Control of Resistant False Cleavers (Galium spurium L.) Population to ALS-Inhibiting Herbicides and Its Impact on the Growth and Yield of Durum Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Main Cultural Practices
2.1.1. Site 1: Domokos
2.1.2. Site 2: Velestino
2.2. Herbicides Application and Doses
2.3. Sampling and Measurements
2.4. Statistical Analysis
3. Results
3.1. Weather Conditions
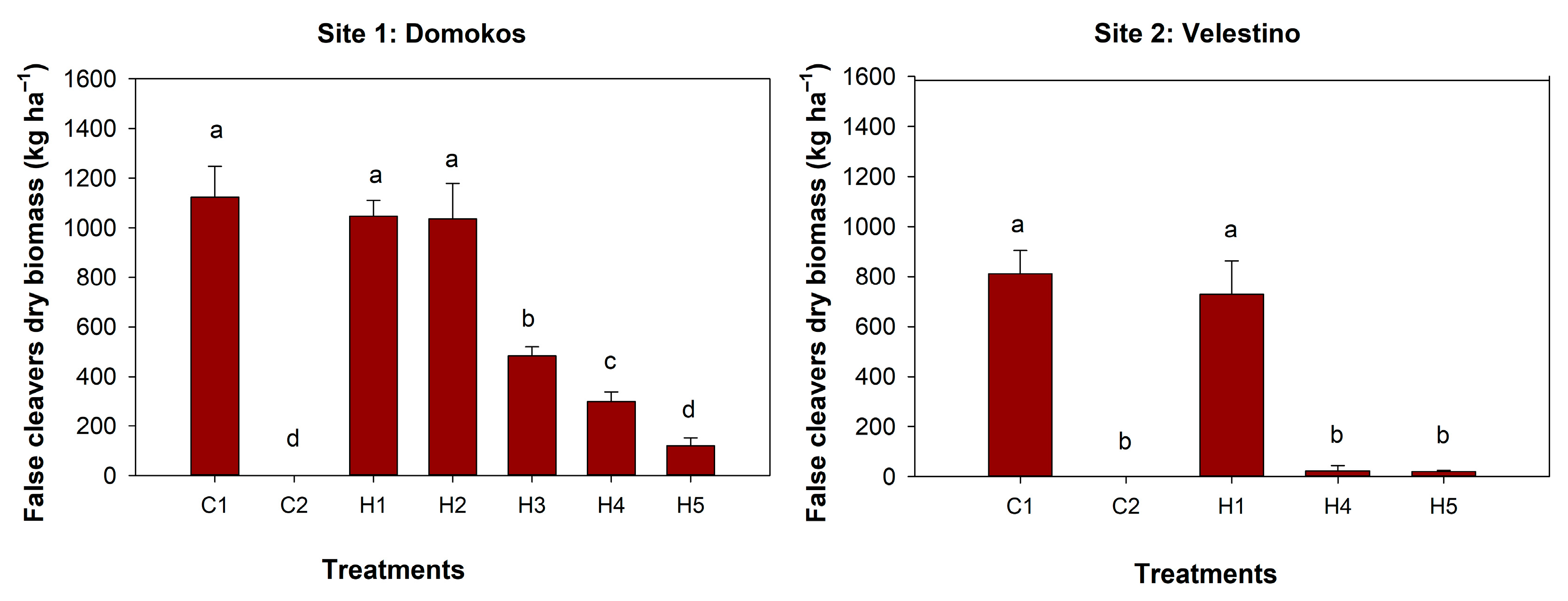
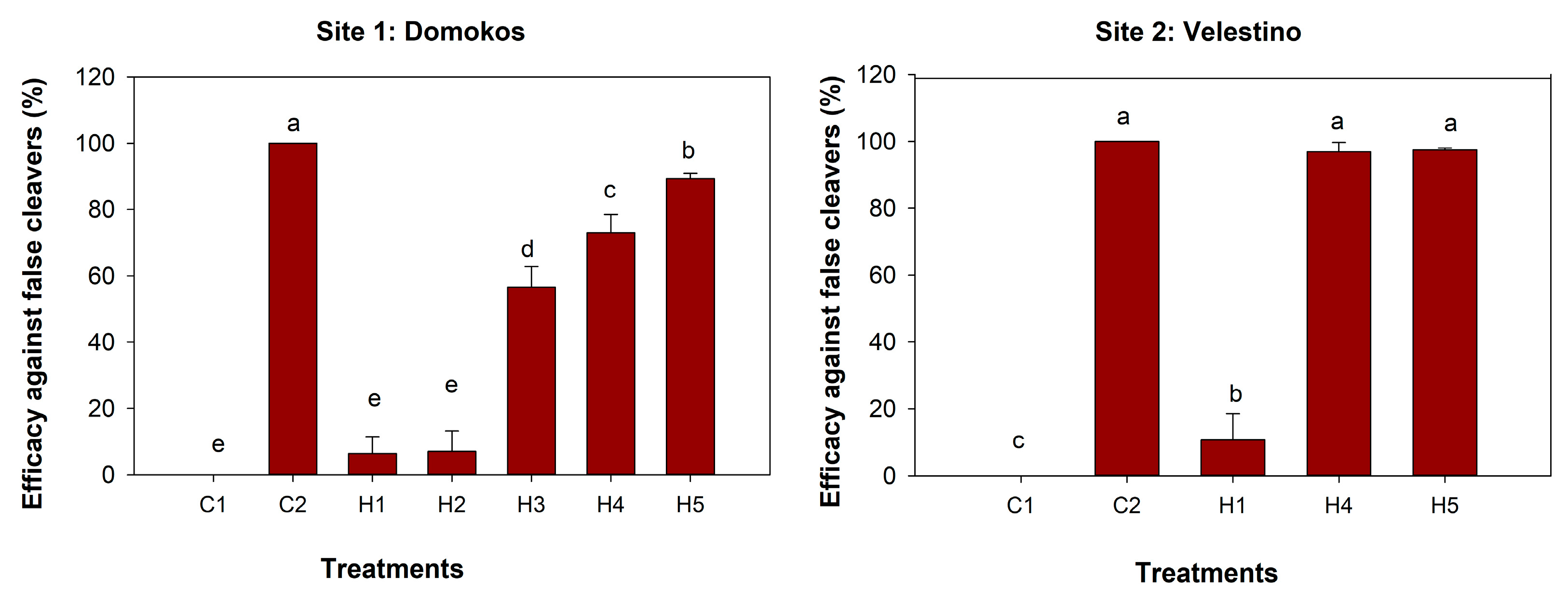
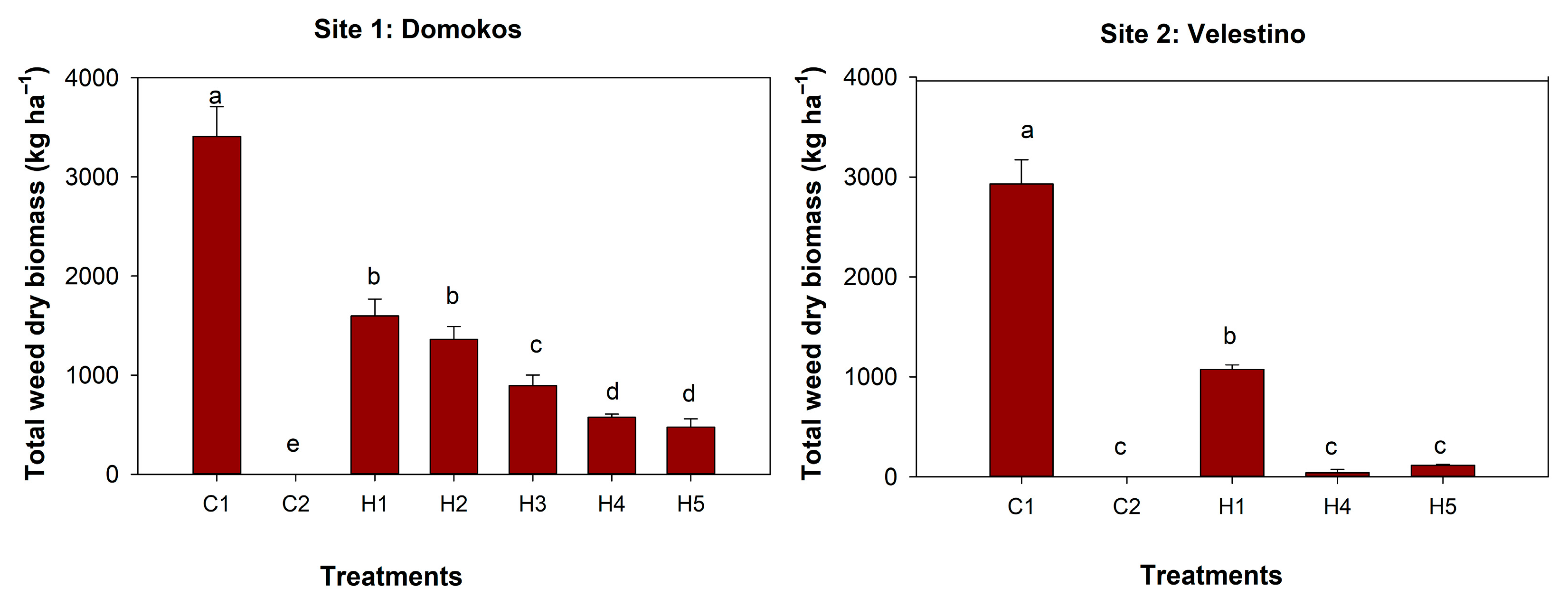
3.2. Weeds Dry Biomass
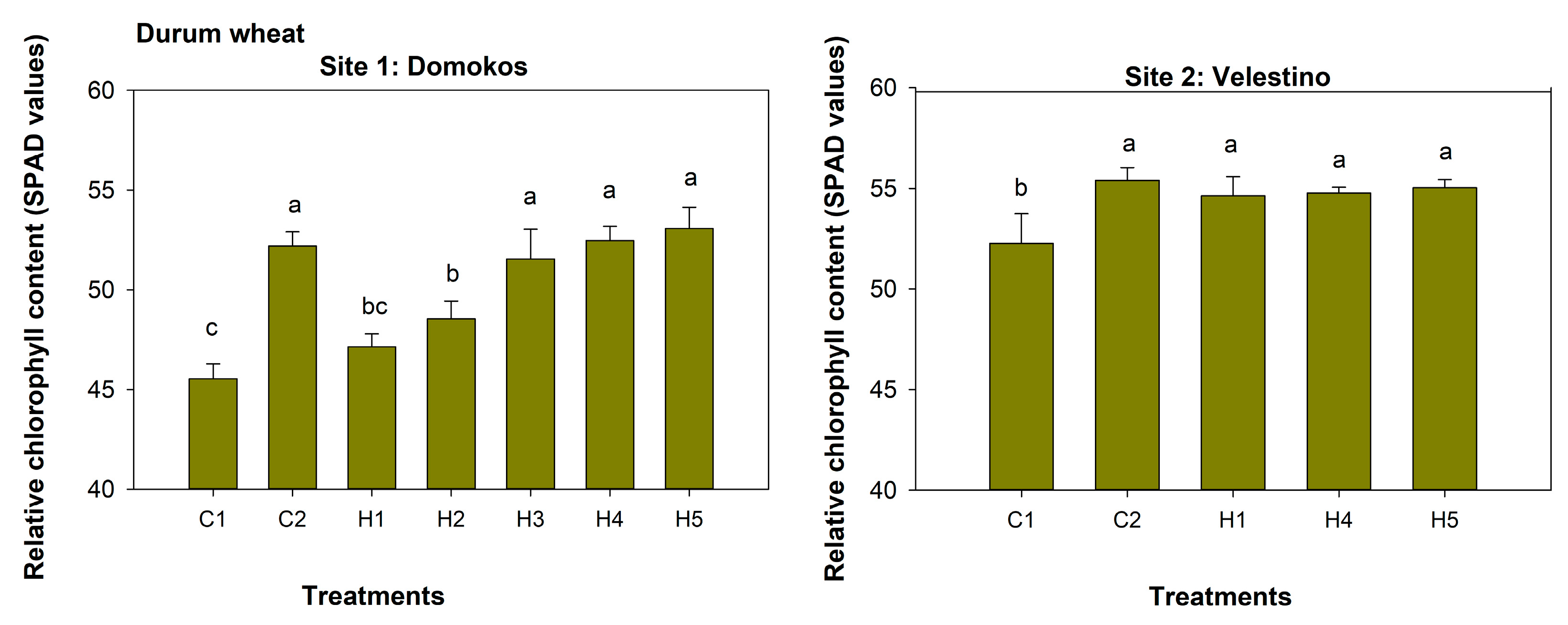
3.3. Relative Chlorophyll Content
3.4. Durum Wheat Growth
3.5. Spike Length, 1000-Seed Weight, and Seed Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- He, H.; Peng, M.; Lu, W.; Hou, Z.; Li, J. Commercial Organic Fertilizer Substitution Increases Wheat Yield by Improving Soil Quality. Sci. Total Environ. 2022, 851, 158132. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of Fertilizer Nitrogen in Cereal Production: Retrospects and Prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing Yield Gaps through Nutrient and Water Management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Umar, M.; Azeem, M.; Uddin, Z.; Siddiqui, Z.S. Salt Tolerance Screening of a Newly Developed Wheat Variety (AZRC-DK-84) in Saline Environment Using Halophytic Grass (Cenchrus penisettiformis) as a Test Model. Acta Physiol. Plant. 2022, 44, 81. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break Crop Benefits in Temperate Wheat Production. Field Crop. Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.; Smith, P.; Zeng, Z.; Olesen, J.E.; Zang, H. Global Systematic Review with Meta-Analysis Reveals Yield Advantage of Legume-Based Rotations and its Drivers. Nat. Commun. 2022, 13, 4926. [Google Scholar] [CrossRef]
- Lemerle, D.; Verbeek, B.; Cousens, R.D.; Coombes, N.E. The Potential for Selecting Wheat Varieties Strongly Competitive against Weeds. Weed Res. 1996, 36, 505–513. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Ann. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Woźniak, A.; Soroka, M. Weed Flora in Crop Rotation and Winter Wheat Monoculture. Span. J. Agric. Res. 2022, 20, e0204. [Google Scholar] [CrossRef]
- Geddes, C.M.; Sharpe, S.M. Crop Yield Losses Due to Kochia (Bassia scoparia) Interference. Crop Prot. 2022, 157, 105981. [Google Scholar] [CrossRef]
- Lemerle, D.; Gill, G.S.; Murphy, C.E.; Walker, S.R.; Consens, R.D.; Mokhtari, S.; Peltzer, S.J.; Coleman, R.; Luckett, D.J. Genetic Improvement and Agronomy for Enhanced Wheat Competitiveness with Weeds. Aust. J. Agric. Res. 2001, 52, 527–548. [Google Scholar] [CrossRef]
- Zargar, M.; Kavhiza, N.J.; Bayat, M.; Pakina, E. Wild Mustard (Sinapis arvensis) Competition and Control in Rain-Fed Spring Wheat (Triticum aestivum L.). Agronomy 2021, 11, 2306. [Google Scholar] [CrossRef]
- Bayat, M.; Zargar, M. Postemergence Herbicide Combinations for Effective Littleseed Canarygrass (Phalaris minor) Control in Winter Wheat (Triticum aestivum L.). Acta Physiol. Plant. 2021, 43, 150. [Google Scholar] [CrossRef]
- Ahmad, I.; Gul, B.; Khan, H.; Fawad, M. Influence of Various Densities of Wild Oat and Holy Thistle on the Productivity of Wheat (Triticum aestivum). Gesunde Pflanz. 2022, 74, 839–852. [Google Scholar] [CrossRef]
- Karkanis, A.; Angou, A.; Athanasiadou, D.; Giannoulis, K.D.; Askianaki, R.; Kousi, N.; Sarridis, A.; Souipas, S.; Karamoutis, C. Using Post-Emergence Herbicides in Combination with the Sowing Date to Suppress Sinapis arvensis and Silybum marianum in Durum Wheat. Agronomy 2022, 12, 2583. [Google Scholar] [CrossRef]
- Beckie, H.J.; Blackshaw, R.E.; Harker, K.N.; Tidemann, B.D. Weed Seed Shatter in Spring Wheat in Alberta. Can. J. Plant Sci. 2017, 98, 107–114. [Google Scholar] [CrossRef]
- Mennan, H.; Streibig, J.C.; Ngouajio, M.; Cankaya, S. Response of Two Catchweed Bedstraw (Galium aparine) Populations to Post-Emergence Herbicides in Winter Wheat. Int. J. Pest Manag. 2011, 57, 347–356. [Google Scholar] [CrossRef]
- Baghestani, M.A.; Zand, E.; Soufizadeh, S.; Beheshtian, M.; Haghighi, A.; Barjasteh, A.; Birgani, D.G.; Deihimfard, R. Study on the Efficacy of Weed Control in Wheat (Triticum aestivum L.) with Tank Mixtures of Grass Herbicides with Broadleaved Herbicides. Crop Prot. 2008, 27, 104–111. [Google Scholar] [CrossRef]
- HRAC, Herbicide Resistance Action Committee. Available online: https://hracglobal.com/tools/hrac-mode-of-action-classification-2022-map (accessed on 9 February 2023).
- Zhou, Q.; Liu, W.; Zhang, Y.; Liu, K.K. Action Mechanisms of Acetolactate Synthase-Inhibiting Herbicides. Pestic. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Saari, L.L.; Cotterman, J.C.; Thill, D.C. Resistance to Acetolactate Synthase Inhibiting Herbicides. In Herbicide Resistance in Plants: Biology and Biochemistry, 1st ed.; (Reissued 2018); Powles, S.B., Holtum, J.A.M., Eds.; CRC Press: New York, NY, USA, 2018; pp. 83–140. [Google Scholar]
- Tranel, P.J.; Wright, T.R. Resistance of Weeds to ALS-Inhibiting Herbicides: What Have We Learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- De Barreda, D.G.; Pardo, G.; Osca, J.M.; Catala-Forner, M.; Consola, S.; Garnica, I.; López-Martínez, N.; Palmerín, J.A.; Osuna, M.D. An Overview of Rice Cultivation in Spain and the Management of Herbicide-Resistant Weeds. Agronomy 2021, 11, 1095. [Google Scholar] [CrossRef]
- Bukun, B.; Gaines, T.A.; Nissen, S.J.; Westra, P.; Brunk, G.; Shaner, D.L.; Sleugh, B.B.; Peterson, V.F. Aminopyralid and Clopyralid Absorption and Translocation in Canada Thistle (Cirsium arvense). Weed Sci. 2009, 57, 10–15. [Google Scholar] [CrossRef]
- Jursík, M.; Soukup, J.; Holec, J.; Andr, J. Herbicide mode of actions and symptoms of plant injury by herbicides: Plant growth regulator herbicides (synthetic auxins). Listy Cukrov. Reparske 2011, 127, 88–92. [Google Scholar]
- McCauley, C.L.; Johnson, W.G.; Young, B.G. Efficacy of Halauxifen-Methyl on Glyphosate-Resistant Horseweed (Erigeron canadensis). Weed Sci. 2018, 66, 758–763. [Google Scholar] [CrossRef]
- Todd, O.E.; Figueiredo, M.R.A.; Morran, S.; Soni, N.; Preston, C.; Kubeš, M.F.; Napier, R.; Gaines, T.A. Synthetic Auxin Herbicides: Finding the Lock and Key to Weed Resistance. Plant Sci. 2020, 300, 110631. [Google Scholar] [CrossRef] [PubMed]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002; pp. 1–671. [Google Scholar]
- Takano, A.; Takahashi, R.; Suzuki, H.; Noguchi, T. Herbicide Effect on the Hydrogen-Bonding Interaction of the Primary Quinone Electron Acceptor QA in Photosystem II as Studied by Fourier Transform Infrared Spectroscopy. Photosynth. Res. 2008, 98, 159–167. [Google Scholar] [CrossRef]
- Malik, N.; Born, W.H.V. The Biology of Canadian Weeds. 86. Galium aparine L. and Galium spurium L. Can. J. Plant Sci. 1988, 68, 481–499. [Google Scholar] [CrossRef]
- Hall, L.M.; Stromme, K.M.; Horsman, G.P.; Devine, M.D. Resistance to Acetolactate Synthase Inhibitors and Quinclorac in a Biotype of False Cleavers (Galium spurium). Weed Sci. 1998, 46, 390–396. [Google Scholar] [CrossRef]
- Papapanagiotou, A.P.; Damalas, C.A.; Bosmali, I.; Madesis, P.; Menexes, G.C.; Eleftherohorinos, I.G. Galium spurium and G. aparine Resistance to Als-Inhibiting Herbicides in Northern Greece. Planta Daninha 2019, 37, e019207288. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Torra, J.; Conesa, J.A.; Recasens, J. Emergence and Early Growth of Galium aparine and Galium spurium. Weed Res. 2012, 52, 458–466. [Google Scholar] [CrossRef]
- Lou, T.; Wang, K.; Chen, J.; Cao, J.; Gu, T.; Jiang, L.; Lou, Y.; Cao, R.; Wang, H. Survey Reveals Frequency of Multiple Resistance to Tribenuron-Methyl, Bensulfuron-Methyl and Halosulfuron-Methyl in Cleavers (Galium aparine L.). Agronomy 2022, 12, 2695. [Google Scholar] [CrossRef]
- Beckie, H.J.; Warwick, S.I.; Sauder, C.A.; Kelln, G.M.; Lozinski, C. Acetolactate Synthase Inhibitor-Resistant False Cleavers (Galium spurium) in Western Canada. Weed Technol. 2012, 26, 151–155. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Stephenson, G.R.; Kwiatkowski, J.; Grossmann, K.; Hall, J.C. Physiological and Biochemical Characterization of Quinclorac Resistance in a False Cleavers (Galium spurium L.) Biotype. J. Agric. Food Chem. 2005, 53, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Lozinski, C.; Shirriff, S.; Brenzil, C.A. Herbicide-Resistant Weeds in the Canadian Prairies: 2007 to 2011. Weed Technol. 2013, 27, 171–183. [Google Scholar] [CrossRef]
- Soltani, N.; Shropshire, C.; Sikkema, P.H. Glyphosate-Resistant Canada Fleabane Control in Winter Wheat with Postemergence Herbicides. Int. J. Agron. 2020, 2020, 8840663. [Google Scholar] [CrossRef]
- Wolf, R.; Clay, S.A.; Wrage, L.J. Herbicide Strategies for Managing Kochia (Kochia scoparia) Resistant to ALS-Inhibiting Herbicides in Wheat (Triticum aestivum) and Soybean (Glycine max). Weed Technol. 2000, 14, 268–273. [Google Scholar] [CrossRef]
- Karkanis, A.; Vellios, E.; Grigoriou, F.; Gkrimpizis, T.; Giannouli, P. Evaluation of Efficacy and Compatibility of Herbicides with Fungicides in Durum Wheat (Triticum durum Desf.) under Different Environmental Conditions: Effects on Grain Yield and Gluten Content. Not. Bot. Horti Agrobot. 2018, 46, 601–607. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Manuchehri, M.R.; Calhoun, J.S.; Childers, J.T.; Merritt, L.H.; Broster, K.L.; Treadway, Z.R.; Ugljic, Z.; Wesley, M.T. Hairy Buttercup (Ranunculus sardous) and Cutleaf Evening Primrose (Oenothera laciniata) Control Using Halauxifen-Methyl Based Programs in Mississippi and Oklahoma Winter Wheat. Weed Technol. 2021, 35, 644–650. [Google Scholar] [CrossRef]

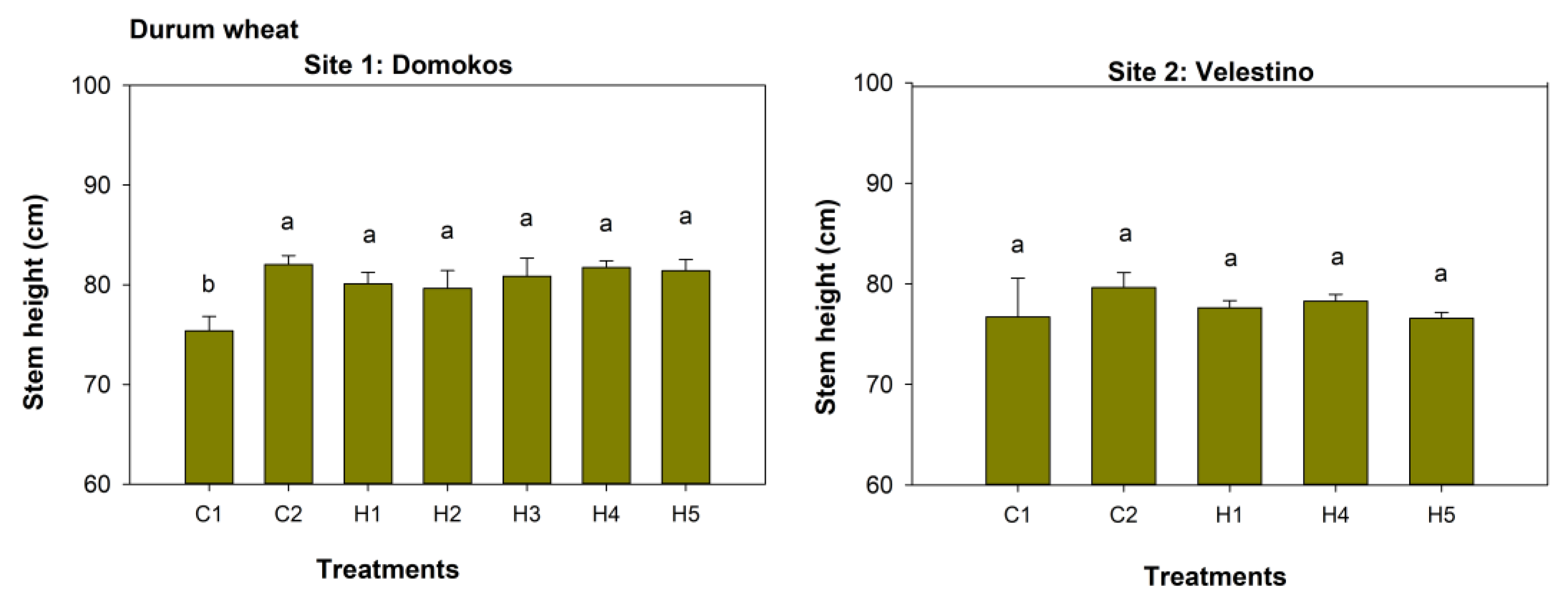
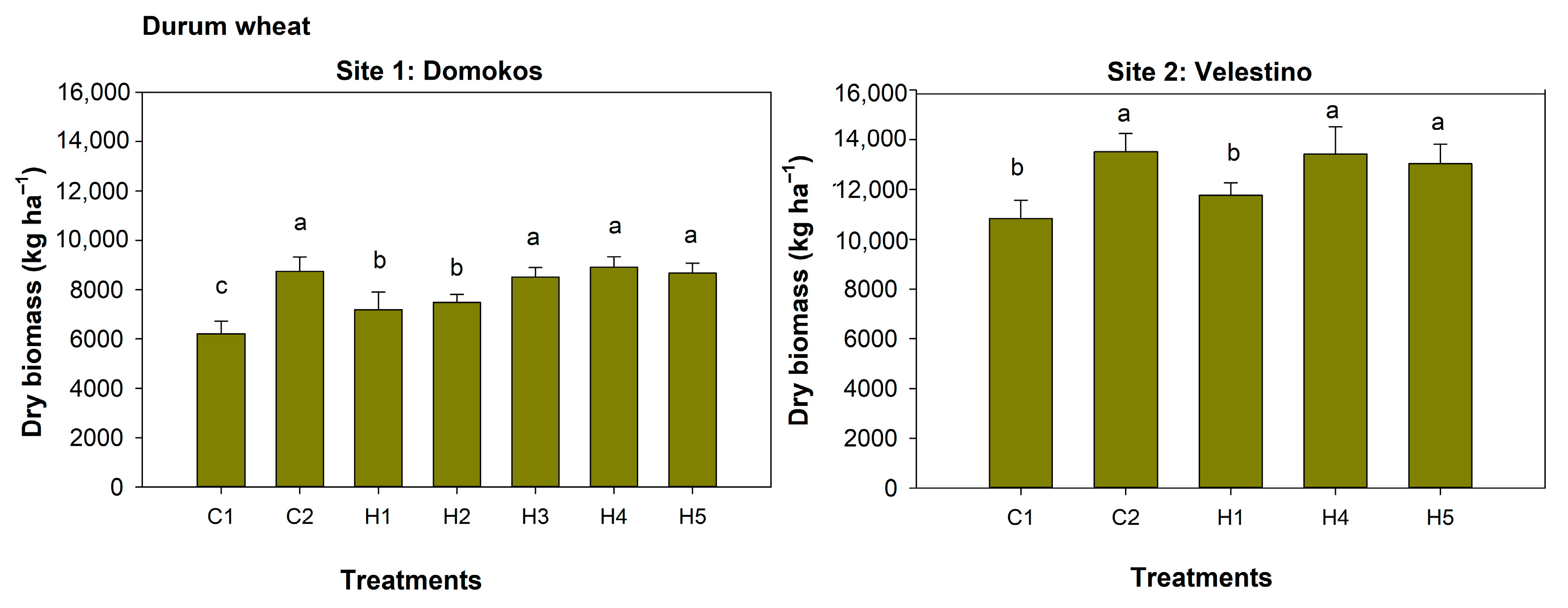
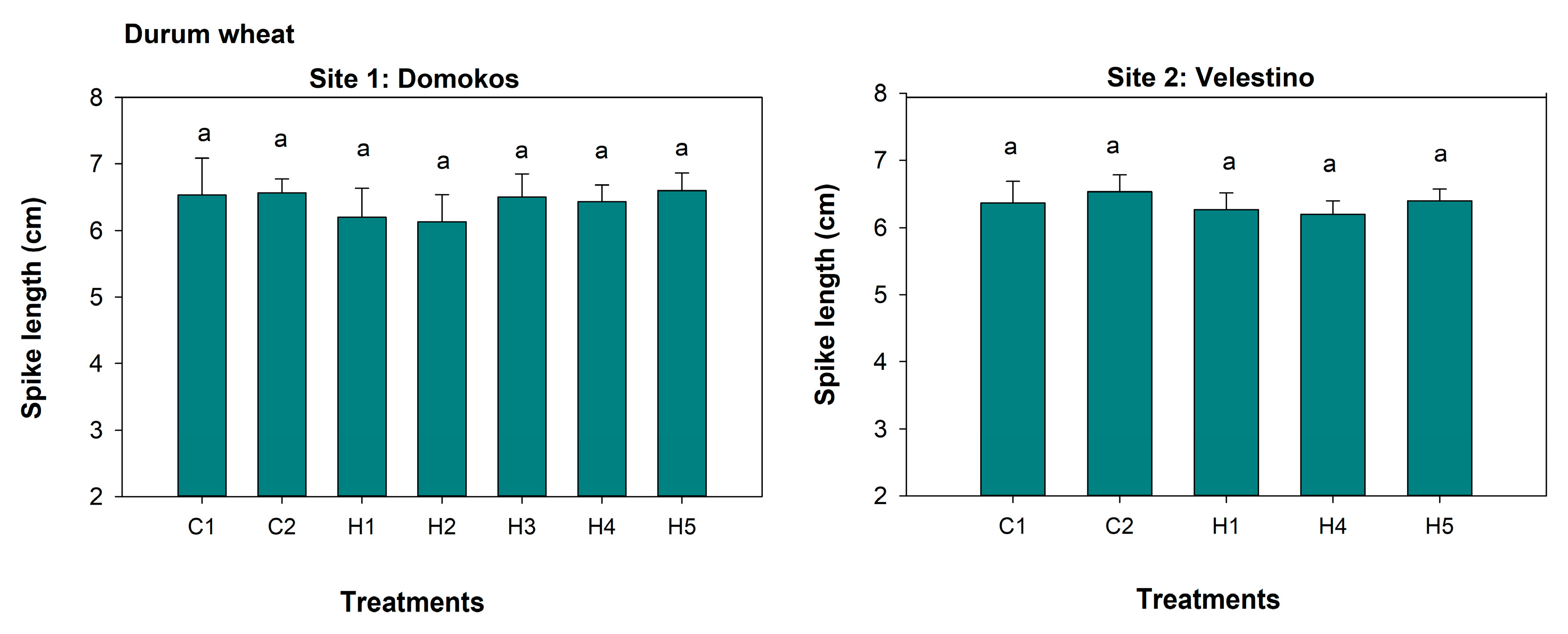

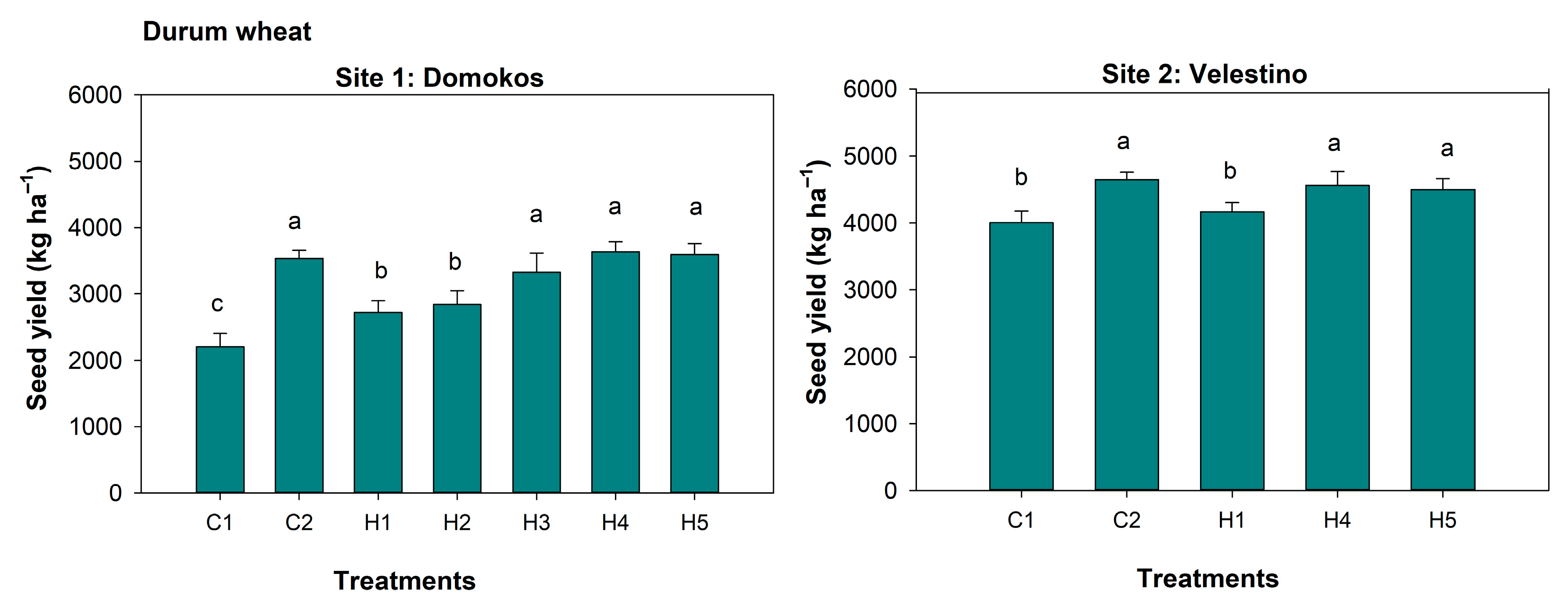

| Site 1: Domokos | SH | WhDB | SPAD | SW | SL | SY | GDB | WeDB |
| SH | 1 | 0.770 *** | 0.705 *** | 0.818 *** | 0.132 | 0.794 *** | −0.639 ** | −0.843 *** |
| WhDB | – | 1 | 0.889 *** | 0.870 *** | 0.158 | 0.870 *** | −0.828 *** | −0.862 *** |
| SPAD | – | – | 1 | 0.827 *** | 0.072 | 0.891 *** | −0.888 *** | −0.864 *** |
| SW | – | – | – | 1 | 0.213 | 0.835 *** | −0.727 *** | −0.852 *** |
| SL | – | – | – | – | 1 | 0.183 | −0.313 | −0.075 |
| SY | – | – | – | – | – | 1 | −0.881 *** | −0.910 *** |
| GDB | – | – | – | – | – | – | 1 | 0.841 *** |
| WeDB | – | – | – | – | – | – | – | 1 |
| Site 2: Velestino | SH | WhDB | SPAD | SW | SL | SY | GDB | WeDB |
| SH | 1 | 0.436 | 0.517 * | 0.033 | 0.423 | 0.258 | −0.326 | −0.350 |
| WhDB | – | 1 | 0.587 * | 0.711 ** | 0.152 | 0.901 *** | −0.812 *** | −0.829 *** |
| SPAD | – | – | 1 | 0.396 | 0.330 | 0.542 * | −0.688 *** | −0.817 *** |
| SW | – | – | – | 1 | −0.142 | 0.694 * | −0.850 *** | −0.653 ** |
| SL | – | – | – | – | 1 | 0.098 | −0.177 | −0.058 |
| SY | – | – | – | – | – | 1 | −0.809 *** | −0.814 *** |
| GDB | – | – | – | – | – | – | 1 | 0.875 *** |
| WeDB | – | – | – | – | – | – | – | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparangis, P.; Efthimiadou, A.; Katsenios, N.; Karkanis, A. Control of Resistant False Cleavers (Galium spurium L.) Population to ALS-Inhibiting Herbicides and Its Impact on the Growth and Yield of Durum Wheat. Agronomy 2023, 13, 1087. https://doi.org/10.3390/agronomy13041087
Sparangis P, Efthimiadou A, Katsenios N, Karkanis A. Control of Resistant False Cleavers (Galium spurium L.) Population to ALS-Inhibiting Herbicides and Its Impact on the Growth and Yield of Durum Wheat. Agronomy. 2023; 13(4):1087. https://doi.org/10.3390/agronomy13041087
Chicago/Turabian StyleSparangis, Panagiotis, Aspasia Efthimiadou, Nikolaos Katsenios, and Anestis Karkanis. 2023. "Control of Resistant False Cleavers (Galium spurium L.) Population to ALS-Inhibiting Herbicides and Its Impact on the Growth and Yield of Durum Wheat" Agronomy 13, no. 4: 1087. https://doi.org/10.3390/agronomy13041087
APA StyleSparangis, P., Efthimiadou, A., Katsenios, N., & Karkanis, A. (2023). Control of Resistant False Cleavers (Galium spurium L.) Population to ALS-Inhibiting Herbicides and Its Impact on the Growth and Yield of Durum Wheat. Agronomy, 13(4), 1087. https://doi.org/10.3390/agronomy13041087









