Tropical Grasses—Annual Crop Intercropping and Adequate Nitrogen Supply Increases Soil Microbial Carbon and Nitrogen
Abstract
1. Introduction
2. Materials and Methods
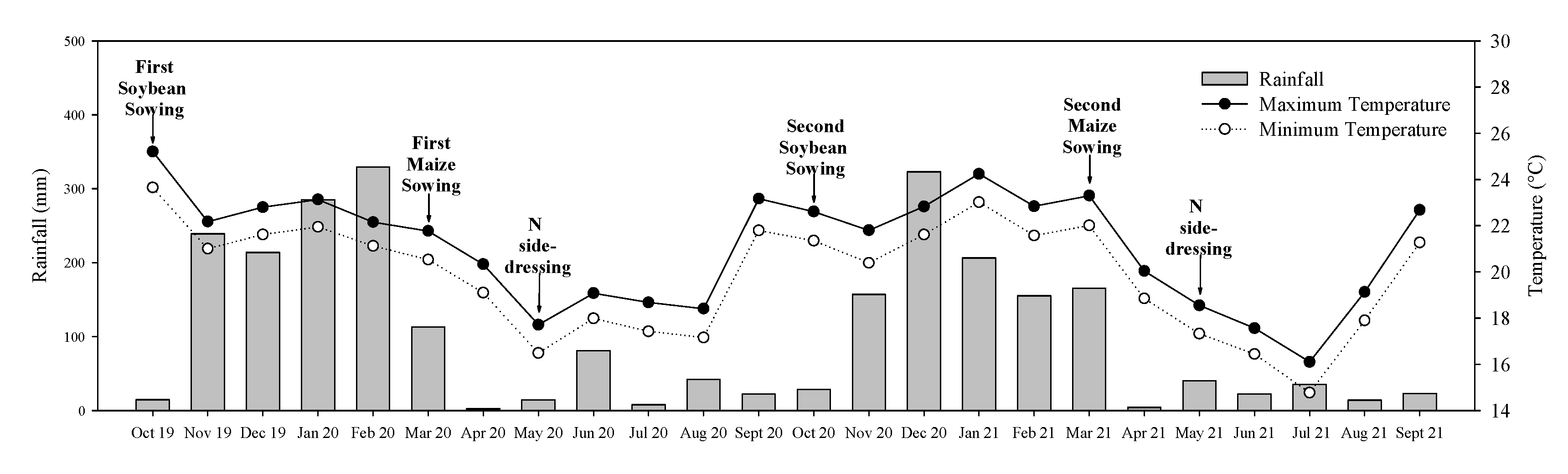
2.1. Characterization and Management of the Experimental Site
2.2. Treatments and Experimental Design
2.3. Soil Sampling and Analysis
2.4. Statistical Analysis
3. Results
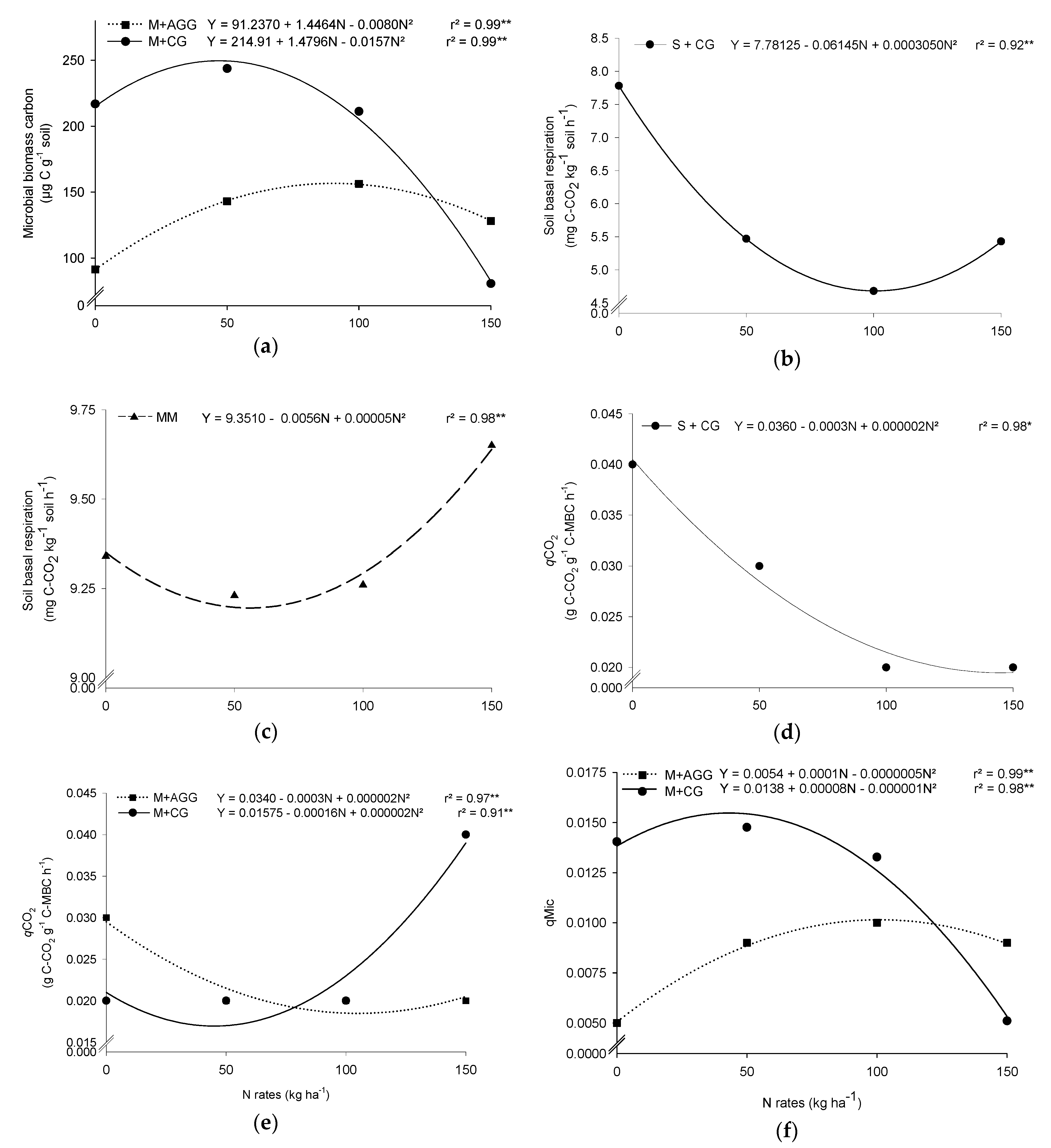
3.1. Microbial Biomass Carbon (MBC)
3.2. Metabolic Quotient (qCO2)
3.3. Microbial Quocient (qMic)
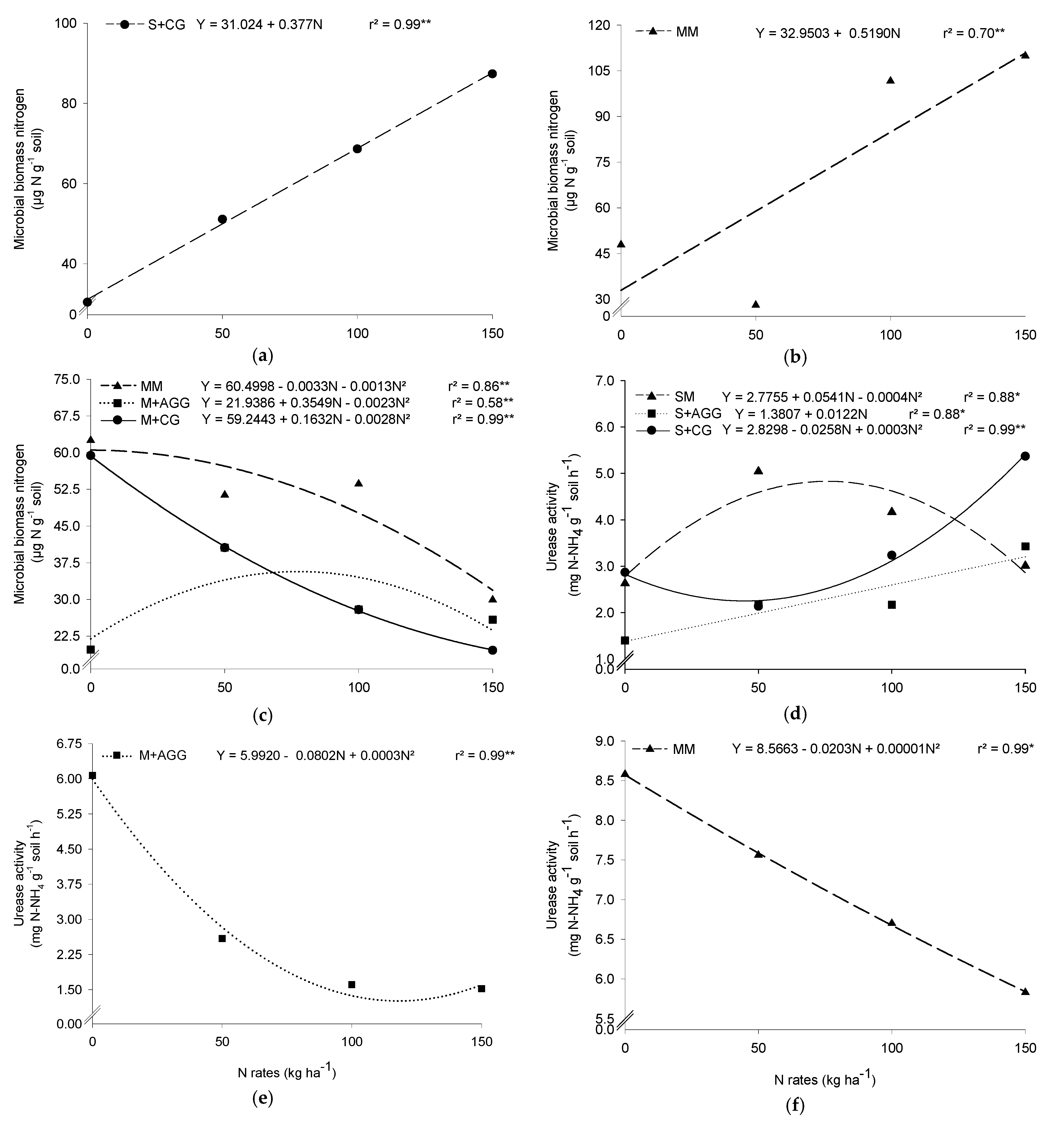
3.4. Microbial Biomass Nitrogen (MBN)
3.5. Urease Activity (UA)
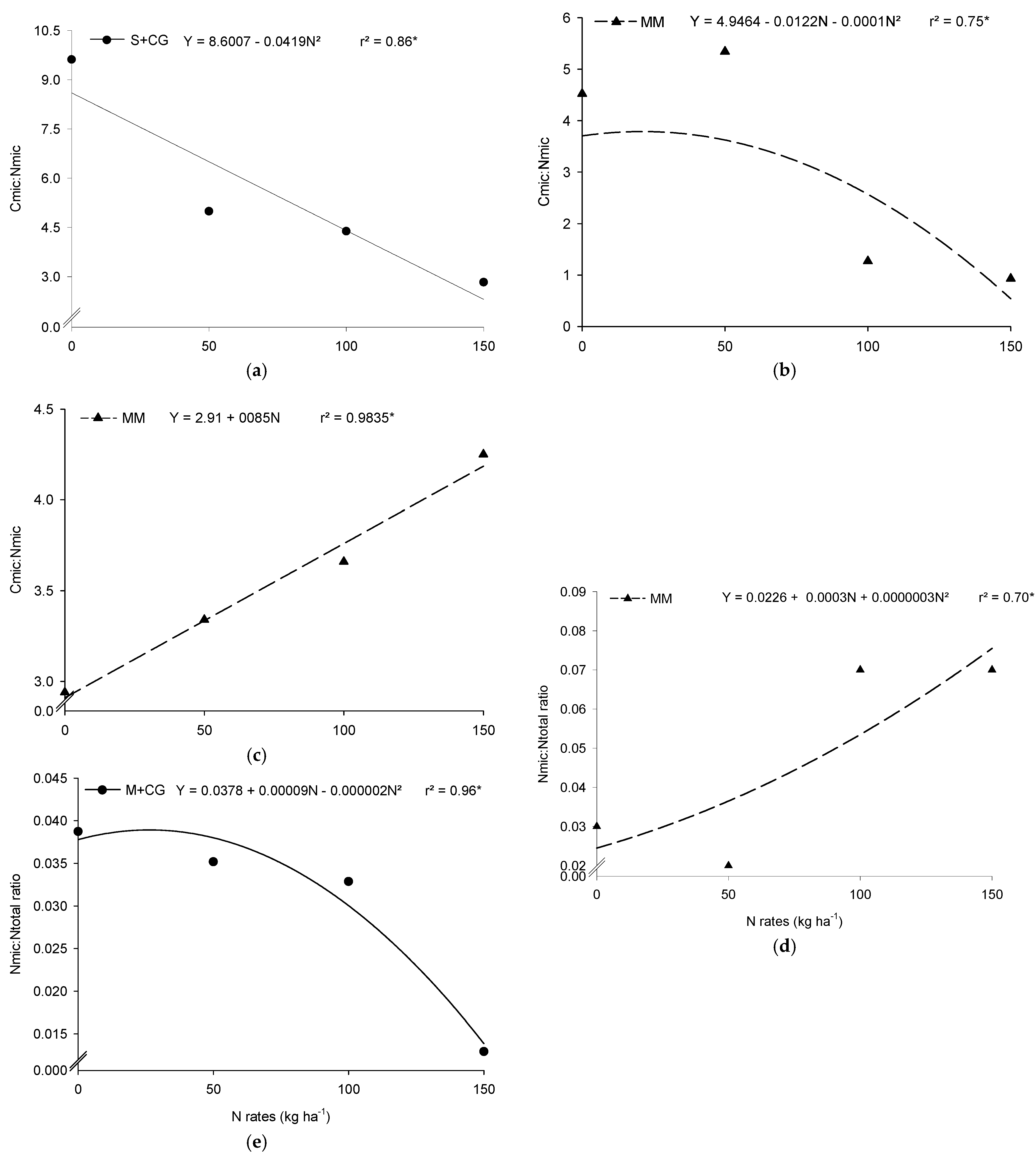
3.6. Microbial Carbon to Microbial Nitrogen Ratio (Cmic:Nmic Ratio)
3.7. Microbial Biomass Nitrogen to Total Soil Nitrogen Ratio (Nmic:Ntotal Ratio)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Li, Z.; Wang, Y.; Zhang, T. Development and prospect of food security cooperation in the BRICS countries. Sustainability 2020, 12, 2125. [Google Scholar] [CrossRef]
- Steinauer, K.; Fischer, F.M.; Roscher, C.; Scheu, S.; Eisenhauer, N. Spatial plant resource acquisition traits explain plant community effects on soil microbial properties. Pedobiologia 2017, 65, 50–57. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dordas, C.A.; Damalas, C.A.; Vlachostergios, D.N. Annual Intercrops: An Alternative Pathway for Sustainable Agriculture. Aust. J. Crop Sci. 2011, 5, 396–410. [Google Scholar]
- Santos, P.R.A.; Chioderoli, C.A.; Silva, A.O.; Nicolau, F.E.A.; Lopes, J.E.L.; Amorim, M.Q.; Mendonça, C.d.A. Physical attributes of the soil and maize productivity under an intercrop system. J. Agric. Sci. 2018, 10, 358–365. [Google Scholar] [CrossRef]
- Soares, D.S.; Ramos, M.L.G.; Marchão, R.L.; Maciel, G.A.; Oliveira, A.D.; Malaquias, J.V.; Carvalho, A.M. How diversity of cropresidues in long-term no-tillage systems affect chemical and microbiological soil properties. Soil Tillage Res. 2019, 194, 104316. [Google Scholar] [CrossRef]
- Curtright, A.J.; Tiemann, L.K. Intercropping increases soil extracellular enzyme activity: A meta-analysis. Agric. Ecosyst. Environ. 2021, 319, 107489. [Google Scholar] [CrossRef]
- Silva, L.S.; Laroca, J.V.S.; Coelho, A.P.; Gonçalves, E.C.; Gomes, R.P.; Pacheco, L.P.; Carvalho, P.C.F.; Pires, G.C.; Oliveira, R.L.; Souza, J.M.A.D.; et al. Does grass-legume intercropping change soil quality and grain yield in integrated crop-livestock systems? Appl. Soil Ecol. 2022, 70, 104257. [Google Scholar] [CrossRef]
- Batista, K.; Giacomini, A.A.; Gerdes, L.; Mattos, W.T.; Otsuk, I.P. Impacts of the nitrogen application on productivity and nutrients concentrations of the corn-Congo grass intercropping system in the dry season. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 567–577. [Google Scholar] [CrossRef]
- Li, X.; Qu, C.; Li, Y.; Liang, Z.; Tian, X.; Shi, J.; Ning, P.; Wei, G. Long-term effects of straw mulching coupled with N application on soil organic carbon sequestration and soil aggregation in an autumn-winter wheat monoculture system. Agron. J. 2021, 113, 2118–2131. [Google Scholar] [CrossRef]
- Gong, J.; Zhu, C.; Yang, L.; Yang, B.; Wang, B.; Baoyin, T.; Liu, M.; Zhang, Z.; Shi, J. Effects of nitrogen addition on above-and belowground litter decomposition and nutrient dynamics in the litter-soil continuum in the temperate steppe of Inner Mongolia; China. J. Arid Environ. 2020, 172, 104036. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; 356p. [Google Scholar]
- United States Department of Agriculture. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture; Natural Resources Conservation Service: Washington, WA, USA, 2014; 359p. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 11–728. [Google Scholar] [CrossRef]
- Van Raij, B.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001; 284p. [Google Scholar]
- Van Raij, B.; Cantarela, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo, 2nd ed.; Instituto Agronômico e Fundação IAC: Campinas, Brazil, 1997; 285p. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil-V: A method for measuring soil biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Anderson, T.; Domsch, K. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions; such as pH; on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2-Microbiological and Biochemical Properties; Weaver, R.W.A., Bottoml, Y.P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide 2009, 2nd ed.; SAS Institute Inc.: Cary, FL, USA, 2009; 176p. [Google Scholar]
- Cordeiro, C.F.S.; Echer, F.R.; Araujo, F.F. Cover crops impact crops yields by improving microbiological activity and fertility in sandy soil. J. Soil Sci. Plant Nutr. 2021, 21, 1968–1977. [Google Scholar] [CrossRef]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India. Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- Simon, C.A.; de Lima, S.F.; Cordeiro, M.S.; Secco, V.A.; Nacata, G.; Silva, A.M.M.; Simon, C.d.C.; Brasil, M.d.S. Cover crops as modifying agents of microbiological soil attribute. Aust. J. Crop Sci. 2019, 13, 1578–1585. [Google Scholar] [CrossRef]
- Lai, H.; Gao, F.; Su, H.; Zheng, P.; Li, Y.; Yao, H. Nitrogen Distribution and Soil Microbial Community Characteristics in a Legume–Cereal Intercropping System: A Review. Agronomy 2022, 12, 1900. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Coelho, M.C.; Dantas, R.A.; Fonseca, O.P.; Guimarães Júnior, R.; Figueiredo, C.C. Chemical composition of cover plants and its effect on maize yield in no-tillage systems in the Brazilian savanna. Crop Pasture Sci. 2013, 63, 1075–1081. [Google Scholar] [CrossRef]
- Zhu, S.G.; Cheng, Z.G.; Batool, A.; Wang, Y.B.; Wang, J.; Zhou, R.; Khan, A.; Zhu, S.Y.; Yang, Y.M.; Wang, W.; et al. Plant facilitation shifts along with soil moisture and phosphorus gradients via rhizosphere interaction in the maize-grass pea intercropping system. Ecol. Indic. 2022, 139, 108901. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The relationship between soil properties; enzyme activity and land use. Lesn. Pract. Badaw. 2017, 78, 39–44. [Google Scholar] [CrossRef]

| Cropping | MBC | SBR | qCO2 | qMic | MBN | UA | Cmic:Nmic |
|---|---|---|---|---|---|---|---|
| µg C g−1 soil | mg C-CO2 kg−1 soil h−1 | g C-CO2 g−1 C-MBC h−1 | µg N g−1 soil | mg N-NH4 g−1 soil h−1 | |||
| SM | 127.76 b | 10.24 a | 0.085 b | 0.010 a | 44.32 a | 4.66 b | 2.90 a |
| S + AGG | 197.34 a | 12.14 a | 0.058 b | 0.010 a | 53.58 a | 7.33 a | 3.68 a |
| S + CG | 74.80 c | 9.97 a | 0.135 a | 0.003 b | 21.13 b | 6.4 a | 3.78 a |
| Means | 133.29 | 10.78 | 0.094 | 0.008 | 39.67 | 6.13 | 3.46 |
| CV | 7.18 (1) | 9.83 (1) | 10.63 (1) | 7.18 (1) | 7.99 (1) | 6.16 (1) | 13.36 (1) |
| Cropping Systems | N Rates (kg ha−1) | Means | F Test for Regression | ||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | Linear | Quadratic | ||
| Microbial biomass carbon content (MBC, µg C g−1 soil) | |||||||
| SM | 236.65 a | 164.18 a | 177.76 a | 131.79 a | 177.60 a | ns | ns |
| S + AGG | 155.29 a | 37.44 b | 326.71 a | 256.39 a | 193.96 a | ns | ns |
| S + CG | 211.23 a | 197.65 a | 297.18 a | 239.89 a | 236.49 a | ns | ns |
| Means | 201.06 | 133.09 | 267.22 | 209.36 | ns | ns | |
| CV | 9.15 (1) | ||||||
| Soil basal respiration (SBR, mg C-CO2 kg−1 soil h−1) | |||||||
| SM | 5.07 b | 3.52 a | 4.91 a | 4.48 a | 4.49 b | ns | ns |
| S + AGG | 5.39 b | 5.48 a | 5.48 a | 4.07 a | 5.05 ab | ns | ns |
| S + CG | 7.78 a | 5.47 a | 4.68 a | 5.43 a | 5.84 a | 0.006 | 0.0010 |
| Means | 6.08 | 4.82 | 5.02 | 4.66 | ns | ns | |
| CV | 8.27 (2) | ||||||
| Metabolic quotient (qCO2, g C-CO2 g−1 C-MBC h−1) | |||||||
| SM | 0.02 a | 0.03 b | 0.03 b | 0.09 a | 0.04 a | ns | ns |
| S + AGG | 0.04 a | 0.15 a | 0.15 a | 0.02 a | 0.06 a | ns | ns |
| S + CG | 0.04 a | 0.03 b | 0.02 b | 0.02 a | 0.03 a | 0.0022 | 0.0003 |
| Means | 0.03 | 0.07 | 0.07 | 0.04 | ns | ns | |
| CV | 32.38 (2) | ||||||
| Microbial biomass nitrogen content (MBN, µg N g−1 soil) | |||||||
| SM | 31.85 a | 119.53 a | 67.89 a | 62.46 a | 70.43 a | ns | ns |
| S + AGG | 32.38 a | 76.66 a | 76.66 a | 78.74 a | 53.38 a | ns | ns |
| S + CG | 30.27 a | 51.09 a | 68.61 a | 87.32 a | 59.32 a | 0.0004 | ns |
| Means | 31.50 | 82.42 | 71.05 | 76.17 | ns | ns | |
| CV | 10.7 (1) | ||||||
| Urease activity (UA, mg N-NH4 g−1 soil h−1) | |||||||
| SM | 2.6 a | 5.04 a | 4.17 a | 3.01 b | 3.71 a | ns | 0.0378 |
| S + AGG | 1.40 a | 2.17 a | 2.17 b | 3.43 b | 2.30 b | 0.0398 | ns |
| S + CG | 2.87 a | 2.14 a | 3.24 ab | 5.37 a | 3.40 ab | 0.0031 | 0.0002 |
| Means | 2.30 | 3.12 | 3.19 | 3.94 | ns | ns | |
| CV | 19.07 (2) | ||||||
| Microbial carbon:microbial nitrogen ratio (Cmic:Nmic) | |||||||
| SM | 8.53 a | 1.30 b | 5.37 b | 2.94 a | 4.53 a | ns | ns |
| S + AGG | 6.99 a | 0.66 b | 15.94 a | 3.60 a | 6.80 a | ns | ns |
| S + CG | 9.62 a | 4.99 a | 4.38 b | 2.83 a | 5.46 a | 0.0225 | ns |
| Means | 8.38 | 2.32 | 8.56 | 3.12 | ns | ns | |
| CV | 30.76 (1) | ||||||
| Cropping Systems | N Rates (kg ha−1) | Means | F Test for Regression | ||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | Linear | Quadratic | ||
| Microbial biomass carbon content (MBC, µg C g−1 soil) | |||||||
| MM | 157.7 b | 121.42 a | 117.12 a | 100.93 a | 124.31 a | ns | ns |
| M + AGG | 281.2 a | 59.53 b | 175.02 a | 103.22 a | 154.75 a | ns | ns |
| M + CG | 149.26 b | 140.66 a | 150.86 a | 197.82 a | 159.65 a | ns | ns |
| Means | 196.08 | 107.21 | 147.67 | 133.99 | ns | ns | |
| CV | 8.45 (1) | ||||||
| Soil basal respiration (SBR, mg C-CO2 kg−1 soil h−1) | |||||||
| MM | 9.34 a | 9.23 a | 9.26 a | 9.65 a | 9.37 a | ns | 0.0117 |
| M + AGG | 9.75 a | 9.17 a | 9.20 a | 9.45 a | 9.39 a | ns | ns |
| M + CG | 9.23 a | 9.04 a | 9.1 a | 8.88 a | 9.07 a | ns | ns |
| Means | 9.44 | 9.15 | 9.19 | 9.33 | ns | ns | |
| CV | 2.54 (1) | ||||||
| Metabolic quotient (qCO2, g C-CO2 g−1 C-MBC h−1) | |||||||
| MM | 0.07 a | 0.08 b | 0.08 a | 0.22 a | 0.11 a | ns | ns |
| M + AGG | 0.03 a | 0.17 a | 0.06 a | 0.09 a | 0.09 a | ns | ns |
| M + CG | 0.06 a | 0.07 b | 0.06 a | 0.05 a | 0.06 a | ns | ns |
| Means | 0.06 | 0.10 | 0.07 | 0.12 | ns | ns | |
| CV | 26.92 (2) | ||||||
| Microbial biomass nitrogen content (MBN, µg N g−1 soil) | |||||||
| MM | 47.90 a | 28.09 b | 101.61 a | 109.88 a | 71.87 a | 0.0091 | ns |
| M + AGG | 23.60 a | 73.46 a | 12.20 b | 33.64 b | 35.72 b | ns | ns |
| M + CG | 25.22 a | 32.32 b | 26.82 b | 23.61 b | 26.99 b | ns | ns |
| Means | 32.24 | 44.62 | 46.88 | 55.71 | ns | ns | |
| CV | 13.76 (1) | ||||||
| Urease activity (UA, mg N-NH4 g−1 soil h−1) | |||||||
| MM | 3.76 ab | 2.64 a | 7.33 a | 3.83 a | 4.39 a | ns | ns |
| M + AGG | 6.07 a | 2.59 a | 1.6 b | 1.5 b | 2.95 ab | 0.0001 | 0.0025 |
| M + CG | 2.63 b | 2.77 a | 2.63 b | 2.87 ab | 2.72 b | ns | ns |
| Means | 4.15 | 2.67 | 3.86 | 2.74 | ns | ns | |
| CV | 16.56 (2) | ||||||
| Microbial biomass nitrogen:total soil nitrogen ratio (Nmic:Ntotal) | |||||||
| MM | 0.03 a | 0.02 b | 0.07 a | 0.07 a | 0.05 a | 0.0035 | 0.0164 |
| M + AGG | 0.02 a | 0.05 a | 0.01 b | 0.03 ab | 0.03 b | ns | ns |
| M + CG | 0.02 a | 0.02 b | 0.02 b | 0.02 b | 0.02 b | ns | ns |
| Means | 0.02 | 0.03 | 0.03 | 0.04 | ns | ns | |
| CV | 26.16 (2) | ||||||
| Microbial carbon:microbial nitrogen ratio (Cmic:Nmic) | |||||||
| MM | 4.52 a | 5.34 a | 1.27 b | 0.93 b | 3.01 b | 0.0079 | 0.0292 |
| M + AGG | 13.98 a | 0.92 a | 14.19 a | 3.88 ab | 8.24 a | ns | ns |
| M + CG | 8.06 a | 4.43 a | 5.65 b | 10.53 a | 7.17 ab | ns | ns |
| Means | 8.85 | 3.56 | 7.04 | 5.11 | ns | ns | |
| CV | 24.62 (3) | ||||||
| Cropping Systems | N Rates (kg ha−1) | Means | F Test for Regression | ||||
|---|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | Linear | Quadratic | ||
| Microbial biomass carbon content (MBC, µg C g−1 soil) | |||||||
| MM | 183.7 ab | 172.93 b | 195.82 ab | 126.18 a | 169.7 ab | ns | ns |
| M + AGG | 91.39 b | 143.11 b | 156.37 b | 128.13 a | 129.75 b | 0.0511 | 0.0001 |
| M + CG | 216.87 a | 243.74 a | 211.18 a | 80.61 b | 188.10 a | 0.0008 | 0.0001 |
| Means | 163.99 | 153.05 | 169.52 | 111.64 | ns | ns | |
| CV | 9.47 (1) | ||||||
| Soil basal respiration (SBR, mg C-CO2 kg−1 soil h−1) | |||||||
| MM | 3.11 a | 3.12 a | 3.08 a | 3.28 a | 3.15 a | ns | ns |
| M + AGG | 3.09 a | 3.02 a | 3.23 a | 3.17 a | 3.13 a | ns | ns |
| M + CG | 3.54 a | 3.02 a | 3.23 a | 3.04 a | 3.42 a | ns | ns |
| Means | 3.25 | 3.05 | 3.18 | 3.17 | ns | ns | |
| CV | 6.43 (2) | ||||||
| Metabolic quotient (qCO2, g C-CO2 g−1 C-MBC h−1) | |||||||
| MM | 0.02 b | 0.02 a | 0.01 b | 0.03 ab | 0.02 a | ns | ns |
| M + AGG | 0.03 a | 0.02 a | 0.02 a | 0.02 b | 0.03 a | 0.0537 | 0.0005 |
| M + CG | 0.02 b | 0.02 a | 0.02 a | 0.04 a | 0.02 a | 0.0040 | 0.0001 |
| Means | 0.02 | 0.02 | 0.02 | 0.03 | ns | ns | |
| CV | 10.17 (2) | ||||||
| Microbial quotient (qMic) | |||||||
| MM | 0.011 ab | 0.009 b | 0.013 a | 0.009 a | 0.012 ab | ns | ns |
| M + AGG | 0.009 b | 0.009 b | 0.010 b | 0.009 a | 0.008 b | 0.0242 | 0.0003 |
| M + CG | 0.0141 a | 0.0148 a | 0.0133 a | 0.0051 b | 0.012 a | 0.0008 | 0.0001 |
| Means | 0.010 | 0.011 | 0.012 | 0.007 | ns | ns | |
| CV | 8.60 (2) | ||||||
| Microbial biomass nitrogen content (MBN, µg N g−1 soil) | |||||||
| MM | 62.47 a | 51.30 a | 53.57 a | 29.90 a | 49.31 a | 0.0001 | 0.0001 |
| M + AGG | 19.73 b | 40.58 b | 27.91 b | 25.85 ab | 28.52 b | ns | 0.0101 |
| M + CG | 59.38 a | 40.58 b | 27.91 b | 19.59 b | 46.60 a | 0.0001 | 0.0001 |
| Means | 47.19 | 44.15 | 36.46 | 25.11 | ns | ns | |
| CV | 5.89 (1) | ||||||
| Urease activity (UA, mg N-NH4 g−1 soil h−1) | |||||||
| MM | 8.58 a | 7.56 a | 6.70 a | 5.83 a | 7.17 a | 0.0057 | 0.0252 |
| M + AGG | 5.30 b | 6.11 a | 4.87 a | 4.94 a | 5.30 b | ns | ns |
| M + CG | 6.79 ab | 6.11 a | 4.87 a | 4.92 a | 5.87 b | ns | ns |
| Means | 6.89 | 6.59 | 5.48 | 5.23 | ns | ns | |
| CV | 11.48 (1) | ||||||
| Microbial biomass nitrogen: total soil nitrogen ratio (Nmic:Ntotal) | |||||||
| MM | 0.05 a | 0.03 a | 0.04 a | 0.02 a | 0.04 a | ns | ns |
| M + AGG | 0.01 b | 0.03 a | 0.02 b | 0.02 a | 0.02 b | ns | ns |
| M + CG | 0.04 a | 0.04 a | 0.03 ab | 0.01 a | 0.03 ab | 0.0001 | 0.0001 |
| Means | 0.03 | 0.03 | 0.03 | 0.02 | ns | ns | |
| CV | 13.54 (2) | ||||||
| Microbial carbon:microbial nitrogen ratio (Cmic:Nmic) | |||||||
| MM | 2.94 b | 3.34 a | 3.66 b | 4.25 a | 3.55 b | 0.0280 | ns |
| M + AGG | 4.72 a | 3.53 a | 5.60 a | 5.07 a | 4.73 a | ns | ns |
| M + CG | 3.66 ab | 4.18 a | 4.46 b | 4.12 a | 4.11 ab | ns | ns |
| Means | 3.77 | 3.68 | 4.57 | 4.48 | ns | ns | |
| CV | 8.91 (2) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, K.; Vilela, L.A.F. Tropical Grasses—Annual Crop Intercropping and Adequate Nitrogen Supply Increases Soil Microbial Carbon and Nitrogen. Agronomy 2023, 13, 1275. https://doi.org/10.3390/agronomy13051275
Batista K, Vilela LAF. Tropical Grasses—Annual Crop Intercropping and Adequate Nitrogen Supply Increases Soil Microbial Carbon and Nitrogen. Agronomy. 2023; 13(5):1275. https://doi.org/10.3390/agronomy13051275
Chicago/Turabian StyleBatista, Karina, and Laíze Aparecida Ferreira Vilela. 2023. "Tropical Grasses—Annual Crop Intercropping and Adequate Nitrogen Supply Increases Soil Microbial Carbon and Nitrogen" Agronomy 13, no. 5: 1275. https://doi.org/10.3390/agronomy13051275
APA StyleBatista, K., & Vilela, L. A. F. (2023). Tropical Grasses—Annual Crop Intercropping and Adequate Nitrogen Supply Increases Soil Microbial Carbon and Nitrogen. Agronomy, 13(5), 1275. https://doi.org/10.3390/agronomy13051275






