Abstract
The input of organic matter in the soil by the no-tillage system (NTS) increases as the system becomes established, raising the levels of organic phosphorus (P) and reducing the P soil adsorption. This study evaluated the availability of organic and inorganic P in areas under different management systems and stages of adoption in the Cerrado. The data were analyzed as a completely randomized design, corresponding to: (1) an NTS after 5 years of its implantation (NTS5); (2) an NTS after 17 years of its implantation (NTS17); (3) a conventional tillage system more than 20 years old (CTS); (4) Native Cerrado (NC). There were five repetitions for all treatments. Depths of 0–5 and 5–10 cm were evaluated for the available P (P-avail), remaining P (P-rem), organic P (oP), and inorganic P (iP) forms extracted with Sodium bicarbonate (NaHCO3) (P-avail), Sulfuric acid (H2SO4) (moderately labile), and Sodium hydroxide (NaOH) (moderately resistant). The P from the sequential extractions accumulated at a depth of 0–5 cm, mostly in the organic form in the NTS17 and NC areas, demonstrating the contribution of the NTS to the conversion of the P reserve in the soil. The CTS treatment greatly accumulated P, especially in the inorganic form, indicating the non-conservationist characteristic of this system. The oP and iP contents in the soil were not affected by age of the NTS, which was similar to the NC. Our results show that the continuous input of organic matter deposited on the soil surface in the NTS17 increased the levels of organic and inorganic P, consequently providing greater availability of P in the soil for cultivated crops.
1. Introduction
The use of new management technologies has improved the yield of crops grown, especially those that promote less soil mobilization and disruption, which increases soil organic matter (SOM) levels after successive crop cycles and improves soil quality, occurring some time after the introduction of the no-tillage system (NTS) in cultivated areas [1,2,3].
In the NTS, there is a considerable supply of organic residues on the surface and in the subsurface from the residues of the coverings used and its root system, which leads to a gradual increase in the SOM contents in the layer from 0 to 10 cm [4,5,6]. This increase in the SOM does not generally occur in the first years of adoption of the NTS; however, it is detectable in the transition phase, starting six years after the start of the system [7,8,9].
The NTS adoption age is an important parameter when evaluating the changes that occur in the contents of SOM, the soil aggregation, and the process of nutrient cycling [10]. Sá et al. [11], Anghinoni [12], and Torres et al. [6,13] have pointed out that in Brazilian regions, in the initial 5 years of the NTS deployment phase, the levels of SOM and the restoration of microbial activity are ongoing. The following phases are the transition phase (5–10 years), the consolidation phase (10–20 years), and the maintenance phase (above 20 years), which are characterized by significate changes in the soil attributes.
The contribution of SOM that occurs in the NTS increases the level of organic P from the organic compounds present in the crop residues added [14], consequently improving the cycling of nutrients to subsequent crops. However, the availability of these nutrients is related to the interaction between climatic factors and the quantity and quality of the plant residue present [10,15].
P is also present in the organic form in the soil, which, in tropical conditions, is an important strategy to preserve the P availability to plants since SOM reduces the effects of soil adsorption by physically and chemically stabilizing the P in the system [14]. Silva and Mendonça [15] observed that the levels of soil organic P ranged from 15 to 80% and that the greatest values were observed in soils and soil depths that were rich in organic matter.
Great levels of organic and inorganic P were observed in the superficial soil layers of conservationist systems that increase the levels of SOM [16]. Systems that improve organic matter accumulation in quality and quantity in the soil minimize problems of P availability to plants [17], especially in humid tropical conditions. Organic P has great importance and is given consideration in the conservation of P available to crops [18].
The role played by SOM has been evaluated in several studies that have highlighted its ambivalent characteristic in relation to P [19,20]. In these studies, the existence of positive correlations between the content of SOM and the P adsorption has been highlighted since the anionic character of SOM allows for the formation of cation bridges with aluminum (Al), iron (Fe), and calcium (Ca) adsorbed to it [21,22]. In addition, SOM increases the soil cation exchange capacity and decreases the point of zero charge [23]. In this way, soils that have naturally low fertility and present elevated acidity also present great potential for P adsorption and are greatly improved by the NTS in the long term [7,8,10,24].
In the Brazilian Cerrado, oxisols predominate, which are poor in fertility, with high acidity and low SOM contents; however, the adsorption of P is one of the most outstanding problems, as it characterizes the chemical process of the passage of labile P conversion to non-labile P [25,26]. This conversion is a strong process for P retention to the point that the P balance with the soil solution disappears and becomes unavailable for plant growth [27]. The fixation of the labile P in weathered soils, such as those found in the Cerrado, causes strong competition between the soil and plants for P applied as an inorganic fertilizer, with a considerably large part of the P retained in the soil mineral fraction via high energy links [28].
The hypothesis tested in this study is that the continuous input of organic matter deposited on the soil surface in the NTS from the residues of cover crops used in the rotation system with commercial crops increases the levels of organic and inorganic P, consequently providing a greater availability of P in the soil for cultivated crops. In this context, this study evaluated the availability of organic and inorganic fractions of P in areas under different management and adoption stages in the Cerrado, Minas Gerais, Brazil.
2. Material and Methods
2.1. Experimental Area
This study was carried out in an experimental area in the municipality of Uberaba, State of Minas Gerais, Brazil (19°39′19″ S, 47°57′27″ W, 790 and 819 m above sea level). Three managed areas and a control area (Native Cerrado without anthropic action) were evaluated between March 2017 and March 2018.
The area under study, with the exception of the Native Cerrado, was managed by conventional tillage, with deep plowing and two harrowing steps (plow and grader) at the beginning of the study. Next, it was separated into three experimental areas, where the no-tillage system (NTS) was introduced to two of them at different times, one 5 years ago (NTS5), and another 17 years ago (NTS17). Another area has been managed by a conventional tillage system (CTS) (Table 1, Figure 1).

Table 1.
Historic description and location of the study areas.
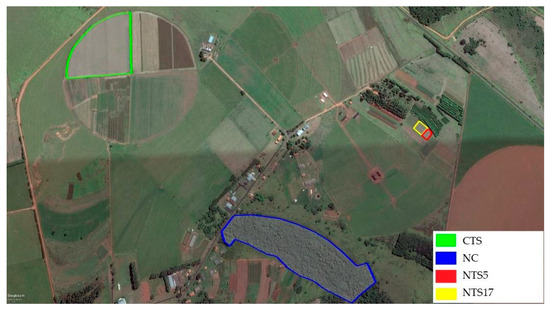
Figure 1.
Areas studied with their respective demarcated perimeters. Source: Google earth (2023).
The Native Cerrado (NC) area is located close to the experimental areas and has been undergoing a natural regeneration process for over twenty years.
The region’s climate is classified as Aw [29], with a rainy season in summer and dryness in winter, presenting an average annual precipitation and temperature of 1600 mm and 22.6 °C, respectively. In the evaluation period, there was accumulated precipitation of 1995.3 mm, well above what is normal for the region (Figure 2) [30].
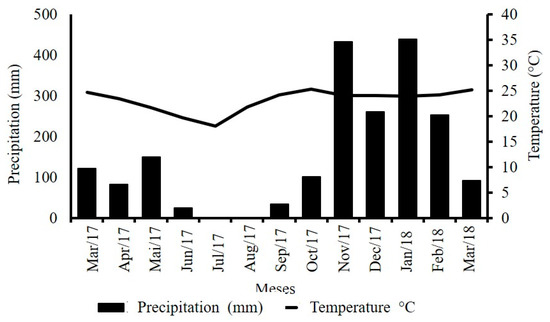
Figure 2.
Climatic variables obtained from the database of meteorological data for the teaching and research of [30], for the period from March 2017 to March 2018.
The soil of the areas was classified as Typic Hapludox [31] with sandy texture. The soil (0–40 cm) presented 220, 720, and 60 g kg−1 of clay, sand, and silt, respectively, according to the soil chemical analysis (Table 2).

Table 2.
Soil chemical attributes in the 0–40 cm soil layer in different systems.
The P, potassium (K), and sodium (Na) contents were extracted by Mehlich 1, and then the P was determined by colorimetry, and the K and Na by flame photometer [32]. The calcium (Ca), magnesium (Mg), and aluminum (Al) contents were extracted by KCl 1.0 mol L−1. The Ca and Mg were determined by atomic absorption spectrophotometry, and the Al by titration with NaOH 0.0125 mol L−1 [33]. The organic matter was determined by the modified Walkley and Black method [34].
Before implementing the experiment, the soil was previously corrected with dolomitic limestone (36.4% calcium oxide, 44% magnesium oxide, 99.87% neutralizing power, and total relative neutralizing power of 90.28%), to raise the baseline saturation to 70%. This correction practice was repeated over the years of cultivation, whenever the potential hydrogen (pH) of the soil in the area was below the values recommended as ideal for the cultivation of plants [35].
2.2. Experimental Design
The experimental design used was the distribution of completely randomized plots, where four soil management systems were evaluated: (1) a no-tillage system (NTS) after 5 years of implantation (NTS5); (2) an NTS after 17 years of implantation (NTS17); (3) a conventional tillage system more than 20 years old (CTS); (4) Native Cerrado (NC) without anthropic action. All samplings were carried out with five repetitions for each area.
The systems were implanted in areas of Typic Hapludox with a sandy texture, with the same granulometric composition and under the same climatic conditions, where the areas are, at most, 400 (CTS) and 200 (NC) meters apart, and the NTS5 and NTS17 are side by side.
Each treatment was implanted in experimental plots measuring seven meters wide by six meters long (7 × 6 m), making a total area of 42 m2, where sunn hemp (Crotalaria juncea L.) or pearl millet (Pennisetum glaucum L., cultivar ADR 500) in each cycle preceded the sowing of commercial crops (corn, soybeans, and beans).
These cover crops were always mechanically sown with a Semina 2 seeder, with five rows spaced 20 cm apart, and using 20 and 50 seeds per meter of sunn hemp and pearl millet. The seeds produced between 4.0 to 9.0 t ha−1 and 7.0 to 12.0 t ha−1 of dry mass, which, after being managed, was deposited on the soil surface, respectively. No type of mineral fertilizer was used in the cultivation of these covers.
The management of the cover crops was carried out using the active ingredient Sal de N-(phosphonomethyl) glycine 792.5 g kg−1 (Roundup WGR) at a dose of 2 kg ha−1 of the commercial product. In general, ten days after handling (desiccation), the commercial crop was mechanically sown.
After managing the coverings, these 42 m2 plots were divided into two, where corn (Zea mays L.) and soybeans (Glycine max L.) (or corn and common beans—Phaseolus vulgaris) were sown, and in the following cycle, these commercial crops were rotated, without repeating planting in the same area.
Fertilization was applied according to the crop culture in the NTS5, NTS17, and CTS areas: corn, with 400 kg ha−1 of 08-28-16 (N-P-K), was applied at sowing, plus 70 kg ha−1 of N and 40 kg ha−1 of K in the top dressing at 20 and 40 days after plant emergence; soybeans generally received 200 kg ha−1 of 0-20-15 + 2.5% Zn + 2.5% of Mn at sowing, corresponding to 40 kg ha−1 of P2O5, 60 kg ha−1 of K2O, 5 kg ha−1 of Zn, and 5 kg ha−1 of Mn, with seed inoculation; common beans generally received 350 kg ha−1 of 8-28-16 (N-P-K) + 0.5% Zn at sowing. All fertilizer doses were determined following the recommendations by Ribeiro et al. [35].
2.3. Soil Sample Collection
In March 2017, for the chemical characterization of the experimental area, an area equivalent in size to the treatment areas (NTS5, NTS17, CTS, and NC) was sampled with the aid of a straight blade. Four composite soil samples formed by 10 simple samples were collected, with five samples collected from the row and five from between rows, at depths of 0–5, 5–10, 10–20, and 20–40 cm. To analyze the total P (tP), P remaining (P-rem), organic P (oP), and inorganic P (iP) fractionation, only the samples collected from the surface layers of the soil (0–5 and 5–10 cm) were used. This depth was chosen due to the deposition of plant residues on the soil surface in the NTS, as well as the greater soil disturbance in the CTS.
These samples were taken to the laboratory, air-dried, and then ground to obtain air-dried fine earth (ADFE), which was later used to carry out chemical and physical analyses and to determine the P fractions of the soil.
2.4. Determination of the Total, Remaining, Organic, and Inorganic Fractions of P
To determine the remaining P (P-rem), 5 cm3 of the ADFE was used, which was placed in a 0.01 mol L−1 CaCl2 solution containing 60 mg L−1 of P for 60 min. Next, 5 mL of the extract was pipetted, where the P concentration was determined from the equilibrium solution with a subsequent reading in a spectrophotometer [36].
The total organic (oP) and inorganic P (iP) fractions were determined using the sequential extraction of P adapted from Bowman [37], where the oP content is obtained by subtracting the total P (tP) from the inorganic P (iP), and recovered in sequential extracts with sodium bicarbonate 0.5 mol L−1 at pH 8.5, acid extraction with sulfuric acid (H2SO4), and alkaline with sodium hydroxide (NaOH) 0.5 mol L−1, according to Bowman and Cole [38], with modifications made by Duda et al. [39].
The soil P fractions were determined using sequential extraction according to Bowman and Cole [38], with modifications made by Duda et al. [39]. For this, first, 40 mL of 0.5 mol L−1 sodium bicarbonate solution (NaHCO3), with a previously controlled pH of 8.5 after correction with NaOH, was added to a 4 g of soil sample and stirred for 16 h. Then the sample was centrifuged at 4000 revolutions per minute (RPM) for 20 min. A 5 mL sample of the supernatant was collected for the determination of the inorganic P extracted with bicarbonate (iP-Bic), where 10 mL of diluted ammonium molybdate was added with the presence of ascorbic acid as a reducer for the development of a blue color and for subsequent determination using spectrophotometry [32], which represents its labile form.
Then, 20 mL of the same supernatant was pipetted, which was placed in digestion tubes together with 2 mL of saturated magnesium chloride (MgCl2) and 3 mL of concentrated perchloric acid (72% P.A.). In a digester block, a gradual increase in temperature was controlled until the formation of a colorless/yellowish gel, at which point the digested material, after being cooled, was transferred to Falcon tubes and calibrated with distilled water to 20 mL to determine the total P extracted with bicarbonate (tP-Bic) [32].
In the same soil sample, 1.5 mL of H2SO4 1.79 mol L−1 was added, and then it was placed in a horizontal shaker for 16 h and centrifuged at 4000 RPM for 20 min, after which the supernatant was collected to determine the inorganic or undigested P (iP-H) and the total or digested P (tP-H) from the extraction with H2SO4, which represents the form of moderately labile P [32].
The last extraction was performed using 40 mL of a 0.5 mol L−1 sodium hydroxide (NaOH) solution, and then the samples were placed in a water bath at 80 °C for 2 h. After cooling, the samples were centrifuged at 4000 RPM for 20 min, where the supernatant was collected to determine the total P (tP) extracted with NaOH (tP-OH), which represents the moderately resistant form of this nutrient [39]. To determine the inorganic P (iP) extracted with NaOH (iP-OH), the pH of each sample was reduced to 1.0 with H2SO4, and 5 mL of the extract was pipetted again to react with ammonium molybdate in the presence of ascorbic acid to form a blue color.
The total P (tP) of the soil was obtained according to Equation (1), where tP-Bic is the total P extracted by NaHCO3, tP-H is the total P extracted by H2SO4, and tP-OH is the total P extracted by NaOH. The total inorganic P (tiP) of soil was obtained according to Equation (2), where iP is the inorganic P extracted by NaHCO3, tP-H is the inorganic P extracted by H2SO4, and tP-OH is the inorganic P extracted by NaOH [38,39]. The organic P (oP) was determined from the difference between the tP and the iP in each extraction, according to Equation (3), and the total organic P (toP) of soil was obtained according to Equation (4). The percentage of organic P (PoP) was calculated using Equation (5).
tP = tP-Bic + tP-H + tP-OH
tiP = iP-Bic + iP-H + iP-OH
oP-Bic/H/OH = tP-Bic/H/OH − iP-Bic/H/OH
toP = oP-Bic + oP-H + oP-OH
PoP = (otP/tP) × 100
2.5. Statistical Analyses
To meet the presumptions for the analyses of variance, the normality of residues (Shapiro–Wilk) and homogeneity of variances (Barttlet) were evaluated for all data. The data were then submitted for analysis of variance with the application of the F test, and when differences were detected, the mean values were compared using the Tukey test at ≤5% probability. The R Core Team software, version 2020 (Viena, Austria), was used to perform these analyses.
3. Results
3.1. Inorganic P Obtained in Routine Analysis via Acid Extraction
The results obtained through routine fertility analysis for the available inorganic P (iP) (iP-avail) and the remaining (iP-rem) levels at depths from 0 to 5 and 5 to 10 cm, show that the highest values were found in the conventional tillage system (CTS) when compared to the other management systems (Table 3).

Table 3.
Inorganic P (iP) available (iP-avail) and remaining (iP-rem) obtained in routine analyses of different systems.
The remaining iP concentrations in the most superficial soil depth (0–5 cm) were similar (p > 0.05) among the NTS5, NTS17, and CTS treatments and above the P level observed in the Native Cerrado (NC). In the 5–10 cm soil depth, the greatest P concentration was found in the CTS compared to the other treatments.
The soil management systems studied resulted in increments of the iP-rem at the 0–5 cm soil depth, which ranged between 35.95 and 40.40 mg kg−1 of P, and at the 5 to 10 cm soil depth, which ranged between 25.36 and 40.59 mg kg−1 of P, greater (p < 0.05) than what was observed in the NC, which ranged between 8.20 and 8.70 mg kg−1 of P. The soil and litter of the native area were responsible for greater nutrient retention, resulting in a significant reduction in the iP-rem, with an average of 8.45 mg kg−1.
3.2. Determination of P Contents using Sequential Extractions with Sodium Bicarbonate, Sulfuric Acid, and Sodium Hydroxide
3.2.1. Labile Portion of Inorganic P, Organic, and Total of Soil Extracted with Sodium Bicarbonate
In analyzing the labile portion of P in the soil, it was observed that the contents of inorganic P (iP-Bic) were similar to each other in the NTS5 and CTS, for organic P (oP-Bic), they were higher in the NTS5 and NTS17, and for the total P (tP-Bic), there were no statistical differences (p > 0.05) among the NTS5, NTS17, and CTS. However, all were higher when compared to the Native Cerrado area (Table 4).

Table 4.
Labile portions of inorganic P (iP-Bic), organic (oP-Bic), and total (tP-Bic) of soil extracted with sodium bicarbonate (NaHCO3) in the different management systems.
The predominant form of P in the labile fraction indicates the contribution of each soil management system to the P content of the soil. The levels of iP-Bic at depths of 0–5 and 5–10 cm were similar in the CTS and NTS5 and lower in the NTS17, while the lowest values were found in NC.
For oP-Bic, it was observed that, compared to the CTS and NC, the NTS17 presented significantly higher values at a depth of 0–5 cm, which also occurred in the NTS5 at 5–10 cm, demonstrating that the phosphate fertilization that was performed superficially in these areas, and which is associated with the mineralization and P cycling of the organic matter, contributed to the soil surface in these systems, increasing the amount of oP-Bic in the soil.
The tP-Bic contents in the soil at a depth of 0–5 cm were similar among the NTS5, NTS17, and CTS, varying between 61.00 and 63.87 mg kg−1 and higher than the value found in the NC (30.41 mg kg−1). However, the same did not occur at a depth of 5–10 cm, as the value observed in the NTS5 (76.35 mg kg−1) was higher than the values of those in the NTS17, CTS, and NC, which ranged from 25.86 to 65.51 mg kg−1.
3.2.2. Moderately Labile Portion of Inorganic P, Organic, and Total of Soil Extracted with Acid Solution (H2SO4)
The less available forms of P in the soil, which are extractable with an acid solution (H2SO4), called moderately labile P, inorganic P (iP-H), organic P (oP -H), and total P (tP-H), had extremely reduced fractions in the NTS5 and NTS17 at 0–10 cm, and at the same time, were abundant in the CTS and intermediate in the NC (Table 5).

Table 5.
Moderately labile portion of inorganic P (iP-H), organic (oP-H), and total (tP-H) from the soil extracted in sulfuric acid solution (H2SO4) in the different management systems.
From the total extracted P in the CTS, 93% at 0–5 cm and 84% at a 5–10 cm soil depth were iP-H. However, in the NTS5, NTS17, and NC, the contents of oP-H were always greater than iP-H. Even with the NTS presenting levels of tP-H much higher than the levels of extracted P, the organic form did not differ from the other treatments.
3.2.3. Moderately Resistant Portion of Inorganic P, Organic, and Total from the Soil Extracting in Alkaline Solution (NaOH)
For products from the basic extraction with sodium hydroxide (NaOH), called moderately resistant P, it was observed that the inorganic P (iP-OH) content in the NTS5 (67.25 mg kg−1) was significantly higher at a depth of 0–5 cm, while at 5–10 cm, there were no differences among the treatments. For organic P (oP-OH), the highest content occurred in the NTS17 (39.31 mg kg−1) at a depth of 0–5 cm, while at 5–10 cm, the same occurred for the NTS5 and NTS17 (29.29 and 36.36 mg kg−1), respectively, which were similar and higher than in the CTS (6.68 mg kg−1) and NC (9.18 mg kg−1). For tP-OH, it was observed that the NTS5 and NTS17 showed statistically equal values at depths of 0–5 and 5–10 cm, both higher than in the CTS and NC (Table 6).

Table 6.
Moderately resistant portion of inorganic P (iP-OH), organic (oP-OH), and total (tP-OH) from the soil extracted in sulfuric acid solution (H2SO4) in the different management systems.
In all management systems, the iP-OH was always higher than the oP-OH due to the low susceptibility of this fraction in the CTS, NTS5, NTS17 and NC.
In analyzing the values and total contributions of the three P extractions (tiP, toP, and tP), it was observed that the highest P contents were obtained in the CTS, NC, and CTS, at depths of 0–5 cm (350.07, 109.85, and 415.62 mg kg−1) and 5–10 cm (366.63, 104.79, and 449.97 mg kg−1, respectively), to the detriment of the other soil systems (NTS5 and NTS17) at both depths. However, a characteristic parameter indicative of the influence of crop management occurred in the NTS17 and NC, as both had total oP values above the total iP (Table 7).

Table 7.
Fractions of the forms of P in soil in function of the management system.
Understanding the relevance of this P behavior in conservation management systems with more time and adoption mainly indicates the importance of P mineralization for plant development, as occurs with the addition of organic fertilizers.
In general, there was an intense accumulation of P in the surface layers of the soil in the NTS and CTS areas from the application of phosphate fertilizers in the sowing line. However, in this study, the accumulation demonstrably only occurred in the CTS area, which, in both studied layers (0–5 and 5–10 cm) presented total P (tP) contents more than 100% higher than any other area, again due to the inorganic P (iP), since the organic form of the element presented values compatible with the other systems and with the NC.
Understanding the importance of this P pattern in ancient conservation systems mainly refers to the importance of P mineralization for plant development, as occurs with the addition of organic fertilizers. Despite the numerical variation presented by the total oP content in the surface soil, values minimums of 64.21 mg kg−1 of P in the NTS5 and 65.55 mg kg−1 in the CTS occurred, an intermediate value occurred in the NTS17 (89.48 mg kg−1), and a maximum value of 109.85 mg kg−1 of P occurred in the NC at a depth of 0–5 cm. Meanwhile, at a depth of 5–10 cm, the lowest values occurred in the NTS5 (76.72 mg kg−1) and NTS17 (71.30 mg kg−1), an intermediate value occurred in the CTS (83.32 mg kg−1), and the maximum value of 104.79 mg kg−1 of P continued to be in the NC.
However, in the NC and NTS17, where the concentrations of the total oP were superior to the total iP in the topsoil, the relationship between toP and tiP presented the following decreasing order: NC (1.18) > NTS17 (1.10) > NTS5 (0.60) > CTS (0.18).
4. Discussion
4.1. Inorganic P Available (iP-ava) and Remaining (iP-rem) Obtained in Routine Soil Fertility Analysis
This higher concentration of iP-avail can be attributed to the high phosphate fertilization used over 20 years in the CTS in the area, where cumulative amounts of P were applied during planting throughout the period. Additionally, the soil in the area has a low clay content (220 g kg−1) as it is an oxisol with high levels of iron (Fe) and aluminum (Al), giving the soil a high P adsorption capacity (P), and consequently, lessening its available to the plants, as was also observed by Loss et al. [7]. Conte et al. [23] and Casali et al. [28] have highlighted that the soil additions of P above the quantities required for plant development increase the fractions of inorganic P (iP), a process that may cause saturation of the adsorptive sites.
With the soil turning that occurs annually in the CTS area, the P comes into contact with the colloids, increasing their adsorption and resulting in weak binding energy, so it can be easily released into the soil solution and increase the P-avail. However, Guppy et al. [20] and Souza [21] have highlighted that, over time, this bond tends to become more stable, and the P is adsorbed with greater energy, making it less available to vegetables.
According to Santos et al. [14], in cultivated soils where there are regular additions of phosphate fertilizers, management provides changes in the fractions and concentrations of P in the soil profile. According to Gatiboni et al. [40], when P is applied in greater amounts compared to what is exported by the crops, this P accumulates in a moderately labile form and acts as a collector. However, when P is applied in small amounts, the P that is accumulated in a moderately labile form can act as a source, supplying the requirements of the culture.
Rheinheimer et al. [41] stated that in areas under an NTS, the highest concentrations of P were observed in the surface layers of the soil, between 5 and 10 cm deep, which is explained by the location of fertilization (no tillage) and nutrient cycling, while in a CTS, the distribution of nutrients followed the depth of the soil plowing—behavior that was also observed in this study, however, the highest accumulation occurred in the CTS areas (Table 3).
In their study, Maia et al. [42] observed that the iP-avail was sensitive to the variation in soil moisture, which was lower in the CTS. This was associated with the lack of vegetation cover or its residues on the soil surface. The cycle of nutrients was the minimum, and thus, the availability of P in the soil decreased when the soil moisture was lower, since the diffusion process depends on water [42]. However, this behavior was not observed in this study, since the CTS presented higher iP-avail and iP-rem values of 0–5 and 5–10 cm compared to the other management systems (Table 3).
This pattern of P-rem at the most superficial depth (0–5 cm), equaling the levels in the NTS5 and NTS17 to the CTS, may have been due to the decomposition of the residues of Poaceae and Fabaceae used in the rotation of cultures in these areas, and the low molecular weight organic acids, which can block P adsorption sites, as highlighted by Bezerra et al. [19].
In studying the maximum P adsorption capacity in an oxisol, Moura et al. [16] observed that the great content of SOM in the topsoil contributed to reducing P retention. However, in the native area, a constant inflow of more lignified plant residues—slow decomposition materials—released small quantities of organic acids of low molecular weight. Based on the results obtained in this study, in analyzing the iP-rem, it is possible to affirm that the NC soil was responsible for the greater fixation action of the nutrient (8.45 mg kg−1).
According to Conte et al. [23] and Rheinheimer et al. [41], the redistribution of P in various forms also occurs in NTS-cultivated soils since the adsorption of P occurs primarily in sites of low lability, and subsequently, the remnant P is redistributed in the forms retained with lower power and higher availability to the plants.
The cover crops used in the crop rotation system for the production of NTS straw effectively contributed to increasing the P-rem content at a depth of 0–5 cm in the soil, which corroborates the statement made by Fernandes et al. [8] that the continuous supply of organic matter increases the P adsorption sites in the soil, which can reduce the adsorption and contributes to the increase in the P content in the soil.
4.2. Labile P
In analyzing the labile portion of P in the soil in this study, it was observed that the P contents (inorganic, organic, and total) were similar in the CTS, NTS5, and NTS17 at the evaluated depths (0–5 and 5–10 cm) and higher when compared to the NC (Table 4), which is in line with the statement made by Santos et al. [14] and Bravo et al. [43] that successive phosphate fertilization promotes increased P lability because the adsorption sites are more avid for this element to be gradually filled, and new fertilizations increase the most labile fractions of P.
Results similar to those obtained in this study for the NTS5 and NTS17 areas were observed by Bezerra et al. [19] and Rodrigues et al. [2], who have also shown increases in the availability of P capable of being absorbed by plants in areas under an NTS when compared to a CTS in an oxisol in the Native Cerrado area.
Considering the initial concentration of P-avail in the areas under study (Table 3), the increase in the levels of tP corresponded to the process of P cycling. This rise in the P content was more intense in areas associated with the use of P fertilizer since the fraction extracted with NaHCO3 demonstrated that the increments in labile P were similar among the soil management systems, as was also observed by Beutler et al. [25].
In the NTS areas, the P tended to accumulate on the soil surface due to fertilizer applications that were carried out at a depth of up to 10 cm, and with this, sorption, cycling, and nutrient recycling reactions occurred at these locations, as well as the mineralization of residues containing P, affecting the distribution of the P in the soil in different ways. Similar results were also observed in the study conducted by Bravo et al. [43].
The labile P fraction shows the contribution of each management system to the P content in the soil, as the Pi-Bic contents in the 0–5 cm layer were similar in the CTS and NTS5, which were higher than in the NTS17 and NC. Meanwhile, from 5 to 10 cm, the CTS, NTS5, and NTS17 showed equal and higher values than the NC. This behavior at both depths allows us to assume that there was a relevant contribution of the phosphate fertilization carried out in areas with Pi-Bic accumulation in the management systems evaluated when compared to the NC.
Generally, only in the CTS, the inorganic forms of labile P were superior to the organic forms by more than 10%. In analyzing the discrepancy between the iP and the oP, the NTS5 presented the greatest similarity of values, having small iP predominance (51%) compared to oP (49%), which are fractions close to those found in the Cerrado biome.
The NTS17 presented 65% of the P in its organic form (0–5 cm), a proportion that was similar to the values found in the native area at the same soil layer (67% iP and 33% oP), which can be attributed to the input of the SOM in the system in the past 17 years, which presented values in the NC > NTS17 > NTS5 > CTS sequence at this same depth. However, these proportions were not maintained at the 5–10 cm soil depth since the levels of iP were superior to the levels of oP under the same conditions. In the native area, the contents of oP were greater than those found for iP, at an approximate ratio of 2:1.
This result reinforces the findings by Gatiboni et al. [40] who, in analyzing the availability of P and its forms accumulated in an NTS, generalized that in the long term, the addition of fertilizers in sufficient quantities to supply plant development equilibrates the capacity of organic and inorganic forms of P to provide this nutrient to crops. Souza et al. [1] and Rodrigues et al. [2] have observed increments of organic and inorganic P in the soil’s superficial layer in an NTS when compared to a CTS, except in treatments where the crop residues were from maize, in which the labile inorganic P was superior in the NTS.
In 5-year NTS areas, Olibone and Rosolem [44] evaluated the organic fractions of P after the application of phosphate fertilizers in the cultivation of soybeans. The authors found that there was an increase in oP after harvest, correlating this increase to the decomposition of the root systems of the cultivated plants.
4.3. Moderately Labile P
The moderately labile forms of P in soil (iP-H, oP-H, and tP-H) were extremely low in the NTS5 and NTS17 and abundant in the CTS and NC. Among the areas cultivated with annual crops, in the CTS, an expressive superiority of tP-H was observed, which may be explained by the heavy P fertilization used in the area at each cultivation cycle, because this area is cultivated only once a year and after is left fallow (native vegetation) until the next cycle.
According to Souza [21] and Beutler et al. [25], the application of annual heavy P fertilization increases the concentration of labile inorganic P fraction (iP). This iP phase is more strongly adsorbed with time with complexes of iron (Fe) and aluminum (Al) oxides, causing an increase in the moderately labile P fraction (iP-H).
In the NC area, where there is continuous deposition of organic material on the soil surface and annual crops are not cultivated, the maintenance of the soil moisture is greater, which favors P cycling, increasing its availability in the soil. According to Costa et al. [45], when there is an increase in humidity in the area, the water film close to the solid soil particles becomes thicker, reducing the ion–colloid interaction.
The differences found in the Pi-H and Po-H values between the systems used are due to the accumulation of the inorganic form of P in the CTS, which reinforces the idea of ion–colloid contact.
Using the same soil chemical extractor, Beutler et al. [25] observed great concentrations of tP-H (moderately labile) when compared to tP extracted with sodium bicarbonate (NaHCO3) (labile fraction), in areas of pasture, integrated crop and livestock, and Cerrado biome (native area). In the present study, this pattern was restricted to the CTS and NC areas, and not observed in the NTS areas.
4.4. Moderately Resistant P
The contents of moderately resistant P (iP-OH, oP-OH, and tP-OH) extracted in alkaline solution (NaOH) were significantly higher in the NTS5, NTS17, and NC compared to the CTS, showing that among the cultivation systems, the forms that tended to have greater P lability were more present in the CTS, which can be explained by the high amounts of P applied to the soil after each cultivation cycle, which were adsorbed on the soil particles.
In this Cerrado region, where the study was conducted, the soils are highly weathered, and the highest proportions of P were found in the extractions with NaOH, probably due to the strong relationship of this fraction with the presence of Fe and Al oxides, kaolinite, and low organic matter content, which favor the adsorption of P in the soil [19,43].
Among the factors that potentiate the difficulty of P extraction, there is reduced P availability due to its fixation with Fe, Al, and Ca, which immobilize P [24]. Corroborating this statement, Souza Júnior et al. [46] observed that in most weathered soils with low pH and low contents of Ca, the majority of inorganic P forms occurred precisely in P-Fe and P-Al, while in alkaline soils, the predominating connection is P-Ca.
In natural conditions of strong P deficiency, Neufeldt et al. [47] reported that more than 60% of the labile portion was derived from organic P, indicating that the primary contribution of organic P concentrates on the more labile fractions, which are also susceptible to rapid mineralization. In this study, even with the highest proportion of organic P in the soil, there was no greater availability for plants.
The NTS5 and CTS differed significantly for iP-OH at the 0–5 cm soil depth, with the area under the no-tillage system presenting 67.25 mg kg−1 of P and the CTS presenting 36.34 mg kg−1 of P. Meanwhile, the accumulation of oP-OH at the 5–10 cm soil depth was superior in both areas under the no-tillage system, even compared to the native area.
The greater accumulation of P in the superficial soil layers of the NTS5 can be explained by the application of phosphate fertilizers in the sowing line or application of spread. According to Redel et al. [48], this accumulation is due to the limited mobility of this element in the soil profile. These results are in line with those presented in the studies by Rheinheimer et al. [41,49], where they highlighted that of the buffer fraction increases when P additions are greater than its production, which decreases in soils cultivated with a low P, and therefore, the accumulation depends entirely on what is returned and removed from the system.
The intense accumulation of P in the superficial soil layers of an NTS from the application of P fertilizers in the sowing line or spread application was reported by Redel et al. [48]. This accumulation was due to the limited mobility of this element in the soil profile. Rheinheimer et al. [41] showed that the magnitude of the buffering fraction increases when the addition of P is superior to its output, which decreases in soils cultivated with a low P reposition of the P exported, and thus, the accumulation depends entirely on what is returned and removed from the system.
According to Gatiboni et al. [40], when the source of fertilizer is of organic origin and easily decomposed, the accumulation of P in the soil may initially be in the organic form, which is subsequently converted to the inorganic form due to microbial mineralization.
Considering the positive correlation between toP and organic C [20], it is possible to observe the contribution generated by organic matter in the long-term cultivation area (NTS17). This proves the hypothesis tested in this study that there is an increase in organic and inorganic P levels, providing greater availability of the nutrient in the soil for crops. The same result was repeated at a 5–10 cm soil depth only in the NC.
In this way, by observing the conditions of the NTS17, it is worth noting that the majority of crops can only use P in its inorganic form, which makes the enzymatic activity of phosphatase crucial in the conversion of organic forms to its inorganic state, capable of absorption [50,51].
5. Conclusions
Our results show that the continuous input of organic matter deposited on the soil surface in the NTS17 from the residues of cover crops used in the rotation system with commercial crops increased the levels of organic and inorganic P, consequently providing greater availability of P in the soil for the cultivated crops.
The P that was the product of sequential extractions accumulated preferentially on the surface in the organic form under a 17-year-old no-tillage system (NTS17) and the Native Cerrado area, demonstrating the contribution of the system in the conversion of the P reserve in the soil.
The conventional tillage system presented the greatest accumulation of P, especially in the inorganic form.
The concentrations of the organic and inorganic P in the soil were not affected by the time of implantation of the no-tillage system, which were similar to those found in the Native Cerrado area.
Author Contributions
Conceptualization, J.L.R.T., M.G.P., and D.D.d.A.C.; Methodology and Experiment Implementation, J.L.R.T., D.D.d.A.C., and M.G.P.; Formal Analysis, J.L.R.T., M.G.P., and A.P.G.; Data Curation, J.L.R.T., M.G.P., A.P.G., A.L., and C.R.L.; Writing—Original Draft, J.L.R.T., D.D.d.A.C., M.C., and C.R.L.; Writing-Review and Editing, J.L.R.T., D.D.d.A.C., A.L., M.C., and C.R.L.; Project Administration, J.L.R.T., D.D.d.A.C., D.M.d.S.V., and L.V.F.G.; Funding Acquisition, J.L.R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq-Process 306151/2020-0), by the Luiz de Queiroz Foundation for Agricultural Studies (AGRISUS FOUNDATION—Project PA 2993-20), and by the State Research Support Foundation of Minas Gerais (FAPEMIG—Project PPM-00560-18), upon signature of the Term of Grant by their legal representatives.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank the Federal Institute of Triângulo Mineiro (IFTM), Uberaba Campus for providing the equipment needed and laboratory space for carrying out the experiments and analyses; the Luiz de Queiroz Foundation for Agricultural Studies (AGRISUS FOUNDATION); and the Brazilian National Council for Scientific and Technological Development (CNPq), State Research Support Foundation of Minas Gerais (FAPEMIG), for funding the research and for granting Research Productivity scholarships to the researchers and Scientific Initiation scholarships to the students involved with this project.
Conflicts of Interest
The authors declare no conflict of interest regarding the data and findings published in this article.
References
- Souza, G.P.; Figueiredo, C.C.; Sousa, D.M.G. Matéria orgânica do solo influenciada por sistemas de manejo, adubação fosfatada e plantas de cobertura. Pesqui. Agropecuária Bras. 2016, 51, 1668–1676. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Mazetto Júnior, J.C.; Torres, J.L.R.; Costa, D.D.d.A.; Silva, V.R.e.; Souza, Z.M.d.; Lemes, E.M. Production and Decomposition of Cover Crop Residues and Associations with Soil Organic Fractions. J. Agric. Sci. 2019, 11, 58–69. [Google Scholar] [CrossRef]
- Silva, V.R.e.; Torres, J.L.R.; Costa, D.D.d.A.; Silveira, B.d.S.; Vieira, D.M.d.S.; Lemes, E.M. Soil Physical Attributes in Long-Term Soil Management Systems (Tillage and No-till). J. Agric. Sci. 2020, 12, 194–207. [Google Scholar] [CrossRef]
- Pinto, L.A.D.S.R.; Torres, J.L.R.; Morais, I.D.S.; Ferreira, R.; da Silva, W.F.; Lima, S.D.S.; Beutler, S.J.; Pereira, M.G. Physicogenic and biogenic aggregates under different management systems in the Cerrado region, Brazil. Rev. Bras. Cienc. Solo 2021, 45, e0200114. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Leal Júnior, A.L.B.; Barreto, A.C.; Carvalho, F.J.; Assis, R.L.; Loss, A.; Lemes, E.M.; Vieira, D.M.S. Mechanical and Biological Soil Decompaction for No-Tillage Maize Production. Agronomy 2022, 12, 2310. [Google Scholar] [CrossRef]
- Loss, A.; Pereira, M.G.; Perin, A.; Beutler, S.J.; Anjos, L.H.C. Oxidizable carbon and humic substances in rotation systems with brachiaria/livestock and pearl millet/no livestock in the Brazilian Cerrado. Span. J. Agric. Res. 2013, 11, 217–231. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Grohskopf, M.A.; Gomes, E.R.; Ferreira, N.R.; Bull, L.T. Fósforo na solução do solo em resposta à aplicação de fertilizantes fluidos mineral e organomineral. Irriga 2015, 1, 14–27. [Google Scholar] [CrossRef]
- Pinto, L.A.D.S.R.; de Lima, S.S.; da Silva, C.F.; Gonçalves, R.G.M.; Morais, I.S.; Ferreira, W.; Silva Junior, W.F.; Torres, J.L.R.; Pereira, M.G. Soil quality indicators in conventional and conservation tillage systems in the Brazilian Cerrado. Env. Earth Sci. 2022, 81, 306–319. [Google Scholar] [CrossRef]
- Schiller, A.P.; Manfrin, J.; Eckhardt, D.C.S.; Seidel, E.P.; Lana, M.C.; Gonçalves, A.C., Jr.; Sampaio, M.C.; Rego, C.A.R.M. Stability of Aggregates and the Processes that Help in Their Formation and Stabilization. Int. J. Plant Soil Sci. 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Sá, J.C.M.; Cerri, C.C.; Piccolo, M.C.; Feigl, B.E.; Fornari, A.; Sá, M.F.M.; Venzke Filho, S.P.; Seguy, L.; Bouzinac, S.; Paulleti, V. O plantio direto como base do sistema de produção visando o sequestro de carbono. Plantio Direto 2004, 84, 45–61. [Google Scholar]
- Anghinoni, I. Fertilidade do solo e seu manejo em sistema de plantio direto. In Fertilidade do Solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS: Viçosa, Brazil, 2007; pp. 873–928. [Google Scholar]
- Torres, J.L.R.; Mazetto Júnior, J.C.; Silva Júnior, J.; Vieira, D.M.d.S.; de Souza, Z.M.; Assis, R.L.d.; Lemes, E.M. Soil physical attributes and organic matter accumulation under no-tillage systems in the Cerrado. Soil Res. 2019, 57, 712–718. [Google Scholar] [CrossRef]
- Santos, D.R.; Gatiboni, L.C.; Kaminski, J. Fatores que afetam a disponibilidade do fósforo e o manejo da adubação fosfatada em solos sob sistema plantio direto. Cienc. Rural 2008, 38, 576–586. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Pereira, M.G.; Rodrigues Junior, D.J.; Loss, A. Production, decomposition of residues and yield of maize and soybeans grown on cover crops. Rev. Cienc. Agron. 2015, 46, 451–459. [Google Scholar] [CrossRef]
- Moura, J.B.; Ventura, M.V.A.; Cabral, J.S.R.; Azevedo, W.R. Adsorção de Fósforo em Latossolo Vermelho Distrófico sob Vegetação de Cerrado em Rio Verde-Go. Front. J. Soc. Technol. Environ. Sci. 2015, 4, 199–208. [Google Scholar] [CrossRef]
- Reis, D.A.; Lima, C.L.R.; Bamberg, A.L. Qualidade física e frações da matéria orgânica de um Planossolo sob sistema plantio direto. Pesqui. Agropecu. Bras. 2016, 51, 1623–1632. [Google Scholar] [CrossRef]
- Devine, S.; Markewitz, D.; Hendrix, P.; Coleman, D. Soil aggregates and associated organic matter under conventional tillage, no-tillage, and forest succession after three decades. PLoS ONE 2014, 9, e84988. [Google Scholar] [CrossRef]
- Bezerra, R.P.M.; Loss, A.; Pereira, M.G.; Perin, A. Frações de fósforo e correlação com atributos edáficos sob sistemas de plantio direto e integração lavoura-pecuária no Cerrado Goiano. Semin. Ciências Agrárias 2015, 36, 1287–1306. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Blamey, F.P.C.; Moody, P.W. Decomposing organic matter residues reduce phosphorus sorption in highly weathered soils? Soil Sci. Soc. Am. J. 2005, 69, 1405–1411. [Google Scholar] [CrossRef]
- Souza, E.D. Evolução Da Matéria Orgânica, Do Fósforo E Da Agregação Do Solo Em Sistema de Integração Agricultura-Pecuária Em Plantio Direto, Submetido a Intensidades de Pastejo. 182f. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2008. [Google Scholar]
- Andrade, F.V.; Mendonça, E.S.; Silva, I.R.; Mateus, R.F. Dry-matter production and phosphorus accumulation by maize plants in response to the addition of organic acids in Oxisols. Commun. Soil Sci. Plant Anal. 2007, 38, 2733–2745. [Google Scholar] [CrossRef]
- Conte, E.; Anghinoni, I.; Rheinheimer, D.S. Frações de fósforo acumuladas em Latossolo argiloso pela aplicação de fosfato no sistema plantio direto. Rev. Bras. Ciênc. Solo 2003, 27, 893–900. [Google Scholar] [CrossRef]
- Camargo, M.S.; Barbosa, D.S.; Resende, R.H.; Korndörfer, G.H.; Pereira, H.S. Fósforo em solos de Cerrado submetidos à calagem. Biosci. J. 2010, 26, 187–194. [Google Scholar]
- Beutler, S.J.; Pereira, M.G.; Loss, A.; Perin, A.; Anjos, L.H.C. Humic substances and phosphorus fractions in areas with crop-livestock integration, pasture and natural Cerrado vegetation in Goiás, Brazil. Trop. Subtrop. Agroecosyst. 2015, 18, 11–25. [Google Scholar]
- Novais, R.F.; Smyth, T.J.; Nunes, F.N. Fósforo. In Fertilidade do solo; Novais, R.F., Alvarez, V.V.H., Barros, N.F., Fontes, R.L.F., Cantarutti, R.B., Neves, J.C.L., Eds.; SBCS: Viçosa, Brazil, 2007; pp. 471–550. [Google Scholar]
- Fernández, R.I.E.; Novais, R.F.; Nunes, F.N.; Ker, J.C. Reversibilidade do fósforo não-lábil em solos submetidos à redução microbiana e química: I-Alterações químicas e mineralógicas. Rev. Bras. Ciênc. Solo 2008, 32, 2319–2330. [Google Scholar] [CrossRef]
- Casali, C.A.; Tiecher, T.; Kaminski, J.; Santos, D.R.D.; Calegari, A.; Piccin, R. Benefícios do uso de plantas de cobertura de solo na ciclagem de fósforo. In Manejo e Conservação do Solo e da Água em Pequenas Propriedades Rurais no Sul do Brasil: Práticas Alternativas de Manejo Visando a Conservação do Solo e da Água; UFRGS: Porto Alegre, Brazil, 2016; pp. 23–33. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180–214. [Google Scholar] [CrossRef] [PubMed]
- Inmte. Gráficos. Available online: http://www.inmet.gov.br/portal/index.php?r=tempo/graficos (accessed on 8 January 2018).
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Cunha, T.J.F.; Oliveira, J.B. Sistema Brasileiro de Classificação de Solos, 5th ed; Brasília, D.F., Ed.; Embrapa: Brasília, DF, Brazil, 2018; 356p. [Google Scholar]
- Bataglia, O.C.; Furlani, A.M.C.; Teixeira, J.P.F.; Furlani, P.R.; Gallo, J.R. Métodos de Análise Química de Plantas; Instituto Agronômico: Campinas, Brasil, 1983; p. 48. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bissani, C.A.; Bohnen, H.; Volkweiss, S.J. Análises de Solo, Plantas e Outros Materiais, 2nd ed.; Boletim Técnico, 5; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995; p. 174. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária—EMBRAPA. Manual de Métodos de Análise de Solo, 2nd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 1997; 212p. [Google Scholar]
- Ribeiro, A.C.; Guimarães, P.T.G.; Alvarez, V.V.H. Recomendações para o Uso de Corretivos e Fertilizantes em Minas Gerais: 5ª Aproximação; UFV: Viçosa, Brazil, 1999; p. 359. [Google Scholar]
- Alvarez, V.V.H.; Fonseca, D.M. Definição de doses de fósforo para determinação da capacidade máxima de adsorção de fosfatos e para ensaios em casa de vegetação. Rev. Bras. Ciênc. Solo 1990, 14, 49–55. [Google Scholar]
- Bowman, R.A. A sequential extraction procedure with concentrated sulfuric acid and diluted base for soil organic phosphorus. Soil Sci. Soc. Am. J. 1989, 53, 326–366. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformation of organic phosphorus substrates in soil as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Duda, G.P.; Guerra, J.G.M.; Pereira, M.G.; Anjos, L.H.C.; Ribeiro, M.R. Avaliação da biodisponibilidade de fósforo em diferentes classes de solos do Brasil. Semin. Cienc. Agrar. 2013, 34, 1563–1576. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Kaminski, J.; Rheinheimer, D.S.; Flores, J.P.C. Biodisponibilidade de formas de fósforo acumuladas em solo sob sistema plantio direto. Rev. Bras. Ciênc. Solo 2007, 31, 691–699. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Anghinoni, I. Accumulation of soil organic phosphorus by soil tillage and cropping systems under subtropical conditions. Com. Soil Sci. Plant Anal. 2003, 34, 2339–2354. [Google Scholar] [CrossRef]
- Maia, R.S.; Vasconcelos, S.S.; Carvalho, C.J.R. Soil phosphorus fractions and mycorrhizal symbiosis in response to the availability of moisture and nutrients at a secondary forest in eastern Amazonia. Acta Amaz. 2015, 45, 255–264. [Google Scholar] [CrossRef]
- Bravo, C.A.; Giraldez, J.V.; Ordoñez, R.; Gonzalez, P.; Torres, F.P. Long term influence of conservation tillage on chemical properties of surface horizon and legume crops yield in a Vertisol of Southern Spain. Soil Sci. 2007, 172, 141–148. [Google Scholar] [CrossRef]
- Olibone, D.; Rosolem, C.A. Phosphate fertilization and phosphorus forms in an Oxisol under no-till. Sci. Agric. 2010, 67, 465–471. [Google Scholar] [CrossRef]
- Costa, J.P.; Barros, N.F.; Albuquerque, A.W.; Moura Filho, G.; Santos, J.R. Fluxo difusivo de fósforo em função de doses e da umidade do solo. Rev. Bras. Eng. Agríc. Ambient. 2006, 10, 828–835. [Google Scholar] [CrossRef]
- Souza Júnior, R.F.; Oliveira, F.H.T.; Santos, H.C.; Freire, F.J.; Arruda, J.A. Frações de fósforo inorgânico do solo e suas correlações com o fósforo quantificado por extratores e pelo milho. Rev. Bras. Ciênc. Solo 2012, 36, 159–169. [Google Scholar] [CrossRef]
- Neufeldt, H.; Silva, J.E.; Ayarza, M.A.; Zech, W. Land-use effects on phosphorus fractions in Cerrado Oxisols. Biol. Fertil. Soils 2000, 31, 30–37. [Google Scholar] [CrossRef]
- Redel, Y.D.; Rubio, R.; Rouanet, J.L.; Borie, F. Phosphorus bioavailability affected by tillage and crop rotation on a Chilean volcanic derived Ultisol. Geoderma 2007, 139, 388–396. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Fornari, M.R.; Bastos, M.C.; Fernandes, G.; Santanna, M.A.; Calegari, A.; Canalli, L.B.S.; Caner, L.; Labanowski, J.; Tiecher, T. Phosphorus distribution after three decades of different soil management and cover crops in subtropical region. Soil Til. Res. 2019, 192, 33–41. [Google Scholar] [CrossRef]
- Suriyagoda, L.D.B.; Ryan, M.H.; Henton, M.; Lambers, H. Plant Responses to Limited Moisture and Phosphorus Availability: A Meta-Analysis. Adv. Agric. 2014, 124, 143–200. [Google Scholar] [CrossRef]
- Borie, F.; Rubio, R. Total and organic phosphorus in chilean volcanic soils. Gayana Bot. 2003, 60, 69–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).