Farm Household Typology Based on Soil Quality and Influenced by Socio-Economic Characteristics and Fertility Management Practices in Eastern Kenya
Abstract
1. Introduction
2. Materials and Methods
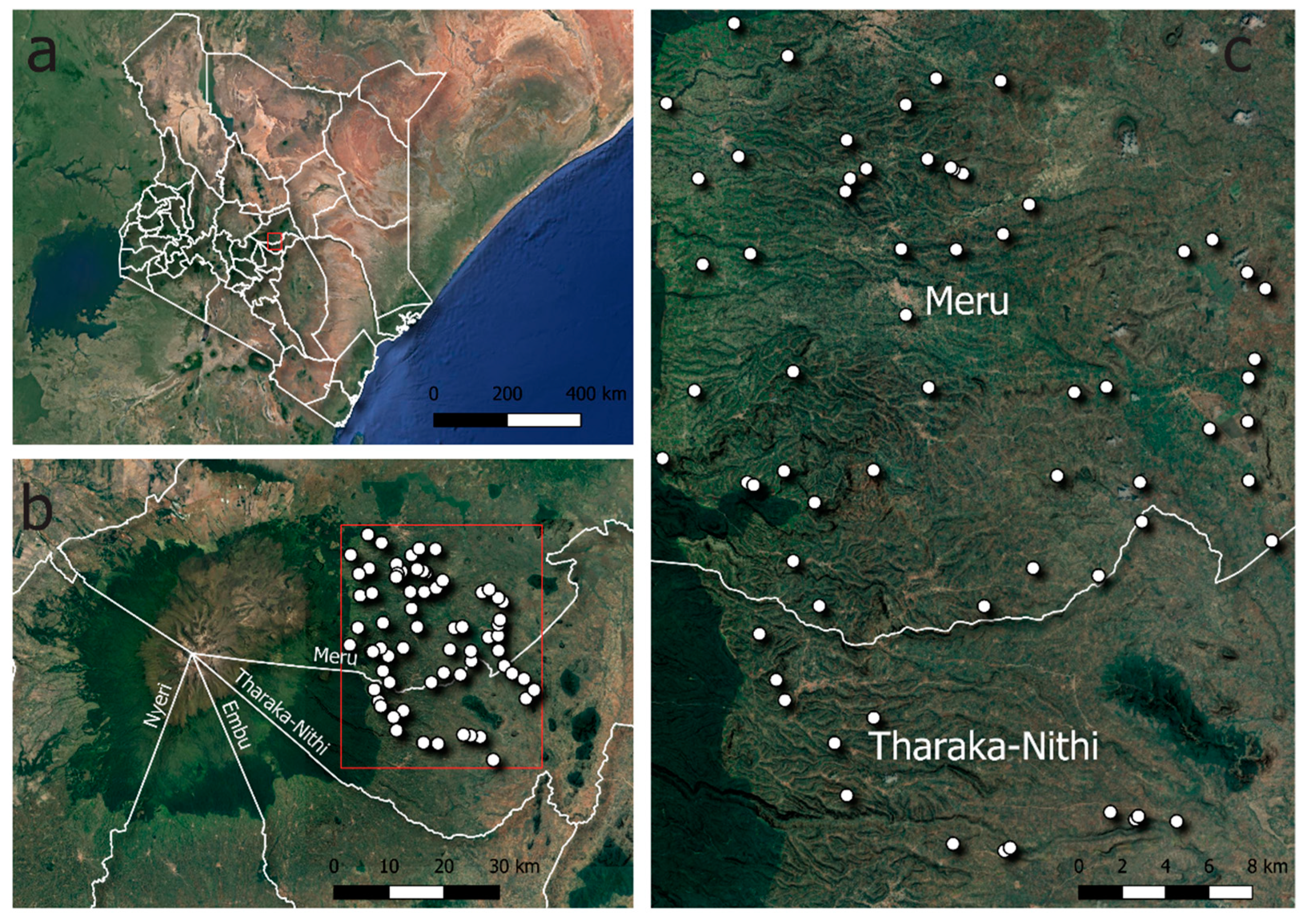
2.1. Study Area Description
2.2. Data Collection Procedures
2.2.1. Soil Sampling
2.2.2. Laboratory Soil Analysis
2.2.3. Sampling for Social Data
2.3. Methods of Data Analysis
2.3.1. Principal Component Analysis (PCA)
2.3.2. Categorical Principal Analysis (CATPCA)
2.3.3. Clustering, Farm Classification, and Characterization
3. Results
3.1. Farm Socio-Economic Characteristics
3.2. Farm Classification
3.2.1. Correlation among Soil Properties
3.2.2. Principal Component Analysis of Socio-Economic Variables
3.2.3. Principal Component Analysis of Soil Fertility Management Practices
3.3. Clustering and Characterization of Farm Types Based on Soil Characteristics
3.3.1. Tendencies of Soil Properties across Farm Types
3.3.2. Socio-Economic Characteristics across the Farm Types
3.3.3. Patterns of Soil Fertility Management Practices across Farm Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tittonell, P.; Vanlauwe, B.; Leffelaar, P.A.; Shepherd, K.D.; Giller, K.E. Exploring diversity in soil fertility management of smallholder farms in western Kenya: II. Within-farm variability in resource allocation, nutrient flows and soil fertility status. Agric. Ecosyst. Environ. 2005, 110, 166–184. [Google Scholar] [CrossRef]
- Zingore, S.; Murwira, H.K.; Delve, R.J.; Giller, K.E. Influence of nutrient management strategies on variability of soil fertility, crop yields and nutrient balances on smallholder farms in Zimbabwe. Agric. Ecosyst. Environ. 2007, 119, 112–126. [Google Scholar] [CrossRef]
- Tittonell, P.; Leffelaar, P.A.; Vanlauwe, B.; van Wijk, M.T.; Giller, K.E. Exploring diversity of crop and soil management within smallholder African farms: A dynamic model for simulation of N balances and use efficiencies at field scale. Agric. Syst. 2006, 91, 71–101. [Google Scholar] [CrossRef]
- Turmel, M.S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2015, 134, 6–16. [Google Scholar] [CrossRef]
- Ojiem, J.O.; de Ridder, N.; Vanlauwe, B.; Giller, K.E. Socio-ecological niche: A conceptual framework for integration of legumes in smallholder farming systems. Int. J. Agric. Sustain. 2006, 4, 79–93. [Google Scholar] [CrossRef]
- Gómez-Limón, J.; Berbel, J.; Arriaza, M. MCDM Farm System Analysis For Public Management Of Irrigated Agriculture. In Handbook On Operations Research In Natural Resources In International Series In Operations Research And Management Science; Weintraub, A., Bjorndal, T., Epstein, R., Romero, Y., Eds.; Páginas, Kluwer: New York, NY, USA, 2007; pp. 93–114. [Google Scholar]
- Sheahan, M.; Olwande, J.; Kirimi, L.; Jayne, T.S. Targeting of Subsidized Fertilizer Under Kenya’s National Accelerated Agricultural Input Access Program (NAAIAP): Food Security Collaborative Working Papers 188572; Michigan State University, Department of Agricultural, Food and Resource Economics: East Lansing, MI, USA, 2014. [Google Scholar]
- Bouma, J.; Reyes-Garcia, V.; Huanca, T.; Arrazola, S. Understanding conditions for co-management: A framed field experiment amongst the Tsimane’, Bolivia. Ecol. Econ. 2017, 141, 32–42. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, X.; Zhu, P.; Singh, A.K.; Zakari, S.; Yang, B.; Chen, C.; Liu, W. Soil quality assessment of different Hevea brasiliensis plantations in tropical China. J. Environ. Manag. 2021, 285, 112147. [Google Scholar] [CrossRef]
- Chikowo, R.; Zingore, S.; Snapp, S.; Johnson, A. Farm typologies, soil fertility variability and nutrient management in smallholder farming in Sub-Saharan Africa Farm typologies, soil fertility variability and nutrient management in smallholder farming in Sub-Saharan Africa. Nutr. Cycl. Agroecosystems 2014, 100, 1–18. [Google Scholar] [CrossRef]
- Braun, A.R.; Smaling, E.M.A.; Muchugu, E.I.; Shepherd, K.D.; Corbett, J.D. Maintenance and improvement of soil productivity in the highlands of Ethiopia, Kenya, Madagascar and Uganda An inventory of spatial and non-spatial survey. Africa Highlands Initiative Techical Report Series; International Centre for Research in Agroforestry: Nairobi, Kenya, 1997; 129p. [Google Scholar]
- Shepherd, K.; Ohisson, E.; Okalebo, J.; Ndufa, J. Potential impact of agroforestry on soil nutrient balances at the farm scale in the East African highlands. Fertil. Res. 1996, 44, 87–99. [Google Scholar] [CrossRef]
- Barrett, C.B.; Marenya, P.P.; Mcpeak, J.; Minten, B.; Murithi, F.; Oluoch-Kosura, W.; Place, F.; Randrianarisoa, J.C.; Rasambainarivo, J.; Wangila, J. Welfare dynamics in rural Kenya and Madagascar. J. Dev. Stud. 2006, 42, 248–277. [Google Scholar] [CrossRef]
- KNBS. 2019 Kenya population and Housing Census. Volume 1: Population by County and Sub-County; KNBS: Nairobi, Kenya, 2019. [Google Scholar]
- Jaetzold, R.; Schmidt, H.; Hornetz, B.; Shisanya, C. Natural conditions and farm management information, part C East Kenya. In Farm Management Handbook of Kenya, 2nd ed.; Ministry of Agriculture: Nairobi, Kenya, 2007; Volume II. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Roudier, P.; Hewitt, A.E.; Beaudette, D.E. A conditioned Latin hypercube sampling algorithm incorporating operational constraints. Digit. Soil Assessments Beyond-Proc. Fifth Glob. Work. Digit. Soil Mapp. 2012, 227–231. [Google Scholar] [CrossRef]
- Mulder, V.L.; de Bruin, S.; Schaepman, M.E. Representing major soil variability at regional scale by constrained Latin Hypercube Sampling of remote sensing data. Int. J. Appl. Earth Obs. Geoinf. 2012, 21, 301–310. [Google Scholar] [CrossRef]
- USDA. Sampling Soils for Nutrient Management; USDA: Washington, DC, USA, 2018. [Google Scholar]
- van Reeuwijk, L. Procedures for Soil Analysis, 6th ed.; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 2022. [Google Scholar]
- Gillman, G.P.; Sumpter, E.A. Modification to the compulsive echange method for measuring exchange characteristics of soils. Aust. J. Soil Res. 1986, 24, 61–66. [Google Scholar] [CrossRef]
- Ross, D.S.; Ketterings, Q. Recommended Methods for Determining Soil Cation Exchange Capacity. In Recommended Soil Testing Procedures for the Northeastern United States, 3rd ed.; Wolf, A., McGrath, J., Eds.; Agricultural Experiment Stations of Connecticut: New Haven, CT, USA, 2011. [Google Scholar]
- Mehlich, A. Mehlich-3 soil test extractant: A modification of Mehlich-2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Carte, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Bremmer, J.M.; Mulvaney, C.S. Nitrogen-Total. Methods of soil anaylsis. Part 2. In Chemical and Biological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 526–595. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nahrstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliumbestimmung. K. Lantbr. Ann. 1960, 26, 199–215. [Google Scholar]
- Haluschak, P.; Laboratory Methods of Soil Analysis. Canada–Manitoba Soil Survey. 2006; 133p. Available online: https://www.gov.mb.ca/agriculture/soil/soil-survey/pubs/laboratory_methods_of_soil_analysis.pdf (accessed on 5 April 2023).
- Giller, K.E.; Tittonell, P.; Rufino, M.C.; van Wijk, M.T.; Zingore, S.; Mapfumo, P.; Adjei-Nsiah, S.; Herrero, M.; Chikowo, R.; Corbeels, M.; et al. Communicating complexity: Integrated assessment of trade-offs concerning soil fertility management within African farming systems to support innovation and development. Agric. Syst. 2011, 104, 191–203. [Google Scholar] [CrossRef]
- Pacini, G.C.; Colucci, D.; Baudron, F.; Righi, E.; Corbeels, M.; Tittonell, P.; Stefanini, F.M. Combining multi-dimensional scaling and cluster analysis to describe the diversity of rural households. Exp. Agric. 2013, 50, 376–397. [Google Scholar] [CrossRef]
- Alary, V.; Messad, S.; Taché, C.; Tillard, E. Approach to the diversity of dairy farm systems in Reunion. Rev. Elev. Méd. Vét. Pays Trop. 2002, 55, 285–297. [Google Scholar] [CrossRef]
- Dossa, L.H.; Abdulkadir, A.; Amadou, H.; Sangare, S.; Schlecht, E. Exploring the diversity of urban and peri-urban agricultural systems in Sudano-Sahelian West Africa: An attempt towards a regional typology. Landsc. Urban Plan. 2011, 102, 197–206. [Google Scholar] [CrossRef]
- Tittonell, P.; Muriuki, A.; Shepherd, K.D.; Mugendi, D.; Kaizzi, K.C.; Okeyo, J.; Verchot, L.; Coe, R.; Vanlauwe, B. The diversity of rural livelihoods and their influence on soil fertility in agricultural systems of East Africa—A typology of smallholder farms. Agric. Syst. 2010, 103, 83–97. [Google Scholar] [CrossRef]
- Hair, J.F.; Black, W.C.; Babin, B.; Anderson, R. Pharmaceutical Quality by Design: A Practical Approach. In Multivariate Data Analysis, 7th ed.; Pearson Education Limited: Essex, UK, 2010. [Google Scholar] [CrossRef]
- Samuels, P. Advice on Exploratory Factor Analysis; Birminghan City University Technical Report; Birminghan City University: Birmingham, UK, 2017. [Google Scholar]
- Linting, M.; Meulman, J.J.; Groenen, P.J.F.; van der Kooij, A.J. Non-linear principal components analysis: Introduction and application. Psychol. Methods 2007, 12, 336–358. [Google Scholar] [CrossRef] [PubMed]
- Meulman, J.J.; Heiser, W. IBM SPSS Categories 19; SPSS Inc.: Armonk, NY, USA, 2010. [Google Scholar]
- Goswami, R.; Chatterjee, S.; Prasad, B. Farm types and their economic characterization in complex agro-ecosystems for informed extension intervention: Study from coastal West Bengal, India. Agric. Food Econ. 2014, 2, 5. [Google Scholar] [CrossRef]
- Jain, R.; Koronios, R. Innovation in the cluster validating techniques. Fuzzy Optim. Decis. Mak. 2008, 7, 233–241. [Google Scholar] [CrossRef]
- Kuria, A.W.; Barrios, E.; Pagella, T.; Muthuri, C.W.; Mukuralinda, A.; Sinclair, F.L. Farmers’ knowledge of soil quality indicators along a land degradation gradient in Rwanda. Geoderma Reg. 2018, 16, 14. [Google Scholar] [CrossRef]
- Wawire, A.W. Analysis of Soil Quality, Farmers’ Knowledge and Management Practices in Mount Kenya East Region. Ph.D. Dissertation, Doctoral School of Environmental Sciences, Hungarian University of Agriculture and Life Sciences, Gödöllő, Hungary, 2021; 208p. [Google Scholar]
- Storck, H.; Emana, B.; Adnew, B.; Borowiccki, A.; Woldehawariat, S. Farming Systems and Resource economics in the Tropics: Farming System and Farm Management Practices of Smallholders in the Hararghe Highland; Wissenschaftsverlag Vauk: Kiel, Germany, 1991; Volume II. [Google Scholar]
- Finch, W.H. Exploratory Factor Analysis. In Handbook of Quantitative Methods for Educational Research; Teo, T., Ed.; Sense Publishers: Rotterdam, The Netherlands, 2013; pp. 1–15. [Google Scholar]
- Stevens, J. Applied Multivariate Statistics for the Social Sciences; Routledge: New York, NY, USA, 1992. [Google Scholar]
- Comrey, L.A.; Lee, H.B. A First Course in Factor Analysis, 2nd ed.; Lawrence Eribaum Associate: Hillside, NJ, USA, 1992. [Google Scholar]
- Field, A. Discovering Statistics Using SPSS; Sage Publications, Inc.: London, UK, 2005. [Google Scholar]
- Amacher, M.C.; O’Neill, K.P.; Perry, C.H. Soil Vital Signs: A New Soil Quality Index (SQI) for Assessing Forest Soil Health; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007. [Google Scholar] [CrossRef]
- Ariga, J.; Jayne, T.S. Fertilizer in Kenya: Factors Driving the Increase in Usage by Smallholder Farmers, 1990–2007. Yes Afr. Can Success Stories A Dyn. Cont. 2011, 269–288. [Google Scholar]
- Tuo, D.; Xu, M.; Gao, G. Relative contributions of wind and water erosion to total soil loss and its effect on soil properties in sloping croplands of the Chinese Loess Plateau. Sci. Total Environ. 2018, 633, 1032–1040. [Google Scholar] [CrossRef]
- Dembe’le’, I.; Kone’, D.; Soumare’, A.; Coulibaly, D.; Kone’, Y.; Ly, B.; Kater, L. Fallows and field systems in Mali. In Nutrients on the Move: Soil Fertility Dynamics in African Farming Systems; Hilhorst, T., Muchena, F., Eds.; IIED: London, UK, 2000; pp. 83–102. [Google Scholar]
- Rotich, D.K.; Ogaro, V.N.; Wabuile, E.; Mulamula, H.H.A.; Wanjala, C.M.M.; Defoer, T. Participatory characterisation and on-farm testing in Mutsulio village, Kakamega District. In Pilot Project on Soil Fertility Replenishment and Recapitalisation in Western Kenya; Report No. 11; Kenya Agricultural Research Institute: Kakamega, Kenya, 1999. [Google Scholar]
- Tully, K.; Sullivan, C.; Weil, R.; Sanchez, P. The State of soil degradation in sub-Saharan Africa: Baselines, trajectories, and solutions. Sustainability 2015, 7, 6523–6552. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Kuivanen, K.S.; Alvarez, S.; Michalscheck, M.; Adjei-Nsiah, S.; Descheemaeker, K.; Mellon-Bedi, S.; Groot, J.C.J. Characterising the diversity of smallholder farming systems and their constraints and opportunities for innovation: A case study from the Northern Region, Ghana. NJAS Wageningen J. Life Sci. 2016, 78, 153–166. [Google Scholar] [CrossRef]
- Hartemink, A.E. Poor soils make poor people and poor people make the soil worse. Manag. Trop. Sandy Soils Sustain. Agric. 2005, 107, 16–20. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Willy, D.K.; Muyanga, M.; Mbuvi, J.; Jayne, T. The effect of land use change on soil fertility parameters in densely populated areas of Kenya. Geoderma 2019, 343, 254–262. [Google Scholar] [CrossRef]
- Bidogeza, J.C.; Berentsen, P.B.M.; De Graaff, J.; Oude Lansink, A.G.J.M. A typology of farm households for the Umutara Province in Rwanda. Food Secur. 2009, 1, 321–335. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Six, J.; Sun, J.H.; Bao, X.G.; Zhang, F.S.; Van Der Werf, W. Intercropping enhances soil carbon and nitrogen. Glob. Chang. Biol. 2015, 21, 1715–1726. [Google Scholar] [CrossRef]
- Segnini, A.; Posadas, A.; da Silva, W.T.L.; Milori, D.M.B.P.; Gavilan, C.; Claessens, L.; Quiroz, R. Quantifying soil carbon stocks and humification through spectroscopic methods: A scoping assessment in EMBU-Kenya. J. Environ. Manag. 2019, 234, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and their contaminants in soils, surface and groundwater, Encyclopedia of the Anthropocene. Encycl. Anthr. 2017, 5, 225–240. [Google Scholar] [CrossRef]
- Köbrich, C.; Rehman, T.; Khan, M. Typification of farming systems for constructing representative farm models: Two illustrations of the application of multi-variate analyses in Chile and Pakistan. Agric. Syst. 2003, 76, 141–157. [Google Scholar] [CrossRef]
- Ndambi, O.A.; Pelster, D.E.; Owino, J.O. Manure Management Practices and Policies in Sub-Saharan Africa: Implications on Manure Quality as a Fertilizer. Front. Sustain. Food Syst. 2019, 3, 29. [Google Scholar] [CrossRef]
- Waithaka, M.M.; Thornton, P.K.; Shepherd, K.D.; Ndiwa, N.N. Factors affecting the use of fertilizers and manure by smallholders: The case of Vihiga, western Kenya. Nutr. Cycl. Agroecosystems 2007, 78, 211–224. [Google Scholar] [CrossRef]
- Mugwe, J.; Mugendi, D.; Mucheru-Muna, M.; Merckx, R.; Chianu, J.; Vanlauwe, B. Determinants of the decision to adopt integrated soil fertility management practices by smallholder farmers in the central highlands of Kenya. Exp. Agric. 2009, 45, 61–75. [Google Scholar] [CrossRef]
- Gondwe, T.M.; Alamu, E.O.; Musonda, M.; Geresomo, N.; Maziya-Dixon, B. The relationship between training farmers in agronomic practices and diet diversification: A case study from an intervention under the Scaling Up Nutrition programme in Zambia. Agric. Food Secur. 2017, 6, 72. [Google Scholar] [CrossRef]



| Variables | Definition | Measurement/Unit |
|---|---|---|
| Ca | Exchangeable Calcium | cmol/kg |
| Mg | Exchangeable Magnesium | cmol/kg |
| Na | Exchangeable Sodium | cmol/kg |
| K | Exchangeable Potassium | cmol/kg |
| pH | pH water | |
| SOC | Total organic carbon | % |
| CEC | Soil CEC | % |
| P2O5 | Plant available P | mg/kg |
| N | Plant available N | mg/kg |
| Clay | Clay content | % |
| Sand | Sand content | % |
| Silt | Silt content | % |
| BS | Base saturation | % |
| K2O | Plant available K | mg/kg |
| Variables | Definition | Measurement/Unit |
|---|---|---|
| Household Socio-Economic Characteristics | ||
| Gender | Gender of the household head | 0 = female, 1 = male |
| Age | Age of household head | Years |
| Education | Household head education level | 1 = below high school, 2 = above high school |
| Farming occupation | Farming as primary occupation | 0 = no, 1 = yes |
| Experience | Years in farming | 1 = below 20, 2 = above 20 |
| Extension contact | Contact with extension in the last 5 years | 0 = no, 1 = yes |
| Soil info | Access to training on soil management | 0 = no, 1 = yes |
| Soil testing | soil analysis has even been undertaken on farm | 0 = no, 1 = yes |
| Credit info | Farmer has ever received training on credit | 0 = no, 1 = yes |
| Crop information | Farmer has ever received training on crop husbandry | 0 = no, 1 = yes |
| Agribusiness info | Farmer has ever received training on agribusiness | 0 = no, 1 = yes |
| Livestock | Livestock ownership | 0 = no, 1 = yes |
| Family size | Number of people in the family | Count |
| Farm size | Total size of landholding cultivated by household | Acres |
| Household income | Annual household income (on-farm and off-farm) | Ksh |
| Work force | Number of household members actively involved in farming | Count |
| TLU | Aggregated livestock assets | standardized value |
| Cropping practices and soil fertility management | ||
| PCrop | Pure crop stands practiced | 0 = no, 1 = yes |
| Mixed | Mixed cropping practiced | 0 = no, 1 = yes |
| Agrof | Agroforestry practiced | 0 = no, 1 = yes |
| IntCrop | Intercropping practiced | 0 = no, 1 = yes |
| Residue | Farm residues applied | 0 = no, 1 = yes |
| Manure | Manure applied | 0 = no, 1 = yes |
| Mintill | Minimum tillage practiced | 0 = no, 1 = yes |
| Fallow | Fallowing practiced | 0 = no, 1 = yes |
| Residue incorp | Incorporation practiced | 0 = no, 1 = yes |
| Burn | Burning residues practiced | 0 = no, 1 = yes |
| Compost | Compost manure applied | 0 = no, 1 = yes |
| Fodder | Farm organic materials used as fodder | 0 = no, 1 = yes |
| Fuel | Farm organic materials used as fuel | 0 = no, 1 = yes |
| Fert. Plant rate | Amount of fertilizer used during planting | kg/ha |
| Fert.Topdress rate | Amount of fertilizer used during for top dressing | kg/ha |
| Parameter | Category | Number of Farmers | ||
|---|---|---|---|---|
| n = 69 | % | Mean | ||
| Gender | Male | 41 | 59.4 | N/A |
| Female | 28 | 40.6 | ||
| Education | Below High school | 38 | 55.1 | N/A |
| High school and above | 31 | 44.9 | ||
| Farming type | Crop | 5 | 7.2 | N/A |
| Crop and livestock | 64 | 92.8 | ||
| Farming as primary occupation | Yes | 64 | 92.8 | N/A |
| No | 5 | 7.2 | ||
| Farming experience (years) | <10 | 18 | 26.1 | N/A |
| 11–20 | 13 | 18.8 | ||
| 21–30 | 24 | 34.8 | ||
| >30 | 14 | 20.3 | ||
| Extension Contact | No | 43 | 62.3 | N/A |
| Yes | 26 | 37.7 | ||
| Soil info | No | 62 | 89.9 | N/A |
| Yes | 7 | 10.1 | ||
| Soil testing | No | 57 | 82.6 | N/A |
| Yes | 12 | 17.4 | ||
| Credit info | No | 64 | 92.8 | N/A |
| Yes | 5 | 7.2 | ||
| Crop husbandry advice | No | 57 | 82.6 | N/A |
| Yes | 12 | 17.4 | ||
| Animal husbandry advice | No | 60 | 87 | N/A |
| Yes | 9 | 13 | ||
| Agribusiness | No | 68 | 98.6 | N/A |
| Yes | 1 | 1.4 | ||
| Age | N/A | N/A | N/A | 46.7 |
| Family size | N/A | N/A | N/A | 5.2 |
| Members active in farming | N/A | N/A | N/A | 3.0 |
| Farm size (Ha) | N/A | N/A | N/A | 3.5 |
| Total income (Ksh *) | N/A | N/A | N/A | 112,512.2 |
| ** TLU (Tropical livestock unit) | N/A | N/A | N/A | 1.8 |
| Variable | Factor | Communalities | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| K | −0.812 | 0.786 | ||
| Na | 0.877 | 0.855 | ||
| CEC | 0.673 | 0.697 | ||
| P2O5 | −0.770 | 0.821 | ||
| K2O | 0.965 | 0.937 | ||
| pH | −0.443 | 0.239 | ||
| Mg | 0.921 | 0.890 | ||
| Ca | 0.736 | 0.623 | ||
| BS | 0.927 | 0.899 | ||
| Sand | 0.857 | 0.848 | ||
| SOC | −0.455 | 0.222 | ||
| N | 0.643 | 0.624 | ||
| Eigenvalues | 3.799 | 2.962 | 1.681 | |
| % of Variance | 31.657 | 24.685 | 14.006 | |
| Cumulative% | 31.657 | 56.342 | 70.348 | |
| Variable | Dimension | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Extension contact | 0.856 | 0.548 | ||
| Soil info | 0.537 | 0.662 | ||
| Soil testing | 0.767 | 0.454 | ||
| Credit INFO | 0.539 | 0.208 | ||
| Crop husbandry advice | 0.733 | 0.628 | ||
| Animal husbandry advice | 0.620 | 0.255 | ||
| Education | 0.690 | 0.389 | ||
| Tot income | 0.444 | 0.773 | ||
| age | −0.477 | 0.302 | ||
| Farm occupation | 0.579 | 0.598 | ||
| Farm size | 0.741 | 0.309 | ||
| TLU | 0.593 | 0.577 | ||
| Workforce | 0.710 | 0.529 | ||
| Cronbach’s alpha | 0.708 | 0.455 | 0.413 | 0.909 a |
| Total (eigenvalue) | 2.889 | 1.724 | 1.617 | 6.231 |
| % of variance | 22. 747 | 14.089 | 13.891 | 50.727 |
| Variable | Dimension | Total | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Pure stand cropping | 0.724 | 0.538 | ||
| Mixed cropping | −0.635 | 0.435 | ||
| Agroforestry | −0.633 | 0.529 | ||
| Minimum tillage | −0.435 | 0.373 | ||
| Fallowing | 0.478 | 0.408 | ||
| Residue incorporation | −0.646 | 0.538 | ||
| Quantity of fertilizer (planting) | 0.740 | 0.713 | ||
| Fertilizer quantity (top dress) | 0.732 | 0.717 | ||
| Residue composted | 0.623 | 0.528 | ||
| Residue used as fodder | −0.696 | 0.657 | ||
| Residue used as fuel | −0.464 | 0.387 | ||
| % of variance | 23.951 | 15.765 | 13.219 | 52.936 |
| Cronbach’s alpha | 0.682 | 0.466 | 0.344 | 0.911 a |
| Cluster (Farm Types) | Total | F | Sig. | |||
|---|---|---|---|---|---|---|
| Variable | 1 (n = 14) | 2 (n = 24) | 3 (n = 30) | |||
| Exch. K | 0.388b | 1.000a | 1.000a | 0.874 | 168.183 | 0.000 |
| Exch. Mg | 0.512b | 0.958a | 0.733ab | 0.767 | 4.995 | 0.010 |
| Exch. Na | 0.059a | 0.000b | 0.000b | 0.013 | 26.188 | 0.000 |
| CEC | 16.448a | 8.167b | 8.033b | 9.813 | 65.407 | 0.000 |
| BS% | 18.730 | 19.083 | 15.633 | 17.488 | 2.074 | 0.132 |
| Sand | 27.857 | 27.958 | 23.333 | 25.897 | 2.159 | 0.124 |
| AL-P2O5 | 5.286c | 828.717a | 740.510b | 620.272 | 348.851 | 0.000 |
| AL-K2O | 195.357a | 13.125b | 9.233b | 48.926 | 42.199 | 0.000 |
| pH.H2O | 4.879b | 5.083b | 6.103a | 5.491 | 38.743 | 0.000 |
| SOC | 0.543bc | 1.398a | 0.835b | 0.974 | 22.797 | 0.000 |
| SQI | 4.286b | 5.291a | 5.233a | 5.059 | 3.468 | 0.037 |
| Variable | Cluster | N | Mean | Std. Dev | Min | Max | F | Sig. |
|---|---|---|---|---|---|---|---|---|
| Family size | 1 | 14 | 4.714 | 1.326 | 3 | 7 | 0.958 | 0.389 |
| 2 | 24 | 5.125 | 1.676 | 1 | 8 | |||
| 3 | 30 | 5.433 | 1.695 | 2 | 11 | |||
| Total | 68 | 5.176 | 1.620 | 1 | 11 | |||
| Farm size | 1 | 14 | 2.482 | 2.202 | 0.25 | 6.00 | 3.692 | 0.030 |
| 2 | 24 | 2.813 | 2.329 | 0.25 | 10.00 | |||
| 3 | 30 | 4.598 | 3.512 | 0.50 | 10.00 | |||
| Total | 68 | 3.532 | 3.011 | 0.25 | 10.00 | |||
| TLU | 1 | 14 | 1.565 | 1.083 | 0.6200 | 4.8500 | 1.497 | 0.232 |
| 2 | 24 | 1.455 | 1.259 | 0.0000 | 5.2000 | |||
| 3 | 30 | 2.133 | 1.845 | 0.0000 | 7.0700 | |||
| Total | 68 | 1.777 | 1.532 | 0.0000 | 7.0700 | |||
| Workforce | 1 | 14 | 3.071 | 1.385 | 1 | 5 | 0.862 | 0.427 |
| 2 | 24 | 2.667 | 1.494 | 1 | 6 | |||
| 3 | 30 | 3.167 | 1.392 | 1 | 6 | |||
| Total | 68 | 2.971 | 1.424 | 1 | 6 | |||
| Age | 1 | 14 | 41.071 | 17.022 | 20 | 73 | 1.617 | 0.206 |
| 2 | 24 | 49.125 | 12.081 | 26 | 75 | |||
| 3 | 30 | 47.867 | 13.627 | 30 | 74 | |||
| Total | 68 | 46.912 | 14.000 | 20 | 75 |
| Variable | Category | Farm Type (Cluster) | Total | % | Coeff | Sig | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 14) | 2 (n = 24) | 3 (n = 30) | |||||||||
| Freq | % | Freq | % | Freq | % | ||||||
| Gender | Female | 5 | 35.7 | 11 | 45.8 | 12 | 40.0 | 28 | 41 | 0.077 | 0.855 |
| Male | 9 | 64.3 | 13 | 54.2 | 18 | 60.0 | 40 | 59 | |||
| Income (Ksh) | <75 | 7 | 53.8 | 10 | 52.6 | 11 | 42.3 | 28 | 48.3 | 0.130 | |
| 75–150 | 1 | 7.7 | 4 | 21.1 | 6 | 23.1 | 11 | 19.0 | |||
| 150–225 | 5 | 38.5 | 4 | 21.1 | 3 | 11.5 | 12 | 20.7 | |||
| >225 | 0 | 0 | 1 | 5.3 | 6 | 23.1 | 7 | 12.1 | |||
| Education | Primary and below | 5 | 35.7 | 14 | 58.3 | 19 | 63.3 | 38 | 56 | 0.207 | 0.218 |
| High school and above | 9 | 64.3 | 10 | 41.7 | 11 | 36.7 | 30 | 44 | |||
| Farm occupation | No | 1 | 7.1 | 2 | 8.3 | 2 | 6.7 | 5 | 7 | 0.029 | 0.973 |
| Yes | 13 | 92.9 | 22 | 91.7 | 28 | 93.3 | 63 | 93 | |||
| Farming experience | <20 | 7 | 50.0 | 8 | 33.3 | 16 | 53.3 | 31 | 46 | 0.180 | 0.318 |
| >20 | 7 | 50.0 | 16 | 66.7 | 14 | 46.7 | 37 | 54 | |||
| Ext contact | No | 10 | 71.4 | 13 | 54.2 | 19 | 63.3 | 42 | 62 | 0.13 | 0.557 |
| Yes | 4 | 28.6 | 11 | 45.8 | 11 | 36.7 | 26 | 38 | |||
| Soil info | No | 13 | 92.9 | 22 | 91.7 | 26 | 86.7 | 61 | 90 | 0.090 | 0.759 |
| Yes | 1 | 7.1 | 2 | 8.3 | 4 | 13.3 | 7 | 10 | |||
| Siol TEST | No | 12 | 85.7 | 8 | 33.3 | 26 | 86.7 | 46 | 68 | 0.141 | 0.500 |
| Yes | 2 | 14.3 | 6 | 25.0 | 4 | 13.3 | 12 | 18 | |||
| Credit INFO | No | 14 | 100.0 | 21 | 87.5 | 28 | 93.3 | 63 | 93 | 0.172 | 0.356 |
| Yes | 0 | 0.0 | 3 | 12.5 | 2 | 6.7 | 5 | 7 | |||
| Crop husbandry advice | No | 12 | 85.7 | 20 | 83.3 | 24 | 80.0 | 56 | 82 | 0.059 | 0.887 |
| Yes | 2 | 14.3 | 4 | 16.7 | 6 | 20.0 | 12 | 18 | |||
| Animal husbandry advice | No | 14 | 100.0 | 21 | 87.5 | 24 | 80.0 | 59 | 87 | 0.216 | 0.188 |
| Yes | 0 | 0.0 | 3 | 12.5 | 6 | 20.0 | 9 | 13 | |||
| Agribiz | No | 14 | 100.0 | 24 | 100.0 | 29 | 96.7 | 67 | 99 | 0.136 | 0.526 |
| Yes | 0 | 0.0 | 0 | 0.0 | 1 | 3.3 | 1 | 1 | |||
| Variable | Cluster (Farm Types) | Total | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 14) | 2 (n = 24) | 3 (n= 30) | |||||||
| freq | % | freq | % | freq | % | ||||
| Pure stand | No | 9a | 64.3 | 14a | 58.3 | 21a | 70.0 | 44 | 0.606 |
| Yes | 5a | 35.7 | 10a | 41.7 | 9a | 30.0 | 24 | ||
| Mixed cropping | No | 3a | 21.4 | 11a | 45.8 | 10a | 33.3 | 24 | 0.308 |
| Yes | 11a | 78.6 | 13a | 54.2 | 20a | 66.7 | 44 | ||
| Agroforestry | No | 10a | 71.4 | 18a | 75.0 | 16a | 53.3 | 44 | 0.255 |
| Yes | 4a | 28.6 | 6a | 25.0 | 14a | 46.7 | 24 | ||
| Intercropping | No | 12a | 85.7 | 21a | 87.5 | 28a | 93.3 | 61 | 0.667 |
| Yes | 2a | 14.3 | 3a | 12.5 | 2a | 6.7 | 7 | ||
| Fallowing | No | 8ab | 57.1 | 13b | 54.2 | 24a | 80.0 | 45 | 0.05 |
| Yes | 6ab | 42.9 | 11b | 45.8 | 6a | 20.0 | 23 | ||
| Residue incorporation | No | 6a | 42.9 | 15a | 62.5 | 17a | 56.7 | 38 | 0.538 |
| Yes | 8a | 57.1 | 9a | 37.5 | 13a | 43.3 | 30 | ||
| Fertilizer planting rate | Low | 7a | 50.0 | 6a | 25.0 | 7a | 23.3 | 20 | 0.043 |
| Moderate | 1ab | 7.1 | 4b | 16.7 | 0a | 0.0 | 5 | ||
| High | 6a | 42.9 | 14ab | 58.3 | 23b | 76.7 | 43 | ||
| Fertilizer top dressing rate | Low | 7a | 50.0 | 6a | 25.0 | 7a | 23.3 | 20 | 0.043 |
| Moderate | 1ab | 7.1 | 4b | 16.7 | 0a | 0.0 | 5 | ||
| High | 6a | 42.9 | 14ab | 58.3 | 23b | 76.7 | 43 | ||
| Residue composting | No | 13a | 92.9 | 19a | 79.2 | 23a | 76.7 | 55 | 0.526 |
| Yes | 1a | 7.1 | 5a | 20.8 | 7a | 23.3 | 13 | ||
| Residue for fodder | No | 2a | 14.3 | 5a | 20.8 | 5a | 16.7 | 12 | 0.921 |
| Yes | 12a | 85.7 | 19a | 79.2 | 25a | 83.3 | 56 | ||
| Residue for fuel | No | 9ab | 64.3 | 18b | 75.0 | 14a | 46.7 | 41 | 0.11 |
| Yes | 5ab | 35.7 | 6b | 25.0 | 16a | 53.3 | 27 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawire, A.; Csorba, Á.; Zein, M.; Rotich, B.; Phenson, J.; Szegi, T.; Tormáné Kovács, E.; Michéli, E. Farm Household Typology Based on Soil Quality and Influenced by Socio-Economic Characteristics and Fertility Management Practices in Eastern Kenya. Agronomy 2023, 13, 1101. https://doi.org/10.3390/agronomy13041101
Wawire A, Csorba Á, Zein M, Rotich B, Phenson J, Szegi T, Tormáné Kovács E, Michéli E. Farm Household Typology Based on Soil Quality and Influenced by Socio-Economic Characteristics and Fertility Management Practices in Eastern Kenya. Agronomy. 2023; 13(4):1101. https://doi.org/10.3390/agronomy13041101
Chicago/Turabian StyleWawire, Amos, Ádám Csorba, Mohammed Zein, Brian Rotich, Justine Phenson, Tamás Szegi, Eszter Tormáné Kovács, and Erika Michéli. 2023. "Farm Household Typology Based on Soil Quality and Influenced by Socio-Economic Characteristics and Fertility Management Practices in Eastern Kenya" Agronomy 13, no. 4: 1101. https://doi.org/10.3390/agronomy13041101
APA StyleWawire, A., Csorba, Á., Zein, M., Rotich, B., Phenson, J., Szegi, T., Tormáné Kovács, E., & Michéli, E. (2023). Farm Household Typology Based on Soil Quality and Influenced by Socio-Economic Characteristics and Fertility Management Practices in Eastern Kenya. Agronomy, 13(4), 1101. https://doi.org/10.3390/agronomy13041101






