Uptake and Translocation of Foliar-Applied L-Proline in Sweet Cherry (Prunus avium L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Trial Design and Treatments
2.3. Sampling and Measurements
2.3.1. Leaf Samples
2.3.2. Fruit Samples
2.3.3. Branch Samples
2.3.4. Sample Preparation and Nitrogen Stable Isotope Analysis
2.4. Statistical Analysis
3. Results
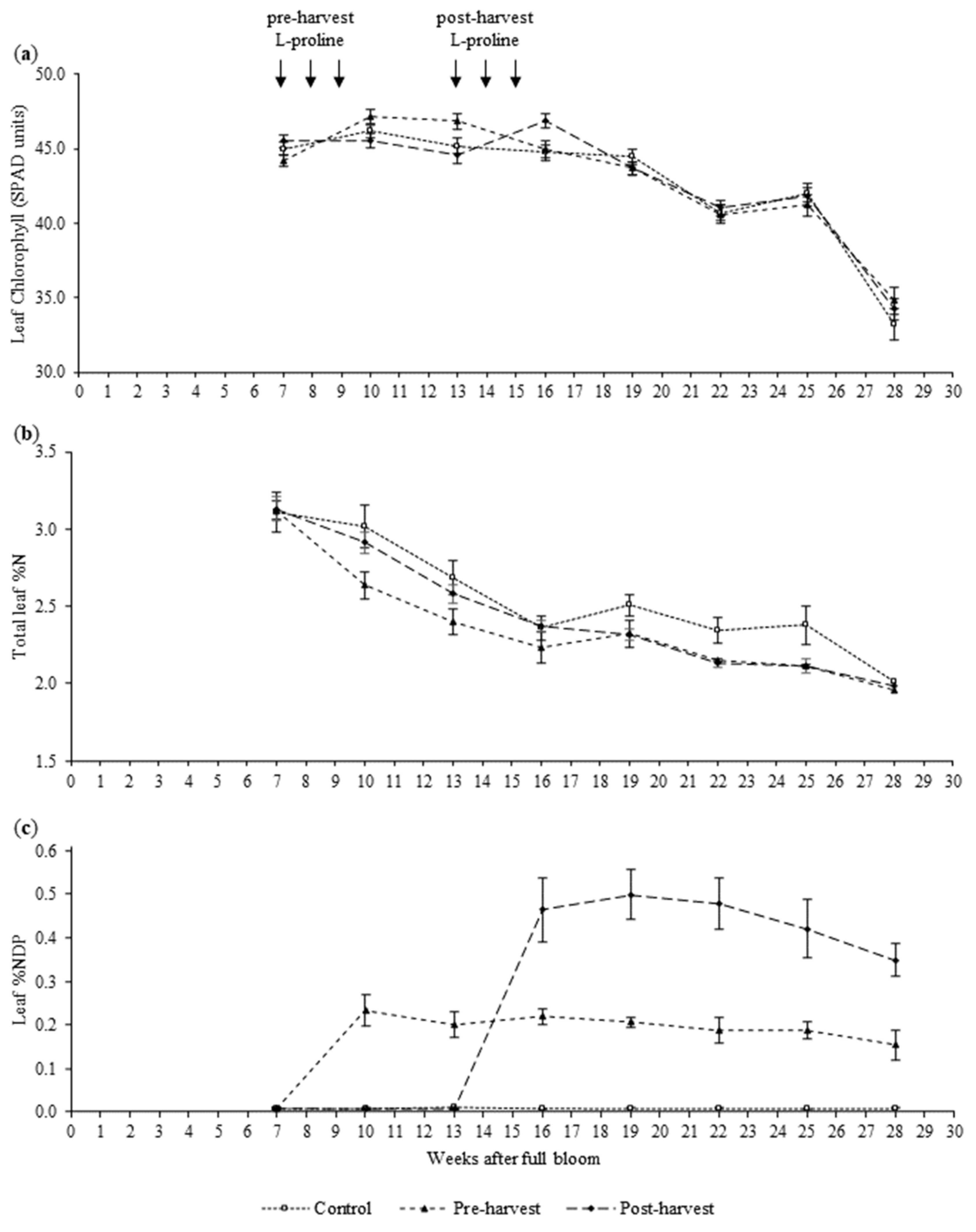
3.1. Chlorophyll Estimates, Total %N and %NDP of Leaves
3.2. Total %N, %NDP, and Quality of Fruit
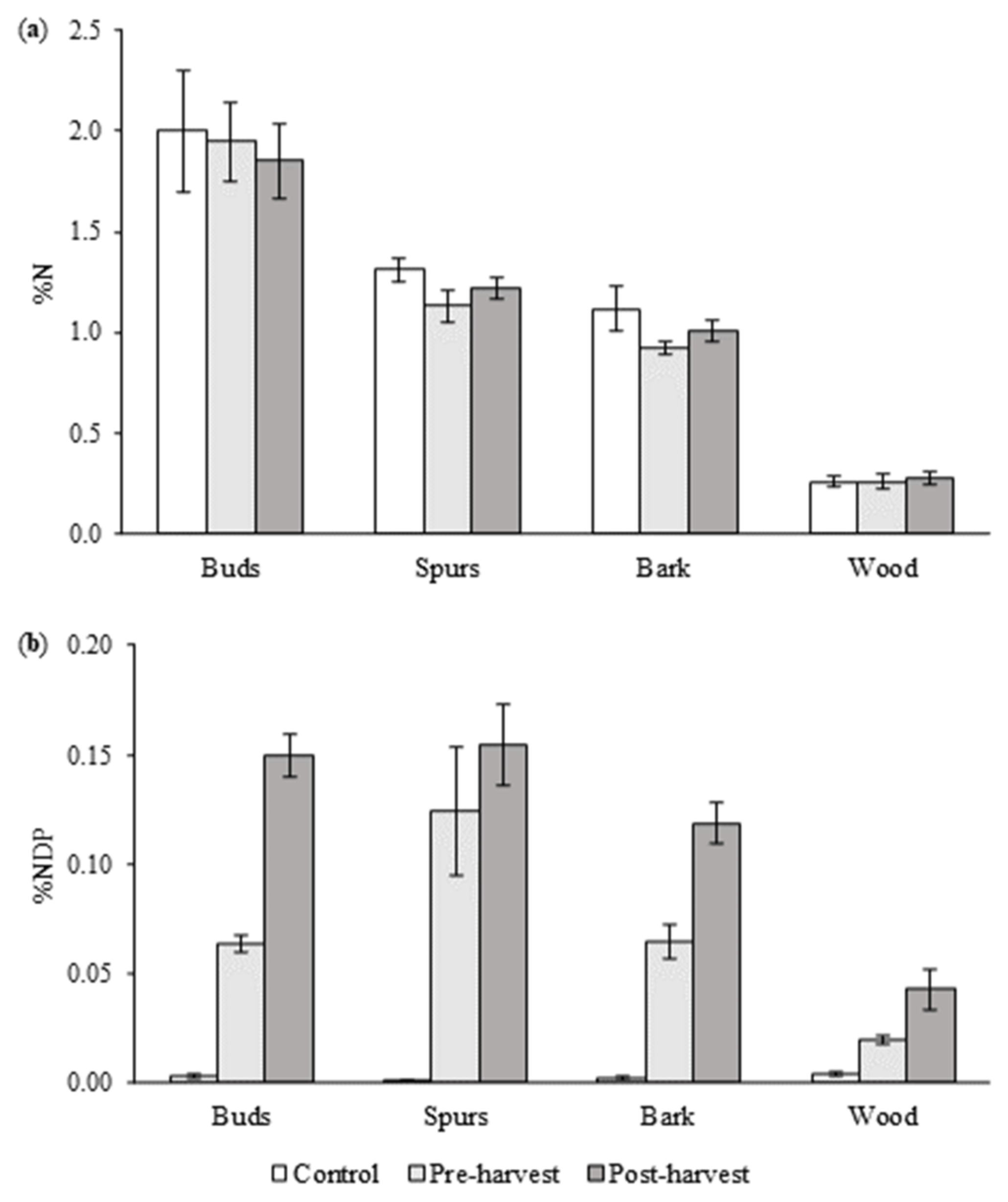
3.3. Total %N and %NDP of Branches
4. Discussion
4.1. Leaves
4.2. Fruit
4.3. Branch
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neilsen, G.; Kappel, F.; Neilsen, D. Fertigation and crop load affect yield, nutrition, and fruit quality of ‘Lapins’ sweet cherry on Gisela 5 rootstock. Hortscience 2007, 42, 1456–1462. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Hardie, M. Two Dimensional Modelling of Nitrate Flux in a Commercial Apple Orchard. Soil Sci. Soc. Am. J. 2017, 81, 1235–1246. [Google Scholar] [CrossRef]
- Quin, P.; Swarts, N.; Oliver, G.; Paterson, S.; Friedl, J.; Rowlings, D. Nitrous oxide emissions from applied nitrate fertiliser in commercial cherry orchards. Soil Res. 2021, 59, 60–67. [Google Scholar] [CrossRef]
- Swarts, N.; Montagu, K.; Oliver, G.; Southam-Rogers, L.; Hardie, M.; Corkrey, R.; Rogers, G.; Close, D. Benchmarking nitrous oxide emissions in deciduous tree cropping systems. Soil Res. 2016, 54, 500–511. [Google Scholar] [CrossRef]
- Millard, P. Ecophysiology of the internal cycling of nitrogen for tree growth. Z. Für Pflanz. Und Bodenkd. 1996, 159, 1–10. [Google Scholar] [CrossRef]
- Artacho, P.; Bonomelli, C. Changes in fine-root production, phenology and spatial distribution in response to N application in irrigated sweet cherry trees. Tree Physiol. 2016, 36, 601–617. [Google Scholar] [CrossRef]
- San-Martino, L.; Sozzi, G.O.; San-Martino, S.; Lavado, R.S. Isotopically-labelled nitrogen uptake and partitioning in sweet cherry as influenced by timing of fertilizer application. Sci. Hortic. 2010, 126, 42–49. [Google Scholar] [CrossRef]
- Tan, B.Z.; Close, D.C.; Quin, P.R.; Swarts, N.D. Nitrogen Use Efficiency, Allocation, and Remobilization in Apple Trees: Uptake Is Optimized With Pre-harvest N Supply. Front. Plant Sci. 2021, 12, 657070. [Google Scholar] [CrossRef]
- Fallahi, E.; Eichert, T. Principles and Practices of Foliar Nutrients with an Emphasis on Nitrogen and Calcium Sprays in Apple. HortTechnology 2013, 23, 542–547. [Google Scholar] [CrossRef]
- Eichert, T.; Fernández, V. Chapter 4-Uptake and Release of Elements by Leaves and Other Aerial Plant Parts. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 71–84. [Google Scholar]
- Ayala, M.; Banados, P.; Thielemann, M.; Toro, R. Distribution and recycling of canopy nitrogen storage reserves in sweet cherry (Prunus avium) fruiting branches following 15N-urea foliar applications after harvest. Cien. Inv. Agr. 2014, 41, 71–79. [Google Scholar] [CrossRef]
- Furuya, S.; Umemiya, Y. The influence of chemical forms on foliar-applied nitrogen absorption for peach trees. Acta Hortic. 2002, 594, 97–103. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Hima Kumari, P.; Sunita, M.S.L.; Sreenivasulu, N. Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 2015, 6, 544. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Arakawa, C.; Kuwahara, Y.; Gemma, H. Effects of L-Proline Foliar Application on the Quality of ‘Kosui’ Japanese Pear. Acta Hortic. 2008, 800, 549–554. [Google Scholar] [CrossRef]
- Caronia, A.; Gugliuzza, G.; Inglese, P. Influence of L-Proline on Citrus sinensis (L.) ‘New Hall’ and ‘Tarocco Scire’ Fruit Quality. Acta Hortic. 2010, 884, 423–426. [Google Scholar] [CrossRef]
- El Sayed, O.M.; El Gammal, O.H.M.; Salama, A.S.M. Effect of proline and tryptophan amino acids on yield and fruit quality of Manfalouty pomegranate variety. Sci. Hortic. 2014, 169, 32–37. [Google Scholar] [CrossRef]
- Green, K. High Density Cherry Systems in Australia. Acta Hortic. 2005, 667, 319–324. [Google Scholar] [CrossRef]
- Luo, M.R. CIELAB. In Encyclopedia of Color Science and Technology; Luo, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 207–212. [Google Scholar]
- Bound, S.A.; Close, D.C.; Quentin, A.G.; Measham, P.F.; Whiting, M.D. Crop Load and Time of Thinning Interact to Affect Fruit Quality in Sweet Cherry. J. Agric. Sci. 2013, 5, 216–230. [Google Scholar] [CrossRef]
- Blackhall, M.L.; Berry, R.; Davies, N.W.; Walls, J.T. Optimized extraction of anthocyanins from Reid Fruits’ Prunus avium ‘Lapins’ cherries. Food Chem. 2018, 256, 280–285. [Google Scholar] [CrossRef]
- Tagliavini, M.; Millard, P.; Quartieri, M.; Marangoni, B. Foliar Nitrogen Uptake and Withdrawal from Peach Leaves during Senescence. Acta Hortic. 1997, 448, 459–466. [Google Scholar] [CrossRef]
- Neilsen, D.; Hogue, E.J.; Neilsen, G.H.; Parchomchuk, P. Using SPAD-502 Values to Assess the Nitrogen Status of Apple Trees. Hortscience 1995, 30, 508–512. [Google Scholar] [CrossRef]
- Thielemann, M.; Toro, R.; Ayala, M. Distribution and Recycling of Canopy Nitrogen Storage Reserves in Sweet Cherry (Prunus avium L.) Fruiting Branches Following N-15-Urea Foliar Applications after Harvest. Acta Hortic. 2014, 1020, 353–361. [Google Scholar] [CrossRef]
- Lea, U.S.; Slimestad, R.; Smedvig, P.; Lillo, C. Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 2007, 225, 1245–1253. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
| Phenological Stage | Date | WAFB | Key Events |
|---|---|---|---|
| Full bloom | 11 October 2018 | 0 | Tree selection |
| Petal Fall | 25 October 2018 | 2 | |
| Pit hardening | 15 November 2018 | 5 | |
| Straw colour | 29 November 2018 | 7 | Start pre-harvest L-proline; initial leaf samples |
| Full maturity | 28 December 2018 | 11 | Fruit harvest; dissection; quality analysis |
| Early leaf yellowing | 10 January 2019 | 13 | Start post-harvest L-proline |
| Leaf yellowing | 7 February 2019 | 17 | Extension growth removal |
| Leaf senescence | 25 April 2019 | 28 | Last leaf samples |
| Late winter dormancy | 22 August 2019 | 46 | Whole branch harvest and dissection |
| Control | Pre-Harvest | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | Cohen’s d | ||
| Pedicel | %N | 1.06 | 0.15 | 1.08 | 0.05 | 0.853 | −0.14 |
| %NDP | 0.00 | 0.00 | 0.21 | 0.05 | <0.001 | 5.72 | |
| Skin | %N | 1.00 | 0.13 | 0.91 | 0.05 | 0.281 | −0.84 |
| %NDP | 0.00 | 0.00 | 0.17 | 0.05 | 0.001 | 4.51 | |
| Flesh | %N | 1.35 | 0.29 | 1.27 | 0.10 | 0.615 | −0.37 |
| %NDP | 0.00 | 0.00 | 0.12 | 0.05 | 0.003 | 3.29 | |
| Stone | %N | 1.05 | 0.33 | 0.94 | 0.05 | 0.542 | −0.46 |
| %NDP | 0.00 | 0.00 | 0.03 | 0.01 | 0.002 | 3.81 | |
| Control | Pre-Harvest | ||||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | Mean | SD | p Value | Cohen’s d | |
| Weight (g) | 80 | 12.9 | 1.6 | 12.2 | 1.7 | 0.006 | −0.44 |
| Size (mm) | 80 | 29.7 | 1.4 | 29.1 | 1.6 | 0.007 | −0.43 |
| Colour (chart) | 80 | 4.5 | 0.8 | 4.9 | 0.8 | 0.005 | 0.45 |
| Lightness L* | 80 | 28.0 | 2.2 | 27.3 | 2.1 | 0.067 | −0.29 |
| Redness a* | 80 | 27.0 | 5.0 | 24.3 | 5.0 | 0.001 | −0.54 |
| Yellowness b* | 80 | 7.5 | 2.5 | 6.2 | 2.4 | 0.002 | −0.50 |
| Compression firmness (g mm−2) | 80 | 304.2 | 37.0 | 293.7 | 37.4 | 0.076 | −0.28 |
| Skin puncture force (kg) | 80 | 0.39 | 0.04 | 0.37 | 0.05 | 0.040 | −0.33 |
| Stem retention force (g) | 80 | 951.8 | 194.2 | 795.8 | 182.1 | <0.001 | −0.83 |
| Total soluble solids (°Brix) | 4 | 15.4 | 0.4 | 15.5 | 0.8 | 0.835 | 0.15 |
| Titratable acidity (g L−1) | 4 | 6.0 | 0.4 | 5.7 | 0.1 | 0.372 | −0.68 |
| Total anthocyanins (mg 100 g−1) | 4 | 15.7 | 6.0 | 18.4 | 4.2 | 0.492 | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hölzel, N.; Close, D.C.; Bound, S.A.; Quin, P.R.; Visentin, D.C.; Swarts, N.D. Uptake and Translocation of Foliar-Applied L-Proline in Sweet Cherry (Prunus avium L.). Agronomy 2023, 13, 958. https://doi.org/10.3390/agronomy13040958
Hölzel N, Close DC, Bound SA, Quin PR, Visentin DC, Swarts ND. Uptake and Translocation of Foliar-Applied L-Proline in Sweet Cherry (Prunus avium L.). Agronomy. 2023; 13(4):958. https://doi.org/10.3390/agronomy13040958
Chicago/Turabian StyleHölzel, Nadine, Dugald C. Close, Sally A. Bound, Peter R. Quin, Denis C. Visentin, and Nigel D. Swarts. 2023. "Uptake and Translocation of Foliar-Applied L-Proline in Sweet Cherry (Prunus avium L.)" Agronomy 13, no. 4: 958. https://doi.org/10.3390/agronomy13040958
APA StyleHölzel, N., Close, D. C., Bound, S. A., Quin, P. R., Visentin, D. C., & Swarts, N. D. (2023). Uptake and Translocation of Foliar-Applied L-Proline in Sweet Cherry (Prunus avium L.). Agronomy, 13(4), 958. https://doi.org/10.3390/agronomy13040958







