Strigolactones in Sugarcane Growth and Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of Plant Expression Vectors
2.3. A Tumefaciens-Mediated Genetic Transformation of ROC22
2.4. PCR Detection of Bar and ScD27.2 Genes in Transgenic Lines
2.5. qRT-PCR
2.6. Phenotypic Analysis of Transgenic Sugarcane Lines
2.7. Simulated Drought Stress Treatment
2.8. Hydroponic Culture
2.9. Analysis of SLs Content in Sugarcane Root Tissue
2.10. Data Analysis
3. Results
3.1. Agrobacterium-Mediated Genetic Transformation of Sugarcane and Detection of Transformed Lines
3.2. Down-Regulation of ScD27.2
3.3. Increased Shoot Branching in ScD27.2R-9
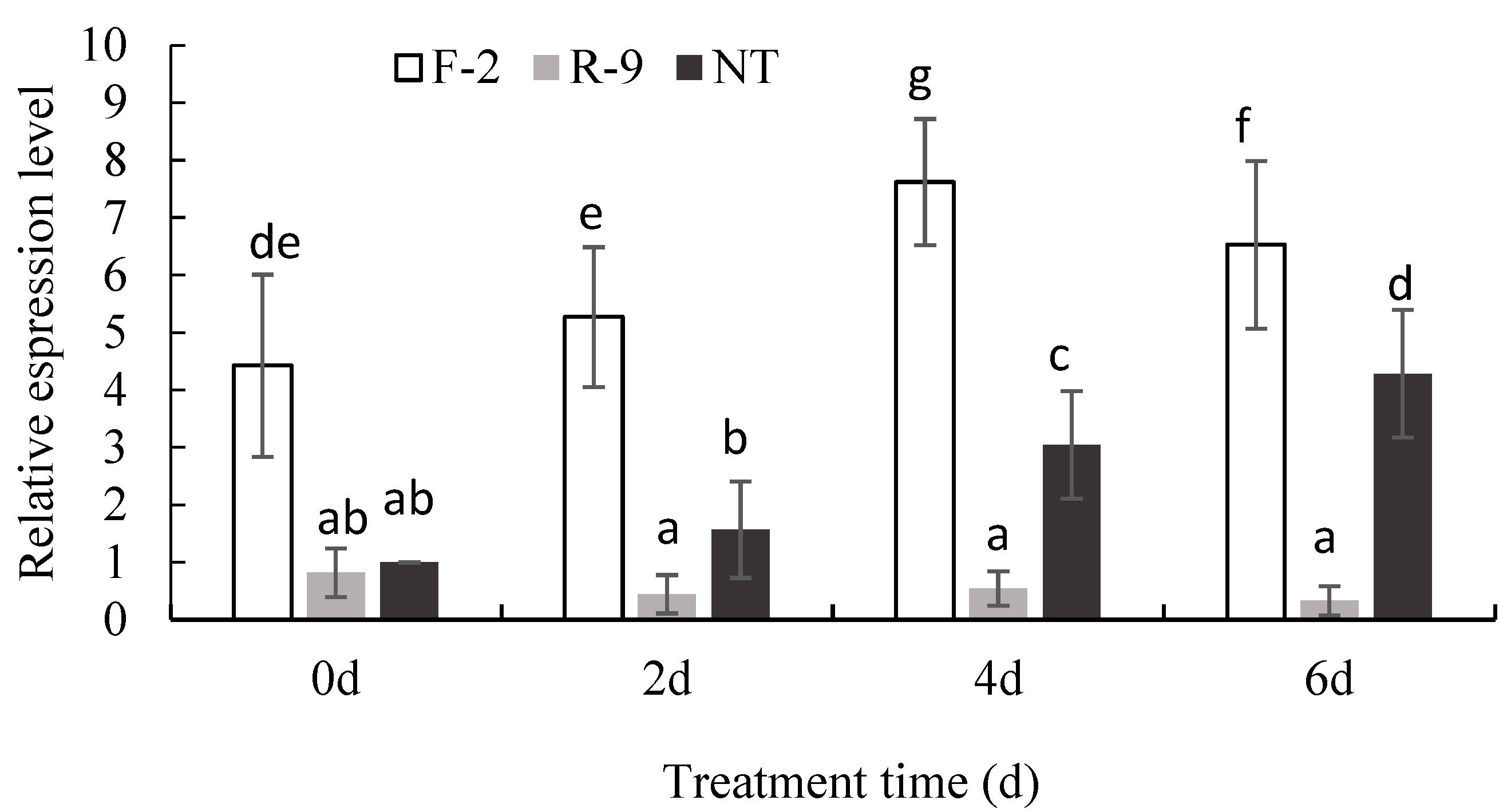
3.4. Transcript Level of the ScD27.2 Gene in ScD27.2R-9
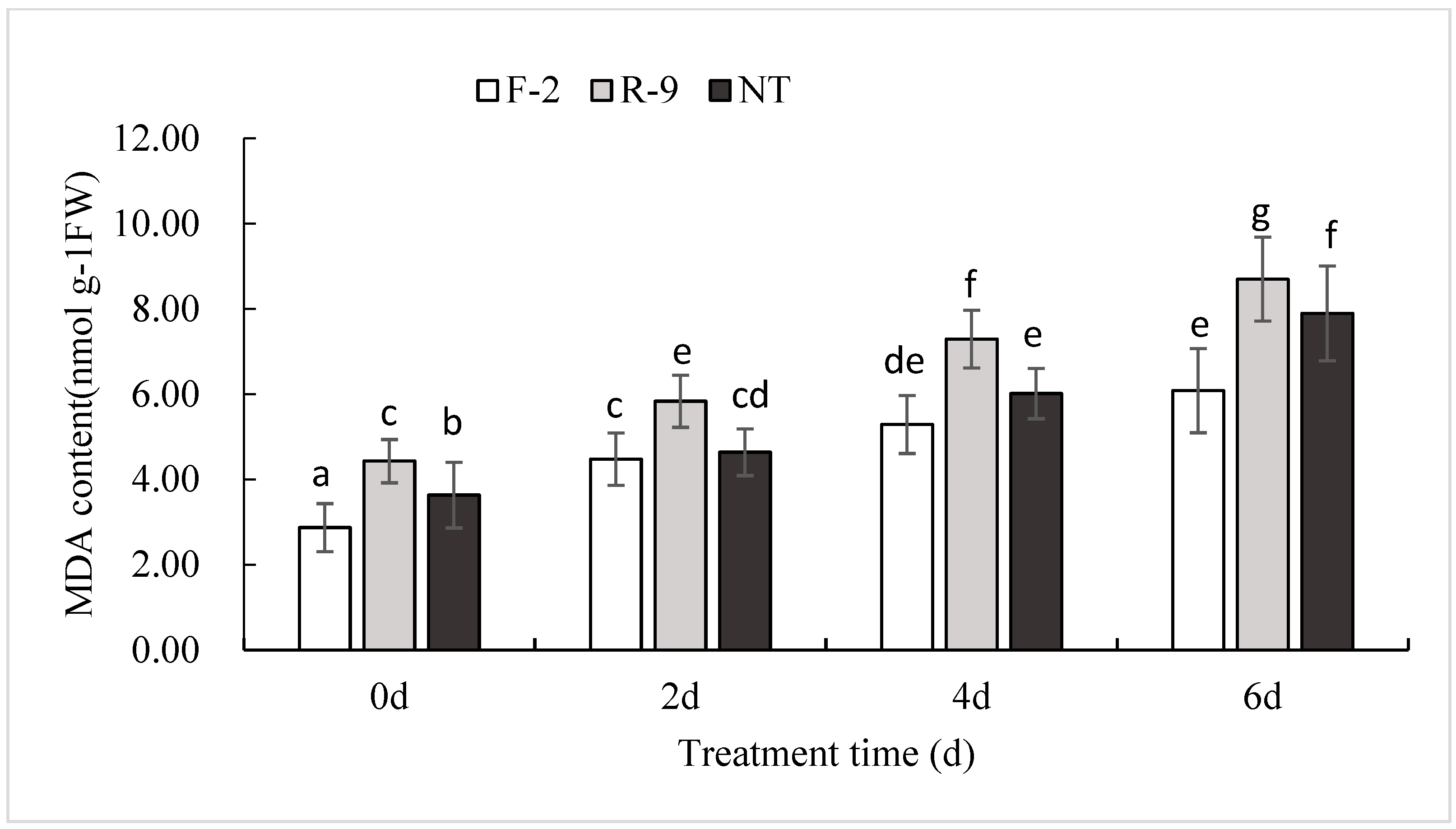
3.5. Increase MDA Content in ScD27.2R-9
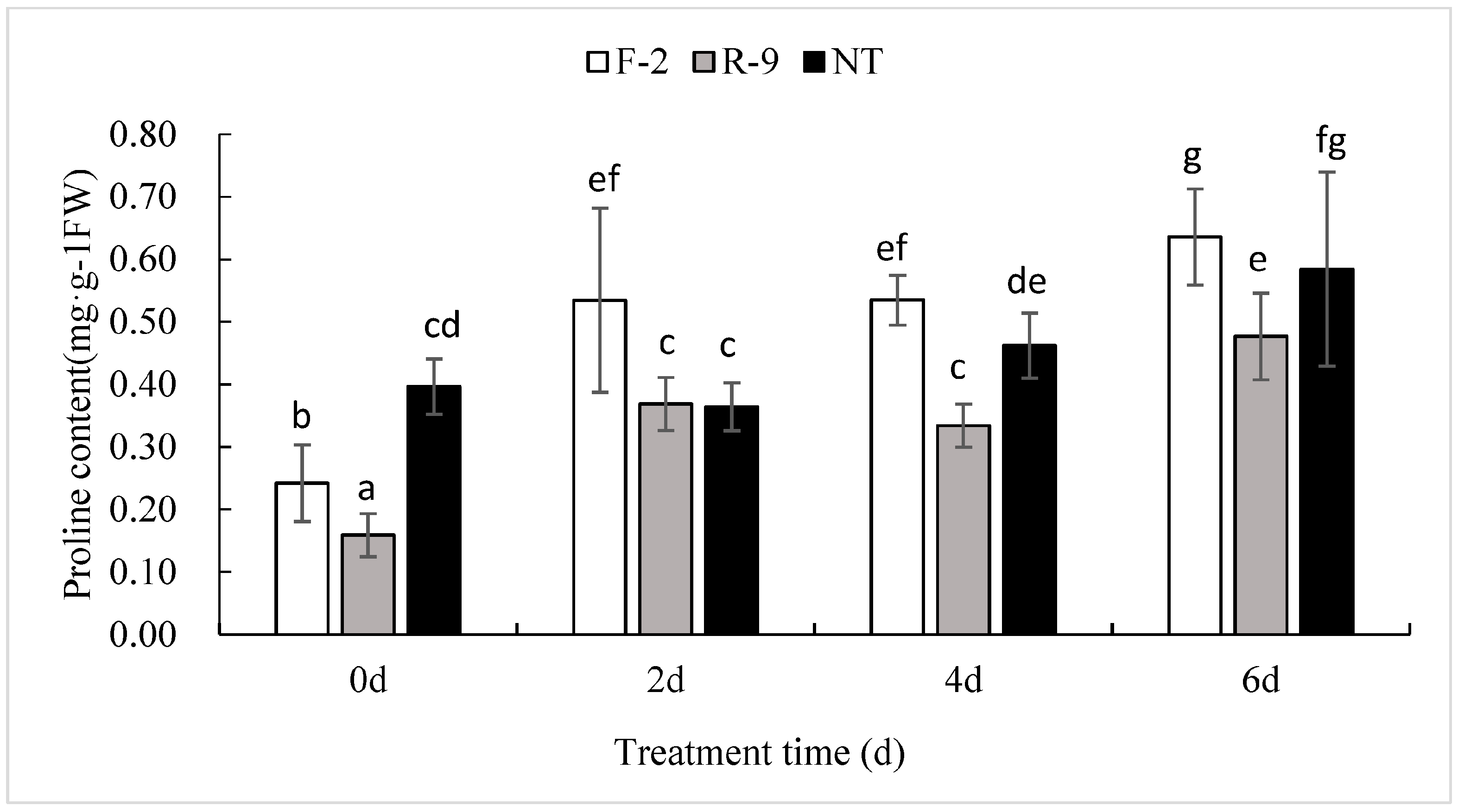
3.6. Lower Free Proline Content in ScD27.2R-9
3.7. The Tiller Number of ScD27.2R-9 after 15 Days of 20% PEG
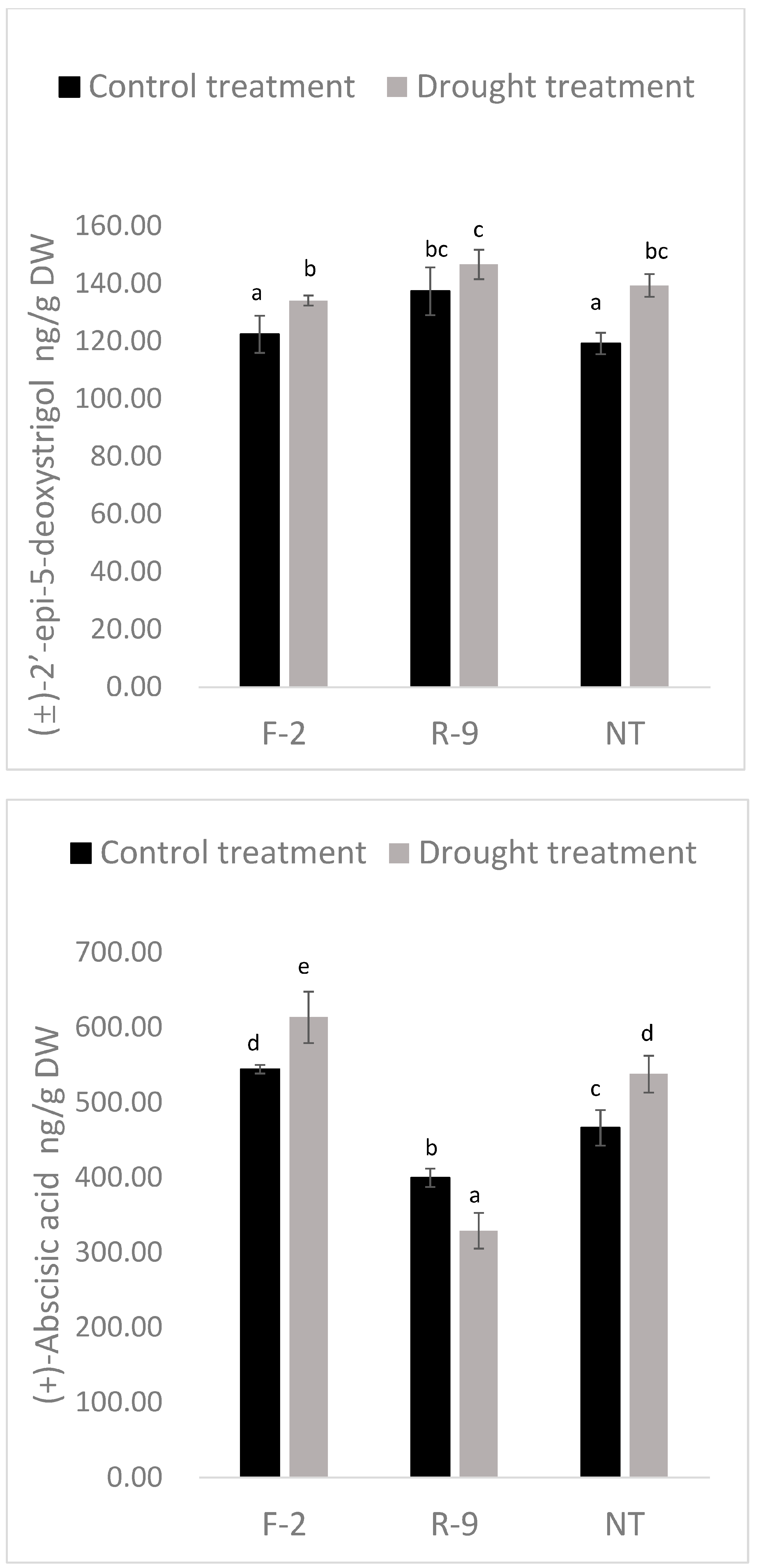
3.8. PEG Increase in the SL Content in Sugarcane Lines
3.9. Lower ABA Levels in ScD27.2R-9
4. Discussion
4.1. ScD27.2R-9 Antisense Line with a Highly Branched Dwarf Phenotype
4.2. Lower ABA Levels Lead to ScD27.2R-9 Drought Sensitivity
4.3. The Fresh Weight of ScD27.2R-9 Lines Was Significantly Lower
4.4. Lower ABA Levels in ScD27.2R-9 Lines Indicated an Interference with the ABA Pathway
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Primer Name. | Accession | Sequence (5′–3′) |
|---|---|---|
| D27FNcoIF | CCCATGGATGGAGGTCGCCGCCACTTGCATGC | |
| D27FBglIIR | GAAGATCTTCAAATAGAGCAATTCACTTGACGG | |
| D27RNcoIF | CCCATGGTCAAATAGAGCAATTCACTTGACG | |
| D27RBglIIR | GAAGATCTGTCGCCGCCACTTGCATGC | |
| QScD27F | GGATGAAGAACGGAAAGGAC | |
| QScD27R | ACGAGCCAAGGGAAGAATAT | |
| GAPDHF | CACGGCCACTGGAAGCA | |
| GAPDHR | TCCTCAGGGTTCCTGATGCC |
References
- Sandhu, H.S.; Nuessly, G.S.; Cherry, R.H.; Gilbert, R.A.; Webb, S.E. Effects of Elasmopalpus lignosellus (Lepidoptera: Pyralidae) damage on sugarcane yield. J. Econ. Èntomol. 2011, 104, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C. Genome Editing in Sugarcane: Challenges Ahead. Front. Plant Sci. 2016, 7, 1542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J.; et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Zalabák, D.; Pospíšilová, H.; Šmehilová, M.; Mrízová, K.; Frébort, I.; Galuszka, P. Genetic engineering of cytokinin metabolism: Prospective way to improve agricultural traits of crop plants. Biotechnol. Adv. 2013, 31, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamaguchi-Shinozaki, K. Metabolic engineering: Towards water deficiency adapted crop plants. J. Plant Physiol. 2021, 258–259, 153375. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Germain, A.D.S.; Braun, N.; Rameau, C. Strigolactones, a novel class of plant hormones controlling branching. Biol. Aujourd’hui 2010, 204, 43–49. [Google Scholar] [CrossRef]
- Brewer, P.B.; Koltai, H.; Beveridge, C.A. Diverse Roles of Strigolactones in Plant Development. Mol. Plant 2013, 6, 18–28. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, T.T.; Ma, S.S.; Jiang, D.J.; Bie, X.M.; Sui, N.; Zhang, X.S.; Wang, F. TaD27-B gene controls the tiller number in hexaploid wheat. Plant Biotechnol. J. 2019, 18, 513–525. [Google Scholar] [CrossRef]
- Qu, B.; Qin, Y.; Bai, Y. From signaling to function: How strigolactones regulate plant development. Sci. China Life Sci. 2020, 63, 1768–1770. [Google Scholar] [CrossRef]
- Cheng, X.; Ruyter-Spira, C.; Bouwmeester, H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 2013, 4, 199. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wan, L.; Zhao, W.; Liu, H.-F.; Li, J.; Zhang, C.-L. Exogenous strigolactones promote lateral root growth by reducing the endogenous auxin level in rapeseed. J. Integr. Agric. 2020, 19, 465–482. [Google Scholar] [CrossRef]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do strigolactones contribute to plant defence? Mol. Plant Pathol. 2013, 15, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Sharma, M.; Pandey, G.K. Emerging Roles of Strigolactones in Plant Responses to Stress and Development. Front. Plant Sci. 2016, 7, 434. [Google Scholar] [CrossRef]
- Li, W.; Herrera-Estrella, L.; Tran, L.-S.P. Do Cytokinins and Strigolactones Crosstalk during Drought Adaptation? Trends Plant Sci. 2019, 24, 669–672. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, Y.; Tong, A.; Yan, B.; Lv, Y.; Wang, S.; Ma, W.; Cui, Z.; Wang, X. LAZY1 Controls Tiller Angle and Shoot Gravitropism by Regulating the Expression of Auxin Transporters and Signaling Factors in Rice. Plant Cell Physiol. 2020, 61, 2111–2125. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Liang, Y.; Li, J.; Wang, Y. Molecular basis underlying rice tiller angle: Current progress and future perspectives. Mol. Plant 2021, 15, 125–137. [Google Scholar] [CrossRef]
- Czarnecki, O.; Yang, J.; Weston, D.J.; Tuskan, G.A.; Chen, J.-G. A Dual Role of Strigolactones in Phosphate Acquisition and Utilization in Plants. Int. J. Mol. Sci. 2013, 14, 7681–7701. [Google Scholar] [CrossRef]
- Gamir, J.; Torres-Vera, R.; Rial, C.; Berrio, E.; Campos, P.M.D.S.; Varela, R.M.; Macías, F.A.; Pozo, M.J.; Flors, V.; López-Ráez, J.A. Exogenous strigolactones impact metabolic profiles and phosphate starvation signalling in roots. Plant Cell Environ. 2020, 43, 1655–1668. [Google Scholar] [CrossRef]
- Yoneyama, K. How Do Strigolactones Ameliorate Nutrient Deficiencies in Plants? Cold Spring Harb. Perspect. Biol. 2019, 11, a034686. [Google Scholar] [CrossRef] [PubMed]
- Shindo, M.; Shimomura, K.; Yamaguchi, S.; Umehara, M. Upregulation of DWARF 27 is associated with increased strigolactone levels under sulfur deficiency in rice. Plant Direct 2018, 2, e00050. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, R.; Qian, Q.; Yan, M.; Meng, X.; Fu, Z.; Yan, C.; Jiang, B.; Su, Z.; Li, J.; et al. DWARF27, an Iron-Containing Protein Required for the Biosynthesis of Strigolactones, Regulates Rice Tiller Bud Outgrowth. Plant Cell 2009, 21, 1512–1525. [Google Scholar] [CrossRef]
- Abuauf, H.W.; Haider, I.; Jia, K.-P.; Ablazov, A.; Mi, J.; Blilou, I.; Al-Babili, S. The Arabidopsis DWARF27 gene encodes an all-trans-/9-cis-β-carotene isomerase and is induced by auxin, abscisic acid and phosphate deficiency. Plant Sci. 2018, 277, 33–42. [Google Scholar] [CrossRef]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; Al-Babili, S.; et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Ma, C.; Su, M.; Wang, J.; Zheng, S.; Zhang, T. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Min, Z.; Li, Z.; Chen, L.; Zhang, Y.; Liu, M.; Yan, X.; Fang, Y. Transcriptome analysis revealed hormone signaling response of grapevine buds to strigolactones. Sci. Hortic.-Amst. 2021, 283, 109936. [Google Scholar] [CrossRef]
- Bruno, M.; Al-Babili, S. On the substrate specificity of the rice strigolactone biosynthesis enzyme DWARF27. Planta 2016, 243, 1429–1440. [Google Scholar] [CrossRef]
- Classic Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wang, W.Z.; Yang, B.P.; Feng, X.Y.; Cao, Z.Y.; Feng, C.L.; Wang, J.G.; Xiong, G.R.; Shen, L.B.; Zeng, J.; Zhao, T.T.; et al. Development and Characterization of Transgenic Sugarcane with Insect Resistance and Herbicide Tolerance. Front. Plant Sci. 2017, 8, 1535. [Google Scholar] [CrossRef]
- Joyce, P.; Hermann, S.; O’Connell, A.; Dinh, Q.; Shumbe, L.; Lakshmanan, P. Field performance of transgenic sugarcane produced using Agrobacterium and biolistics methods. Plant Biotechnol. J. 2013, 12, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.J. A reformulation of Murashige and Skoog medium (WPBS medium) improves embryogenesis, morphogenesis and transformation efficiency in temperate and tropical grasses and cereals. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Wu, Q.; Guo, J.; Xu, L.; Que, Y. Comprehensive Selection of Reference Genes for Gene Expression Normalization in Sugarcane by Real Time Quantitative RT-PCR. PLoS ONE 2014, 9, e97469. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.L.D.O.; Silva, M.D.; Neto, J.R.C.F.; de Nardi, C.H.; Chabregas, S.M.; Burnquist, W.L.; Kahl, G.; Benko-Iseppon, A.M.; Kido, E.A. Validation of Novel Reference Genes for Reverse Transcription Quantitative Real-Time PCR in Drought-Stressed Sugarcane. Sci. World J. 2014, 2014, 357052. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.G.; Rosa-Santos, T.M.; França, S.D.C.; Kottapalli, P.; Kottapalli, K.R.; Zingaretti, S.M. Microtranscriptome analysis of sugarcane cultivars in response to aluminum stress. PLoS ONE 2019, 14, e0217806. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Warthmann, N.; Chen, H.; Ossowski, S.; Weigel, D.; Hervé, P. Highly Specific Gene Silencing by Artificial miRNAs in Rice. PLoS ONE 2008, 3, e1829. [Google Scholar] [CrossRef]
- Diouf, I.; Albert, E.; Duboscq, R.; Santoni, S.; Bitton, F.; Gricourt, J.; Causse, M. Integration of QTL, Transcriptome and Polymorphism Studies Reveals Candidate Genes for Water Stress Response in Tomato. Genes 2020, 11, 900. [Google Scholar] [CrossRef]
- Selim, D.A.-F.H.; Nassar, R.M.A.; Boghdady, M.S.; Bonfill, M. Physiological and anatomical studies of two wheat cultivars irrigated with magnetic water under drought stress conditions. Plant Physiol. Biochem. 2018, 135, 480–488. [Google Scholar] [CrossRef]
- López-Ráez, J.A.; Shirasu, K.; Foo, E. Strigolactones in plant interactions with beneficial and detrimental organisms: The yin and yang. Trends Plant Sci. 2017, 6, 527–537. [Google Scholar] [CrossRef]
- Peccoux, A.; Loveys, B.; Zhu, J.; Gambetta, G.; Delrot, S.; Vivin, P.; Schultz, H.R.; Ollat, N.; Dai, Z. Dissecting the rootstock control of scion transpiration using model-assisted analyses in grapevine. Tree Physiol. 2017, 38, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Comstock, J.P. Hydraulic and chemical signalling in the control of stomatal conductance and transpiration. J. Exp. Bot. 2002, 53, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, L.; Liu, Y.; Yu, Y.; Li, H.; Wang, X.; Pang, Y.; Cao, H.; Sun, Q. Molecular and physiological mechanisms of strigolactones-mediated drought stress in crab apple (Malus hupehensis Rehd.) seedlings. Sci. Hortic. 2023, 311, 111800. [Google Scholar] [CrossRef]
- Christmann, A.; Moes, D.; Himmelbach, A.; Yang, Y.; Tang, Y.; Grill, E. Integration of Abscisic Acid Signalling into Plant Responses. Plant Biol. 2006, 8, 314–325. [Google Scholar] [CrossRef]
- Van Ha, C.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Van Dong, N.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef]
- Liu, J.; Novero, M.; Charnikhova, T.; Ferrandino, A.; Schubert, A.; Ruyter-Spira, C.; Bonfante, P.; Lovisolo, C.; Bouwmeester, H.J.; Cardinale, F. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013, 64, 1967–1981. [Google Scholar] [CrossRef]
- Iqbal, R.; Habib-Ur-Rahman, M.; Raza, M.A.S.; Waqas, M.; Ikram, R.M.; Ahmed, M.Z.; Toleikiene, M.; Ayaz, M.; Mustafa, F.; Ahmad, S.; et al. Assessing the potential of partial root zone drying and mulching for improving the productivity of cotton under arid climate. Environ. Sci. Pollut. Res. 2021, 28, 66223–66241. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.; Franssen, M.C.; Beale, M.H.; Bouwmeester, H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche spp. Are Derived from the Carotenoid Pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta 2015, 241, 1435–1451. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Saab, A.; McErlean, C.S.; Lumba, S.; Savchenko, A.; Stogios, P.J.; McCourt, P. A novel strigolactone receptor antagonist provides insights into the structural inhibition, conditioning, and germination of the crop parasite Striga. J. Biol. Chem. 2022, 298, 101734. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, L.; Long, H.; Su, X.; Wang, Y.; Xing, Q.; Yao, R.; Zhang, M.; Chen, L. Chapter Eighteen—Strigolactone signaling complex formation in yeast: A paradigm for studying hormone-induced receptor interaction with multiple downstream proteins. In Methods in Enzymology; Wurtzel, E.T., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 519–541. [Google Scholar]
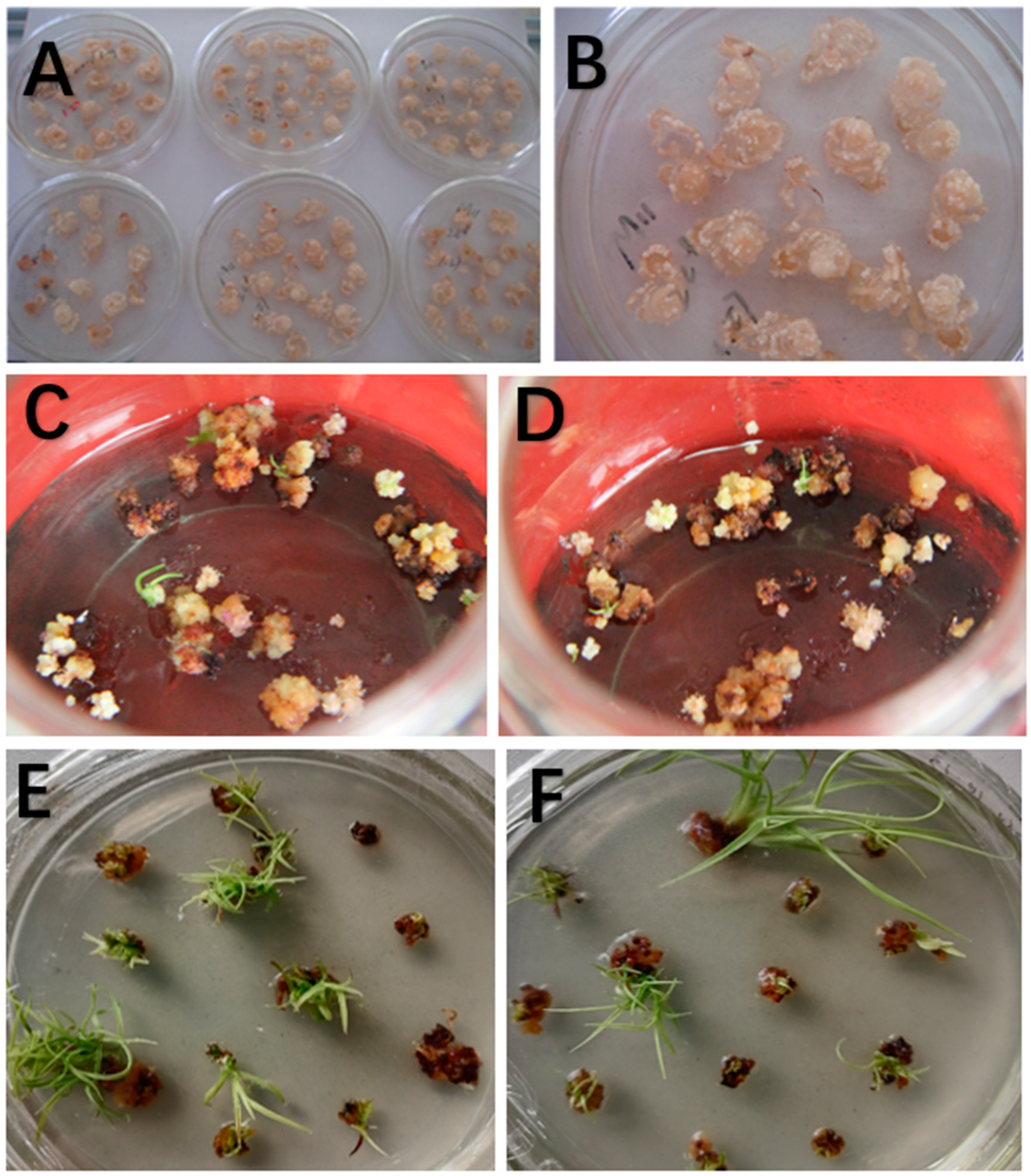
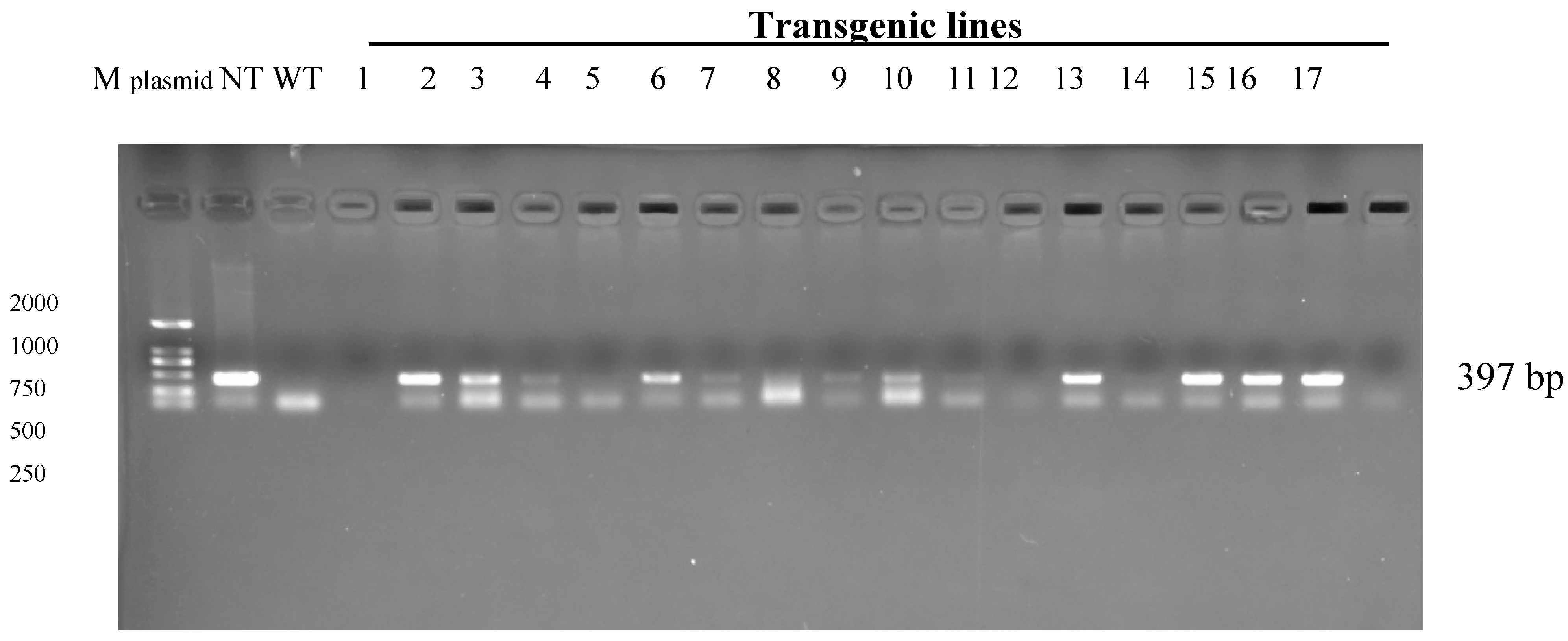
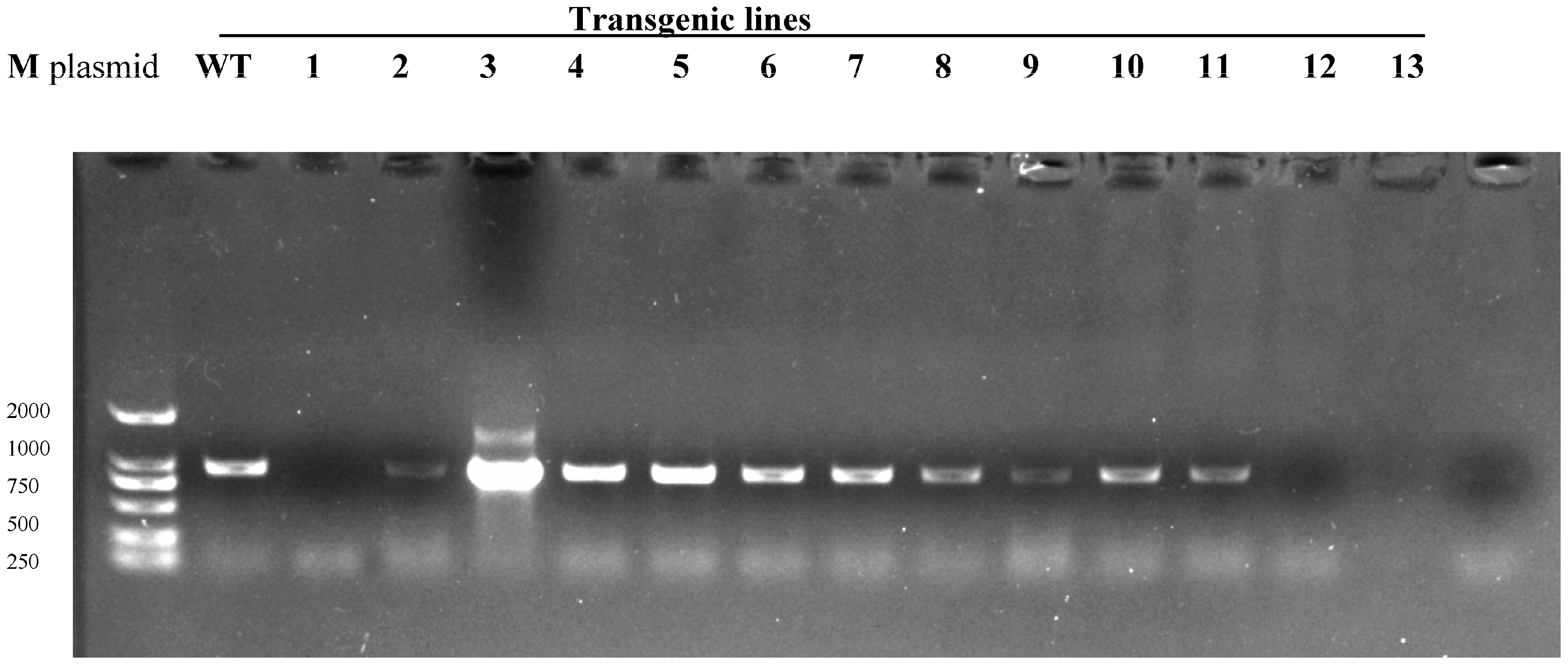

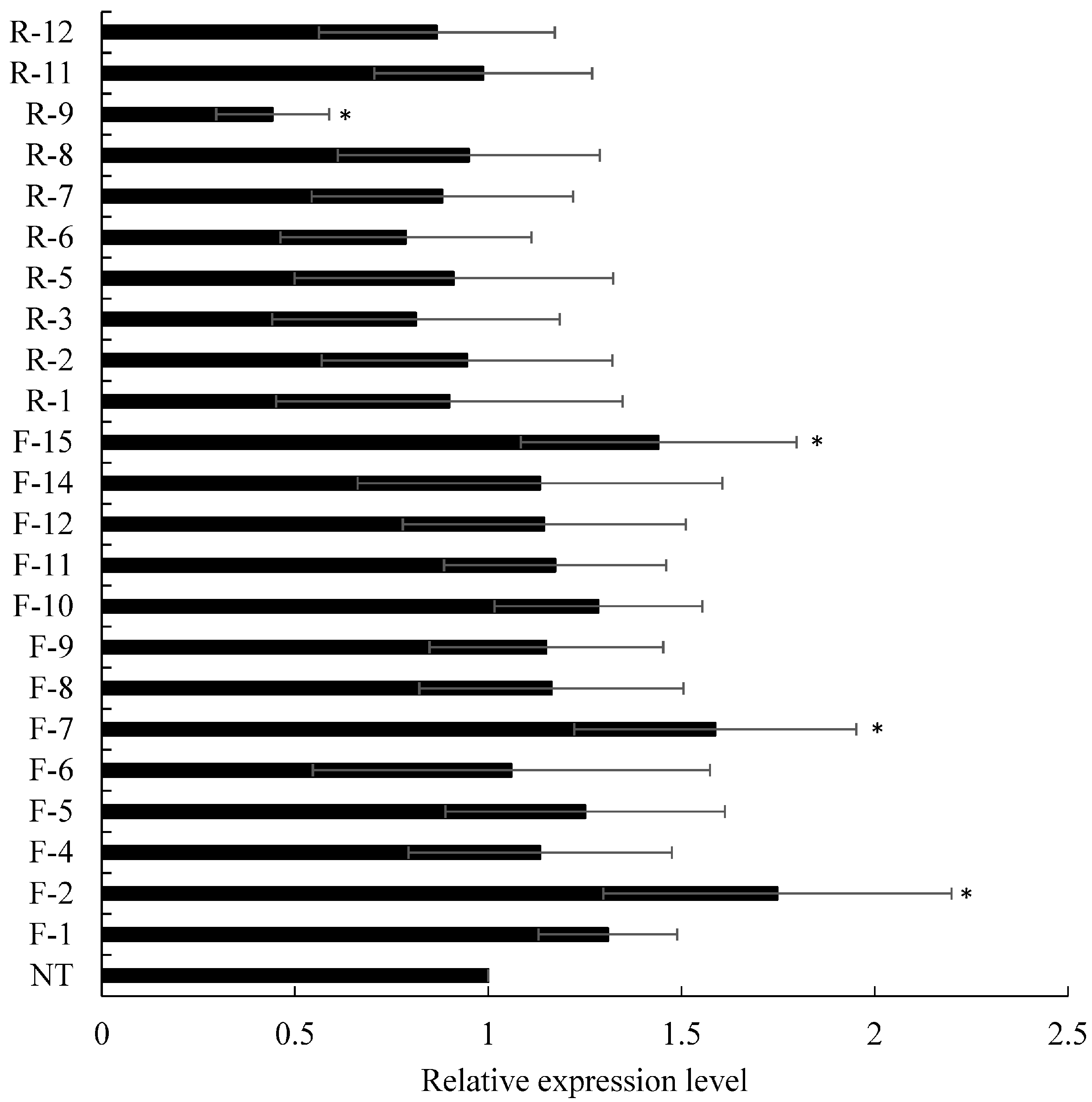


| Bar | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCAMBIA3301-ScD27 sense lines | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| PCAMBIA3301-ScD27 antisense lines | + | + | + | + | + | + | + | + | + | + | + | + | |||
| ScD27.2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| PCAMBIA3301-ScD27 sense lines | + | + | − | + | + | + | + | + | + | + | + | + | − | + | + |
| PCAMBIA3301-ScD27 antisense lines | + | + | + | − | + | + | + | + | + | − | + | + |
| Plant Line | Tillering Number | Plant Height (mm) | Stem Thickness (cm) | Fresh Weight (g) |
|---|---|---|---|---|
| ScD27.2F-2 | 1.33 ± 0.47 a | 47.99 ± 3.69 a | 15.65 ± 0.50 a | 239.06 ± 26.01 a |
| ScD27.2F-3 | 2.1 ± 0.58 b | 51.23 ± 3.56 a | 15.42 ± 0.60 a | 245.36 ± 28.36 a |
| ScD27.2R-5 | 2.5 ± 0.21 b | 48.68 ± 3.25 a | 16.35 ± 0.85 a | 262.53 ± 27.51 a |
| ScD27.2R-7 | 3.0 ± 0.24 b | 45.36 ± 2.36 a | 16.53 ± 0.75 a | 255.59 ± 12.36 a |
| ScD27.2R-9 | 4.67 ± 1.25 c | 18.01 ± 2.06 b | 14.53 ± 0.88 a | 198.08 ± 9.40 b |
| NT | 2.00 ± 0.82 b | 50.98 ± 2.51 a | 15.07 ± 0.89 a | 244.94 ± 6.18 a |
| Plant Line | Tillering Number | Plant Height (cm) | Stem Thickness (cm) | Root Length (cm) | Fresh Weight (g) |
|---|---|---|---|---|---|
| ScD27.2F-2 | 1.20 ± 0.40 a | 10.77 ± 1.35 b | 0.30 ± 0.05 b | 2.24 ± 0.67 b | 4.17 ± 0.53 b |
| ScD27.2R-9 | 5.30 ± 1.10 b | 6.12 ± 0.74 a | 0.25 ± 0.03 a | 1.36 ± 0.25 a | 2.12 ± 0.41 a |
| NT | 1.40 ± 0.49 a | 11.80 ± 0.91 c | 0.31 ± 0.05 b | 2.22 ± 0.20 b | 4.24 ± 0.87 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zan, F.; Wu, Z.; Wang, W.; Hu, X.; Feng, L.; Liu, X.; Liu, J.; Zhao, L.; Wu, C.; Zhang, S.; et al. Strigolactones in Sugarcane Growth and Development. Agronomy 2023, 13, 1086. https://doi.org/10.3390/agronomy13041086
Zan F, Wu Z, Wang W, Hu X, Feng L, Liu X, Liu J, Zhao L, Wu C, Zhang S, et al. Strigolactones in Sugarcane Growth and Development. Agronomy. 2023; 13(4):1086. https://doi.org/10.3390/agronomy13041086
Chicago/Turabian StyleZan, Fenggang, Zhuandi Wu, Wenzhi Wang, Xin Hu, Lu Feng, Xinlong Liu, Jiayong Liu, Liping Zhao, Caiwen Wu, Shuzhen Zhang, and et al. 2023. "Strigolactones in Sugarcane Growth and Development" Agronomy 13, no. 4: 1086. https://doi.org/10.3390/agronomy13041086
APA StyleZan, F., Wu, Z., Wang, W., Hu, X., Feng, L., Liu, X., Liu, J., Zhao, L., Wu, C., Zhang, S., & Guo, J. (2023). Strigolactones in Sugarcane Growth and Development. Agronomy, 13(4), 1086. https://doi.org/10.3390/agronomy13041086




