Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
3. Results
3.1. Soil Characterization
3.2. Morphological Measurement
3.3. Plant Biomass Production
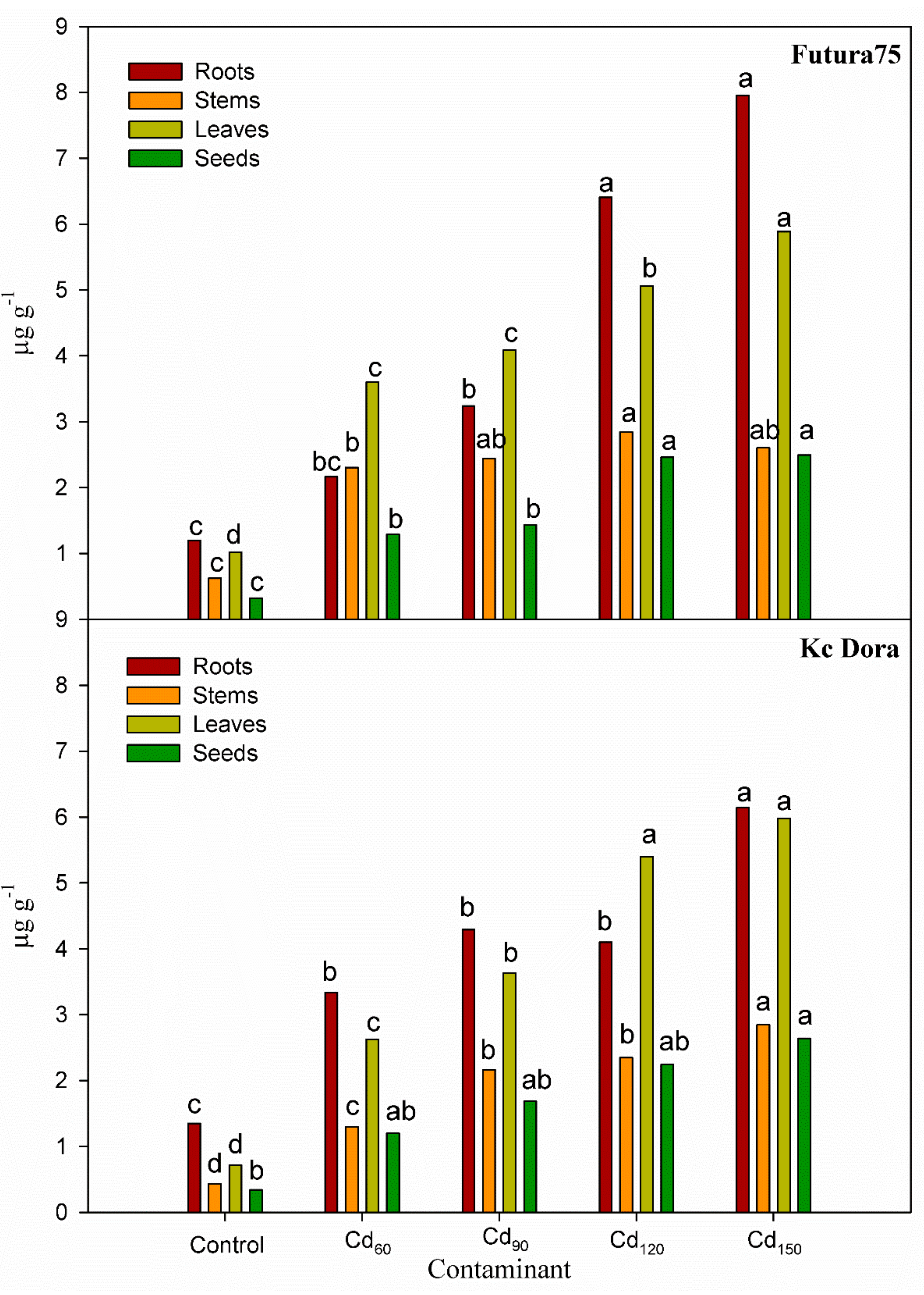
3.4. The Concentration of Heavy Metals in the Different Parts of the Plants
3.5. Evaluating the Tolerance and the Potential Phytoextraction by Phytoremediation Index and Factors
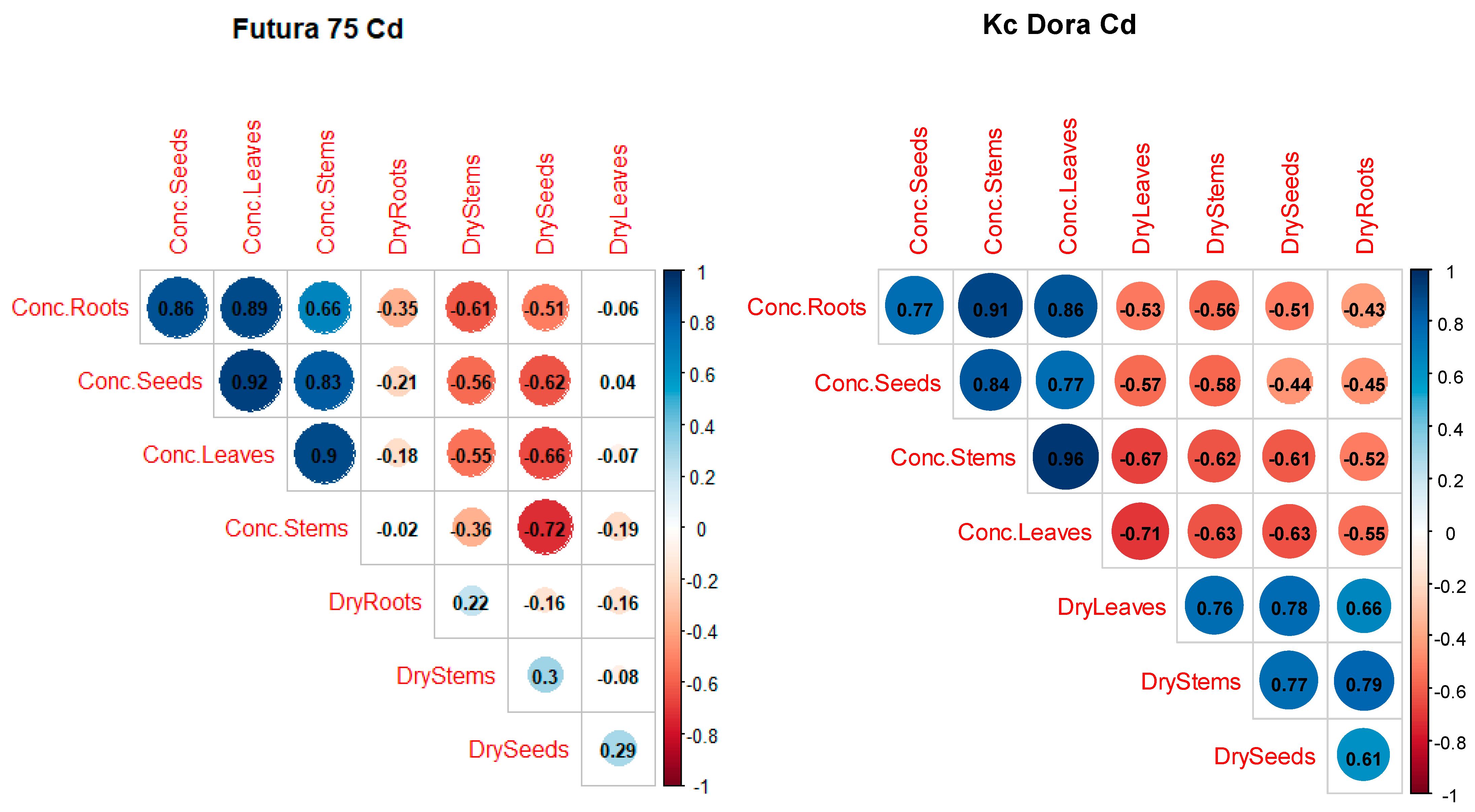
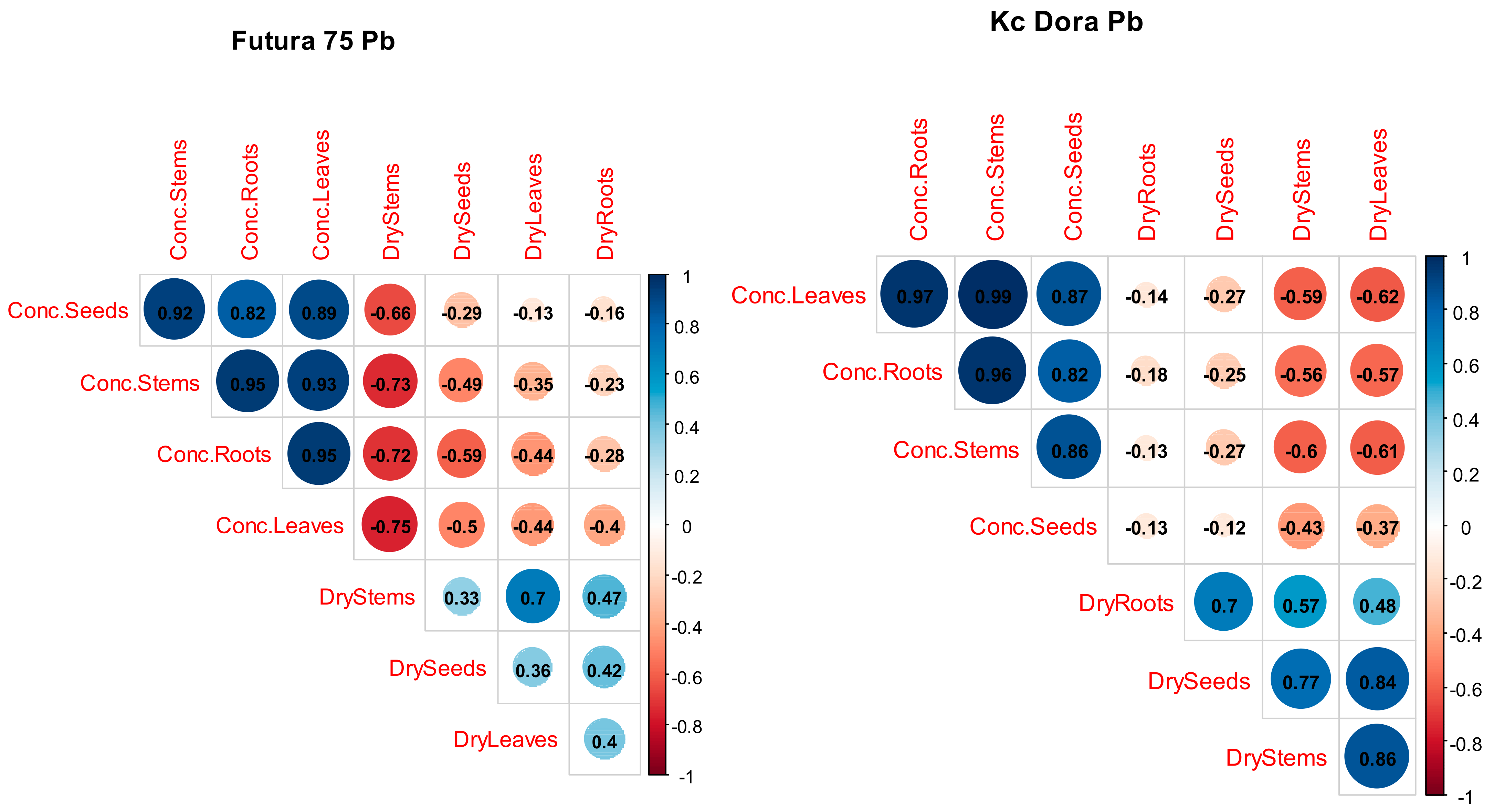
3.6. Correlation of the Main Factor between the Two Varieties of Industrial Hemp
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A Critical Review on the Phytoremediation of Heavy Metals from Environment: Performance and Challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in Agricultural Soils of the European Union with Implications for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Jaskulak, M.; Grobelak, A.; Vandenbulcke, F. Modelling Assisted Phytoremediation of Soils Contaminated with Heavy Metals –Main Opportunities, Limitations, Decision Making and Future Prospects. Chemosphere 2020, 249, 126196. [Google Scholar] [CrossRef] [PubMed]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy Metal Tolerance and Accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Khan, N.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochemical. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Manno, E.; Varrica, D.; Dongarrà, G. Metal Distribution in Road Dust Samples Collected in an Urban Area Close to a Petrochemical Plant at Gela, Sicily. Atmos. Environ. 2006, 40, 5929–5941. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic Metal Compounds: Recent Insight into Molecular and Cellular Mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Cristaldi, A.; Oliveri, G.; Hea, E.; Zuccarello, P. Environmental Technology & Innovation Phytoremediation of Contaminated Soils by Heavy Metals and PAHs. A Brief Review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- EEA (European Environment Agency). Progress in Management of Contaminated Sites; European Environment Agency: Copenhagen, Denmark, 2014; ISBN 9789279348464. [Google Scholar]
- Fagnano, M.; Visconti, D.; Fiorentino, N. Agronomic Approaches for Characterization, Remediation, and Monitoring of Contaminated Sites. Agronomy 2020, 10, 1335. [Google Scholar] [CrossRef]
- Sur, I.M.; Micle, V.; Polyak, E.T.; Gabor, T. Assessment of Soil Quality Status and the Ecological Risk in the Baia Mare, Romania Area. Sustainability 2022, 14, 3739. [Google Scholar] [CrossRef]
- Shah, V.; Daverey, A. Phytoremediation: A Multidisciplinary Approach to Clean up Heavy Metal Contaminated Soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. Bioenergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Ciaramella, B.R.; Corinzia, S.A.; Cosentino, S.L.; Testa, G. Phytoremediation of Heavy Metal Contaminated Soils Using Safflower. Agronomy 2022, 12, 2302. [Google Scholar] [CrossRef]
- Fernando, A.L.; Duarte, M.P.; Vatsanidou, A.; Alexopoulou, E. Environmental Aspects of Fiber Crops Cultivation and Use. Ind. Crops Prod. 2015, 68, 105–115. [Google Scholar] [CrossRef]
- EU Directive (EU) 2018/2001 of the European Parliament and of the Council on the Promotion of the Use of Energy from Renewable Sources. Off. J. Eur. Union 2018, 2018, 82–209.
- Papazoglou, E.G.; Arundo Donax, L. Stress Tolerance under Irrigation with Heavy Metal Aqueous Solutions. Desalination 2007, 211, 304–313. [Google Scholar] [CrossRef]
- Gomes, L.; Costa, J.; Moreira, J.; Cumbane, B.; Abias, M.; Santos, F.; Zanetti, F.; Monti, A.; Fernando, A.L. Switchgrass and Giant Reed Energy Potential When Cultivated in Heavy Metals Contaminated Soils. Energies 2022, 15, 5538. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Growth, Tolerance Efficiency and Phytoremediation Potential of Ricinus communis (L.) and Brassica juncea (L.) in Salinity and Drought Affected Cadmium Contaminated Soil. Ecotoxicol. Environ. Saf. 2012, 85, 13–22. [Google Scholar] [CrossRef]
- Dimitriu, D. Restoration of heavy metals polluted soils case study—Camelina. AgroLife Sci. J. 2014, 3, 29–38. [Google Scholar]
- Zhao, X.; Guo, Y.; Papazoglou, E.G. Screening Flax, Kenaf and Hemp Varieties for Phytoremediation of Trace Element-Contaminated Soils. Ind. Crops Prod. 2022, 185, 115121. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Li, Z.; Jia, R.; Luo, D.; Tang, M.; Lu, H.; Hu, Y.; Yue, J.; Huang, Z. Transcriptome Analysis Revealed Key Genes and Pathways Related to Cadmium-Stress Tolerance in Kenaf (Hibiscus cannabinus L.). Ind. Crops Prod. 2020, 158, 112970. [Google Scholar] [CrossRef]
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.G.; Komnou, A.A.; Vasilou, C. Investigating the Potential of Heavy Metal Accumulation from Hemp. The Use of Industrial Hemp (Cannabis sativa L.) for Phytoremediation of Heavily and Moderated Polluted Soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Mihoc, M.; Pop, G.; Alexa, E.; Radulov, I. Nutritive Quality of Romanian Hemp Varieties (Cannabis sativa L.) with Special Focus on Oil and Metal Contents of Seeds. Chem. Cent. J. 2012, 6, 122. [Google Scholar] [CrossRef]
- Canu, M.; Mulè, P.; Spanu, E.; Fanni, S.; Marrone, A.; Carboni, G. Hemp Cultivation in Soils Polluted by Cd, Pb and Zn in the Mediterranean Area: Sites Characterization and Phytoremediation in Real Scale Settlement. Appl. Sci. 2022, 12, 3548. [Google Scholar] [CrossRef]
- De Vos, B.; De Souza, M.F.; Michels, E.; Meers, E. Industrial Hemp (Cannabis sativa L.) Field Cultivation in a Phytoattenuation Strategy and Valorization Potential of the Fibers for Textile Production. Environ. Sci. Pollut. Res. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Shi, G.; Liu, C.; Cui, M.; Ma, Y.; Cai, Q. Cadmium Tolerance and Bioaccumulation of 18 Hemp Accessions. Appl. Biochem. Biotechnol. 2012, 168, 163–173. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A Comprehensive Study of Planting Density and Nitrogen Fertilization Effect on Dual-Purpose Hemp (Cannabis sativa L.) Cultivation. Ind. Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Rheay, H.T.; Omondi, E.C.; Brewer, C.E. Potential of Hemp (Cannabis sativa L.) for Paired Phytoremediation and Bioenergy Production. GCB Bioenergy 2021, 13, 525–536. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New Developments in Fiber Hemp (Cannabis sativa L.) Breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.X.; Heidel, M.; Wu, Z.; Yoshimoto, A.; Leong, G.; Pan, D.; Ako, H. Comparative Evaluation of Industrial Hemp Varieties: Field Experiments and Phytoremediation in Hawaii. Ind. Crops Prod. 2021, 170, 113683. [Google Scholar] [CrossRef]
- Fiorentino, N.; Mori, M.; Cenvinzo, V.; Duri, L.G.; Gioia, L.; Visconti, D.; Fagnano, M. And Degraded Land on Er Al. Ital. J. Agron. 2018, 13, 34–44. [Google Scholar]
- Fagnano, M. The Ecoremed Protocol for an Integrated Agronomic Approach to Characterization and Remediation of Contaminated Soils. Definition of a Site as Contaminated: Problems Related to Agricultural Soils. Ital. J. Agron. 2018, 13, 1–68. [Google Scholar]
- della Repubblica, I.P. DECRETO LEGISLATIVO 3 Aprile 2006, n. 152 Norme in Materia Ambientale. Gazz. Uff. 2006, 1, 172. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 539–579. ISBN 9780891189770. [Google Scholar]
- ISO 11047; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc—Flame and Electrothermal Atomic Absorption Spectrometric Methods. Vernier: Geneva, Switzerland, 1998.
- 61010-1©Iec2001; ISO 707: 2008 International Standard International Standard. Vernier: Geneva, Switzerland, 2003.
- Yadav, S.K.; Juwarkar, A.A.; Kumar, G.P.; Thawale, P.R.; Singh, S.K.; Chakrabarti, T. Bioaccumulation and Phyto-Translocation of Arsenic, Chromium and Zinc by Jatropha curcas L.: Impact of Dairy Sludge and Biofertilizer. Bioresour. Technol. 2009, 100, 4616–4622. [Google Scholar] [CrossRef]
- Mattina, M.J.I.; Lannucci-Berger, W.; Musante, C.; White, J.C. Concurrent Plant Uptake of Heavy Metals and Persistent Organic Pollutants from Soil. Environ. Pollut. 2003, 124, 375–378. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy Metal Contamination and Accumulation in Soil and Wild Plant Species from Industrial Area of Islamabad, Pakistan. Pakistan J. Bot. 2010, 42, 291–301. [Google Scholar]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Bio/Technology 1995, 13, 468–474. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Erickson, L.; Stefanovska, T.; Popelka, J.; Hettiarachchi, G.; Davis, L.; Trögl, J. Potential Phytomanagement of Military Polluted Sites and Biomass Production Using Biofuel Crop Miscanthus x Giganteus. Environ. Pollut. 2019, 249, 330–337. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Copani, V. Sowing Time and Prediction of Flowering of Different Hemp (Cannabis sativa L.) Genotypes in Southern Europe. Ind. Crops Prod. 2012, 37, 20–33. [Google Scholar] [CrossRef]
- Pietrini, F.; Passatore, L.; Patti, V.; Francocci, F.; Giovannozzi, A.; Zacchini, M. Morpho-Physiological and Metal Accumulation Responses of Hemp Plants (Cannabis sativa L.) Grown on Soil from an Agro-Industrial Contaminated Area. Water 2019, 11, 808. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Palm, E.; Mancuso, S.; Azzarello, E. Trace Element Phytoextraction from Contaminated Soil: A Case Study under Mediterranean Climate. Environ. Sci. Pollut. Res. 2018, 25, 9114–9131. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European Developed Fibre Hemp Genotypes (Cannabis sativa L.) in Semi-Arid Mediterranean Environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Afzal, O.; Hassan, F.; Ahmed, M.; Shabbir, G.; Ahmed, S. Determination of Stable Safflower Genotypes in Variable Environments by Parametric and Non-Parametric Methods. J. Agric. Food Res. 2021, 6, 100233. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Bio-Accumulation and Distribution of Heavy Metals in Fibre Crops (Flax, Cotton and Hemp). Ind. Crops Prod. 2004, 19, 197–205. [Google Scholar] [CrossRef]
- Ferrarini, A.; Fracasso, A.; Spini, G.; Fornasier, F.; Taskin, E.; Fontanella, M.C.; Beone, G.M.; Amaducci, S.; Puglisi, E. Bioaugmented Phytoremediation of Metal-Contaminated Soils and Sediments by Hemp and Giant Reed. Front. Microbiol. 2021, 12, 645893. [Google Scholar] [CrossRef]




| Contaminant | Cd | Pb | Ni |
|---|---|---|---|
| Legal limit (mg kg−1) | 15 | 1000 | 500 |
| Concentration I (mg kg−1) | 60 | 1000 | 500 |
| Concentration II (mg kg−1) | 90 | 1500 | 1000 |
| Concentration III (mg kg−1) | 120 | 2000 | 1500 |
| Concentration IV (mg kg−1) | 150 |
| Physical Characteristics | |
|---|---|
| Clay (%) | 3.0 |
| Silt (%) | 4.1 |
| Sand (%) | 92.9 |
| Texture | Sandy |
| Conductivity (μS/cm) | 34.2 |
| Chemical Characteristics | |
| pH | 7.4 |
| Organic matter (%) | 0.86 |
| Fe (mg kg−1) | 23.6 |
| P (mg kg−1) | 7 |
| Mn (mg kg−1) | 0.1 |
| Cu (mg kg−1) | 21.8 |
| Total | Available | ||
|---|---|---|---|
| H.M. in soil (mg kg−1) | H.M. in soil (mg kg−1) | ||
| Cd | Control | 1.7 ± 0.1 | 1.1 ± 0.1 |
| 60 | 59.0 ± 2.3 | 36.0 ± 2.1 | |
| 90 | 88.2 ± 1.4 | 55.3 ± 2.0 | |
| 120 | 119.4 ± 1.4 | 80.2 ± 2.3 | |
| 150 | 150.5 ± 2.6 | 112.9 ± 1.5 | |
| Pb | Control | 39.6 ± 0.0 | 19.3 ± 0.0 |
| 1000 | 1075.5 ± 46.9 | 570.9 ± 7.1 | |
| 1500 | 1546.6 ± 11.9 | 1116.2 ± 57.9 | |
| 2000 | 1808.1 ± 32.3 | 1465.9 ± 53.7 | |
| Ni | Control | 40.3 ± 5.7 | 8.7 ± 1.9 |
| 500 | 508.2 ± 43.1 | 331.0 ± 14.4 | |
| 1000 | 1047.3 ± 44.5 | 753.6 ± 29.5 | |
| 1500 | 1491.5 ± 18.7 | 1153.9 ± 16.1 |
| Variety | Cont | Conc. | Plant Survival (%) | Average Height (cm) | Avarage Diameter (mm) |
|---|---|---|---|---|---|
| Futura 75 | Control | 100 a | 81.9 ± 9.6 a | 4.8 ± 1.0 a | |
| Cd | 60 | 100 a | 88.3 ± 7.3 a | 4.6 ± 0.2 a | |
| Cd | 90 | 93 ab | 75.4 ± 7.7 a | 4.6 ± 0.3 a | |
| Cd | 120 | 73 ab | 80.1 ± 10.3 a | 4.5 ± 0.8 a | |
| Cd | 150 | 57 b | 72.3 ± 7.3 a | 4.3 ± 0.4 a | |
| Ni | 500 | 93 ab | 66.3 ± 4.1 a | 3.6 ± 0.3 a | |
| Ni | 1000 | 87 ab | 63.0 ± 2.3 a | 3.7 ± 0.3 a | |
| Ni | 1500 | 53 b | 64.9 ± 18.6 a | 3.7 ± 0.8 a | |
| Pb | 1000 | 87 ab | 78.3 ± 16.4 a | 4.8 ± 1.5 a | |
| Pb | 1500 | 80 ab | 60.4 ± 3.4 a | 3.7 ± 0.6 a | |
| Pb | 2000 | 73 ab | 63.2 ± 9.5 a | 3.5 ± 0.6 a | |
| KC Dora | Control | 100 a | 77.9 ± 10.2 a | 4.2 ± 1.9 a | |
| Cd | 60 | 93 a | 76.7 ± 5.1 a | 4.9 ± 0.5 a | |
| Cd | 90 | 93 a | 77.3 ± 14.9 a | 4.5 ± 1.1 a | |
| Cd | 120 | 73 ab | 65.3 ± 2.3 a | 4.1 ± 0.8 a | |
| Cd | 150 | 6 ab | 55.3 ± 16.2 a | 3.6 ± 1.3 a | |
| Ni | 500 | 87 a | 71.0 ± 8.4 a | 4.4 ± 0.9 a | |
| Ni | 1000 | 87 a | 62.1 ± 12.7 a | 4.0 ± 0.5 a | |
| Ni | 1500 | 47 b | 47.4 ± 11.4 a | 3.0 ± 0.6 a | |
| Pb | 1000 | 80 ab | 76.5 ± 8.9 a | 4.8 ± 0.9 a | |
| Pb | 1500 | 87 a | 75.4 ± 11.4 a | 4.9 ± 0.2 a | |
| Pb | 2000 | 73 ab | 69.9 ± 4.6 a | 3.8 ± 2.5 a |
| Variety | Cont. | Conc. | Average Roots Biomass (g) | Average Stems Biomass (g) | Average Leaves Biomass (g) | Average Seeds Biomass (g) |
|---|---|---|---|---|---|---|
| Futura 75 | Control | 1.6 a | 6.4 ab | 3.5 a | 1.5 a | |
| Cd | 60 | 3.2 a | 6.9 a | 2.7 ab | 0.6 a | |
| 90 | 1.4 a | 5.2 ab | 3.8 ab | 0.9 a | ||
| 120 | 1.2 a | 5.3 ab | 3.3 b | 0.4 a | ||
| 150 | 1.3 a | 4.3 ab | 3.1 ab | 0.7 a | ||
| Ni | 500 | 1.1 a | 4.0 ab | 3.8 ab | 0.6 a | |
| 1000 | 1.2 a | 4.1 ab | 1.9 ab | 0.9 a | ||
| 1500 | 0.9 a | 3.0 b | 1.6 ab | 0.7 a | ||
| Pb | 1000 | 1.2 a | 5.7 ab | 2.7 ab | 0.7 a | |
| 1500 | 1.6 a | 3.9 ab | 2.5 ab | 0.8 a | ||
| 2000 | 1.0 a | 3.4 b | 2.8 ab | 0.8 a | ||
| KC Dora | Control | 2.0 a | 6.6 a | 4.0 a | 1.2 a | |
| Cd | 60 | 1.6 a | 5.5 ab | 3.3 ab | 1.3 a | |
| 90 | 1.6 a | 5.2 ab | 2.3 ab | 0.8 a | ||
| 120 | 0.9 a | 4.2 ab | 1.7 ab | 0.8 a | ||
| 150 | 1.1 a | 3.9 ab | 2.1 ab | 0.3 a | ||
| Ni | 500 | 0.9 a | 2.8 ab | 2.2 ab | 0.6 a | |
| 1000 | 0.7 a | 2.5 b | 1.5 ab | 0.6 a | ||
| 1500 | 0.6 a | 2.0 b | 1.2 b | 0.3 a | ||
| Pb | 1000 | 1.8 a | 4.2 ab | 2.0 ab | 0.7 a | |
| 1500 | 1.6 a | 4.3 ab | 2.3 ab | 1.0 a | ||
| 2000 | 1.6 a | 3.8 ab | 2.0 ab | 0.9 a |
| Varieties | H.M.—Conc | TI | mAI | mBCF Aboveground | TF | mBCF Belowground | |
|---|---|---|---|---|---|---|---|
| Futura 75 | Cd | 60 | 0.90 | 3.66 | 0.28 | 3.32 | 0.09 |
| 90 | 0.87 | 4.06 | 0.20 | 2.47 | 0.08 | ||
| 120 | 0.79 | 5.26 | 0.16 | 1.62 | 0.10 | ||
| 150 | 0.72 | 5.58 | 0.13 | 1.38 | 0.09 | ||
| Ni | 500 | 0.73 | 6.55 | 0.34 | 1.76 | 0.20 | |
| 1000 | 0.60 | 10.90 | 0.26 | 2.07 | 0.13 | ||
| 1500 | 0.46 | 14.92 | 0.16 | 2.38 | 0.07 | ||
| Pb | 1000 | 0.81 | 3.68 | 0.05 | 0.50 | 0.10 | |
| 1500 | 0.64 | 5.71 | 0.03 | 0.52 | 0.07 | ||
| 2000 | 0.62 | 8.94 | 0.05 | 0.55 | 0.08 | ||
| KC Dora | Cd | 60 | 0.84 | 3.45 | 0.14 | 1.54 | 0.09 |
| 90 | 0.63 | 5.03 | 0.14 | 1.74 | 0.08 | ||
| 120 | 0.57 | 6.72 | 0.15 | 2.44 | 0.06 | ||
| 150 | 0.53 | 8.40 | 0.15 | 2.04 | 0.07 | ||
| Ni | 500 | 0.62 | 6.02 | 0.12 | 0.71 | 0.17 | |
| 1000 | 0.51 | 12.27 | 0.11 | 1.14 | 0.10 | ||
| 1500 | 0.35 | 26.79 | 0.15 | 1.41 | 0.11 | ||
| Pb | 1000 | 0.75 | 8.12 | 0.04 | 0.49 | 0.09 | |
| 1500 | 0.64 | 13.39 | 0.04 | 0.58 | 0.07 | ||
| 2000 | 0.56 | 20.19 | 0.06 | 0.60 | 0.09 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, G.; Corinzia, S.A.; Cosentino, S.L.; Ciaramella, B.R. Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp. Agronomy 2023, 13, 995. https://doi.org/10.3390/agronomy13040995
Testa G, Corinzia SA, Cosentino SL, Ciaramella BR. Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp. Agronomy. 2023; 13(4):995. https://doi.org/10.3390/agronomy13040995
Chicago/Turabian StyleTesta, Giorgio, Sebastiano Andrea Corinzia, Salvatore Luciano Cosentino, and Barbara Rachele Ciaramella. 2023. "Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp" Agronomy 13, no. 4: 995. https://doi.org/10.3390/agronomy13040995
APA StyleTesta, G., Corinzia, S. A., Cosentino, S. L., & Ciaramella, B. R. (2023). Phytoremediation of Cadmium-, Lead-, and Nickel-Polluted Soils by Industrial Hemp. Agronomy, 13(4), 995. https://doi.org/10.3390/agronomy13040995







