturae 2021, 7, 124. [Google Scholar] [CrossRef]
- Basak, B.B.; Maity, A.; Biswas, D.R. Cycling of natural sources of phosphorus and potassium for environmental sustainability. In Biogeochemical Cycles: Ecological Drivers and Environmental Impact; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 285–299. ISBN 978-1-119-413332. [Google Scholar]
- Kumar, R.R.; Cho, J.Y. Reuse of hydroponic waste solution. Environ. Sci. Pollut. Res. 2014, 21, 9569–9577. [Google Scholar] [CrossRef] [PubMed]
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Nerg, A.M.; Sjöblom, A.; Holopainen, J.K.; Heinonen-Tanski, H. Use of human urine fertilizer in cultivation of cabbage (Brassica oleracea)—Impacts on chemical, microbial, and flavor quality. J. Agric. Food Chem. 2007, 55, 8657–8663. [Google Scholar] [CrossRef]
- Songthanasak, K.; Klongtroujrok, C.; Swattdipat, Y.; Sumphunprateep, S.; Lawchod, T. Preliminary Study on Urine-Compost Extract as Bio-Liquid Fertilizer for Hydroponics. 4th Int. Dry. Toilet. Conf. 2012, 1–7. Available online: http://resources.cwis.com.s3.amazonaws.com/evidence/files/2-1618-44-preliminary-study-on-urine-compost-extract-as-bio-liquid-fertiliser-for-hydroponics-k-songthanasak-c-klongtroujrok-y-swattdipat-s-sumphunprateep-t-lawchod-.pdf (accessed on 5 June 2021).
- Dumitrescu, V.A. Comparative Analysis of Biogas Slurry and Urine as Sustainable Nutrient Sources for Hydroponic Vertical Farming. Master’s Thesis, Linköping University, Linköping, Sweden, 2013. [Google Scholar]
- Monsees, H.; Suhl, J.; Paul, M.; Kloas, W.; Dannehl, D.; Würtz, S. Lettuce (Lactuca sativa, variety Salanova) production in decoupled aquaponic systems: Same yield and similar quality as in conventional hydroponic systems but drastically reduced greenhouse gas emissions by saving inorganic fertilizer. PLoS ONE 2019, 14, e0218368. [Google Scholar] [CrossRef]
- Ferreira, F.P.; Caldart, E.T.; Freire, R.L.; Mitsuka-Breganó, R.; de Freitas, F.M.; Miura, A.C.; Mareze, M.; Martins, F.D.C.; Urbano, M.R.; Seifert, A.L.; et al. The effect of water source and soil supplementation on parasite contamination in organic vegetable gardens. Braz. J. Vet. Parasitol. 2018, 27, 327–337. [Google Scholar] [CrossRef]
- Bourn, D.; Prescott, J. A comparison of the nutritional value, sensory qualities, and food safety of organically and conventionally produced foods. Crit. Rev. Food Sci. Nutr. 2002, 42, 1–34. [Google Scholar] [CrossRef]
- Gorenjak, A.H.; Koležnik, U.R.; Cencič, A. Nitrate content in dandelion (Taraxacum officinale) and lettuce (Lactuca sativa) from organic and conventional origin: Intake assessment. Food Addit. Contam. Part. B Surveill. 2012, 5, 93–99. [Google Scholar] [CrossRef]
- Stoknes, K.; Beyer, D.M.; Norgaard, E. Anaerobically digested food waste in compost for Agaricus bisporus and Agaricus subrufescens and its effect on mushroom productivity. J. Sci. Food Agric. 2013, 93, 2188–2200. [Google Scholar] [CrossRef]
- Stoknes, K.; Scholwin, F.; Krzesiński, W.; Wojciechowska, E.; Jasińska, A. Efficiency of a novel “Food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Manag. 2016, 56, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Dasgan, H.Y.; Yucebilgic, A.; Altuntas, O. The organic nutrition on hydroponically grown greenhouse melon. Acta Hortic. 2017, 1164, 549–553. [Google Scholar] [CrossRef]
- Rogers, M.A. Organic vegetable crop production in controlled environments using soilless media. Horttechnology 2017, 27, 166–170. [Google Scholar] [CrossRef]
- Texier, W. Hydroponic Plant Cultivation Procedure Uses Nutrient Liquid Containing Biological Fertilizer Seeded with Bacteria or Enzymes. Patent FR2893226A1, 18 May 2007. [Google Scholar]
- Williams, K.A.; Nelson, J.S. Challenges of using organic fertilizers in hydroponic production systems. Acta Hortic. 2016, 1112, 365–370. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215. [Google Scholar] [CrossRef]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial ecosystem constructed in water for successful organic hydroponics. Soil. Sci. Plant. Nutr. 2011, 3, 1–14. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Junge, R.; Schmautz, Z.; Sambo, P.; Borin, M. Hydroponic systems and water management in aquaponics: A review. Ital. J. Agron. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Schmautz, Z.; Espinal, C.A.; Smits, T.H.M.; Frossard, E.; Junge, R. Nitrogen transformations across compartments of an aquaponic system. Aquac. Eng. 2021, 92, 102145. [Google Scholar] [CrossRef]
- Reinhold, G.; König, V.; Herold, L. Auswirkungen der Biogaserzeugung auf die Eigenschaften der Gärsubstrate. 116 VDLUFA-Kongr. 2004, p. 8. Available online: www.tll.de/ainfo/pdf/bio10904.pdf (accessed on 2 May 2020).
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient recovery from digestate: Systematic technology review and product classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef]
- Paradelo, R.; Villada, A.; Barral, M.T. Heavy metal uptake of lettuce and ryegrass from urban waste composts. Int. J. Environ. Res. Public. Health 2020, 17, 2887. [Google Scholar] [CrossRef]
- Michalska, M.; Asp, H. Influence of lead and cadmium on growth, heavy metal uptake, and nutrient concentration of three lettuce cultivars grown in hydroponic culture. Commun. Soil. Sci. Plant. Anal. 2001, 32, 571–583. [Google Scholar] [CrossRef]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil. Sci. Plant. Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
- National Organic Standards Board. Organic Hydroponic and Aquaponic Task. Force Report. 2016. Available online: https://www.ams.usda.gov/content/organic-hydroponic-and-aquaponic-task-force-report (accessed on 4 April 2021).
- Sanosil AG: Hydrokultur, Hydroponic, Pflanzenzüchtung. Available online: https://www.sanosil.com/de/anwendungsbereiche/landwirtschaft/agrikultur/homegrow-hydrokultur/ (accessed on 2 May 2021).
- Paulitz, T.C. Biological Control of Root Pathogens in Soilless and Hydroponic Systems. HortScience 1997, 32, 193–195. [Google Scholar] [CrossRef]
- Martin, F.N.; Loper, J.E. Soil-Borne Plant Diseases Caused by Pythium spp.: Ecology, Epidemiology, and Prospects for Biological Control; Taylor Francis: Abingdon, UK, 1999; Volume 18, ISBN 0735268999130. [Google Scholar]
- Schnitzler, W.H. Pest and disease management of soilless culture. Acta Hortic. 2004, 648, 191–203. [Google Scholar] [CrossRef]
- Zinnen, T.M. Assessment of plant diseases in hydroponic culture. Plant. Dis. 1988, 72, 96–100. [Google Scholar] [CrossRef]
- Jenkins, J.S.F.; Averre, C.W. Root diseases of vegetables in hydroponic culture systems in north carolina greenhouses. Plant. Dis. 1983, 67, 968–970. [Google Scholar] [CrossRef]
- Whipps, J.M.; Lumsden, R.D. Biological control of Pythium species. Biocontrol Sci. Technol. 1991, 1, 75–90. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Stewart, A.; Hill, R. Biotechnology and Biology of Trichoderma—Applications of Trichoderma in Plant Growth Promotion; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780444595768. [Google Scholar]
- Sirakov, I.; Lutz, M.; Graber, A.; Mathis, A.; Staykov, Y.; Smits, T.H.M.; Junge, R. Potential for Combined Biocontrol Activity against Fungal Fish and Plant Pathogens by Bacterial Isolates from a Model Aquaponic System. Water 2016, 8, 518. [Google Scholar] [CrossRef]
- Burr, T.J. Increased potato yields by treatment of seedpieces with specific strains of Pseudomonas fluorescens and P. putida. Phytopathology 1978, 68, 1377–1383. [Google Scholar] [CrossRef]
- Mazzola, M.; Cook, R.J.; Thomashow, L.S.; Weller, D.M.; Pierson, L.S. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl. Environ. Microbiol. 1992, 58, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Hagn, A.; Engel, M.; Kleikamp, B.; Munch, J.C.; Schloter, M.; Bruns, C. Microbial community shifts in Pythium ultimum-inoculated suppressive substrates. Biol. Fertil. Soils 2008, 44, 481–490. [Google Scholar] [CrossRef]
- Lutz, M.P.; Michel, V.; Martinez, C.; Camps, C. Lactic acid bacteria as biocontrol agents of soil-borne pathogens. IOBC-WPRS Bull. 2012, 78, 285–288. [Google Scholar]
- Chinta, Y.D.; Kano, K.; Widiastuti, A.; Fukahori, M.; Kawasaki, S.; Eguchi, Y.; Misu, H.; Odani, H.; Zhou, S.; Narisawa, K.; et al. Effect of corn steep liquor on lettuce root rot (Fusarium oxysporum f.sp. lactucae) in hydroponic cultures. J. Sci. Food Agric. 2014, 94, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- MycoSolutions, AG. Treating Roses with Trichoderma atrobrunneum increases the Size of Flower Heads Significantly. St. Gallen, Switzerland, 2021; unpublished. [Google Scholar]
- MycoSolutions, AG. Effect of Trichoderma atrobrunneum Strain T-720 on Growth of Lettuce (Lactuca sativa L.) under Greenhouse Conditions. St. Gallen, Switzerland, 2020; unpublished. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiol. 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil. Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Combalbert, S.; Capdeville, M.J.; Bellet, V.; Rajagopal, R.; Motte, J.C.; Balaguer, P.; Béline, F. Fate of antibiotics, steroid hormones and multiple endocrine activities during biological treatment of swine manure under anaerobic and aerobic/anoxic conditions. In Proceedings of the 13th World Congress on Anaerobic Digestion, Santiago de Compostela, Spain, 25–28 June 2018; pp. 1–4. Available online: https://agris.fao.org/agris-search/search.do?recordID=FR2021080384 (accessed on 5 April 2021).
- Visca, A.; Barra Caracciolo, A.; Grenni, P.; Patrolecco, L.; Rauseo, J.; Massini, G.; Mazzurco Miritana, V.; Spataro, F. Anaerobic digestion and removal of Sulfamethoxazole, Enrofloxacin, Ciprofloxacin and their antibiotic resistance fenes in a full-scale biogas plant. Antibiotics 2021, 10, 502. [Google Scholar] [CrossRef]
- Landesamt Umwelt Landwirtschaft und Geologie Sachsen. Hygienisierung von Wirtschaftsdünger und Gärresten. Schriftenr. Des. LfULG 2014, 37, 1–109. [Google Scholar]
- Schwermer, C.U.; Krzeminski, P.; Wennberg, A.C.; Vogelsang, C.; Uhl, W. Removal of antibiotic resistant E. coli in two Norwegian wastewater treatment plants and by nano- and ultra-filtration processes. Water Sci. Technol. 2018, 77, 1115–1126. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating aquaculture tank production systems: Aquaponics- integrating fish and plant culture. SRAC Publ. South. Reg. Aquac. Cent. 2006, 454, 16. [Google Scholar]
- Fernandez, D. HydroBuddy, v1.62; An Open Source Nutrient Calculator for Hydroponics and General Agriculture. 2017. Available online: https://scienceinhydroponics.com/2016/03/the-first-free-hydroponic-nutrient-calculator-program-o.html (accessed on 15 January 2020).
- Metrohm AG Switzerland. IC Application Note No. S-173: Nitrite and Nitrate in A Plant Extract. 2004. Available online: https://www.metrohm.com/de_ch/applications/application-notes/aa-s-001-100/an-s-173.html (accessed on 17 April 2021).
- Zhang, W.; Tu, J.C. Effect of ultraviolet disinfection of hydroponic solutions on Pythium root rot and non-target bacteria. Eur. J. Plant. Pathol. 2000, 106, 415–421. [Google Scholar] [CrossRef]
- Sametz-Baron, L.; Berrocal, T.G.M.; Amit, R.; Herrera-Estrella, A.; Horwitz, B.A. Photoreactivation of UV-inactivated spores of Trichoderma harzianum. Photochem. Photobiol. 1997, 65, 849–854. [Google Scholar] [CrossRef]
- Sutton, J.C.; Yu, H.; Grodzinski, B.; Johnstone, M. Relationships of ultraviolet radiation dose and inactivation of pathogen propagules in water and hydroponic nutrient solutions. Can. J. Plant. Pathol. 2000, 22, 300–309. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; pp. 235–276. [Google Scholar]
- Robinson, D. Broom: An R Package for Converting Statistical Analysis Objects Into Tidy Data Frames. arXiv 2014, arXiv:1412.3565. [Google Scholar] [CrossRef]
- Searle, S.R.; Speed, F.M.; Milliken, G.A. Population Marginal Means in the Linear Model: An Alternative to Least Squares Means. Am. Stat. 1980, 34, 216–221. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr: Ggplot2 Based Publication Ready Plots. 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/readme/README.html (accessed on 6 November 2020).
- Auguie, B. GridExtra: Miscellaneous Functions for “Grid” Graphics. 2017. Available online: https://rdrr.io/cran/gridExtra/ (accessed on 6 November 2020).
- Lenth, R.V. Least-Squares Means: The R package lsmeans v2.30-0. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Grolemund, G.; Wickham, H. Dates and Times Made Easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Spencer, G.; Piepho, H.-P.; Dorai-Raj, L.; Selzer, S. MultcompView: Visualizations of Paired Comparison. R package v0.1-8. 2019. Available online: https://cran.r-project.org/web/packages/multcompView/ (accessed on 6 November 2020).
- Schauberger, P.; Walker, A. Openxlsx: Read, Write and Edit xlsx Files. 2021. Available online: r-project.org (accessed on 6 November 2020).
- Neuwirth, E. RColorBrewer: ColorBrewer palettes. R package v1.1-2. 2014. Available online: https://cran.r-project.org/web/packages/RColorBrewer/index.html (accessed on 6 November 2021).
- Bryan, H.W.; Readxl, J. Read Excel Files. R package v1.3.1. 2019. Available online: https://cran.r-project.org/web/packages/readxl/index.html (accessed on 6 November 2020).
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package v0.7.0. 2021. Available online: https://cran.r-project.org/web/packages/rstatix/index.html (accessed on 6 November 2020).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open. Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- XLSTAT: Excel Statistik Software. Available online: https://www.xlstat.com/de/ (accessed on 21 March 2021).
- Lucas, J.S.; Southgate, P.C.; Aquaculture, T.C.S. Farming Aquatic Animals and Plants, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-1-119-23086-1. [Google Scholar]
- El Sheshtawy, A.; Salah, A.; Rahman Ibrahim, M.A.; Mocuta, D.N.; Turek Rahoveanu, A.; Hossu, A.M. The nitrification capacity of different types of biological filters—An overview. Risk Contemp. Econ. 2017, 1, 321–328. [Google Scholar] [CrossRef]
- Joshi, J.A.; Hogan, J.A.; Cowan, R.M.; Strom, P.F.; Finstein, M.S. Biological removal of gaseous ammonia in biofilters: Space travel and earth-based applications. J. Air Waste Manag. Assoc. 2000, 50, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Voogt, W.; Diaz-Ismael, A.; Nina Oud, R.L. Dealing with Na accumulation in soilless grown crops with recirculation of drainwater: A case study with sweet pepper (Capsicum annuum). In Proceedings of the ISHS hydro2020, Lemesos, Cyprus, 19–22 March 2021. [Google Scholar]
- Han, H.-J.; Kim, H.J.; Jung, D.-H.; Cho, W.-J.; Cho, Y.-Y.; Lee, G.-I. Real-time Nutrient Monitoring of Hydroponic Solutions Using an Ion-selective Electrode-based Embedded System. Prot. Hortic. Plant. Factory. 2020, 29, 141–152. [Google Scholar] [CrossRef]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.; Wuertz, S.; Junge, R. The effect of anaerobic and aerobic fish sludge supernatant on hydroponic lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef]
- Wongkiew, S.; Hu, Z.; Lee, J.W.; Chandran, K.; Nhan, H.T. Nitrogen recovery via aquaponics—Bioponics: Engineering considerations and perspectives. ACS EST Eng. 2021, 1, 326–339. [Google Scholar] [CrossRef]
- Sörensen, M.; Zurell, S.; Frimmel, F.H. Degradation pathway of the photochemical oxidation of Ethylenediaminetetraacetate (EDTA) in the UV/H2O2-process. Acta Hydrochim. Hydrobiol. 1998, 26, 109–115. [Google Scholar] [CrossRef]
- Sörensen, V.M.; Weckenmann, J.; Hofmann, R.; Pagel, J.; Weber, A. Konzept, Technologie und Praxisbericht der Referenzkunden Multek und Shipley Zerstörung von komplexem Cu-EDTA Konzept, Technologie und Praxisbericht. Galvanotechnik 2002, 93, 1–8. [Google Scholar]
- Pérez-gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Pushnik, J.C.; Miller, G.W.; Manwaring, J.H. The role of iron in higher plant chlorophyll biosynthesis, maintenance and chloroplast biogenesis. J. Plant. Nutr. 1984, 7, 733–758. [Google Scholar] [CrossRef]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef]
- Atkin, K.; Nichols, M.A. Organic hydroponics. In Acta Horticulturae, Proceedings of the International Society for Horticultural Science (ISHS), Leuven, Belgium, 10 February 2003; Acta Horticulturae: Liège, Belgium, 2004; pp. 121–127. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Tahboub, A.B.; Abu-Zahra, T.R.; Al-Abbadi, A.A. Chemical composition of lettuce (Lactuca sativa) grown in soils amended with different sources of animal manure to simulate organic farming conditions. J. Food Agric. Environ. 2010, 8, 736–740. [Google Scholar]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Yield and quality of leafy lettuce in response to nutrient solution composition and growing season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant. Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Plant Strategies and the Dynamics and Structure of Plant Communities; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Fernandez, D. Inner Leaf Tipzburn in Hydroponic Lettuce. Available online: https://scienceinhydroponics.com/2020/11/inner-leaf-tipburn-in-hydroponic-lettuce.html (accessed on 16 June 2021).
- Sago, Y. Effects of light intensity and growth rate on tipburn development and leaf calcium concentration in butterhead lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Hoque, M.; Ajwa, H.; Othman, M.; Smith, R.; Cahn, M. Yield and postharvest quality of lettuce in response to nitrogen, phosphorus, and potassium fertilizers. HortScience 2010, 45, 1539–1544. [Google Scholar] [CrossRef]
- Swiss Confederation. Gesetzgebung—Anhang 2 der Verordnung des EDI über die Höchstgehalte für Pestizidrückstände in Oder auf Erzeugnissen Pflanzlicher und Tierischer Herkunft (VPRH) (XLS, 4 MB, 29 June 2020). Available online: https://www.blv.admin.ch/blv/de/home/lebensmittel-und-ernaehrung/rechts-und-vollzugsgrundlagen/gesetzgebung-lme.html (accessed on 1 May 2021).
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of Silicon With Essential and Beneficial Elements in Plants. Front. Plant. Sci. 2021, 12, 697592. [Google Scholar] [CrossRef]
- Voogt, W.; Sonneveld, C. Chapter 6 Silicon in Horticultural Crops Grown in Soilless Culture; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 115–131. [Google Scholar] [CrossRef]
- Weightman, R.M.; Dyer, C.; Buxton, J.; Farrington, D.S. Effects of light level, time of harvest and position within field on the variability of tissue nitrate concentration in commercial crops of lettuce (Lactuca sativa) and endive (Cichorium endiva). Food Addit. Contam. 2006, 23, 462–469. [Google Scholar] [CrossRef]
- Alexander, J.; Benford, D.; Cockburn, A.; Cravedi, J.; Dogliotti, E.; Domenico, A.D.; Fernández-Cruz, M.L.; Fink-Gremmels, J.; Fürst, P.; Galli, C.; et al. Nitrate in vegetables—Scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008, 6, 1–79. [Google Scholar] [CrossRef]
- Europäische Kommission. Verordnung (EG) Nr. 1881/2006 der Kommission vom 19. Dezember 2006 zur Festsetzung der Höchstgehalte für bestimmte Kontaminanten in Lebensmitteln. Amtsbl. Der Eur. Union. 2006, 1881, 5–24. [Google Scholar]
- Kanton Thurgau. Nitrat in Wintersalaten Untersuchungsziele und Methodik Proben und Resultate Beurteilung und Zusammenfassung. 2021. Available online: https://kantlab.tg.ch/public/upload/assets/64524/201804A_Nitrat_in_Wintersalaten.pdf (accessed on 8 December 2020).
- Kappel, N.; Boros, I.F.; Ravelombola, F.S.; Sipos, L. EC sensitivity of hydroponically-grown lettuce (Lactuca sativa L.) types in terms of nitrate accumulation. Agriculture 2021, 11, 315. [Google Scholar] [CrossRef]
- Manzocco, L.; Foschia, M.; Tomasi, N.; Maifreni, M.; Dalla Costa, L.; Marino, M.; Cortella, G.; Cesco, S. Influence of hydroponic and soil cultivation on quality and shelf life of ready-to-eat lamb’s lettuce (Valerianella locusta L. Laterr). J. Sci. Food Agric. 2011, 91, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Prinsi, B.; Espen, L.; Ferrante, A. Influence of different ammonium and nitrate ratio on yield and quality of rocket. In Proceedings of the ISHS Hydro 2020, Lemesos, Cyprus, 19–22 March 2021. [Google Scholar]
- Blom-Zandstra, G.; Lampe, J.E.M. The effect of chloride and sulphate salts on the nitrate content in lettuce plants (Lactuca sativa L.). J. Plant. Nutr. 1983, 6, 611–628. [Google Scholar] [CrossRef]
- Giuffrida, F.; Scuderi, D.; Noto, G. Effects of nutrient solution EC on yield, quality and shelf-life of lettuce grown in floating system. Acta Hortic. 2009, 807, 221–226. [Google Scholar]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef]
- De Gelder, L.; Audenaert, K.; Willems, B.; Schelfhout, K.; De Saeger, S.; De Boevre, M. Processing of mycotoxin contaminated waste streams through anaerobic digestion. Waste Manag. 2018, 71, 122–128. [Google Scholar] [CrossRef]
- Congilosi, J.L.; Aga, D.S. Review on the fate of antimicrobials, antimicrobial resistance genes, and other micropollutants in manure during enhanced anaerobic digestion and composting. J. Hazard. Mater. 2021, 405. [Google Scholar] [CrossRef]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: A review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Al-Huqail, A.; El-Dakak, R.M.; Sanad, M.N.; Badr, R.H.; Ibrahim, M.M.; Soliman, D.; Khan, F. Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum Basilicum L.) Leafy Vegetable. Scientifica 2020, 2020. [Google Scholar] [CrossRef]


| Mixture of Tap Water and Deionized Water (1:1) | Biogas Digestate Concentrate | HP Nutrient Solution | |
|---|---|---|---|
| TOC [mg L−1] | 1.5 | 1722 | - |
| TN [mg L−1] | 1.1 | 5434 | - |
| NO3−-N [mg L−1] | 0.6 | <LOD | 66.9 |
| NH4+-N [mg L−1] | <LOD | 5730 | 5.0 |
| PO43−-P [mg L−1] | <LOD | 113 | 17.0 |
| K+ [mg L−1] | <LOD | 11,589 | 96.5 |
| Na+ [mg L−1] | 4.4 | 2897 | - |
| Ca2+ [mg L−1] | 9.3 | 267 | 152.3 |
| Mg2+ [mg L−1] | 6.0 | <LOD | 19.5 |
| Cl− [mg L−1] | 3.9 | 7969 | 8.5 |
| S [mg L−1] | - | - | 39.0 |
| Fe [mg L−1] | - | - | 4.0 |
| Mn [mg L−1] | - | - | 0.45 |
| Zn [mg L−1] | - | - | 0.3 |
| B [mg L−1] | - | - | 0.05 |
| Cu [mg L−1] | - | - | 0.03 |
| Mo [mg L−1] | - | - | 0.02 |
| First Addition | Second Addition | Third Addition | ||||
|---|---|---|---|---|---|---|
| Trial A | 800 mL | Day 1 | 1150 mL | Day 14 | 1150 mL | Day 16 |
| Trial B | 800 mL | Day 1 | 1150 mL | Day 10 | 1150 mL | Day 15 |
| Trial C | 1150 mL | Day 1 | 800 mL | Day 12 | 1150 mL | Day 15 |
| Parameter | System | Analyzed Sample | Place of Measurement | Measurement Interval | Sample Preparation | Lab Equipment | Company |
|---|---|---|---|---|---|---|---|
| pH [-], T [°C] | BP, HP | Nutrient solution | Direct, in the sump | Daily | - | Probe PHC10103 and HQ40d portable multimeter | Hach Lange, Loveland, CO, USA |
| Dissolved oxygen [mg L−1] | All | Nutrient solution | Direct, in the sump | Three times per week | - | Probe LDO10101 and HQ40d portable multimeter | Hach Lange, Loveland, CO, USA |
| Electrical conductivity [μS cm−1] | All | Nutrient solution | Direct, in the sump | Three times per week | - | Probe CDC40103 and HQ40d portable multimeter | Hach Lange, Loveland, CO, USA |
| NH4+-N, NO2−-N, NO3−-N, TN, PO43−-P [mg L−1] | BP, HP | Nutrient solution | Direct, in the laboratory | Trial A: At system start and after 7 and 12 days | Filtered with 0.45 μm (not for TN) | LCK tests no. 304, 341, 339, 138, 349; DR 3800 VIS Spectrophotometer | Whatman Maidstone, UK; Hach Lange, Loveland, CO, USA |
| TN [mg L−1] | BP, HP | Nutrient solution | Direct, in the laboratory | Trial B and C: At system start | - | LCK tests no. 138; DR 3800 VIS Spectrophotometer | Hach Lange, Loveland, CO, USA |
| TOC and TN [mg L−1] | BP, HP | Nutrient solution | Direct, in the laboratory | Three times per week | 1:2 diluted | TOC-L Analyser and ASI-L | Shimadzu Europa GmbH, Duisburg, Germany |
| NH4+, Na+, K+, Ca2+, Mg2+ [mg L−1] | BP, HP | Nutrient solution | Stored at −20 °C in 15 mL falcon tube, laboratory | Three times per week | Filtered with 0.45 μm, 1 μL 2 M HNO3 per 1 mL sample | 930 Compact IC flex | Whatman Maidstone, UK; Metrohm Schweiz AG, Zofingen, Switzerland |
| Cl−, NO2−, NO3−, PO43−, SO42− [mg L−1] | BP, HP | Nutrient solution | Stored in 15 mL falcon tube, laboratory | Three times per week | Filtered with 0.45 μm | 930 Compact IC flex | Metrohm Schweiz AG, Zofingen, Switzerland |
| Fe, Mn [mg L−1] | BP, HP | Nutrient solution | Stored in 50 mL falcon tube, laboratory | Three times per week | Filtered with 0.45 μm, 5% HNO3 (end concentration) | ICP-OES, Varian Vista AX CCD Simultaneous | Agilent Technologies, Santa Clara, CA, USA |
| Chlorophyll, Flavonoid, Anthocyanin [µg cm−2] | All | Lettuce leaves | Directly on living plant, epidermis of lettuce | At end of each trial | - | Dualex® Scientific | ForceA, Université Paris Sud, Orsay Cedex, France |
| NO3− [mg kg−1] | All | Lettuce leaves | Direct, in the laboratory | At end of each trial | Lettuce leave incubated in hot water for 15 min, extract measured | 930 Compact IC flex | Metrohm Schweiz AG, Zofingen, Switzerland |
| K+, Ca2+, Mg2+, Na+, NH4+, NO3−, TN, Cl−, S, P, Si, Fe, Mn, Zn, B, Cu, Mo, Al [mg kg−1] | All | Lettuce leaves | In external laboratory | At start, middle and end of each trial | Plant sap analysis | Confidential | NovaCropControl, Oisterwijk, The Netherlands |
| Transmission | BP2, BP4, HP1, HP3 | Nutrient solution | Stored at −20 °C in 50 mL falcon tube, laboratory | Trial A: middle and end of trial; Trial B and C: start, middle, and end of trials | - | UV-1600 PC Spectrophotometer | VWR International, Radnor, PA, USA |
| Presence/absence of T. atrobrunneum T720 | All | Nutrient solution and soil | In external laboratory | At start, middle and end of each trial | - | Agar plates, Visual inspection | MycoSolutions AG, St. Gallen, Switzerland |
| Trial A | Trial B | Trial C | ||
|---|---|---|---|---|
| n | 15 June–10 July 2020 | 13 July–7 August 2020 | 10 August–4 September 2020 | |
| Air temperature [°C] | 208 | 24.2 (6.0–41.4) a | 25.2 (7.1–45.1) b | 24.0 (14.2–44.4) c |
| Air humidity [%] | 478 | 59.7 (24.9–86.2) a | 59.4 (24.1–86.0) a | 61.1 (25.2–86.0) b |
| Daily radiation | 27 | 1088.5 (348.7–1520.5) a | 977.2 (168.8–1430.3) ab | 856.1 (82.8–1237.2) b |
| BP | HP | |
|---|---|---|
| Temp [°C] | 23.8 ± 3.5 b | 21.1 ± 3.3 a |
| pH [-] | 6.9 ± 1.1 | 7.4 ± 0.4 |
| EC [μS cm−1] | 846.4 ± 282.5 | 832.6 ± 212.9 |
| Oxygen [%] | 96.8 ± 6.6 a | 100.5 ± 2.2 b |
| Height/Length | Diameter | Fresh Weight | Yield per Trail | Dry Matter Fraction | Shelf-Life | ||
|---|---|---|---|---|---|---|---|
| [cm] | [cm] | [g] | [kg m−2] | [%] | [% Remaining] | ||
| n = BP/HP/SO | 108/108/54 | 108/108/54 | 108/107/54 | 3/3/3 | 108/107/54 | 24/24/12 | |
| Shoot | BP | 15.0 ± 1.9 b | 22.1 ± 2.2 b | 186.5 ± 39.3 b | 3.09 ± 0.46 b | 3.7 ± 0.7 b | 83.1 ± 11.1 |
| HP | 15.4 ± 1.5 b | 22.1 ± 1.9 b | 257.7 ± 40.9 c | 4.26 ± 0.27 c | 2.9 ± 0.5 a | 79.1 ± 12.8 | |
| SO | 12.4 ± 2.3 a | 21.3 ± 2.4 a | 147.6 ± 26.5 a | 2.83 ± 0.82 a | 3.9 ± 0.5 b | 81.4 ± 9.6 | |
| n = BP/HP | 108/108 | - | 108/108 | 12/12 | 12/12 | - | |
| Root | BP | 30.0 ± 9.0 a | - | 18.8 ± 7.2 b | 0.35 ± 0.09 | 3.5 ± 0.4 | - |
| HP | 47.5 ± 13.3 b | - | 15.9 ± 10.5 a | 0.30 ± 0.08 | 3.3 ± 0.6 | - | |
| SO | - | - | - | - | - | - | |
| pH | EC | Sugar | TN | Si | ||
|---|---|---|---|---|---|---|
| [-] | [µS cm−2] | [%] | [mg kg−1] | [mg kg−1] | ||
| n = BP/HP/SO | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | |
| Young leaves | BP | 6.02 ± 0.07 b | 8.37 ± 1.01 b | 1.16 ± 0.25 | 1328.59 ± 203.19 | 4.04 ± 1.19 b |
| HP | 5.93 ± 0.09 a | 6.46 ± 1.14 a | 1.45 ± 0.31 | 1243.91 ± 111.01 | 0.79 ± 0.33 a | |
| SO | 5.91 ± 0.07 a | 8.63 ± 1.73 b | 1.38 ± 0.74 | 1169.04 ± 277.61 | 4.79 ± 1.55 b | |
| n = BP/HP | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | |
| Old leaves | BP | 5.82 ± 0.16 | 12.75 ± 1.44 | 0.39 ± 0.12 | 852.35 ± 191.22 a | 7.85 ± 2.00 b |
| HP | 5.84 ± 0.13 | 12.12 ± 1.70 | 0.41 ± 0.20 | 1313.95 ± 132.24 b | 2.28 ± 1.25 a | |
| SO | 5.81 ± 0.11 | 11.83 ± 0.58 | 0.55 ± 0.22 | 1022.58 ± 317.54 a | 8.88 ± 2.74 b | |
| NO3− | NH4+ | Cl | ||
|---|---|---|---|---|
| [mg kg−1] | [mg kg−1] | [mg kg−1] | ||
| n = BP/HP/SO | 12/12/6 | 12/12/6 | 12/12/6 | |
| Young leaves | BP | 983.61 ± 267.07 a | 79.80 ± 32.51 | 1474.33 ± 229.59 c |
| HP | 1997.79 ± 629.54 b | 61.78 ± 15.32 | 307.57 ± 69.46 a | |
| SO | 1848.42 ± 1189.95 ab | 49.72 ± 13.97 | 619.76 ± 171.83 b | |
| n = BP/HP | 12/12/6 | 12/12/6 | 12/12/6 | |
| Old leaves | BP | 1442.923 ± 526.13 a | 33.51 ± 5.28 b | 2441.71 ± 414.57 c |
| HP | 4173.663 ± 470.762 b | 25.44 ± 3.35 a | 609.08 ± 232.91 a | |
| SO | 3010.397 ± 1197.552 ab | 20.70 ± 5.45 a | 1087.75 ± 323.12 b | |
| Cd | Mn | Pb | Cu | Al | ||
|---|---|---|---|---|---|---|
| [mg kg−1] | [mg kg−1] | [mg kg−1] | [mg kg−1] | [mg kg−1] | ||
| n = BP/HP/SO | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | |
| Young leaves | BP | <LOD | <LOD | <LOD | 0.65 ± 0.15 b | 0.14 ± 0.07 |
| HP | <LOD | <LOD | <LOD | 0.45 ± 0.28 b | 0.08 ± 0.03 | |
| SO | <LOD | <LOD | <LOD | 0.19 ± 0.07 a | 0.14 ± 0.07 | |
| n = BP/HP | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | 12/12/6 | |
| Old leaves | BP | <LOD | <LOD | <LOD | 0.42 ± 0.21 c | 0.33 ± 0.17 b |
| HP | <LOD | <LOD | <LOD | 0.24 ± 0.11 b | 0.13 ± 0.04 a | |
| SO | <LOD | <LOD | <LOD | 0.12 ± 0.03 a | 0.14 ± 0.04 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartmann, F.; Hügly, J.; Krähenbühl, N.; Brinkmann, N.; Schmautz, Z.; Smits, T.H.M.; Junge, R. Bioponics—An Organic Closed-Loop Soilless Cultivation System: Yields and Characteristics Compared to Hydroponics and Soil Cultivation. Agronomy 2023, 13, 1436. https://doi.org/10.3390/agronomy13061436
Gartmann F, Hügly J, Krähenbühl N, Brinkmann N, Schmautz Z, Smits THM, Junge R. Bioponics—An Organic Closed-Loop Soilless Cultivation System: Yields and Characteristics Compared to Hydroponics and Soil Cultivation. Agronomy. 2023; 13(6):1436. https://doi.org/10.3390/agronomy13061436
Chicago/Turabian StyleGartmann, Florentina, Julian Hügly, Nikita Krähenbühl, Nadine Brinkmann, Zala Schmautz, Theo H. M. Smits, and Ranka Junge. 2023. "Bioponics—An Organic Closed-Loop Soilless Cultivation System: Yields and Characteristics Compared to Hydroponics and Soil Cultivation" Agronomy 13, no. 6: 1436. https://doi.org/10.3390/agronomy13061436
APA StyleGartmann, F., Hügly, J., Krähenbühl, N., Brinkmann, N., Schmautz, Z., Smits, T. H. M., & Junge, R. (2023). Bioponics—An Organic Closed-Loop Soilless Cultivation System: Yields and Characteristics Compared to Hydroponics and Soil Cultivation. Agronomy, 13(6), 1436. https://doi.org/10.3390/agronomy13061436









