Preliminary Results on the Effects of Initial Stand Density and Supplemental Irrigation on Biomass of Eucalyptus camaldulensis Dehn. Grown as a Short Rotation Woody Crop under Semi-Arid Conditions
Abstract
1. Introduction
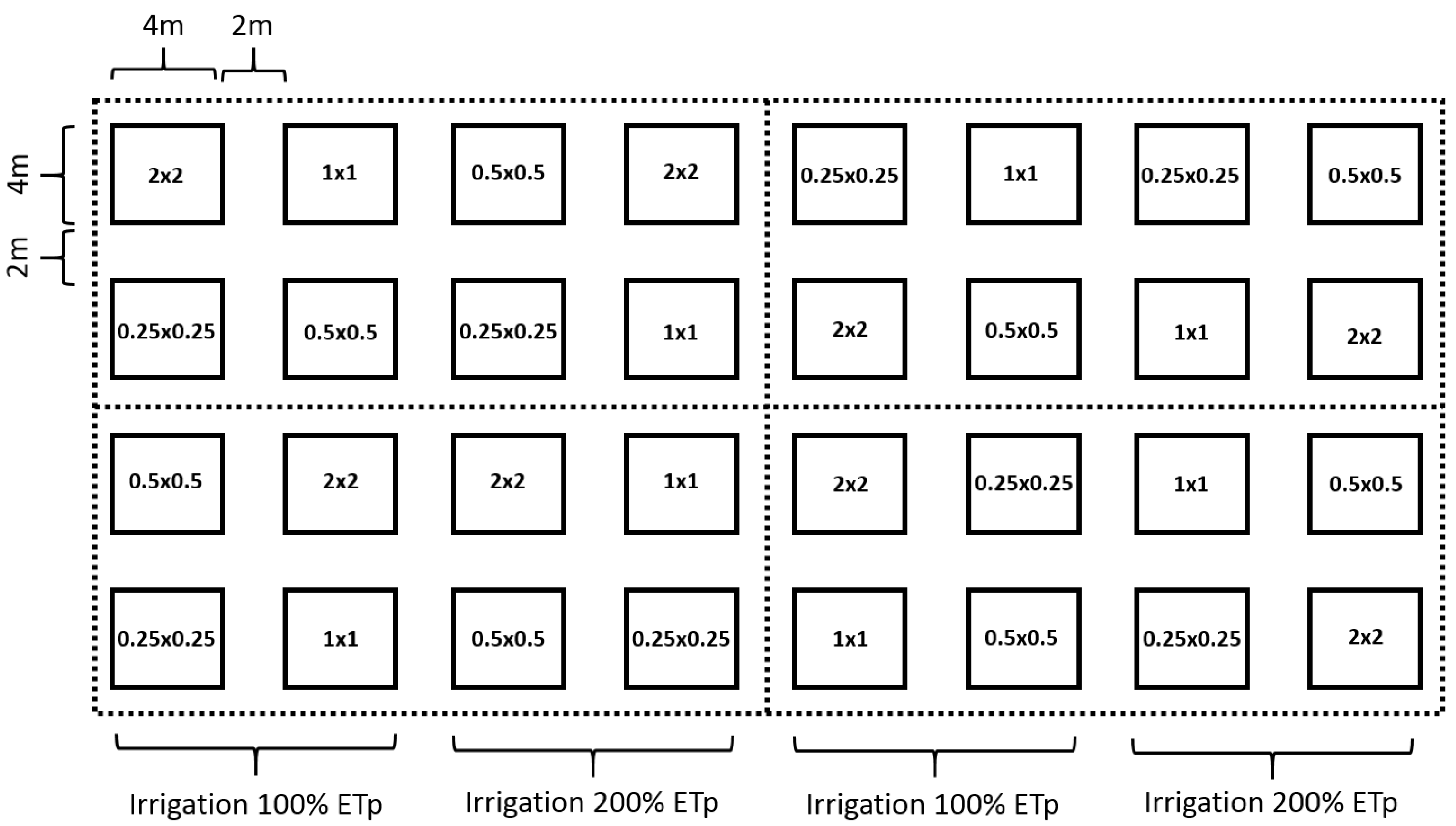
2. Materials and Methods
- (1)
- Tree height (cm);
- (2)
- Stem diameter (cm) at 50 cm;
- (3)
- Number of stems counted at 50 cm height;
- (4)
- Dry weight (%)—measured on one random tree per 4 × 4 m plot;
- (5)
- Dry weight per hectare—Fresh 200–500 g samples of stems and leaves were oven-dried at 60 °C for 48 h, and dry weight per hectare was calculated using average green biomass per hectare and percentage dry biomass.
3. Results
3.1. Vegetative Growth and Biomass Production
3.1.1. Establishment and Acclimation (Year I)
3.1.2. SRC Effects on Vegetative Growth and Biomass Production
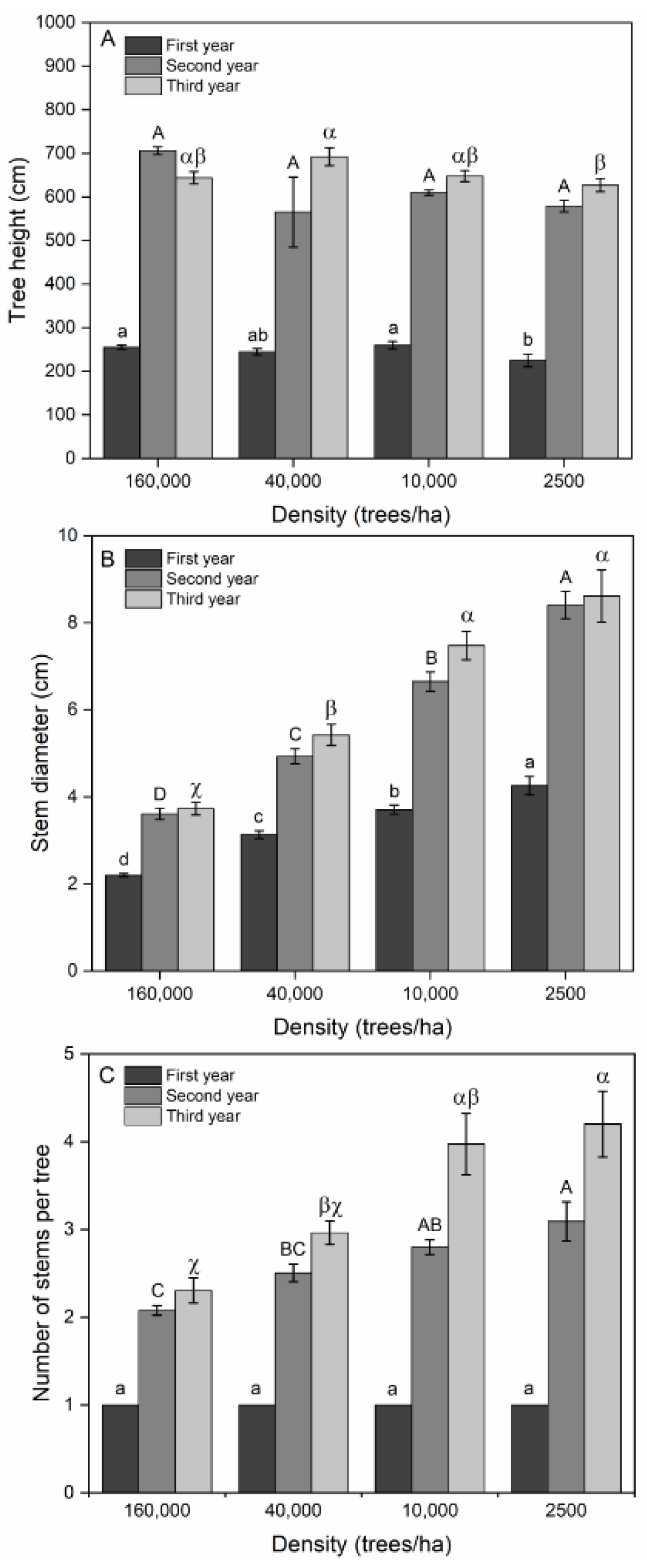
Tree Height
Stem Diameter
Number of Stems per Tree
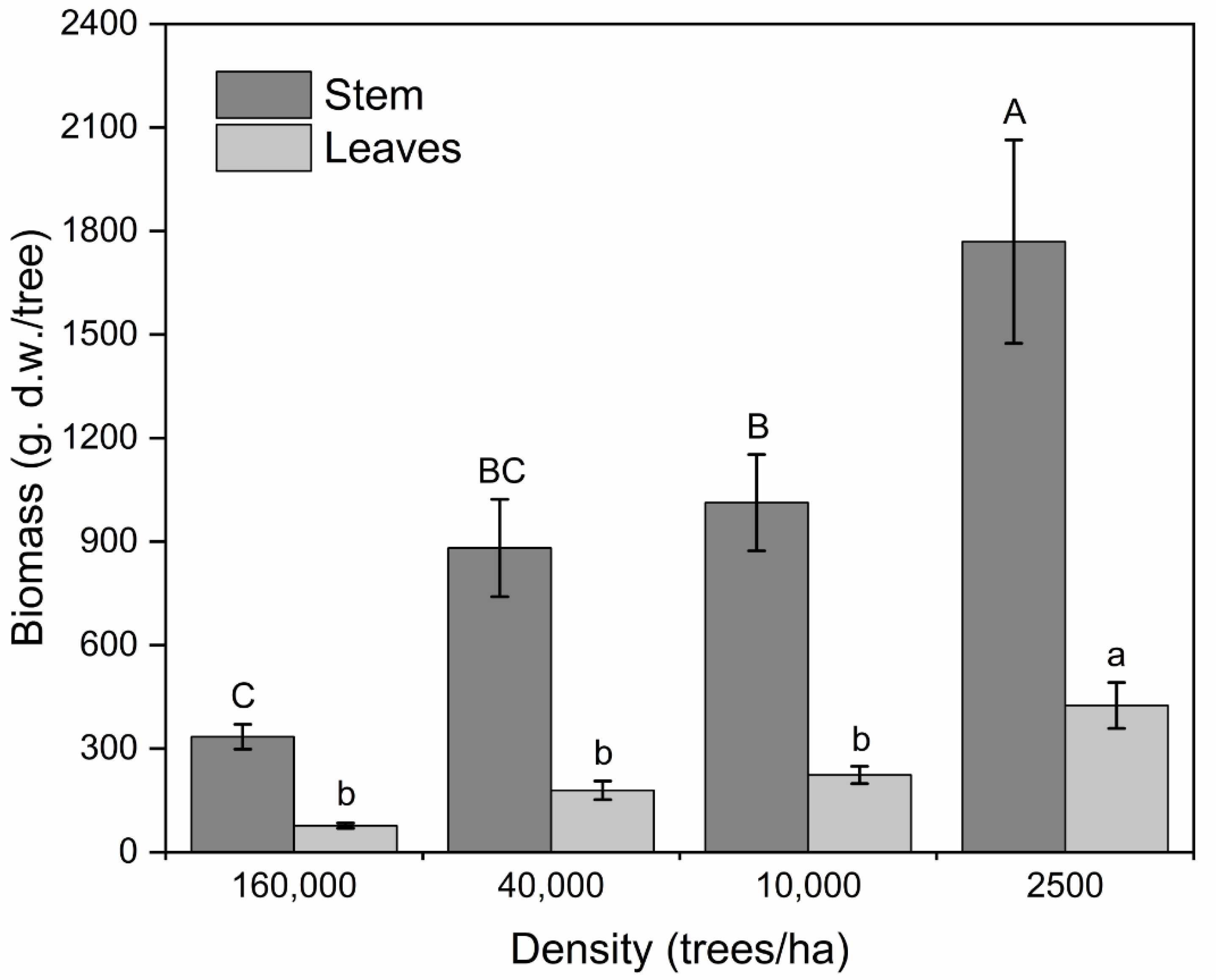
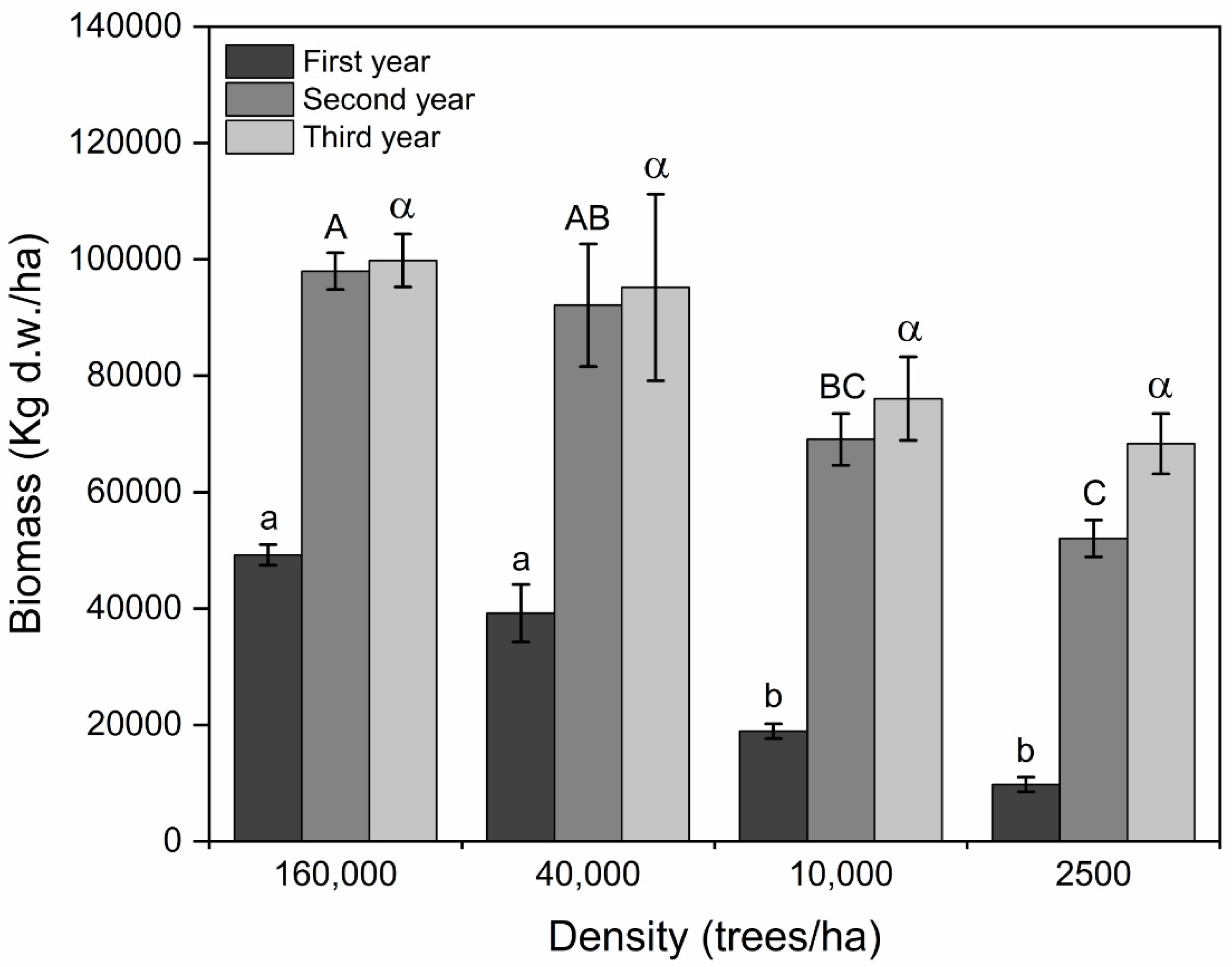
Biomass Production
4. Discussion
- We grew Eucalyptus trees under Mediterranean semi-arid conditions.
- We planted Eucalyptus trees at extremely high densities starting at 2500 and reaching 160,000 TPH.
- We provided supplemental irrigation during the dry summer months.
- We cut down the trees at extremely short rotations of once a year.
4.1. Year I—Establishment and Acclimation
4.2. SRC Effects on Vegetative Growth and Biomass Production
4.2.1. Tree Height
4.2.2. Stem Diameter
4.2.3. Number of Stems per Tree
4.2.4. Biomass Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakravarty, S.; Ghosh, S.K.; Suresh, C.P.; Dey, A.N.; Shukla, G. Deforestation: Causes, effects and control strategies. In Global Perspectives on Sustainable Forest Management; Okia, C.A., Ed.; InTech: Rang-Du-Fliers, France, 2012; Volume 1, pp. 1–26. ISBN 978-953-51-0569-5. Available online: http://www.intechopen.com/books/globalperspectives-on-sustainable-forest-management/deforestation-causes-effects-and-control-strategies (accessed on 1 March 2023).
- Huang, J.; Ji, M.; Xie, Y.; Wang, S.; He, Y.; Ran, J. Global semi-arid climate change over last 60 years. Clim. Dynam. 2016, 46, 1131–1150. [Google Scholar] [CrossRef]
- IEA. Analysis and Forecast to 2026; International Energy Agency: Paris, France, 2021; Available online: https://iea.blob.core.windows.net/assets/5ae32253-7409-4f9a-a91d-1493ffb9777a/Renewables2021-Analysisandforecastto2026.pdf (accessed on 16 April 2023).
- Vogel, K.P.; Brejda, J.J.; Walters, D.T.; Buxton, D.R. Switchgrass biomass production in the Midwest USA. Agron. J. 2002, 94, 413–420. [Google Scholar] [CrossRef]
- Adler, P.R.; Sanderson, M.A.; Boateng, A.A.; Weimer, P.J.; Jung, H.J.G. Biomass yield and biofuel quality of switchgrass harvested in fall or spring. Agron. J. 2006, 98, 1518–1525. [Google Scholar] [CrossRef]
- Lemus, R.; Brummer, E.C.; Burras, C.L.; Moore, K.J.; Barker, M.F.; Molstad, N.E. Effects of nitrogen fertilization on biomass yield and quality in large fields of established switchgrass in southern Iowa, USA. Biomass Bioenergy 2008, 32, 1187–1194. [Google Scholar] [CrossRef]
- Kauter, D.; Lewandowski, I.; Claupein, W. Quantity and quality of harvestable biomass from Populus short rotation coppice for solid fuel use—A review of the physiological basis and management influences. Biomass Bioenergy 2003, 24, 411–427. [Google Scholar] [CrossRef]
- Amichev, B.Y.; Johnston, M.; Van Rees, K.C. Hybrid poplar growth in bioenergy production systems: Biomass prediction with a simple process-based model (3PG). Biomass Bioenergy 2010, 34, 687–702. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J.; Waliszewska, B.; Szczukowski, S.; Tworkowski, J.; Załuski, D.; Śnieg, M. Willow biomass as feedstock for an integrated multi-product biorefinery. Ind. Crop. Prod. 2014, 58, 230–237. [Google Scholar] [CrossRef]
- Eastham, J.; Scott, P.R.; Steckis, R.A.; Barton, A.F.M.; Hunter, L.J.; Sudmeyer, R.J. Survival, growth and productivity of tree species under evaluation for agroforestry to control salinity in the Western Australian wheatbelt. Agrofor. Syst. 1993, 21, 223–237. [Google Scholar] [CrossRef]
- Du Toit, B. Effects of site management on growth, biomass partitioning and light use efficiency in a young stand of Eucalyptus grandis in South Africa. For. Ecol. Manag. 2008, 255, 2324–2336. [Google Scholar] [CrossRef]
- Stape, J.L.; Binkley, D.; Ryan, M.G.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Claudio, R.S.; Sergio, R.S.; Rodrigo, E.H.; Ferreira, J.M.D.A.; et al. The Brazil Eucalyptus Potential Productivity Project: Influence of water, nutrients and stand uniformity on wood production. For. Ecol. Manag. 2010, 259, 1684–1694. [Google Scholar] [CrossRef]
- Barreiro, S.; Tomé, M. Analysis of the impact of the use of eucalyptus biomass for energy on wood availability for eucalyptus forest in Portugal: A simulation study. Ecol. Soc. 2012, 17, 14. Available online: https://www.jstor.org/stable/26269037 (accessed on 1 March 2023). [CrossRef]
- Borralho, N.M.G.; Cotterill, P.P.; Kanowski, P.J. Breeding objectives for pulp production Of Eucalyptus globulus under different industrial cost structures. Can. J. For. Res. 1993, 23, 648–656. [Google Scholar] [CrossRef]
- Raymond, C.A.; Schimleck, L.R.; Muneri, A.; Michell, A.J. Genetic parameters and genotype-by-environment interactions for pulp-yield predicted using near infrared reflectance analysis and pulp productivity in Eucalyptus globulus. For. Genet. 2001, 8, 213–224. [Google Scholar]
- Almeida, A.C.; Soares, J.V.; Landsberg, J.J.; Rezende, G.D. Growth and water balance of Eucalyptus grandis hybrid plantations in Brazil during a rotation for pulp production. For. Ecol. Manag. 2007, 251, 10–21. [Google Scholar] [CrossRef]
- Eldridge, K.; Davidson, J.; Harwood, C.; VanWyk, G. Eucalypt Domestication and Breeding; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Niknam, S.R.; McComb, J. Salt tolerance screening of selected Australian woody species—A review. For. Ecol. Manag. 2000, 139, 1–19. [Google Scholar] [CrossRef]
- Zohar, Y.; Gafni, A.; Morris, J.; Shalhevet, S. Eucalyptus plantations in Israel: An assessment of economic and environmental viability. New For. 2008, 36, 135–157. [Google Scholar] [CrossRef]
- Kheshgi, H.S.; Prince, R.C.; Marland, G. The potential of biomass fuels in the context of global climate change: Focus on transportation fuels. Annu. Rev. Energy Environ. 2000, 25, 199–244. [Google Scholar] [CrossRef]
- Harper, R.J.; Sochacki, S.J.; Smettem, K.R.J.; Robinson, N. Bioenergy feedstock potential from short-rotation woody crops in a dryland environment. Energy Fuels 2010, 24, 225–231. [Google Scholar] [CrossRef]
- Antal, M.J.; Croiset, E.; Dai, X.; DeAlmeida, C.; Mok, W.S.L.; Norberg, N.; Al Majthoub, M. High-yield biomass charcoal. Energy Fuels 1996, 10, 652–658. [Google Scholar] [CrossRef]
- Norgate, T.; Langberg, D. Environmental and economic aspects of charcoal use in steelmaking. ISIJ Int. 2009, 49, 587–595. [Google Scholar] [CrossRef]
- Piketty, M.G.; Wichert, M.; Fallot, A.; Aimola, L. Assessing land availability to produce biomass for energy: The case of Brazilian charcoal for steel making. Biomass Bioenergy 2009, 33, 180–190. [Google Scholar] [CrossRef]
- Inoue, H.; Yano, S.; Endo, T.; Sakaki, T.; Sawayama, S. Combining hot compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol. Biofuels 2008, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Kaida, R.; Kaku, T.; Baba, K.; Oyadomari, M.; Watanabe, T. Enzymatic saccharification and ethanol production of Acacia mangium and Paraserianthes falcataria wood, and Elaeis guineensis trunk. J. Wood Sci. 2009, 55, 381–386. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Wang, Q.; Qi, W. Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2010, 101, 4895–4899. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, W.L.; Helm, C.; Silva, P.; Lima, E.; Hoffman, K. Pretreatment of Eucalypts biomass towards enzymatic saccharification. BMC Proc. 2011, 5, P116. [Google Scholar] [CrossRef]
- Shepherd, M.; Bartle, J.; Lee, D.J.; Brawner, J.; Bush, D. Eucalypts as a biofuel feedstock. Biofuels 2011, 2, 639–657. [Google Scholar] [CrossRef]
- Karschon, R. The Effect of Irrigation Upon the Growth of Eucalyptus camaldulensis Dehn: Report to 4th Session, Committee on Mediterranean Forest Research, Ankara; FAO: Rome, Italy, 1970; p. 4. [Google Scholar]
- Grunwald, C.; Karschon, R. Variation of Eucalyptus camaldulensis from north Australia grown in Israel. Silvae Genet. 1983, 5, 165–173. [Google Scholar]
- Zohar, Y. Biomass production of short rotation Eucalyptus camaldulensis Dehn. stands growing on peat soil under a high water table in Israel. S. Afr. For. J. 1989, 149, 54–57. [Google Scholar] [CrossRef]
- Zohar, Y.; Karschon, R. Above-ground biomass of Eucalyptus camaldulensis Dehn. in Israel. S. Afr. For. J. 1984, 128, 26–29. [Google Scholar] [CrossRef]
- Zohar, Y.; Moreshet, S. Provenances of Eucalyptus occidentalis in the arid zone of Israel. For. Ecol. Manag. 1987, 22, 71–77. [Google Scholar] [CrossRef]
- Zohar, Y.; Schiller, G. Growth and water use by selected seed sources of eucalyptus under high water table and saline conditions. Agric. Ecosyst. Environ. 1998, 69, 265–277. [Google Scholar] [CrossRef]
- Korol, L.E.; Atzmon, N.; Shkliar, G.; Moshe, Y. Genetic Characterization of Basic Eucalyptus Species in Israel; KKL: Jerusalem, Israel, 2010. [Google Scholar]
- Korol, L.E.; Riev, Y.; Shkliar, G.; Moshe, Y.; Sitbon, R. Genetic Diversity in the River Red Gum (Eucalyptus camaldulensis) Growing in Israel; KKL: Jerusalem, Israel, 2012. [Google Scholar]
- Prober, S.J.; Bell, C.; Moran, G. A phylogenetic and allozyme approach to understanding rarity in three “green ash” eucalypts (Myrtaceae). Plant Syst. Evol. 1990, 172, 99–118. [Google Scholar] [CrossRef]
- Brooker, M.I.H.; Kleinig, D.A. Field Guide to Eucalypts: Vol. 1. South-Western and Southern Australia, Revised ed.; Bloomings Books: Melbourne, Australia, 1999. [Google Scholar]
- Butcher, P.A.; McDonald, M.W. Congruence between environmental parameters, morphology and genetic structure in Australia’s most widely distributed eucalypt, Eucalyptus camaldulensis. Tree Genet. Genomes 2009, 5, 189–210. [Google Scholar] [CrossRef]
- Potts, B.M.; Wiltshire, R.J.E. Eucalypt genetics and genecology. In Eucalypt Ecology: Individuals to Ecosystems; Williams, J., Woinarski, J., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 56–91. [Google Scholar]
- Zahid, D.M.; Shah, F.U.R.; Majeed, A. Planting Eucalyptus camaldulensis in arid environment—Is it useful species under water deficit system. Pak. J. Bot. 2010, 42, 1733–1744. [Google Scholar]
- Nagar, B.; Rawat, S.; Rathiesh, P.; Sekar, I. Impact of initial spacing on growth and yield of Eucalyptus camaldulensis in arid region of India. World Appl. Sci. J. 2015, 33, 1362–1368. [Google Scholar]
- Beck, H.; Zimmermann, N.; McVicar, T.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; p. 15. [Google Scholar]
- Venendaal, R.; Jørgensen, U.; Foster, C.A. European energy crops: A synthesis. Biomass Bioenergy 1997, 13, 147–185. [Google Scholar] [CrossRef]
- Sims, R.E.; Handford, P.A.; Bell, T. Wood Fuel Supply and Utilization from Short Rotation Energy Plantations; Agronomy Department, Massey University: Palmerston North, New Zealand, 1990. [Google Scholar]
- Rockwood, D.; Rudie, A.; Ralph, S.; Zhu, J.; Winandy, J. Energy product options for Eucalyptus species grown as short rotation woody crops. Int. J. Mol. Sci. 2008, 9, 1361–1378. [Google Scholar] [CrossRef]
- Gabrielle, B.; Nguyen The, N.; Maupu, P.; Vial, E. Life cycle assessment of eucalyptus short rotation coppices for bioenergy production in southern France. Gcb Bioenergy 2013, 5, 30–42. [Google Scholar] [CrossRef]
- Bernardo, A.L.; Reis, M.G.; Reis, G.G.; Harrison, R.B.; Firme, D.J. Effect of spacing on growth and biomass distribution in Eucalyptus camaldulensis, E. pellita and E. urophylla plantations in southeastern Brazil. For. Ecol. Manag. 1998, 104, 1–13. [Google Scholar] [CrossRef]
- Schönau, A.P.G.; Coetzee, J. Initial spacing, stand density and thinning in eucalypt plantations. For. Ecol. Manag. 1989, 29, 245–266. [Google Scholar] [CrossRef]
- King, D.A. The adaptive significance of tree height. Am. Nat. 1990, 135, 809–828. [Google Scholar] [CrossRef]
- Alcorn, P.J.; Pyttel, P.; Bauhus, J.; Smith, R.G.B.; Thomas, D.; James, R.; Nicotra, A. Effects of initial planting density on branch development in 4-year-old plantation grown Eucalyptus pilularis and Eucalyptus cloeziana trees. For. Ecol. Manag. 2007, 252, 41–51. [Google Scholar] [CrossRef]
- Henskens, F.L.; Battaglia, M.; Cherry, M.L.; Beadle, C.L. Physiological basis of spacing effects on tree growth and form in Eucalyptus globulus. Trees 2001, 15, 365–377. [Google Scholar] [CrossRef]
- Xue, L.; Pan, L.; Zhang, R.; Xu, P.B. Density effects on the growth of self-thinning Eucalyptus urophylla stands. Trees 2011, 25, 1021–1031. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Neilsen, W.A. Crown and stand characteristics of Eucalyptus nitens in response to initial spacing: Implications for thinning. For. Ecol. Manag. 2003, 172, 215–227. [Google Scholar] [CrossRef]
- Kearney, D.; James, R.; Montagu, K.; Smith, R.G.B. The effect of initial planting density on branching characteristics of Eucalyptus pilularis and E. grandis. Aust. For. 2007, 70, 262–268. [Google Scholar] [CrossRef]
- Thoranisorn, S.; Sahunalu, P.; Yoda, K. Density effects and self-thinning in even-aged pure stands of Eucalyptus camaldulensis Dehn. Bot. Mag. 1990, 103, 283–295. [Google Scholar] [CrossRef]
- Neilsen, W.A.; Gerrand, A.M. Growth and branching habit of Eucalyptus nitens at different spacing and the effect on final crop selection. For. Ecol. Manag. 1999, 123, 217–229. [Google Scholar] [CrossRef]
- Paris, P.; Mareschi, L.; Sabatti, M.; Tosi, L.; Scarascia-Mugnozza, G. Nitrogen removal and its determinants in hybrid Populus clones for bioenergy plantations after two biennial rotations in two temperate sites in northern Italy. iFor.-Biogeosci. For. 2015, 8, 668. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaknin, Y.; Korol, L. Preliminary Results on the Effects of Initial Stand Density and Supplemental Irrigation on Biomass of Eucalyptus camaldulensis Dehn. Grown as a Short Rotation Woody Crop under Semi-Arid Conditions. Agronomy 2023, 13, 1216. https://doi.org/10.3390/agronomy13051216
Vaknin Y, Korol L. Preliminary Results on the Effects of Initial Stand Density and Supplemental Irrigation on Biomass of Eucalyptus camaldulensis Dehn. Grown as a Short Rotation Woody Crop under Semi-Arid Conditions. Agronomy. 2023; 13(5):1216. https://doi.org/10.3390/agronomy13051216
Chicago/Turabian StyleVaknin, Yiftach, and Leonid Korol. 2023. "Preliminary Results on the Effects of Initial Stand Density and Supplemental Irrigation on Biomass of Eucalyptus camaldulensis Dehn. Grown as a Short Rotation Woody Crop under Semi-Arid Conditions" Agronomy 13, no. 5: 1216. https://doi.org/10.3390/agronomy13051216
APA StyleVaknin, Y., & Korol, L. (2023). Preliminary Results on the Effects of Initial Stand Density and Supplemental Irrigation on Biomass of Eucalyptus camaldulensis Dehn. Grown as a Short Rotation Woody Crop under Semi-Arid Conditions. Agronomy, 13(5), 1216. https://doi.org/10.3390/agronomy13051216




