First Report of Resistance to Glyphosate in Several Species of the Genus Echinochloa in Argentina
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Survival
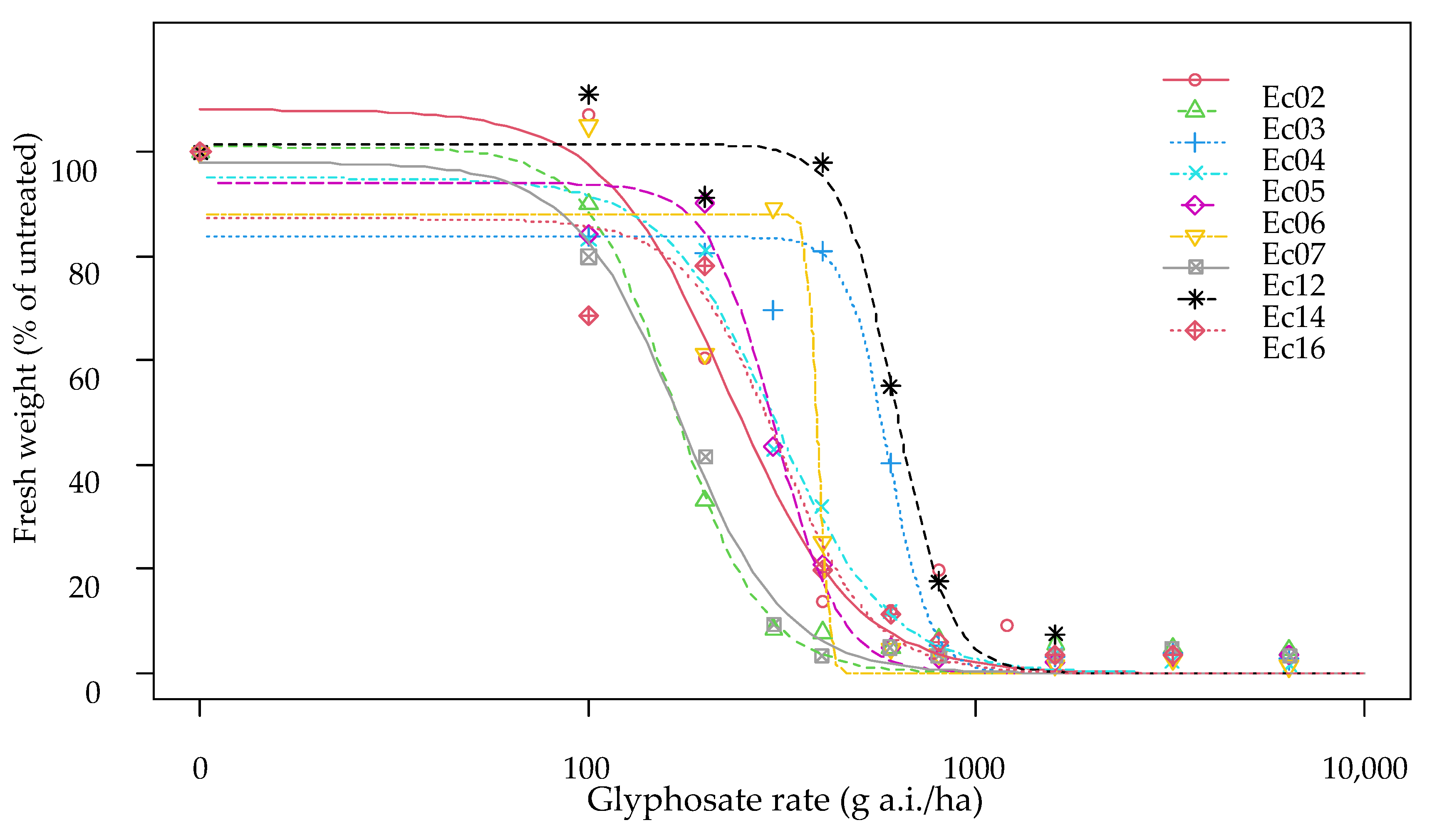
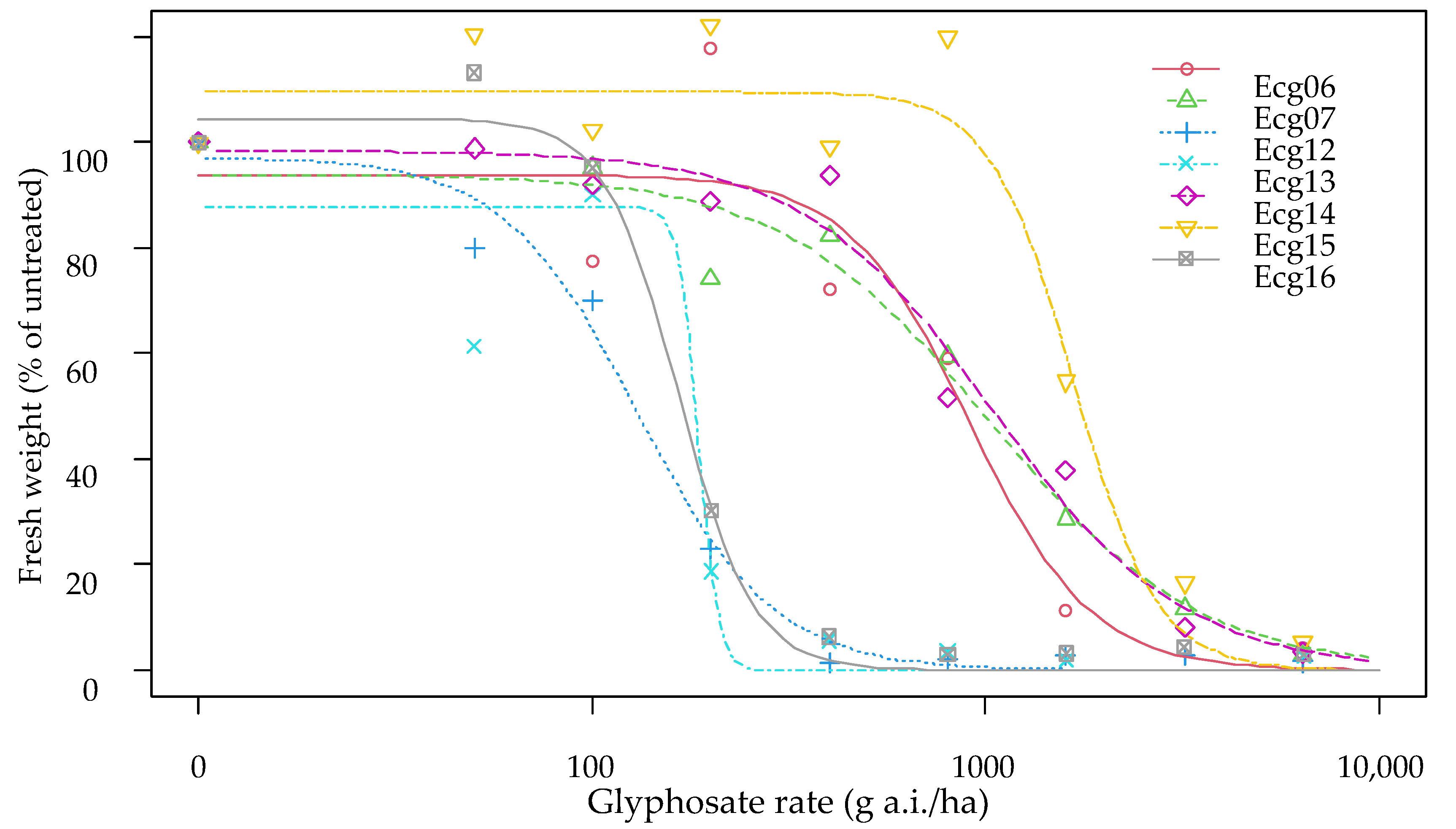
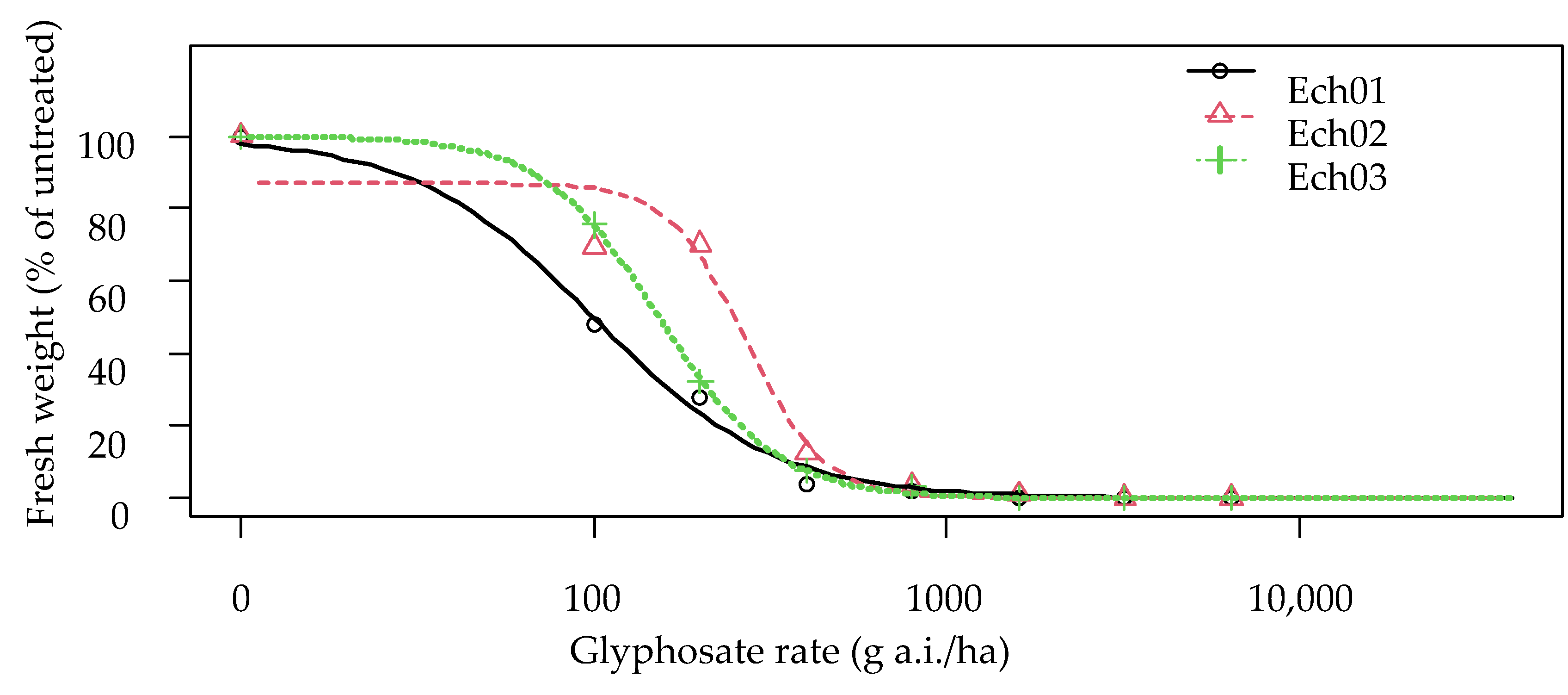
3.2. Dose Response Trials
3.2.1. Echinochloa colona
3.2.2. Echinochloa crus-galli
3.2.3. Echinochloa oryzoides
3.2.4. Echinochloa chacoensis
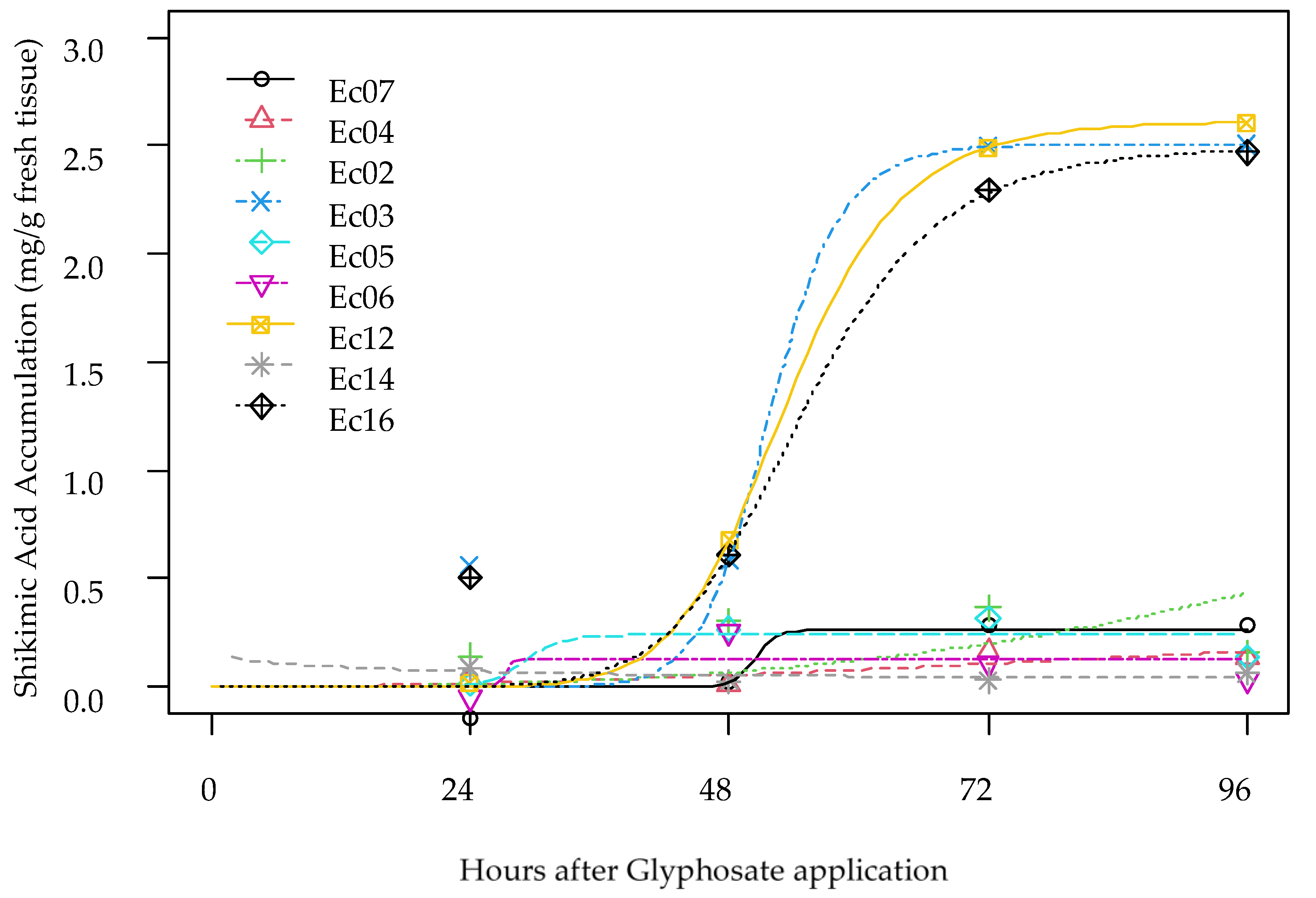
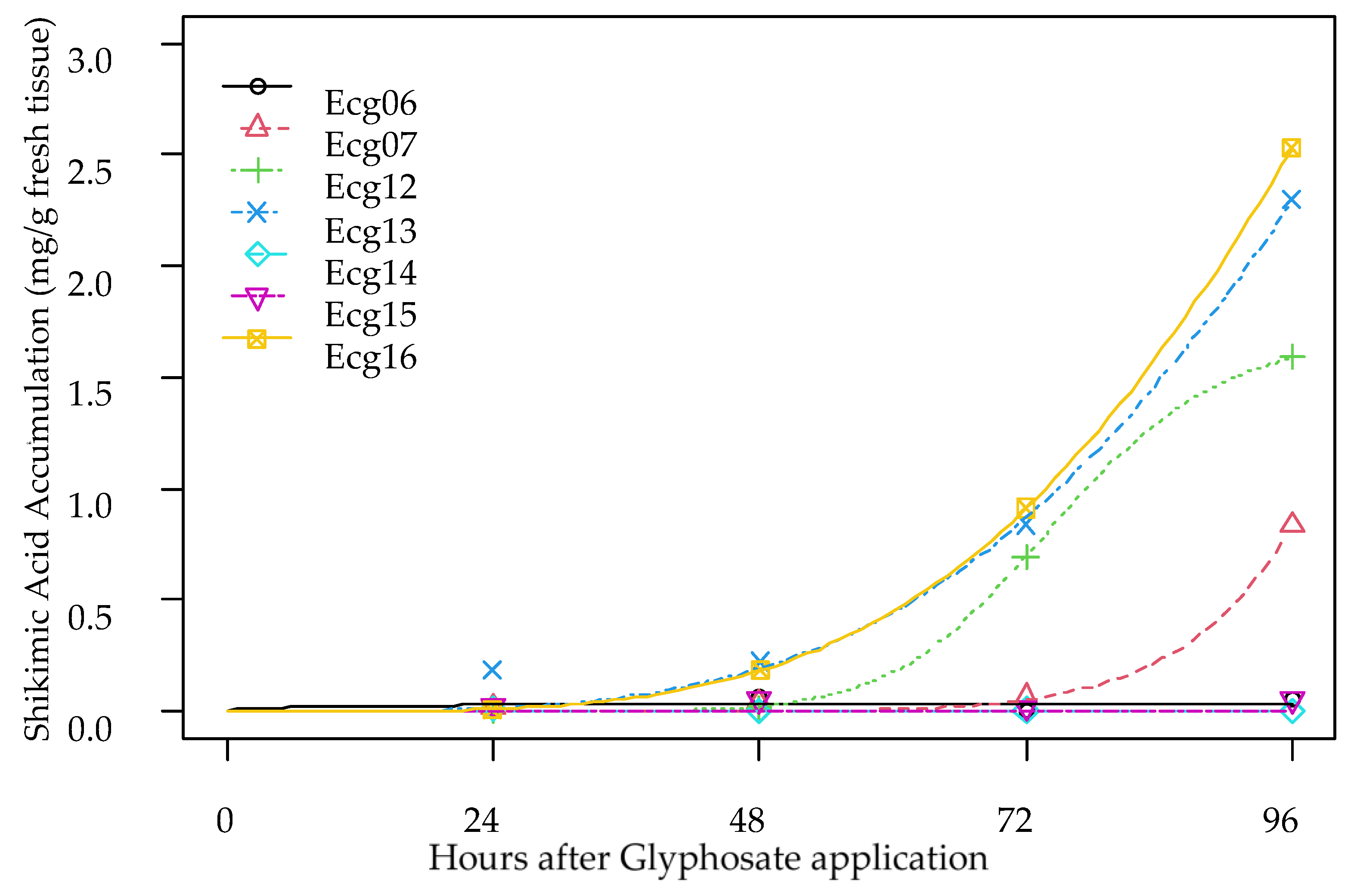
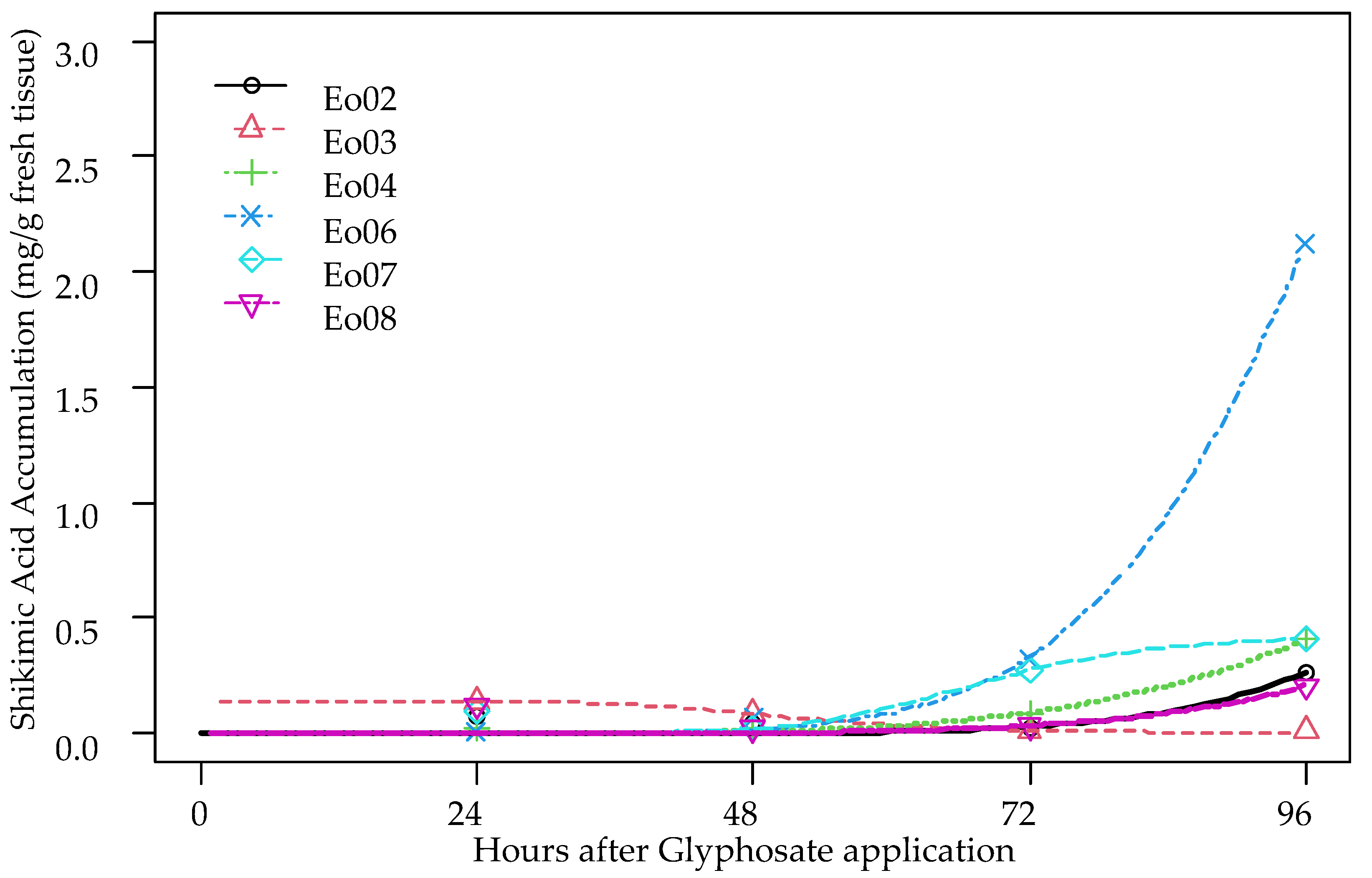
3.3. Shikimic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Teisher, J.K.; Clark, L.G.; Barberá, P.; Gillespie, L.J.; Zuloaga, F.O. A Worldwide Phylogenetic Classification of the Poaceae (Gramineae) II: An Update and a Comparison of Two 2015 Classifications: Phylogenetic Classification of the Grasses II. Jnl. Sytematics Evol. 2017, 55, 259–290. [Google Scholar] [CrossRef]
- Belgrano, M.J.; Morrone, O.; Zuloaga, F.O. Catálogo de Las Plantas Vasculares del Cono Sur: (Argentina, Sur de Brasil, Chile, Paraguay y Uruguay); Catalog of the Vascular Plants of the Southern Cone; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; ISBN 978-1-930723-76-4. [Google Scholar]
- Zuloaga, F.O.; Belgrano, M.J.; Zanotti, C.A. Actualización del Catálogo de las Plantas Vasculares del Cono Sur. Darwiniana Nueva Ser. 2019, 7, 208–278. [Google Scholar] [CrossRef]
- Picapietra, G.; Ponsa, J.C. Competencia y Manejo de Capín de Arroz En El Cultivo de Soja|Instituto Nacional de Tecnología Agropecuaria. Informe Técnico INTA. Ediciones INTA. 2015. [Google Scholar]
- Holm, L.G. The World’s Worst Weeds: Distribution and Biology; Krieger: Malabar, FL, USA, 1991; ISBN 978-0-89464-415-3. [Google Scholar]
- Pautasso, L. Pérdida del Rendimiento del Cultivo de Soja por la Presencia de Rama Negra y Capín; Serie Extensión No 76 Actualización Técnica Soja; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2015; pp. 87–91. [Google Scholar]
- Chin, D.V. Biology and Management of Barnyardgrass, Red Sprangletop and Weedy Rice. Weed Biol. Manag. 2001, 1, 37–41. [Google Scholar] [CrossRef]
- REM Malezas: Continúa El Avance de Las Resistencias. Available online: https://www.aapresid.org.ar (accessed on 14 October 2022).
- Papa, J.C.; Tuesca, D.; Bacigaluppo, D. Detección Reciente en la Provincia de Santa Fe de Biotipos de Echinochloa colona Sospechosos de Presentar Resistencia a Glifosato. Para Mejorar la Producción 45. Revista Cultivos Estivales. Inta Oliveros; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2010; pp. 91–94. [Google Scholar]
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladha, J.K.; Mortimer, A.M. Weed Management in Direct-Seeded Rice. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2007; Volume 93, pp. 153–255. [Google Scholar]
- Damalas, C.A.; Dhima, K.V.; Eleftherohorinos, I.G. Morphological and Physiological Variation among Species of the Genus Echinochloa in Northern Greece. Weed Sci. 2008, 56, 416–423. [Google Scholar] [CrossRef]
- Yabuno, T. Biology of Echinochloa Species. In Proceedings of the Conference on Weed Control in Rice, Los Baños, Philippines, 31 August–4 September 1981; International Rice Research Institute: Los Baños, Philippines, 1983; pp. 307–318. [Google Scholar]
- Pfitscher, E.M.; Barreto, I.L. Species of the genus Echinochloa (Gramineae) occurring in Rio Grande do Sul (Brazil). Anu. Tec. Do Inst. De Pesqui. Zootec. Fr. Osorio 1976, 3, 245–289. [Google Scholar]
- Martínez Crovetto, R. Las Gramíneas Argentinas Del Género Echinochloa. Rev. Argent. Agron. 1942, 9, 310–342. [Google Scholar]
- Renvoize, S. A new species of Echinochloa (Poaceae) from Bolivia and Northern Argentina. Kurtziana 1995, 24, 161–163. [Google Scholar]
- Sudershan, M.; Guru, S.K.; Nitin, K.; Prinsa, R.; Babita, J. Herbicide Resistance: A Threat to Herbicide Utility and Sustainability. In Phytoremediation of Heavy Metals: A Green Technology to Clean Environment; Volume Advances in Agriculture Sciences; Anik Publication: New Delhi, India, 2020; pp. 115–129. ISBN 978-93-88112-21-5. [Google Scholar]
- WFO. World Flora Online. Available online: http://www.worldfloraonline.org (accessed on 20 December 2022).
- Heap, I.M. The International Survey of Herbicide Resistant Weeds. Available online: weedscience.org (accessed on 14 October 2022).
- Leguizamón, E. Rama Negra Conyza bonariensis (L. Cronquist) Bases para su Manejo y Control en Sistemas de Producción; Manejo de Malezas Problema; REM Aapresid: Rosario, Argentina, 2011. [Google Scholar]
- Panigo, E.S.; Dellaferrera, I.M.; Acosta, J.M.; Bender, A.G.; Garetto, J.I.; Perreta, M.G. Glyphosate-Induced Structural Variations in Commelina Erecta, L. (Commelinaceae). Ecotoxicol. Environ. Saf. 2012, 76, 135–142. [Google Scholar] [CrossRef]
- González-Torralva, F.; Norsworthy, J.K.; Piveta, L.B.; Varanasi, V.K.; Barber, T.; Brabham, C. Susceptibility of Arkansas Palmer Amaranth Accessions to Common Herbicide Sites of Action. Weed Technol. 2020, 34, 770–775. [Google Scholar] [CrossRef]
- Busi, R.; Beckie, H.J. Are Herbicide Mixtures Unaffected by Resistance? A Case Study with Lolium Rigidum. Weed Res. 2021, 61, 92–99. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J.C. Bioassay Analysis Using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R Software Package for Dose-Response Studies: The Concept and Data Analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Singh, B.; Shaner, D. Rapid Determination of Glyphosate Injury to Plants and Identification of Glyphosate-Resistant Plants. Weed Technol. 1998, 12, 527–530. [Google Scholar] [CrossRef]
- Streibig, J.C.; Kudsk, P. Herbicide Bioassays; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Alarcón-Reverte, R.; García, A.; Urzúa, J.; Fischer, A.J. Resistance to Glyphosate in Junglerice (Echinochloa Colona) from California. Weed Sci. 2013, 61, 48–54. [Google Scholar] [CrossRef]
- Alarcón-Reverte, R.; García, A.; Watson, S.B.; Abdallah, I.; Sabaté, S.; Hernández, M.J.; Dayan, F.E.; Fischer, A.J. Concerted Action of Target-Site Mutations and High EPSPS Activity in Glyphosate-Resistant Junglerice (Echinochloa Colona) from California. Pest Manag. Sci. 2015, 71, 996–1007. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Koetz, E.; Wu, H.; Hopwood, M.; Shephard, A. Fate and Adaptive Plasticity of Heterogeneous Resistant Population of Echinochloa Colona in Response to Glyphosate. Sci. Rep. 2021, 11, 14858. [Google Scholar] [CrossRef]
- Burgos, N.R.; Tranel, P.J.; Streibig, J.C.; Davis, V.M.; Shaner, D.; Norsworthy, J.K.; Ritz, C. Review: Confirmation of Resistance to Herbicides and Evaluation of Resistance Levels. Weed Sci. 2013, 61, 4–20. [Google Scholar] [CrossRef]
- Gaines, T.A.; Cripps, A.; Powles, S.B. Evolved Resistance to Glyphosate in Junglerice (Echinochloa Colona) from the Tropical Ord River Region in Australia. Weed Technol. 2012, 26, 480–484. [Google Scholar] [CrossRef]
- Morran, S.; Moretti, M.L.; Brunharo, C.A.; Fischer, A.J.; Hanson, B.D. Multiple Target Site Resistance to Glyphosate in Junglerice (Echinochloa Colona) Lines from California Orchards. Pest Manag. Sci. 2018, 74, 2747–2753. [Google Scholar] [CrossRef]
- Nguyen, T.H. Evolution and Spread of Glyphosate Resistant Barnyard Grass (Echinochloa Colona (L.) Link) from Australia; School of Agriculture, Food and Wine Faculty of Sciences, The University of Adelaide: Adelaide, Australia, 2015. [Google Scholar]
- Travlos, I.; Kanatas, P.; Tsekoura, A.; Gazoulis, I.; Papastylianou, P.; Kakabouki, I.; Antonopoulos, N. Efficacy of Different Herbicides on Echinochloa Colona (L.) Link Control and the First Case of Its Glyphosate Resistance in Greece. Agronomy 2020, 10, 1056. [Google Scholar] [CrossRef]
- Mahajan, G.; Kaur, V.; Thompson, M.; Chauhan, B.S. Growth Behavior and Glyphosate Resistance Level in 10 Populations of Echinochloa Colona in Australia. PLoS ONE 2020, 15, e0221382. [Google Scholar] [CrossRef] [PubMed]
- Talbert, R.E.; Burgos, N.R. History and Management of Herbicide-Resistant Barnyardgrass (Echinochloa Crus-Galli) in Arkansas Rice. Weed Technol. 2007, 21, 324–331. [Google Scholar] [CrossRef]
- Vázquez-García, J.G.; Rojano-Delgado, A.M.; Alcántara-de la Cruz, R.; Torra, J.; Dellaferrera, I.; Portugal, J.; De Prado, R. Distribution of Glyphosate-Resistance in Echinochloa crus-galli across Agriculture Areas in the Iberian Peninsula. Front. Plant Sci. 2021, 12, 33. [Google Scholar] [CrossRef]


| Population | Species | Crop in Which it Was Found | Location (Town) | Province | GPS Location | Years of Agriculture | Date of Harvest |
|---|---|---|---|---|---|---|---|
| Ec02 | E. colona | Soybean-Corn | Manfredi | Córdoba | −63.76988 −31.84076 | 20 | 2015 |
| Ec03 | E. colona | Soybean-Corn | Colonia Marina | Córdoba | −62.41800 −31.21785 | 20 | 2017 |
| Ec04 | E. colona | Soybean-Corn | Freyre | Córdoba | −62.0710 −31.17283 | 20 | 2015 |
| Ec05 | E. colona | Soybean-Corn | Luxardo | Córdoba | −62.25967 −31.27033 | 10 | 2015 |
| Ec06 | E. colona | Soybean-Corn | Estación Luxardo | Córdoba | 62.09588 −31.31240 | 20 | 2017 |
| Ec07 | E. colona | Soybean-Corn | Colonia Castelar | Santa Fe | 62.13270 −31.65483 | 20 | 2015 |
| Ec12 | E. colona | Soybean-Corn | San Francisco | Córdoba | −62.18506 −31.39578 | 20 | 2016 |
| Ec14 | E. colona | Soybean-Corn | Arias | Córdoba | −62.45239 −33.62968 | 15 | 2017 |
| Ec16 | E. colona | Soybean-Corn | El Paraíso | Buenos Aires | −59.96448 −33.58911 | 15 | 2017 |
| Ecg06 | E. crus-galli | Soybean-Corn | Colonia Mascias | Santa Fe | −60.00472 −30.78352 | 10 | 2016 |
| Ecg07 | E. crus-galli | Soybean-Corn | Saladero Cabal | Santa Fe | −60.05598 −30.88797 | 10 | 2016 |
| Ecg12 | E. crus-galli | Soybean | Santa Anita | Entre Ríos | −58.86091 −32.15184 | 10 | 2016 |
| Ecg13 | E. crus-galli | Rice | Villa Elisa | Entre Ríos | −58.38793 −32.17088 | 15 | 2016 |
| Ecg14 | E. crus-galli | Corn | Chajarí | Entre Ríos | −57.95368 −30.76185 | 15 | 2016 |
| Ecg15 | E. crus-galli | Soybean | San Salvador | Entre Ríos | −58.54736 −31.64929 | 15 | 2016 |
| Ecg16 | E. crus-galli | Not agricultural | C. del Uruguay | Entre Ríos | −58.24531 −32.42552 | ---- | 2016 |
| Eo02 | E. oryzoides | Soybean-Corn | Colonia Mascías | Santa Fe | −60.02201 −30.83483 | 15 | 2015 |
| Eo03 | E. oryzoides | Not agricultural | Colonia Mascías | Santa Fe | −59.99370 −30.83492 | ----- | 2015 |
| Eo04 | E. oryzoides | Soybean-Corn | Colonia Mascías | Santa Fe | −60.10391 −30.75559 | 15 | 2016 |
| Eo06 | E. oryzoides | Rice | Colonia Mascías | Santa Fe | −60.1063 −30.79600 | 15 | 2016 |
| Eo07 | E. oryzoides | Soybean-Corn | Colonia Mascías | Santa Fe | −60.1063 −30.67708 | 15 | 2016 |
| Eo08 | E. oryzoides | Soybean-Corn | Colonia Mascías | Santa Fe | −60.20002 −30.81921 | 15 | 2016 |
| Ech01 | E. chacoensis | Soybean-Corn | Colonia Mascías | Santa Fe | −59.99849 −30.83808 | 10 | 2016 |
| Ech02 | E. chacoensis | Soybean-Corn | Colonia Mascías | Santa Fe | −60.12071 −30.8313 | 10 | 2016 |
| Ech03 | E. chacoensis | Not agricultural | Colonia Mascías | Santa Fe | −60.08638 −30.7233 | 10 | 2016 |
| Population | b | d | GR50 | se | RF 1 | p-Value * | GR80 | se |
|---|---|---|---|---|---|---|---|---|
| Ec02 | 2.585585 | 1.065524 | 224.276 | 23.825 | 1.35 | 0.0218 | 383.39 | 61.27 |
| Ec03 | 3.726338 | 1.008724 | 165.993 | 18.160 | 1.00 | - | 240.80 | 26.87 |
| Ec04 | 10.74425 | 0.746329 | 610.123 | 30.627 | 3.68 | <0.0001 | 694.15 | 78.72 |
| Ec05 | 2.569705 | 0.957845 | 292.053 | 40.473 | 1.76 | <0.0001 | 500.90 | 76.89 |
| Ec06 | 5.147392 | 0.933955 | 300.983 | 19.148 | 1.81 | <0.0001 | 394.01 | 38.05 |
| Ec07 | 24.22164 | 0.871925 | 385.122 | 39.361 | 2.32 | <0.0001 | 407.81 | 22.27 |
| Ec12 | 3.294016 | 0.984736 | 175.349 | 23.188 | 1.06 | 0.7428 | 267.10 | 29.37 |
| Ec14 | 5.708362 | 0.926718 | 608.017 | 24.187 | 3.66 | <0.0001 | 775.15 | 47.18 |
| Ec16 | 1.635326 | 0.985857 | 187.777 | 37.313 | 1.13 | 0.5632 | 438.33 | 84.83 |
| Population | b | d | GR50 | se | RF 1 | p-Value * | GR80 | se |
|---|---|---|---|---|---|---|---|---|
| Ecg06 | 2.657 | 91.137 | 884.881 | 138.486 | 6.91 | <0.0001 | 1491.045 | 359.492 |
| Ecg07 | 1.595 | 93.014 | 984.309 | 241.805 | 7.72 | <0.0001 | 2347.048 | 600.342 |
| Ecg12 | 2.405 | 97.351 | 127.408 | 20.451 | 1.00 | - | 226.77 | 44.102 |
| Ecg13 | 17.329 | 87.430 | 185.437 | 127.837 | 1.45 | 0.62128 | 200.881 | 11.082 |
| Ecg14 | 1.776 | 98.269 | 1039.178 | 172.755 | 8.15 | <0.0001 | 2267.936 | 524.427 |
| Ecg15 | 4.070 | 97.405 | 1667.596 | 190.313 | 13.09 | <0.0001 | 2344.365 | 615.555 |
| Ecg16 | 4.145 | 102.580 | 160.410 | 17.114 | 1.26 | 0.18881 | 224.119 | 29.17 |
| Population | b | d | GR50 | se | RF 1 | p-Value * | GR80 | se |
|---|---|---|---|---|---|---|---|---|
| Eo02 | 1.400 | 95.449 | 3.006.63 | 615.244 | 8.53 | 0.0003 | 8090.58 | 1.798.65 |
| Eo03 | 0.988 | 98.007 | 3.312.02 | 856.805 | 9.39 | 0.0023 | 13463.87 | 3.820.10 |
| Eo04 | 1.984 | 112.915 | 804.93 | 117.447 | 2.28 | 0.0045 | 1619.15 | 450.23 |
| Eo06 | 4.295 | 104.756 | 352.54 | 45.943 | 1 | - | 486.83 | 83.65 |
| Eo07 | 1.282 | 96.955 | 3.070.23 | 981.319 | 8.71 | 0.00001 | 9055.23 | 3.455.12 |
| Eo08 | 1.488 | 96.658 | 5.107.50 | 1.050.115 | 14.49 | 0.00001 | 12969.33 | 3.078.01 |
| Population | b | d | GR50 | se | RF 1 | p-Value * | GR80 | se |
|---|---|---|---|---|---|---|---|---|
| Ech01 | 1.708 | 99.45 | 99.67 | 13.13 | 1.00 | - | 224.420 | 31.045 |
| Ech02 | 4.004 | 86.91 | 269.36 | 21.08 | 2.70 | <0.0001 | 380.785 | 39.791 |
| Ech03 | 2.622 | 99.93 | 152.44 | 12.63 | 1.53 | 0.00101 | 258.623 | 27.930 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, E.; Schneider, A.; Panigo, E.; Perreta, M.; De Prado, R.; Dellaferrera, I. First Report of Resistance to Glyphosate in Several Species of the Genus Echinochloa in Argentina. Agronomy 2023, 13, 1219. https://doi.org/10.3390/agronomy13051219
Cortés E, Schneider A, Panigo E, Perreta M, De Prado R, Dellaferrera I. First Report of Resistance to Glyphosate in Several Species of the Genus Echinochloa in Argentina. Agronomy. 2023; 13(5):1219. https://doi.org/10.3390/agronomy13051219
Chicago/Turabian StyleCortés, Eduardo, Ana Schneider, Elisa Panigo, Mariel Perreta, Rafael De Prado, and Ignacio Dellaferrera. 2023. "First Report of Resistance to Glyphosate in Several Species of the Genus Echinochloa in Argentina" Agronomy 13, no. 5: 1219. https://doi.org/10.3390/agronomy13051219
APA StyleCortés, E., Schneider, A., Panigo, E., Perreta, M., De Prado, R., & Dellaferrera, I. (2023). First Report of Resistance to Glyphosate in Several Species of the Genus Echinochloa in Argentina. Agronomy, 13(5), 1219. https://doi.org/10.3390/agronomy13051219





