The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
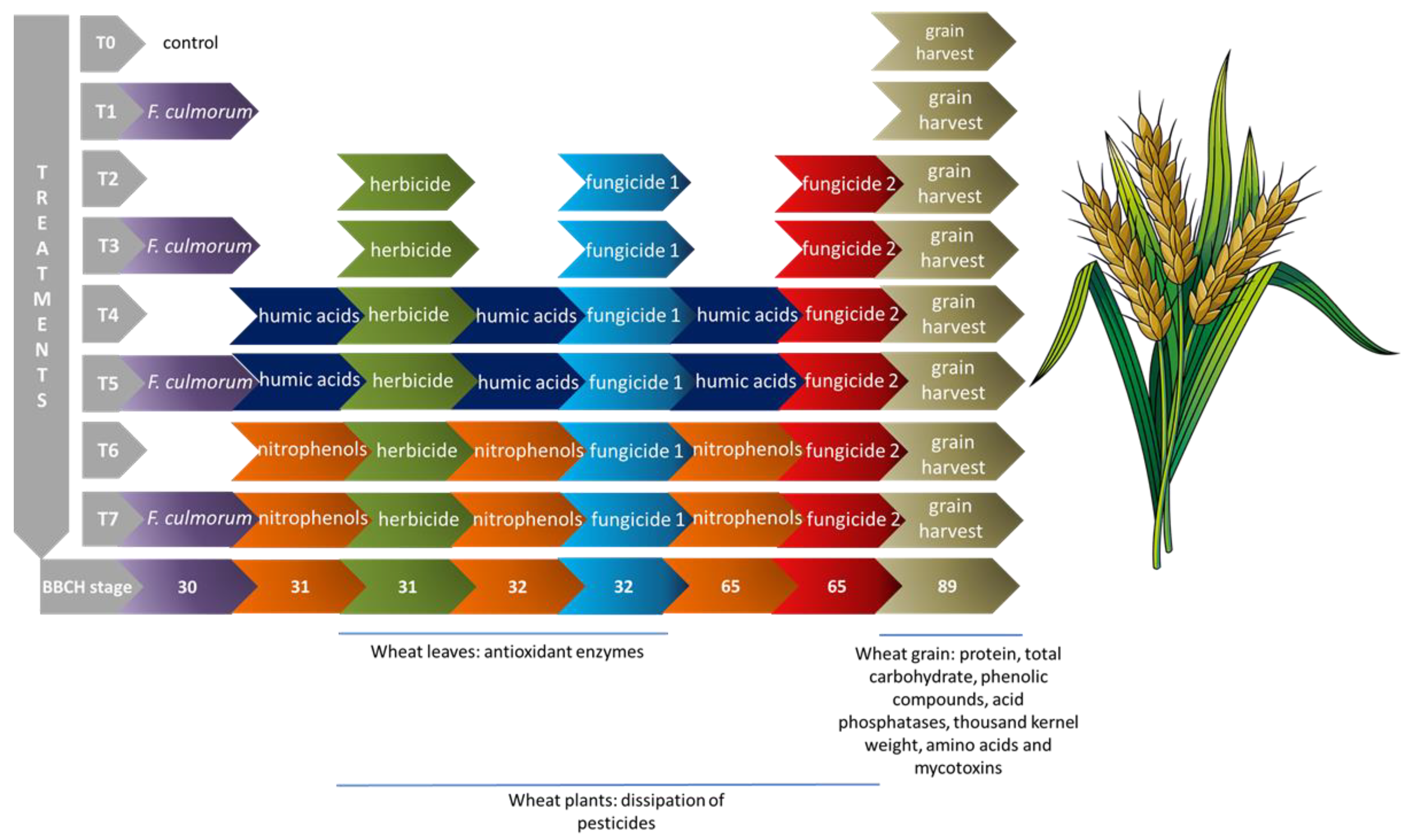
2.2. Design of Experiment under Controlled Conditions
2.3. Determination of the Activity of Antioxidant Enzymes in Wheat Leaves
2.4. Pesticide Extraction in Wheat Plants Using the QuEChERS Technique and a Validation of the LC-MS/MS Method
2.5. Dissipation Kinetics under Controlled Conditions in Wheat Plants
2.6. Determination of Wheat Metabolites in Grain
2.7. Determination of Mycotoxins in Wheat Grain
2.8. Determination of Thousand Kernel Weight (TKW)
2.9. Determination of the Antifungal Activities of Biostimulators, Fusarium Head Blight Severity, and Morphological Parameters of Wheat
2.10. Statistical Analysis
3. Results
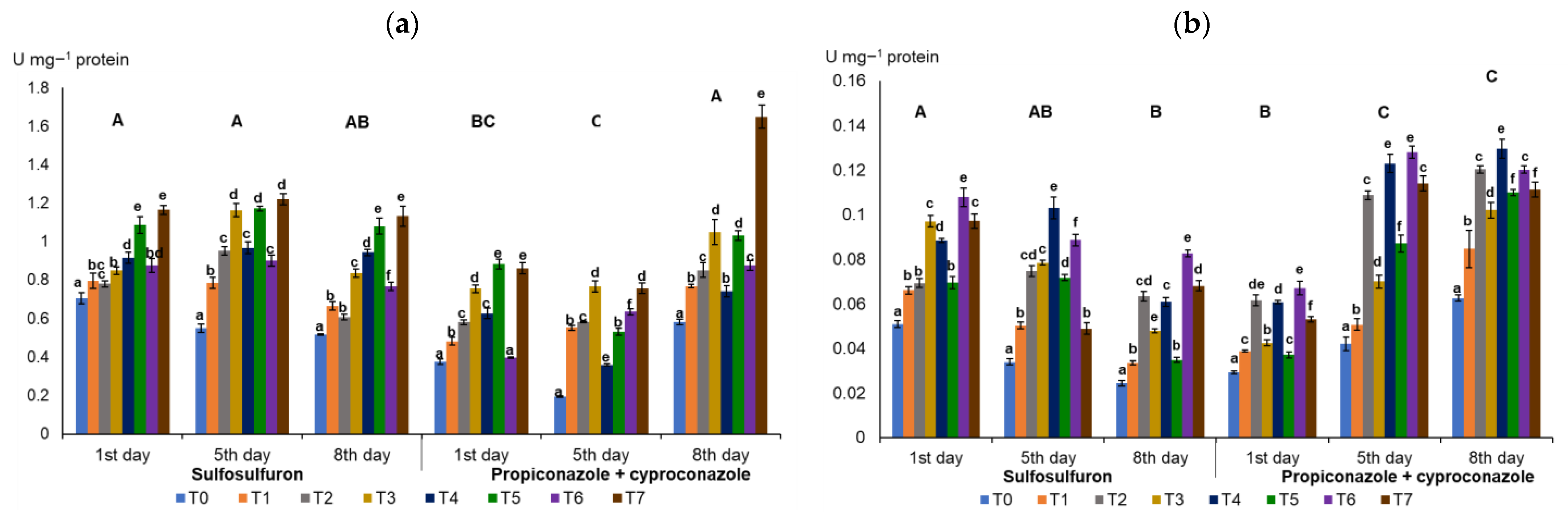
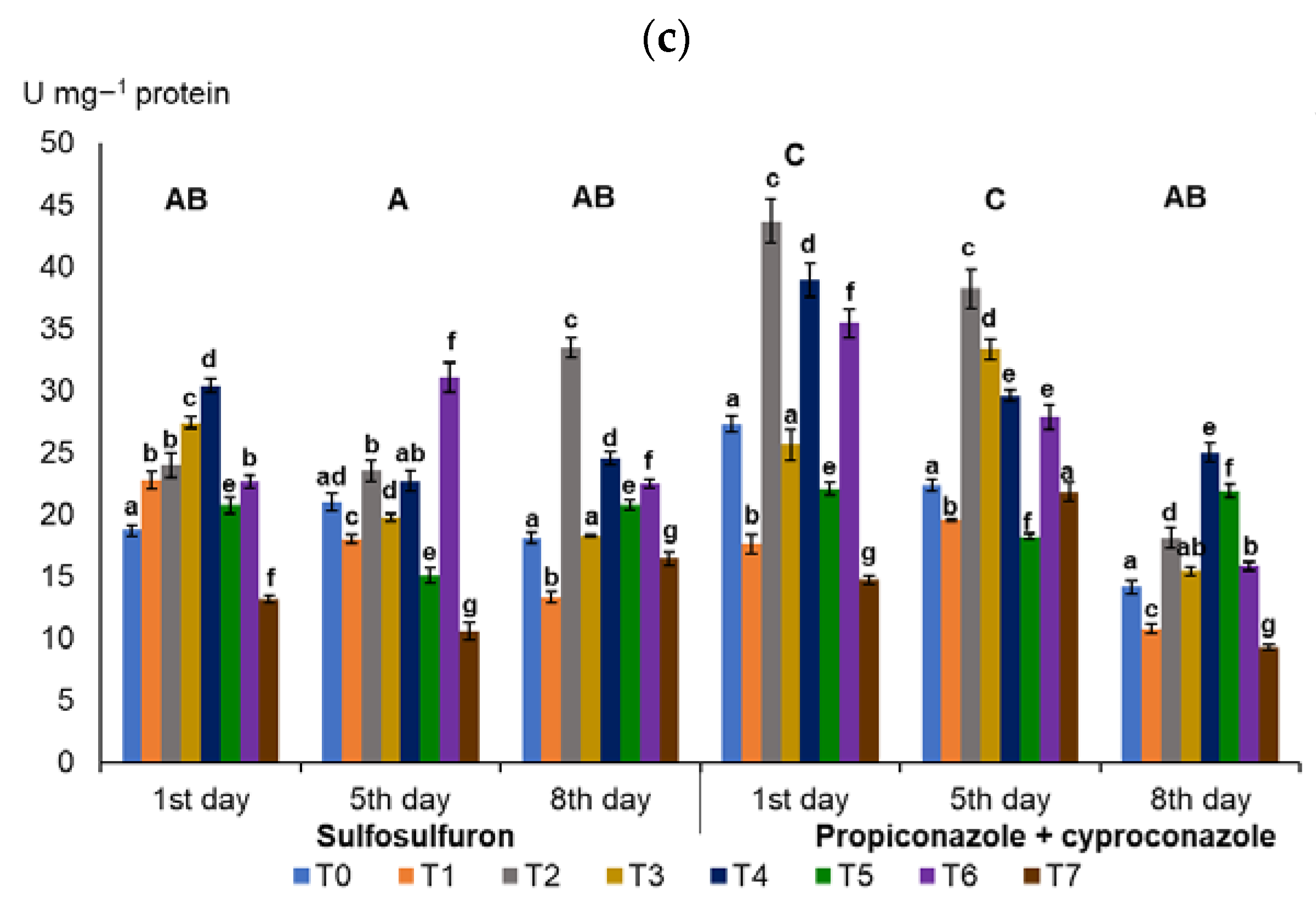
3.1. Activities of Antioxidant Enzymes in Wheat Leaves
3.2. Dissipation of Pesticides in Wheat Plants under the Influence of Biostimulators and Validation of the LC-MS/MS Technique
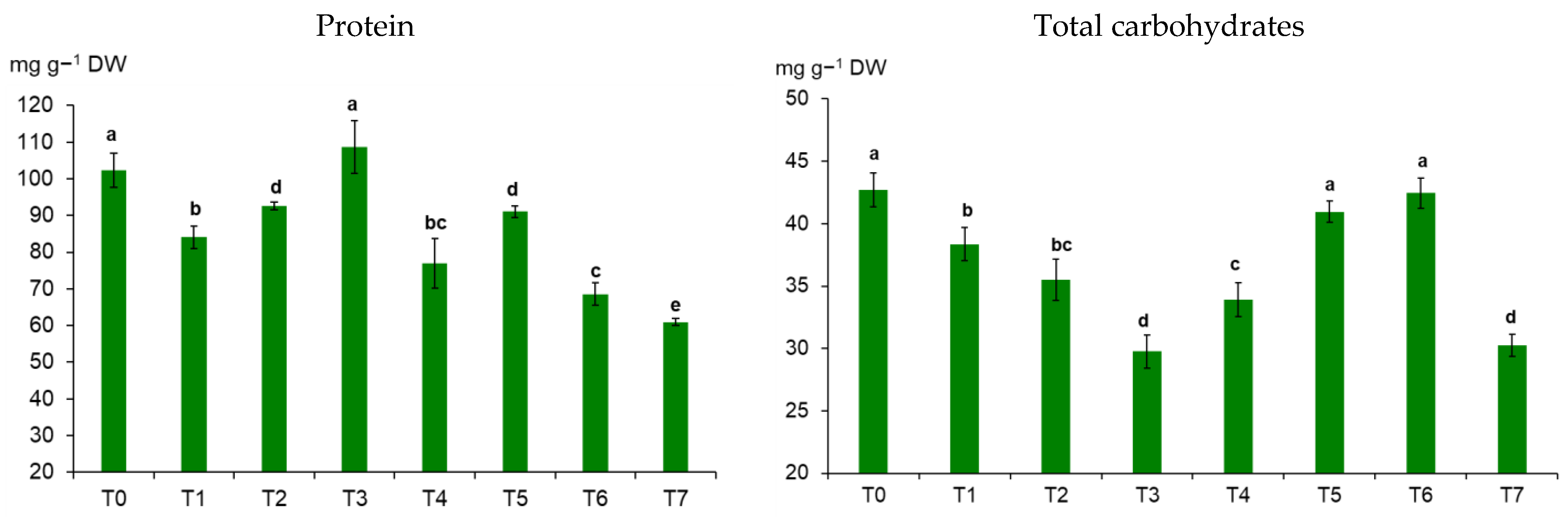
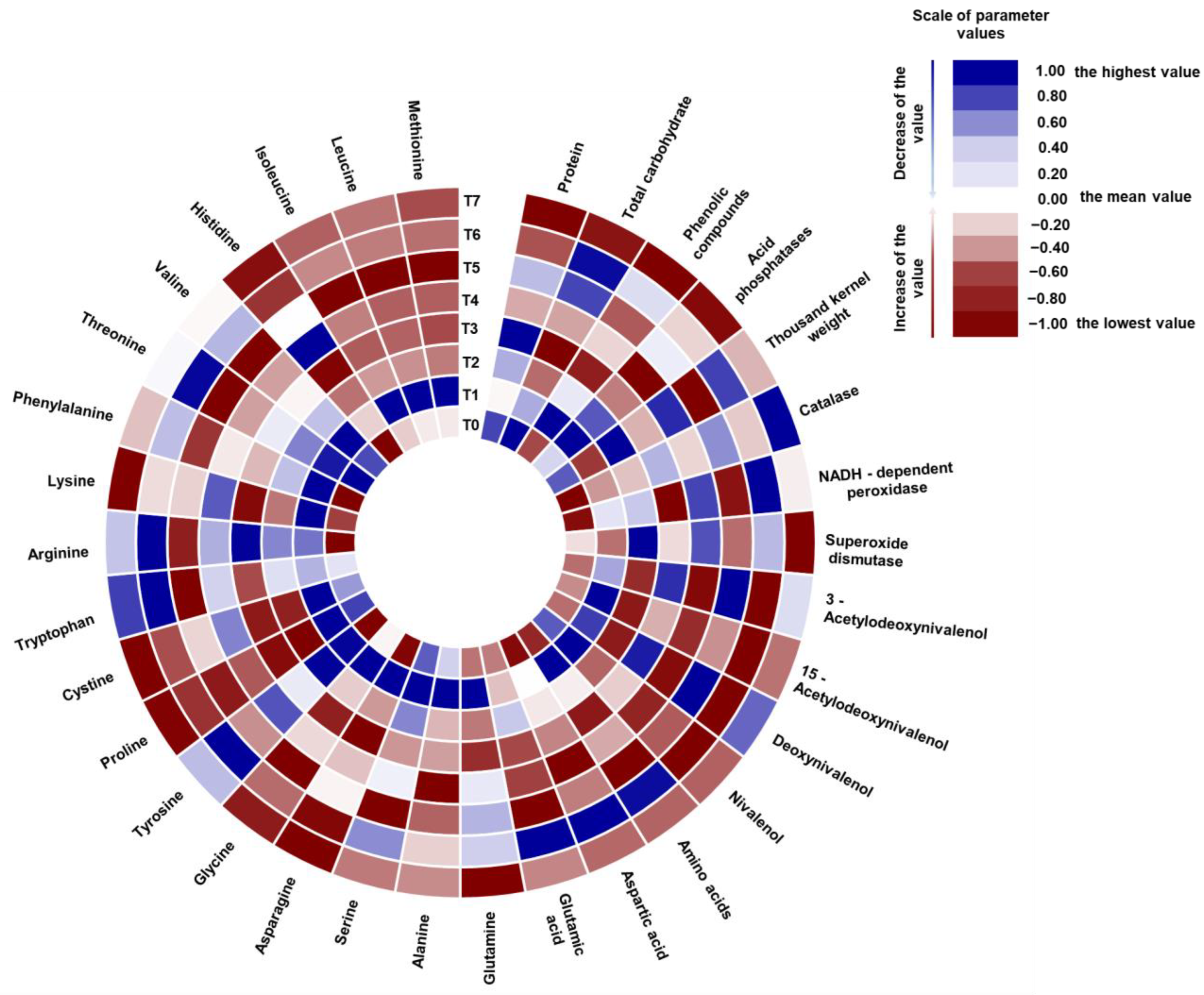
3.3. Wheat Metabolites, TKW, and Mycotoxins in the Grain under the Influence of Biostimulators
3.4. Antifungal Activity of Biostimulators, Fusarium Head Blight Severity, and Morphological Parameters of Wheat
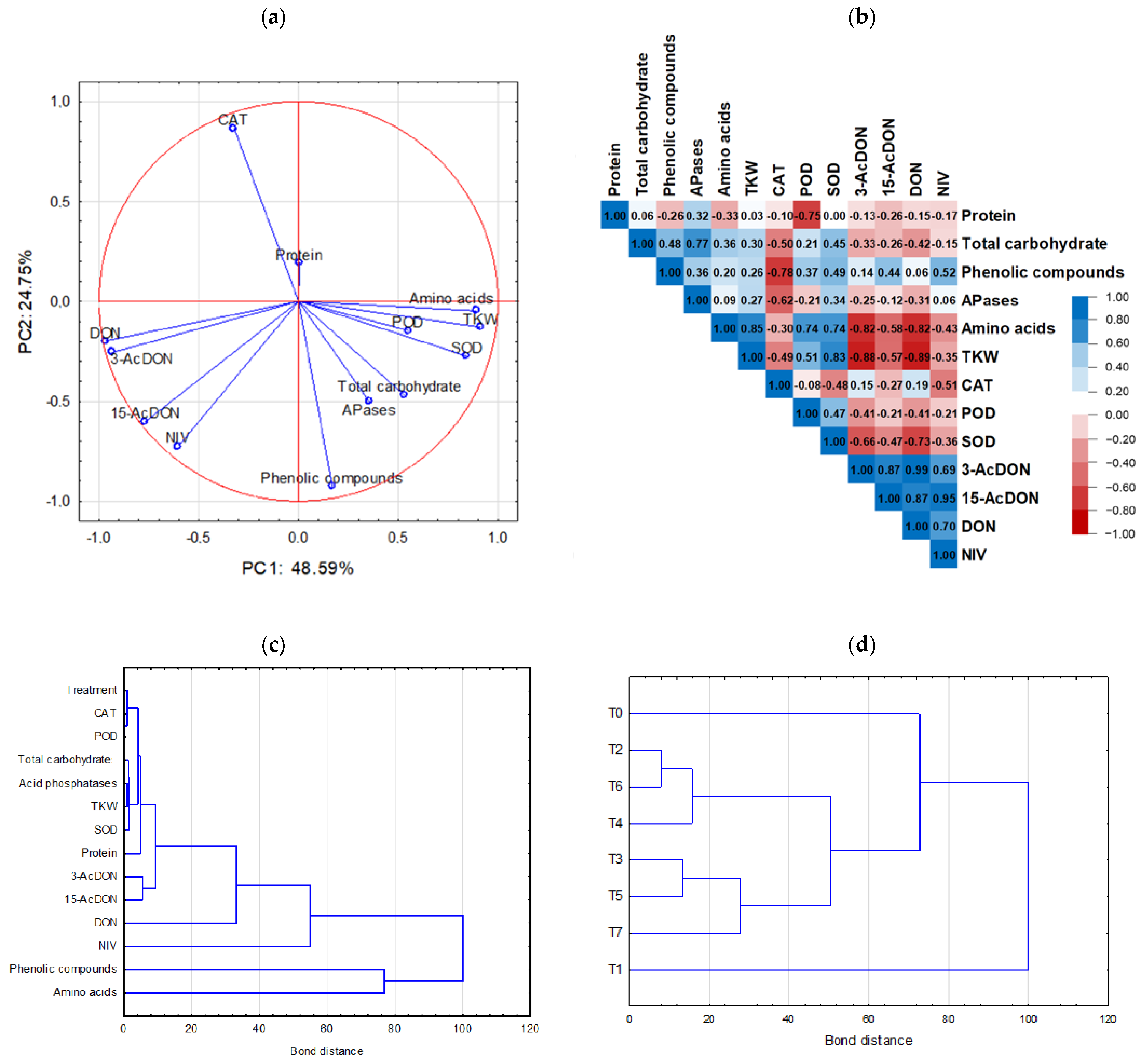
3.5. Chemometric Analysis
4. Discussion
4.1. Influence of Humic Acids and Nitrophenols on the Activities of Antioxidant Enzymes in Wheat Leaves
4.2. Dissipation of Pesticides in Wheat Plants under the Influence of Humic Acids and Nitrophenols
4.3. Influence of Humic Acids and Nitrophenols on Wheat Metabolites and Mycotoxins in Grain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Łozowicka, B.; Iwaniuk, P.; Konecki, R.; Kaczyński, P.; Kuldybayev, N.; Dutbayev, Y. Impact of diversified chemical and biostimulator protection on yield, health status, mycotoxin level, and economic profitability in spring wheat (Triticum aestivum L.) cultivation. Agronomy 2022, 12, 258. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium head blight from a microbiome perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Spanic, V.; Maricevic, M.; Ikic, I.; Sulyok, M.; Sarcevic, H. Three-year survey of Fusarium multi-metabolites/mycotoxins contamination in wheat samples in potentially epidemic FHB conditions. Agronomy 2023, 13, 805. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; Ur Rehman, S.; Zhaorong, D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L. Metabolomic and transcriptomic investigation of metabolic perturbations in Oryza sativa L. triggered by three pesticides. Environ. Sci. Technol. 2020, 54, 6115–6124. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugala, M.; Sikorska, A.; Mystkowska, I.; Baranowska, A.; Nieweglowski, M.; Dolega, H. The effect of herbicides and biostimulants on polyphenol content of potato (Solanum tuberosum L.) tubers and leaves. J. Saudi Soc. Agric. Sci. 2019, 18, 102–106. [Google Scholar] [CrossRef]
- Amiri, O.; Nemmiche, S.; Hennia, A.; Bentamra, Z.; Benhachem, I.; Benkhelifa, M. Effect of the pesticide treatment dose on the biochemical response of the potato plants (Solanum tuberosum L.). Plant Arch. 2022, 22, 261–267. [Google Scholar] [CrossRef]
- Clement, J.; Delisle-Houde, M.; Nguyen, T.T.A.; Dorais, M.; Tweddell, R.J. Effect of biostimulants on leafy vegetables (baby leaf lettuce and batavia lettuce) exposed to abiotic or biotic stress under two different growing systems. Agronomy 2023, 13, 879. [Google Scholar] [CrossRef]
- Goni, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.L.; Garcia-Machado, F.J.; Borges, A.A.; Morales-Sierra, S.; Boto, A.; Jimenez-Arias, D. Pure organic active compounds against abiotic stress: A biostimulant overview. Front. Plant. Sci. 2020, 11, 575829. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Latef, A.A.H.A. Fluoride mitigates aluminum-toxicity in barley: Morpho-physiological responses and biochemical mechanisms. BMC Plant Biol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Campos, C.N.S.; da Silva Junior, G.B.; de Prado, R.M.; de David, C.H.O.; de Souza Junior, J.P.; Teodoro, P.E. Silicon mitigates ammonium toxicity in plants. Agron. J. 2020, 112, 635–647. [Google Scholar] [CrossRef]
- Amin, M.; Gurmani, A.R.; Rafique, M.; Khan, S.U.; Mehmood, A.; Muhammad, D.; Syed, J.H. Investigating the degradation behavior of cypermethrin (CYP) and chlorpyrifos (CPP) in peach orchard soils using organic/inorganic amendments. Saudi J. Biol. Sci. 2021, 28, 5890–5896. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Biostimulation as a process aiding tebuconazole degradation in soil. J. Soils Sediments 2019, 19, 3728–3741. [Google Scholar] [CrossRef]
- Carpio, M.J.; Marin-Benito, J.M.; Sanchez-Marin, M.J.; Rodriguez-Cruz, M.S. Accelerated dissipation of two herbicides after repeated application in field experiments with organically-amended soil. Agronomy 2021, 11, 1125. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J.; Borowik, A.; Kaczyński, P. Possibilities of restoring homeostasis of soil exposed to terbuthylazine by its supplementation with HumiAgra preparation. Appl. Soil Ecol. 2022, 178, 104582. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Borusiewicz, A.; Łozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hortic. 2022, 298, 110988. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Łozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Łozowicka, B.; Mojsak, P.; Kaczyński, P.; Konecki, R.; Borusiewicz, A. The fate of spirotetramat and dissipation metabolites in Apiaceae and Brassicaceae leaf-root and soil system under greenhouse conditions estimated by modified QuEChERS/LC–MS/MS. Sci. Total Environ. 2017, 603, 178–184. [Google Scholar] [CrossRef]
- SANTE. Document No. SANTE/12682/2019. Analytical quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. 2019. Available online: https://www.eurlpesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 8 December 2021).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Jain, V.M.; Karibasappa, G.N.; Dodamani, A.S.; Mali, G.V. Estimating the carbohydrate content of various forms of tobacco by phenol-sulfuric acid method. J. Educ. Health Prom. 2017, 6, 90. [Google Scholar] [CrossRef]
- Alvarez, R.; Araya, H.; Navarro-Lisboa, R.; de Dicastillo, C.L. Evaluation of polyphenol content and antioxidant capacity of fruits and vegetables using a modified enzymatic extraction. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef]
- Ciereszko, I.; Balwicka, H.; Zebrowska, E. Acid phosphatases activity and growth of barley, oat, rye and wheat plants as affected by Pi deficiency. Open Plant Sci. J. 2017, 10, 110–122. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Konecki, R.; Kaczyński, R.; Rysbekova, A.; Łozowicka, B. Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat. Crop J. 2022, 10, 1198–1206. [Google Scholar] [CrossRef]
- SANTE. Document No. SANTE/12089 /2016. Guidance Document on Identification of Mycotoxins in Food and Feed. 2016. Available online: https://ec.europa.eu/food/system/files/2017-05/cs_contaminants_sampling_guid-doc-ident-mycotoxins.pdf (accessed on 8 December 2021).
- Iwaniuk, P.; Łozowicka, B.; Kaczyński, P.; Konecki, R. Multifactorial wheat response under Fusarium culmorum, herbicidal, fungicidal and biostimulator treatments on the biochemical and mycotoxins status of wheat. J. Saudi Soc. Agric. Sci. 2021, 20, 443–453. [Google Scholar] [CrossRef]
- Yu, G.-B.; Chen, R.-N.; Chen, Q.-S.; Chen, F.-Q.; Liu, H.-L.; Ren, C.-Y.; Zhang, Y.-X.; Yang, F.-J.; Wei, J.-P. Jasmonic acid promotes glutathione assisted degradation of chlorothalonil during tomato growth. Ecotoxicol. Environ. Saf. 2022, 233, 113296. [Google Scholar] [CrossRef]
- Li, X.; Riaz, M.; Song, B.; Liang, X.; Liu, H. Exogenous salicylic acid alleviates fomesafen toxicity by improving photosynthetic characteristics and antioxidant defense system in sugar beet. Ecotoxicol. Environ. Saf. 2022, 238, 113587. [Google Scholar] [CrossRef]
- Marsik, P.; Zunova, T.; Vanek, T.; Podlipna, R. Metazachlor effect on poplar—Pioneer plant species for riparian buffers. Chemosphere 2021, 274, 129711. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Nair, A.M.; Verma, P.K. Surviving the odds: From perception to survival of fungal phytopathogens under host-generated oxidative burst. Plant Commun. 2021, 2, 100142. [Google Scholar] [CrossRef] [PubMed]
- Matic, M.; Vukovic, R.; Vrandecic, K.; Camagajevac, I.S.; Cosic, J.; Vukovic, A.; Sabljic, K.; Sabo, N.; Dvojkovic, K.; Novoselovic, D. Oxidative status and antioxidative response to fusarium attack and different nitrogen levels in winter wheat varieties. Plants 2021, 10, 611. [Google Scholar] [CrossRef]
- Singh, P.; Prasad, S.M. Antioxidant enzyme responses to the oxidative stress due to chlorpyrifos, dimethoate and dieldrin stress in palak (Spinacia oleracea L.) and their toxicity alleviation by soil amendments in tropical croplands. Sci. Total Environ. 2018, 630, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Alsamadany, H.; El Desoky, S.M.; Rady, M.M. Silymarin-enriched biostimulant foliar application minimizes the toxicity of cadmium in maize by suppressing oxidative stress and elevating antioxidant gene expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 255, 459–469. [Google Scholar] [CrossRef]
- Baia, D.C.; Olivares, F.L.; Zandonadi, D.B.; Soares, C.P.; Spaccini, R.; Canellas, L.P. Humic acids trigger the weak acids stress response in maize seedlings. Chem. Biol. Technol. Agric. 2020, 7, 31. [Google Scholar] [CrossRef]
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-tolerance to biotic and abiotic stresses in plants: A focus on resistance to aphid infestation. J. Exp. Bot. 2016, 67, 2025–2037. [Google Scholar] [CrossRef]
- Shakir, S.K.; Irfan, S.; Akhtar, B.; Rehman, S.U.; Daud, M.K.; Taimur, N.; Azizullah, A. Pesticide-induced oxidative stress and antioxidant responses in tomato (Solanum lycopersicum) seedlings. Ecotoxicology 2018, 27, 919–935. [Google Scholar] [CrossRef]
- Garcia-Delgado, C.; Barba-Vicente, V.; Marin-Benito, J.M.; Igual, J.M.; Sanchez-Martin, M.J.; Rodriguez-Cruz, M.S. Influence of different agricultural management practices on soil microbial community over dissipation time of two herbicides. Sci. Total Environ. 2018, 646, 1478–1488. [Google Scholar] [CrossRef]
- Yavari, S.; Sapari, N.B.; Malakahmad, A.; Yavari, S. Degradation of imazapic and imazapyr herbicides in the presence of optimized oil palm empty fruit bunch and rice husk biochars in soil. J. Hazard. Mater. 2019, 366, 636–642. [Google Scholar] [CrossRef]
- Tang, F.; Xu, Z.; Gao, M.; Li, L.; Li, H.; Cheng, H.; Tian, G. The dissipation of cyazofamid and its main metabolite in soil response oppositely to biochar application. Chemosphere 2019, 218, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kaczyński, P.; Łozowicka, B.; Wołejko, E.; Iwaniuk, P.; Konecki, R.; Drągowski, W.; Łozowicki, J.; Amanbek, N.; Rusiłowska, J.; Pietraszko, A. Complex study of glyphosate and metabolites influence on enzymatic activity and microorganisms association in soil enriched with Pseudomonas fluorescens and sewage sludge. J. Hazard. Mater. 2020, 393, 122443. [Google Scholar] [CrossRef]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Łozowicki, J.; Tujtebajeva, G.; Wydro, U.; Jabłońska-Trypuć, A. Effect of microorganism on behavior of two commonly used herbicides in wheat/soil system. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Bhatt, P.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Chen, S. Biodegradation of allethrin by a novel fungus Fusarium proliferatum strain CF2, isolated from contaminated soils. Microorganisms 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Jamiołkowska, A.; Skwaryło-Bednarz, B.; Mielniczuk, E.; Bisceglie, F.; Pelosi, G.; Degola, F.; Galazka, A.; Grzeda, E. Effect of thiosemicarbazone derivatives and Fusarium culmorum (Wm.G. Sm.) Sacc. infection of winter wheat seedlings on their health status and soil biological activity. Agronomy 2022, 12, 116. [Google Scholar] [CrossRef]
- Ćwieląg-Piasecka, I.; Medyńska-Juraszek, A.; Jerzykiewicz, M.; Dębicka, M.; Bekier, J.; Jamroz, E.; Kawałko, D. Humic acid and biochar as specific sorbents of pesticides. J. Soils Sediments 2018, 18, 2692–2702. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bhat, W.F.; Bano, B. Spectroscopic evaluation of the interaction between pesticides and chickpea cystatin: Comparative binding and toxicity analyses. Environ. Sci. Process. Impacts 2016, 18, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Matysiak, K.; Miziniak, W.; Kaczmarek, S.; Kierzek, R. Herbicides with natural and synthetic biostimulants in spring wheat. Cienc. Rural 2018, 48, e20180405. [Google Scholar] [CrossRef]
- Spanic, V.; Horvat, D.; Drezner, G.; Zdunic, Z. Changes in protein composition in the grain and malt after fusarium infection dependently of wheat resistance. Pathogens 2019, 8, 112. [Google Scholar] [CrossRef]
- Nosenko, T.; Hanke-Uhe, M.; Heine, P.A.; Shahid, A.; Dübel, S.; Rennenberg, H.; Schumacher, J.; Winkler, J.B.; Schnitzler, J.-P.; Hänsch, R.; et al. Plant defense proteins as potential markers for early detection of forest damage and diseases. Front. For. Glob. Chang. 2021, 4, 654032. [Google Scholar] [CrossRef]
- Fukui, K.; Hayashi, K. Manipulation and sensing of auxin metabolism, transport and signaling. Plant Cell Physiol. 2018, 59, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Revi. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Manes, N.; Brauer, E.K.; Hepworth, S.; Subramaniam, R. MAMP and DAMP signaling contributes resistance to Fusarium graminearum in Arabidopsis. J. Exp. Bot. 2021, 72, 6628–6639. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhael, C.; Rouphael, Y.; Ritieni, A.; di Vaio, C. Biostimulants improve plant growth and bioactive compounds of young olive trees under abiotic stress conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.A. Phenolic compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef]
- Silva, B.; Souza, M.M.; Badiale-Furlong, E. Antioxidant and antifungal activity of phenolic compounds and their relation to aflatoxin B1 occurrence in soybeans (Glycine max L.). J. Sci. Food Agric. 2020, 100, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, J.; Szczerba, A.; Dziurka, M.; Hornyak, M.; Kopec, P.; Szklarczyk, M.; Plazek, A. Physiological and biochemical response to Fusarium culmorum infection in three durum wheat genotypes at seedling and full anthesis stage. Int. J. Mol. Sci. 2021, 22, 7433. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef]
- Hoelscher, M.P.; Forner, J.; Calderone, S.; Kramer, C.; Taylor, Z.; Loiacono, F.V.; Agrawal, S.; Karcher, D.; Moratti, F.; Kroop, X.; et al. Expression strategies for the efficient synthesis of antimicrobial peptides in plastids. Nat. Commun. 2022, 13, 5856. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. Excli J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhou, Y.; Lv, G.; Zhou, R. Simultaneous detoxification of aflatoxin B1, zearalenone and deoxynivalenol by modified montmorillonites. Molecules 2022, 27, 315. [Google Scholar] [CrossRef]
- Maguey-González, J.A.; Nava-Ramírez, M.d.J.; Gómez-Rosales, S.; Ángeles, M.d.L.; Solís-Cruz, B.; Hernández-Patlán, D.; Merino-Guzmán, R.; Hernández-Velasco, X.; Figueroa-Cárdenas, J.d.D.; Vázquez-Durán, A.; et al. Humic Acids preparation, characterization, and their potential adsorption capacity for aflatoxin B1 in an in vitro poultry digestive model. Toxins 2023, 15, 83. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A plant biostimulant made from the marine brown algae Ascophyllum nodosum and chitosan reduced Fusarium head blight and mycotoxin contamination in wheat. PLoS ONE 2019, 14, e0220562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nie, D.; Fan, K.; Yang, J.; Guo, W.; Meng, J.; Zhao, Z.; Han, Z. A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit. Rev. Food Sci. Nutr. 2020, 60, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
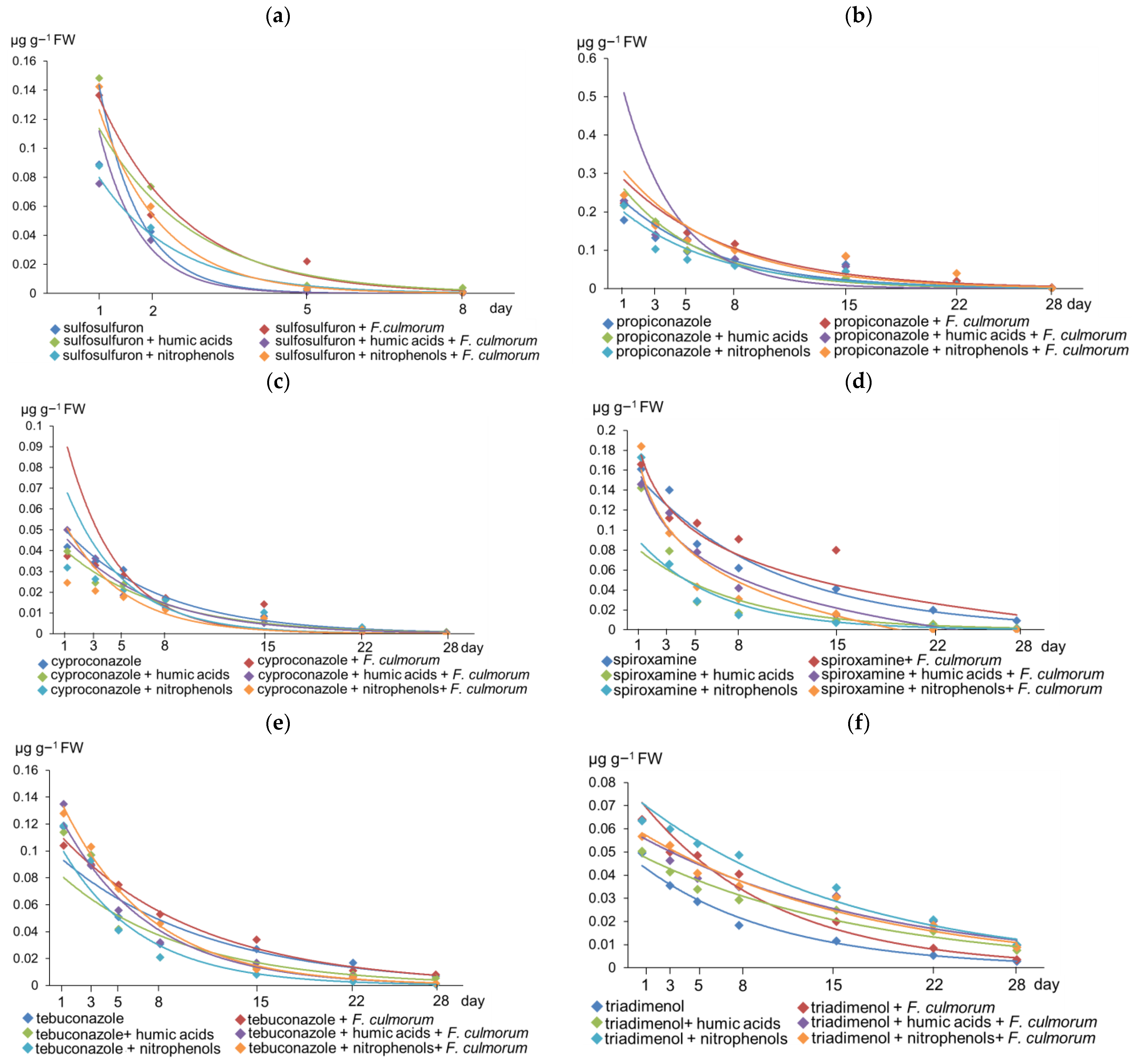

| Pesticide | Treatment | Equation of DegRadation | R2 | DT50 1 | DT99 1 |
|---|---|---|---|---|---|
| sulfosulfuron | sulfosulfuron | y = 0.519e−1.287x | 0.9567 | 0.54 | 3.07 |
| sulfosulfuron + F. culmorum | y = 0.245e−0.601x | 0.9588 | 1.15 | 5.33 | |
| sulfosulfuron + humic acids | y = 0.196e−0.544x | 0.9894 | 1.27 | 5.47 | |
| sulfosulfuron + humic acids + F. culmorum | y = 0.402e−1.280x | 0.9543 | 0.54 | 2.89 | |
| sulfosulfuron + nitrophenols | y = 0.156e−0.674x | 0.9991 | 1.03 | 4.80 | |
| sulfosulfuron + nitrophenols + F. culmorum | y = 0.288e−0.825x | 0.9996 | 0.84 | 4.08 | |
| propiconazole | propiconazole | y = 0.266e−0.164x | 0.9427 | 4.23 | 20.08 |
| propiconazole + F. culmorum | y = 0.327e−0.143x | 0.9371 | 4.85 | 24.41 | |
| propiconazole + humic acids | y = 0.315e−0.194x | 0.9852 | 3.58 | 17.81 | |
| propiconazole + humic acids + F. culmorum | y = 0.686e−0.296x | 0.9091 | 2.34 | 14.27 | |
| propiconazole + nitrophenols | y = 0.234e−0.165x | 0.9028 | 4.20 | 19.14 | |
| propiconazole + nitrophenols + F. culmorum | y = 0.358e−0.159x | 0.9329 | 4.37 | 22.55 | |
| cyproconazole | cyproconazole | y = 0.056e−0.146x | 0.9747 | 5.70 | 27.32 |
| cyproconazole + F. culmorum | y = 0.118e−0.275x | 0.9168 | 2.52 | 8.97 | |
| cyproconazole + humic acids | y = 0.045e−0.146x | 0.9773 | 4.75 | 10.37 | |
| cyproconazole + humic acids + F. culmorum | y = 0.053e−0.163x | 0.9756 | 4.24 | 10.59 | |
| cyproconazole + nitrophenols | y = 0.086e−0.240x | 0.8634 | 2.88 | 8.96 | |
| cyproconazole + nitrophenols + F. culmorum | y = 0.064e−0.244x | 0.8684 | 2.92 | 9.11 | |
| spiroxamine | spiroxamine | y = 0.165e−0.100x | 0.9645 | 6.20 | 26.35 |
| spiroxamine + F. culmorum | y = 0.813e−0.399x | 0.9117 | 1.74 | 11.02 | |
| spiroxamine + humic acids | y = 0.089e−0.138x | 0.9186 | 4.25 | 15.91 | |
| spiroxamine + humic acids + F. culmorum | y = 0.536e−0.398x | 0.9713 | 1.70 | 10.02 | |
| spiroxamine + nitrophenols | y = 0.102e−0.172x | 0.9656 | 4.04 | 13.58 | |
| spiroxamine + nitrophenols + F. culmorum | y = 0.441e−0.391x | 0.9860 | 1.77 | 11.26 | |
| tebuconazole | tebuconazole | y = 0.102e−0.093x | 0.8890 | 6.11 | 25.09 |
| tebuconazole + F. culmorum | y = 0.120e−0.098x | 0.9918 | 7.04 | 25.86 | |
| tebuconazole + humic acids | y = 0.089e−0.110x | 0.8829 | 5.30 | 19.87 | |
| tebuconazole + humic acids + F. culmorum | y = 0.141e−0.155x | 0.9773 | 4.47 | 17.06 | |
| tebuconazole + nitrophenols | y = 0.118e−0.174x | 0.9609 | 3.99 | 14.25 | |
| tebuconazole + nitrophenols + F. culmorum | y = 0.155e−0.157x | 0.9953 | 4.41 | 16.96 | |
| triadimenol | triadimenol | y = 0.048e−0.102x | 0.9789 | 6.83 | 19.88 |
| triadimenol + F. culmorum | y = 0.079e−0.104x | 0.9675 | 4.42 | 15.63 | |
| triadimenol + humic acids | y = 0.051e−0.061x | 0.9587 | 11.28 | 26.63 | |
| triadimenol + humic acids + F. culmorum | y = 0.059e−0.057x | 0.9129 | 12.06 | 31.11 | |
| triadimenol + nitrophenols | y = 0.076e−0.065x | 0.9655 | 10.67 | 25.12 | |
| triadimenol + nitrophenols + F.culmorum | y = 0.062e−0.062x | 0.9624 | 11.15 | 26.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. https://doi.org/10.3390/agronomy13051378
Iwaniuk P, Łuniewski S, Kaczyński P, Łozowicka B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy. 2023; 13(5):1378. https://doi.org/10.3390/agronomy13051378
Chicago/Turabian StyleIwaniuk, Piotr, Stanisław Łuniewski, Piotr Kaczyński, and Bożena Łozowicka. 2023. "The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress" Agronomy 13, no. 5: 1378. https://doi.org/10.3390/agronomy13051378
APA StyleIwaniuk, P., Łuniewski, S., Kaczyński, P., & Łozowicka, B. (2023). The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy, 13(5), 1378. https://doi.org/10.3390/agronomy13051378







