Effects of Biodegradable Plastic Mulch Film on Cabbage Agronomic and Nutritional Quality Traits, Soil Physicochemical Properties and Microbial Communities
Abstract
1. Introduction
2. Material and Methods
2.1. Plastic Mulch Films and Sample Collection
2.2. Determination of Agronomic and Nutritional Quality Traits and Physicochemical Properties
2.3. Soil DNA Extraction, PCR, and Sequencing
2.4. Bioinformatics Analysis
3. Results
3.1. Effects of BDM and PEM on Agronomic and Nutritional Quality Traits of Cabbage
3.2. Analysis of the Physicochemical Properties of Soil
3.3. Analysis of Soil Microbial Community Diversity
3.4. Alpha Diversity Comparison among Different Soil Samples
3.5. Beta Diversity Comparison among Different Soil Samples
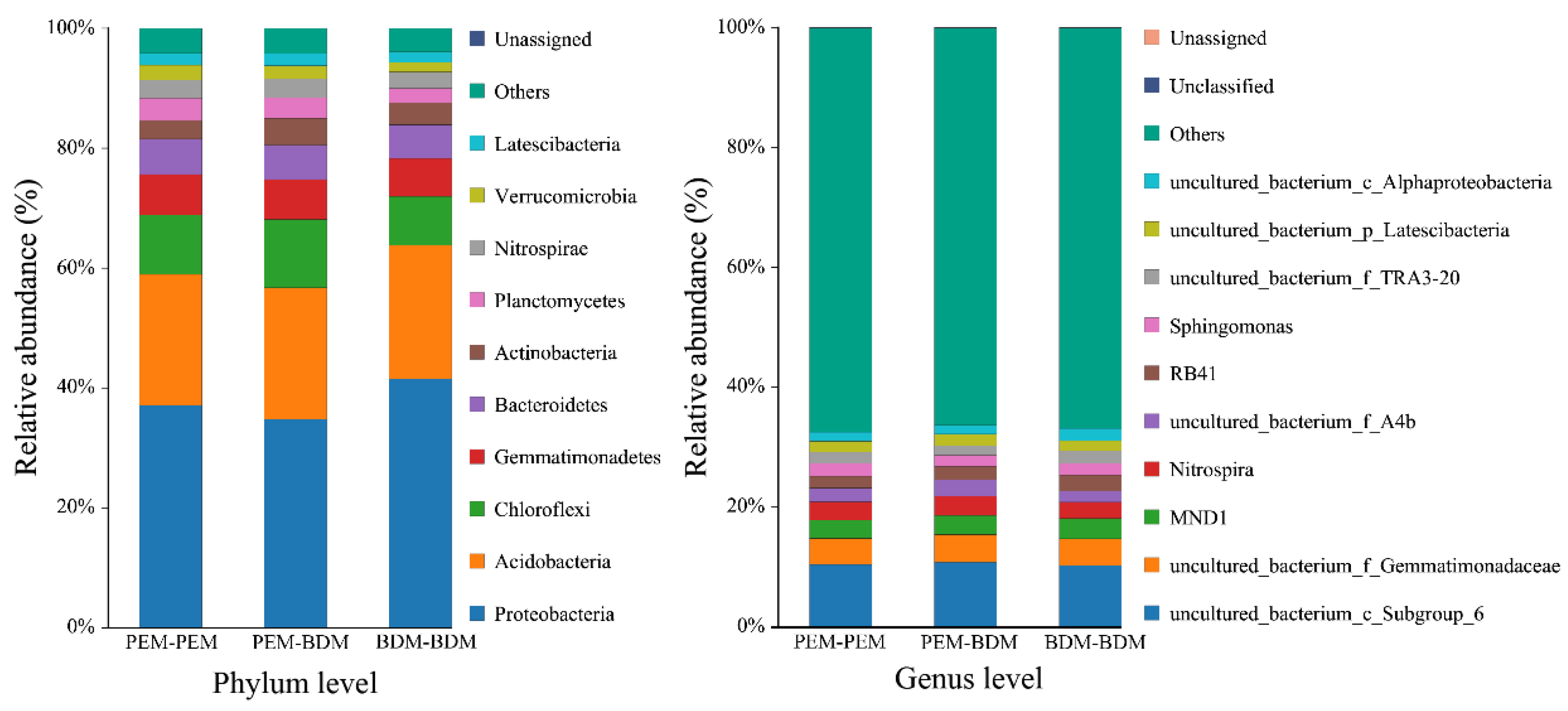
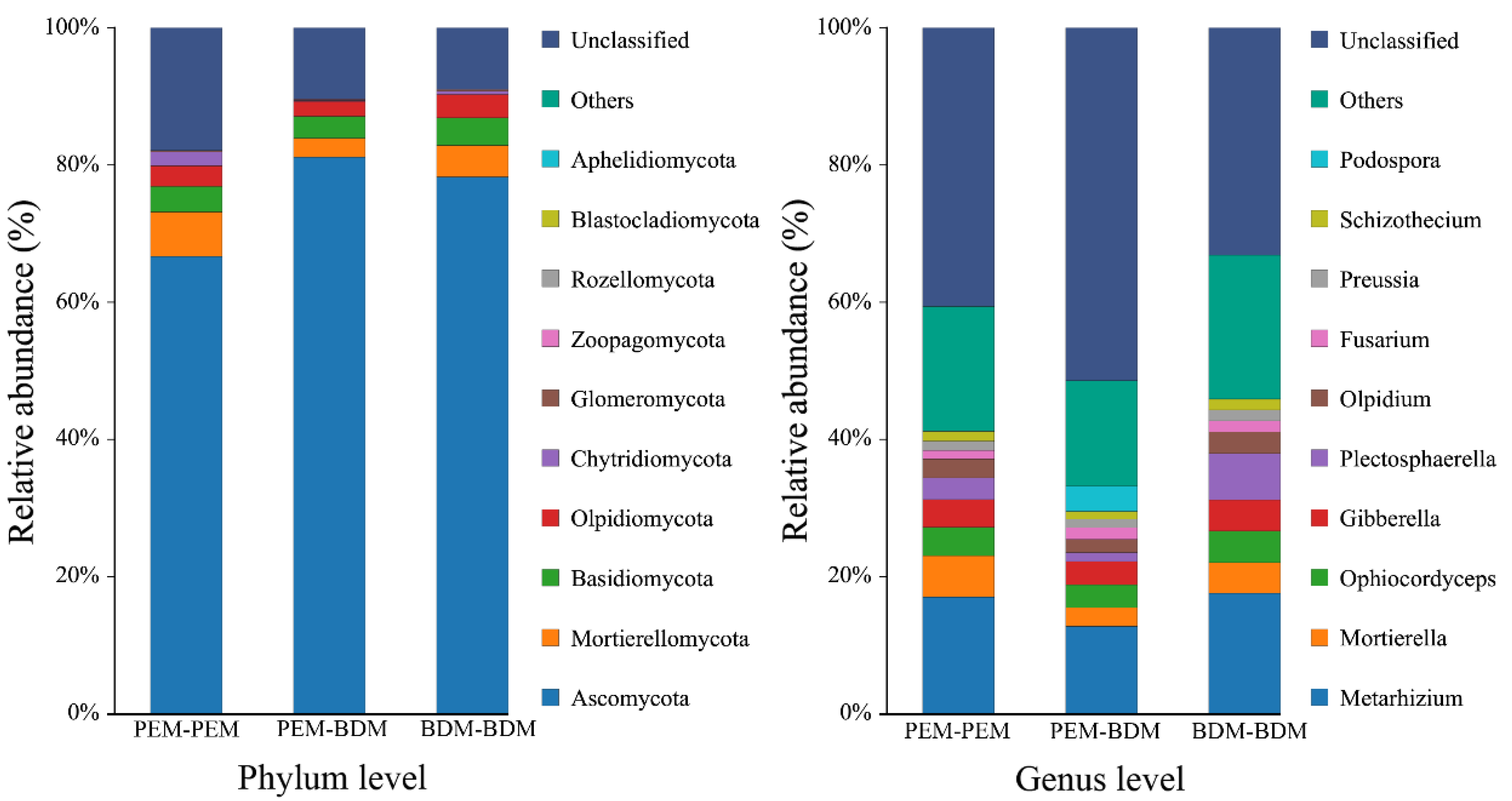
3.6. Effects of BDM and PEM on Soil Microbial Community Composition
3.7. The Cost–Benefit Analysis of Cabbage Production Covered with BDM and PEM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lamont, W.J. Plastics: Modifying the microclimate for the production of vegetable crops. HortTechnology 2005, 15, 477–481. [Google Scholar] [CrossRef]
- Kasirajan, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 168, 155–166. [Google Scholar] [CrossRef]
- Zong, R.; Han, H.; Li, Q. Grain yield and water-use efficiency of summer maize in response to mulching with different plastic films in the North China Plain. Exp. Agric. 2021, 57, 33–44. [Google Scholar] [CrossRef]
- Ngouajio, M.; Auras, R.; Fernandez, R.T.; Rubino, M.; Counts, J.W.; Kijchavengkul, T. Field performance of aliphatic-aromatic copolyester biodegradable mulch films in a fresh market tomato production system. HortTechnology 2008, 18, 605–610. [Google Scholar] [CrossRef]
- Jiang, R.; Li, X.; Zhou, M.; Li, H.J.; Zhao, Y. Plastic film mulching on soil water and maize (Zea mays L.) yield in a ridge cultivation system on Loess Plateau of China. Soil Sci. Plant Nutr. 2016, 621, 1–15. [Google Scholar] [CrossRef]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 99, 091001. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Wollmann, C.; Schaefer, M.; Buchmann, C.; David, J.; Tröger, J.; Muñoz, K.; Frör, O.; Schaumann, G.E. Plastic mulching in agriculture. Trading short-term agronomic benefits for long-term soil degradation? Sci. Total Environ. 2016, 550, 690–705. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable plastic mulch films: Impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Han, Y.; Teng, Y.; Wang, X.; Ren, W.; Wang, X.; Luo, Y.; Zhang, H.; Christie, P. Soil type driven change in microbial community affects poly(butylene adipate-co-terephthalate) degradation potential. Environ. Sci. Technol. 2021, 55, 4648–4657. [Google Scholar] [CrossRef]
- Yan, C.; He, W.; Turner, N. Plastic-film mulch in Chinese agriculture: Importance and problems. World Agric. 2014, 4, 32–36. [Google Scholar]
- Albertsson, A.C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Haider, T.P.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. Engl. 2019, 58, 50–62. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is biodegradable plastic mulch the solution to agriculture’s plastic problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Muroi, F.; Tachibana, Y.; Kobayashi, Y.; Sakurai, T.; Kasuya, K. Influences of poly(butylene adipate-co-terephthalate) on soil microbiota and plant growth. Polym. Degrad. Stab. 2016, 129, 338–346. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Mariappan Kumaresan, S.; Muthuraman, M.S.; Park, S.U. An update on polyethylene and biodegradable plastic mulch films and their impact on the environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef]
- Xue, Y.; Jin, T.; Gao, C.; Li, C.; Zhou, T.; Wan, D.; Yang, M. Effects of biodegradable film mulching on bacterial diversity in soils. Arch. Microbiol. 2022, 204, 195. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, W.; Dai, Z.; Li, J.; Mao, W.; Yu, F.; Ma, J.; Wang, S.; Zeng, X. Comparative analysis of the effects of plastic mulch films on soil nutrient, yields and soil microbiome in three vegetable fields. Agronomy 2022, 12, 506. [Google Scholar] [CrossRef]
- Li, C.; Moore-Kucera, J.; Miles, C.; Leonas, K.; Lee, J.; Corbin, A. Degradation of potentially biodegradable plastic mulch films at three diverse US locations. Agroecol. Sustain. Food Syst. 2014, 38, 861–889. [Google Scholar] [CrossRef]
- Sander, M. Biodegradation of polymeric mulch films in agricultural soils: Concepts, knowledge gaps, and future research directions. Environ. Sci. Technol. 2019, 53, 2304–2315. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Liquet, Y. González. J.E.; Henderson, K.B.; Anunciado, M.B.; Hayes, D.G.; DeBruyn, J.M. Soil microbial communities associated with biodegradable plastic mulch films. Front. Microbiol. 2020, 11, 587074. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.J. Biological degradation of synthetic polyesters-Enzymes as potential catalysts for polyester recycling. Process. Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Schintlmeister, A.; Nelson, T.F.; Baumgartner, R.; Woebken, D.; Wagner, M.; Kohler, H.E.; McNeill, K.; Sander, M. Biodegradation of synthetic polymers in soils: Tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018, 4, eaas9024. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Mo, A.; Jiang, J.; He, D. Degradation of polylactic acid/polybutylene adipate films in different ratios and the response of bacterial community in soil environments. Environ. Pollut. 2022, 313, 120167. [Google Scholar] [CrossRef] [PubMed]
- Saadi, Z.; Cesar, G.; Bewa, H.; Benguigui, L. Fungal degradation of poly(butylene adipate-co-terephthalate) in soil and in compost. J. Polym. Environ. 2013, 21, 893–901. [Google Scholar] [CrossRef]
- Šerá, J.; Stloukal, P.; Jancova, P.; Verney, V.; Pekarova, S.; Koutny, M. Accelerated biodegradation of agriculture film based on aromatic-aliphatic copolyester in soil under mesophilic conditions. J. Agric. Food Chem. 2016, 64, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.A.; Gauthier, E.; Colwell, J.M.; Halley, P.; Bottle, S.E.; Laycock, B.; Truss, R. The challenges in lifetime prediction of oxodegradable polyolefin and biodegradable polymer films. Polym. Degrad. Stabil. 2017, 145, 102–119. [Google Scholar] [CrossRef]
- Li, X.; Fang, Z. Descriptors and Data Standards for Cabbage (Brassica oleracea L. var. capitata L. and Brassica oleracea L. var. gemmifera Zenk); China Agriculture Press: Beijing, China, 2007. [Google Scholar]
- Pearson, D. The Chemical Analysis of Foods; Longman Group Ltd.: London, UK, 1976. [Google Scholar]
- Lu, R. Analysis Method of Agricultural Chemistry in Soil; Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Norman, R.J.; Edberg, J.C.; Stucki, J.W. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 1985, 49, 1182–1185. [Google Scholar] [CrossRef]
- Sinclair, L.; Osman, O.A.; Bertilsson, S.; Eiler, A. Microbial community composition and diversity via 16S rRNA gene amplicons: Evaluating the illumina platform. PLoS ONE 2015, 10, e0116955. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved bacterial 16S rRNA gene (V4 and V4–5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. Msystems 2015, 1, e00009-15. [Google Scholar] [CrossRef]
- Edgar, R. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.W.; Roberts, M.D.W. Package ‘labdsv’. Ordination Multivar. 2016, 775, 1–68. [Google Scholar]
- Kader, M.A.; Singha, A.; Begum, M.A.; Jewel, A.; Khan, F.H.; Khan, N.I. Mulching as water-saving technique in dryland agriculture. Bull. Natl. Res. Cent. 2019, 43, 147. [Google Scholar] [CrossRef]
- Hanson, B.D.; Gerik, J.S.; Schneider, S.M. Effects of reduced-rate methyl bromide applications under conventional and virtually impermeable plastic film in perennial crop field nurseries. Pest Manag. Sci. 2010, 66, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Feng, F.; Zhao, C.; Yu, A.; Hu, F.; Chai, Q.; Gan, Y.; Guo, Y. Integrated double mulching practices optimizes soil temperature and improves soil water utilization in arid environments. Int. J. Biometeorol. 2016, 60, 1423–1437. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of microplastics and plastic film residues in the soil environment: A critical review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Tofanelli, M.B.D.; Wortman, S.E. Benchmarking the agronomic performance of biodegradable mulches against polyethylene mulch film: A meta-analysis. Agronomy 2020, 10, 1618. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Flury, M.; Schaeffer, S.M.; Chang, Y.; Tao, Z.; Jia, Z.; Li, S.; Ding, F.; Wang, J. Agronomic performance of polyethylene and biodegradable plastic film mulches in a maize cropping system in a humid continental climate. Sci. Total Environ. 2021, 786, 147460. [Google Scholar] [CrossRef]
- Li, F.M.; Song, Q.H.; Jjemba, P.K.; Shi, Y.C. Dynamics of soil microbial biomass C and soil fertility in cropland mulched with plastic film in a semiarid agro-ecosystem. Soil Biol. Biochem. 2004, 3611, 1893–1902. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, J.; Yao, Z.; Luo, S.; Tian, L.; Tian, C.; Sun, Y. Preliminary findings of polypropylene carbonate (PPC) plastic film mulching effects on the soil microbial community. Agriculture 2022, 12, 406. [Google Scholar] [CrossRef]
- Kapanen, A.; Schettini, E.; Giuliano, V.; Itävaara, M. Performance and environmental impact of biodegradable films in agriculture: A field study on protected cultivation. J. Polym. Environ. 2008, 16, 109–122. [Google Scholar] [CrossRef]
- Moreno, M.M.; Moreno, A. Effect of different biodegradable and polyethylene mulches on soil properties and production in a tomato crop. Sci. Hortic. 2008, 116, 256–263. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bandopadhyay, S.; English, M.E.; Bary, A.I.; DeBruyn, J.M.; Schaeffer, S.M.; Miles, C.A.; Reganold, J.P.; Flury, M. Impacts of biodegradable plastic mulches on soil health. Agric. Ecosyst. Environ. 2019, 273, 36–49. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, Y.; Jin, T.; Zhang, K.; Li, Z.; Sun, C.; Mi, Q.; Li, Q. Effect of long-term biodegradable film mulch on soil physicochemical and microbial properties. Toxics 2022, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, H.; Gu, J.; Liao, B.; Zhang, J.; Wu, P. Application of rapeseed residue increases soil organic matter, microbial biomass, and enzyme activity and mitigates cadmium pollution risk in paddy fields. Environ. Pollut. 2020, 264, 114681. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crops Res. 2006, 95, 115–125. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Xing, Y. Effects of mulching and nitrogen on soil temperature, water content, nitrate-N content and maize yield in the Loess Plateau of China. Agric. Water Manag. 2015, 161, 53–64. [Google Scholar]
- Müller, C.A.; Perz, V.; Provasnek, C.; Quartinello, F.; Guebitz, G.M.; Berg, G. Discovery of polyesterases from moss-associated microorganisms. Appl. Environ. Microbiol. 2017, 83, e02641-16. [Google Scholar] [CrossRef] [PubMed]
- Perz, V.; Hromic, A.; Baumschlager, A.; Steinkellner, G.; Pavkov-Keller, T.; Gruber, K.; Bleymaier, K.; Zitzenbacher, S.; Zankel, A.; Mayrhofer, C.; et al. An esterase from anaerobic Clostridium hathewayi can hydrolyze aliphatic-aromatic polyesters. Environ. Sci. Technol. 2016, 50, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Sintim, H.Y.; Debruyn, J.M. Structural and functional responses of soil microbial communities to biodegradable plastic film mulching in two agroecosystems. PeerJ 2020, 8, e9015. [Google Scholar] [CrossRef] [PubMed]

| Treatment | First Year (2021) | Second Year (2022) |
|---|---|---|
| PEM-PEM | PEM covered (removed after cabbage harvest) | PEM covered (removed after cabbage harvest) |
| PEM-BDM | PEM covered (removed after cabbage harvest) | BDM covered (tilled after cabbage harvest) |
| BDM-BDM | BDM covered (tilled after cabbage harvest) | BDM covered (tilled after cabbage harvest) |
| Trait | PEM-PEM | PEM-BDM | BDM-BDM |
|---|---|---|---|
| Head weight (kg) | 2.32 ± 0.16 a | 2.21 ± 0.12 a | 2.19 ± 0.08 a |
| Head vertical diameter (cm) | 17.50 ± 1.06 a | 18.37 ± 0.85 a | 19.07 ± 1.26 a |
| Head transverse diameter (cm) | 20.50 ± 0.66 a | 19.50 ± 1.35 a | 18.90 ± 1.67 a |
| Core length (cm) | 6.77 ± 0.91 a | 7.20 ± 0.46 a | 6.97 ± 0.57 a |
| Core width (cm) | 3.37 ± 0.40 a | 3.53 ± 0.32 a | 3.67 ± 0.31 a |
| Yield (ton/ha) | 87.84 ± 1.31 a | 84.68 ± 1.76 a | 86.74 ± 1.55 a |
| Total soluble solids (%) | 5.86 ± 0.11 a | 5.57 ± 0.32 a | 6.05 ± 0.22 a |
| Soluble sugar (mg/g DW) | 251.70 ± 14.88 a | 245.30 ± 13.12 a | 254.13 ± 1.67 a |
| Soluble protein (mg/g FW) | 1.32 ± 0.12 a | 1.43 ± 0.14 a | 1.57 ± 0.10 a |
| Vitamin C (mg/100g FW) | 74.66 ± 4.21 a | 78.13 ± 5.87 a | 81.07 ± 3.98 a |
| Treatment | pH Value | Nitrate Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) | Organic Matter (g/kg) | Total Nitrogen (%) |
|---|---|---|---|---|---|---|
| PEM-PEM | 5.93 ± 0.29 a | 37.20 ± 6.06 a | 24.00 ± 4.03 a | 64.33 ± 9.50 a | 24.03 ± 0.55 b | 0.18 ± 0.04 a |
| PEM-BDM | 5.27 ± 0.31 b | 30.47 ± 6.23 a | 33.57 ± 6.84 a | 59.33 ± 7.02 a | 30.47 ± 1.42 a | 0.20 ± 0.03 a |
| BDM-BDM | 5.27 ± 0.23 b | 33.37 ± 7.60 a | 30.93 ± 6.34 a | 56.00 ± 8.72 a | 32.23 ± 1.44 a | 0.21 ± 0.03 a |
| Type | Sample | ACE | Chao1 | Simpson | Shannon |
|---|---|---|---|---|---|
| Bacteria | PEM-PEM | 2192.48 ± 4.89 a | 2201.92 ± 7.68 a | 0.0022 ± 0.0001 a | 6.87 ± 0.01 a |
| PEM-BDM | 2191.97 ± 20.84 a | 2200.62 ± 18.80 a | 0.0022 ± 0.0003 a | 6.87 ± 0.07 a | |
| BDM-BDM | 2178.28 ± 20.62 a | 2191.58 ± 15.57 a | 0.0026 ± 0.0002 a | 6.75 ± 0.06 a | |
| Fungi | PEM-PEM | 843.36 ± 17.82 b | 849.02 ± 15.89 a | 0.0433 ± 0.0133 a | 4.58 ± 0.08 a |
| PEM-BDM | 877.66 ± 13.75 a | 884.97 ± 7.82 a | 0.0820 ± 0.0694 a | 4.26 ± 0.76 a | |
| BDM-BDM | 863.75 ± 15.86 ab | 877.72 ± 29.57 a | 0.0553 ± 0.0051 a | 4.44 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ma, J.; Cui, Z.; Xu, L.; Liu, Q.; Li, J.; Wang, S.; Zeng, X. Effects of Biodegradable Plastic Mulch Film on Cabbage Agronomic and Nutritional Quality Traits, Soil Physicochemical Properties and Microbial Communities. Agronomy 2023, 13, 1220. https://doi.org/10.3390/agronomy13051220
Zhang W, Ma J, Cui Z, Xu L, Liu Q, Li J, Wang S, Zeng X. Effects of Biodegradable Plastic Mulch Film on Cabbage Agronomic and Nutritional Quality Traits, Soil Physicochemical Properties and Microbial Communities. Agronomy. 2023; 13(5):1220. https://doi.org/10.3390/agronomy13051220
Chicago/Turabian StyleZhang, Wei, Jinjun Ma, Zhongli Cui, Langtao Xu, Qian Liu, Jianbin Li, Shenyun Wang, and Xiaoping Zeng. 2023. "Effects of Biodegradable Plastic Mulch Film on Cabbage Agronomic and Nutritional Quality Traits, Soil Physicochemical Properties and Microbial Communities" Agronomy 13, no. 5: 1220. https://doi.org/10.3390/agronomy13051220
APA StyleZhang, W., Ma, J., Cui, Z., Xu, L., Liu, Q., Li, J., Wang, S., & Zeng, X. (2023). Effects of Biodegradable Plastic Mulch Film on Cabbage Agronomic and Nutritional Quality Traits, Soil Physicochemical Properties and Microbial Communities. Agronomy, 13(5), 1220. https://doi.org/10.3390/agronomy13051220





