CT-Based Phenotyping and Genome-Wide Association Analysis of the Internal Structure and Components of Maize Kernels
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Data Acquisition
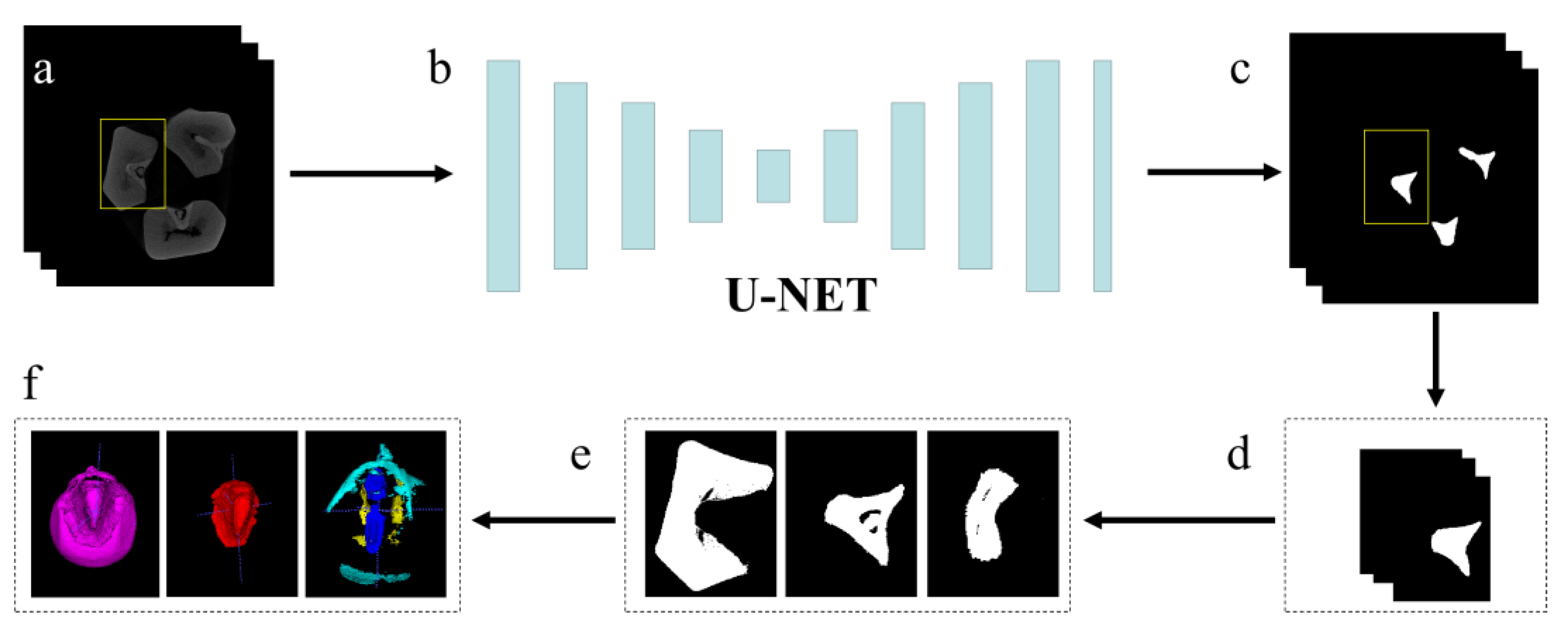
2.2. Kernel Phenotyping Pipeline
2.3. Genome-Wide Association Analysis
2.4. Functional Analysis of Candidate Genes
3. Result
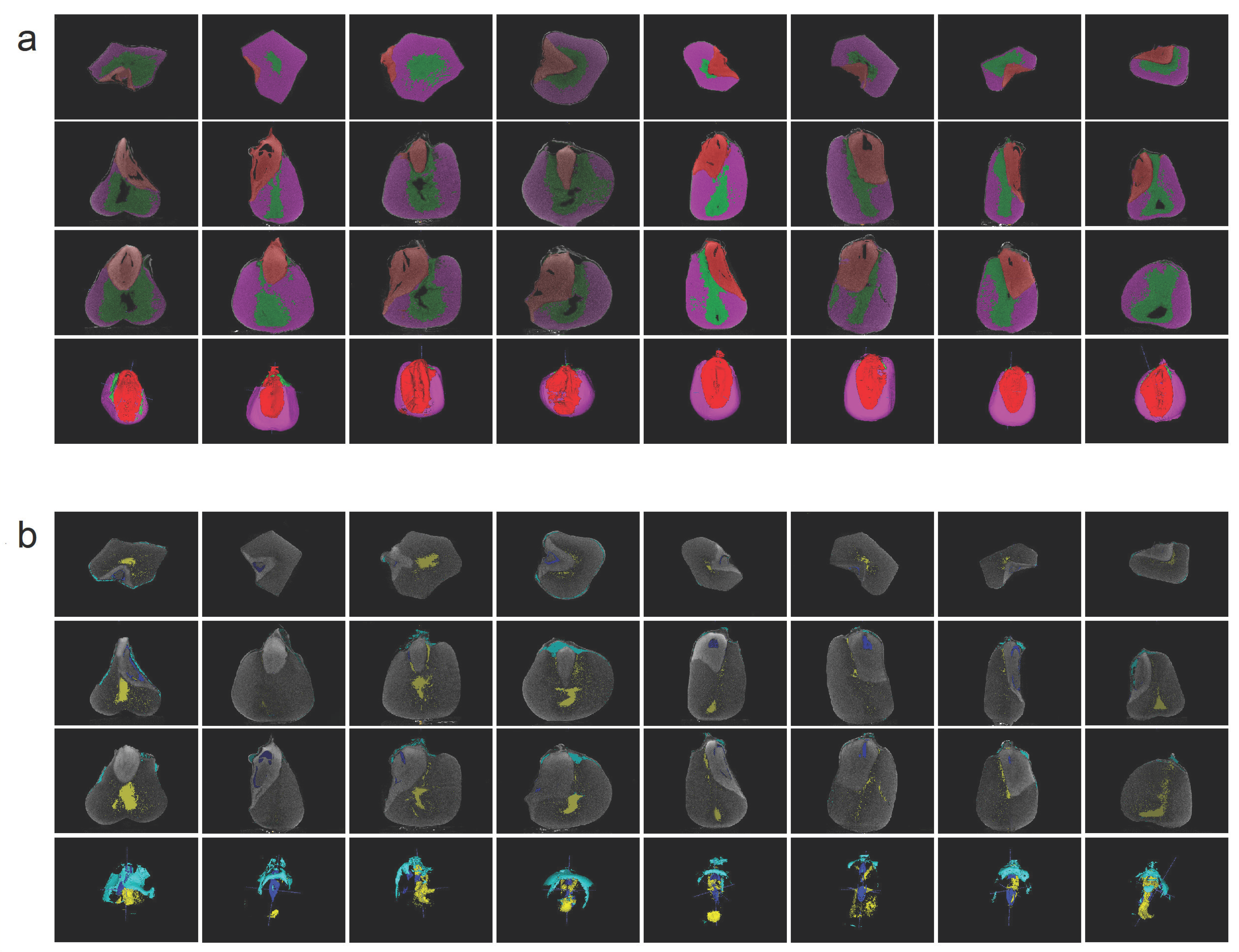
3.1. Phenotypes Accuracy Evaluation
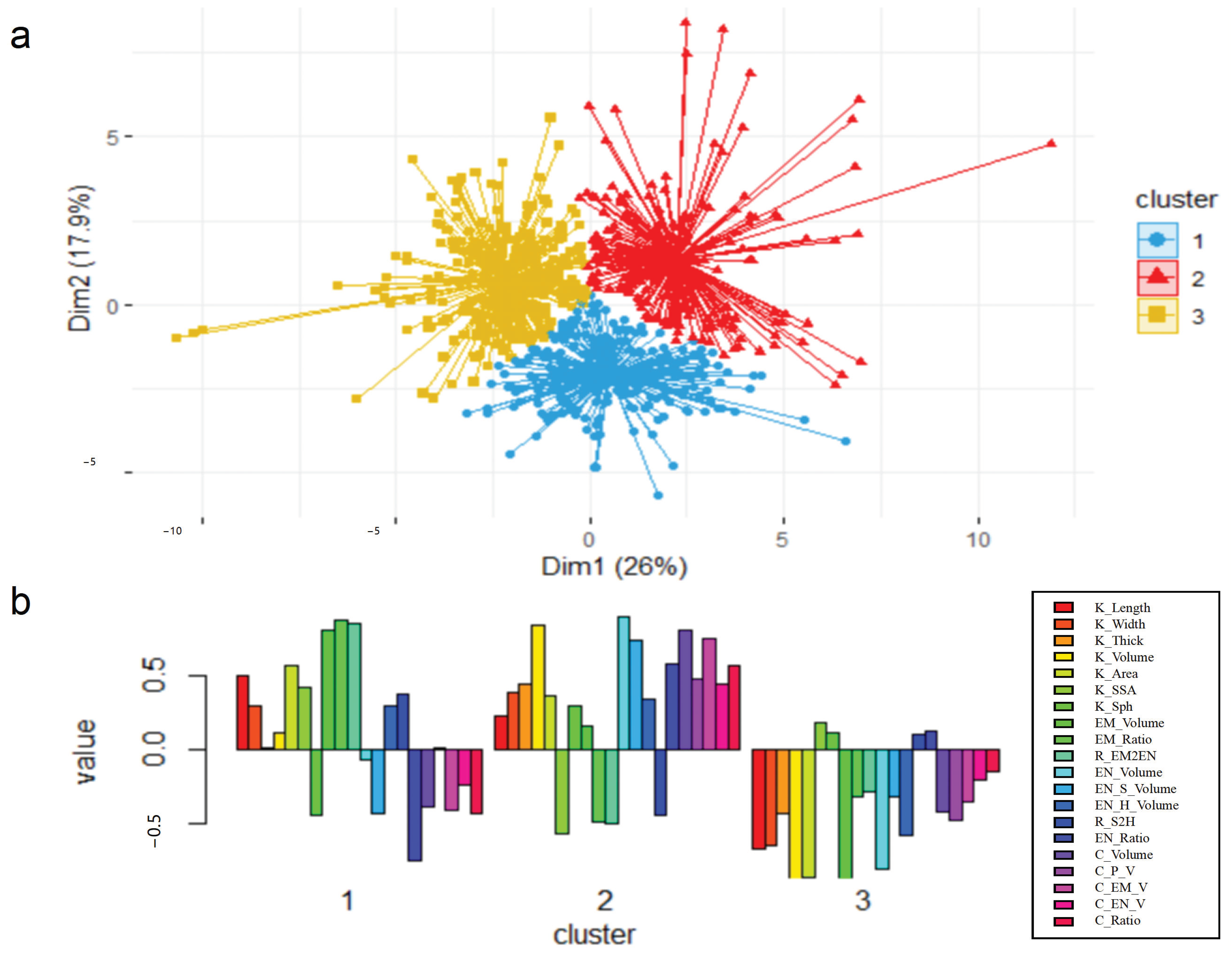
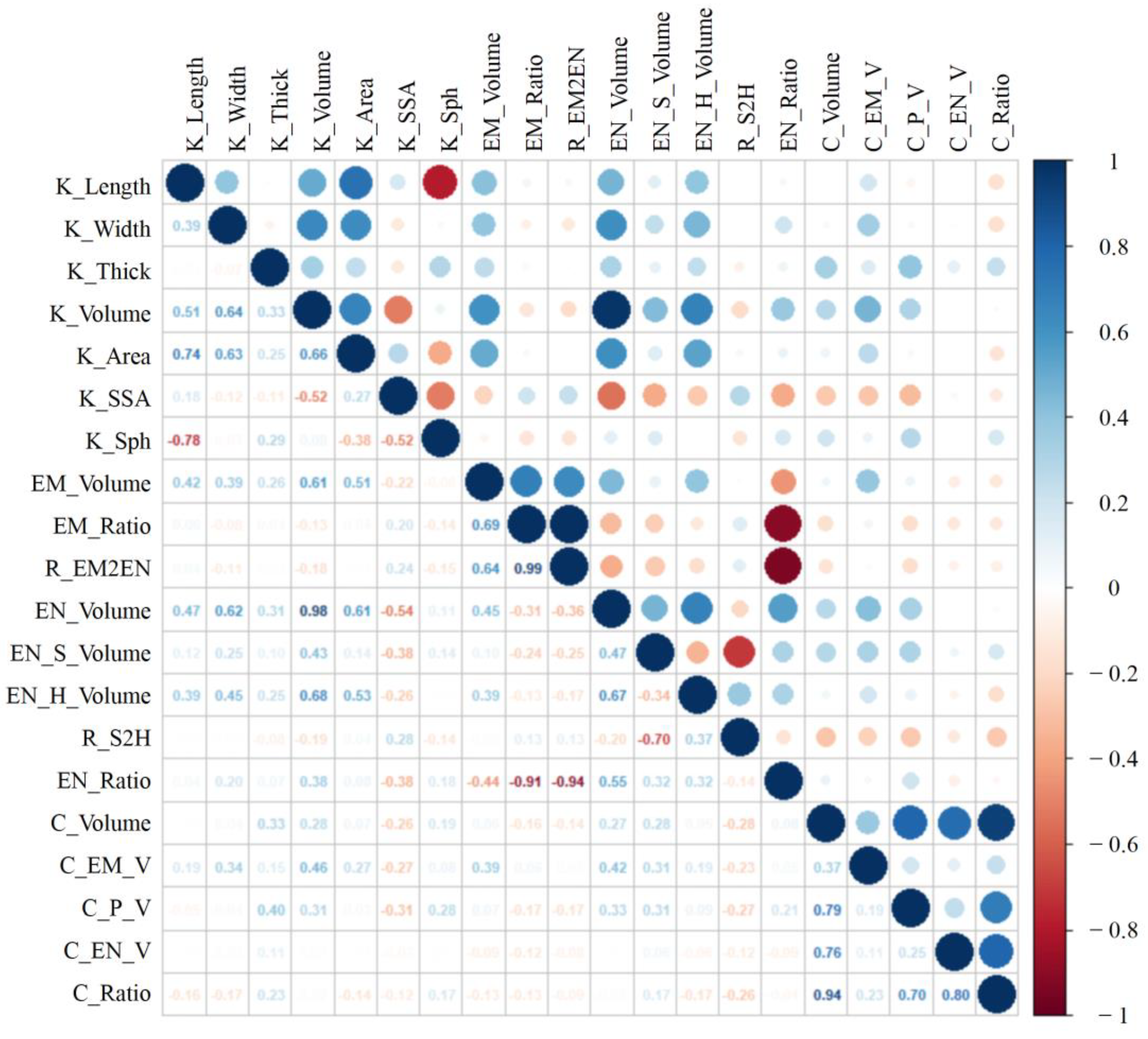
3.2. Statistical Analysis of Kernel Phenotypes
3.3. Significant SNPs and Candidate Genes Identified by GWAS
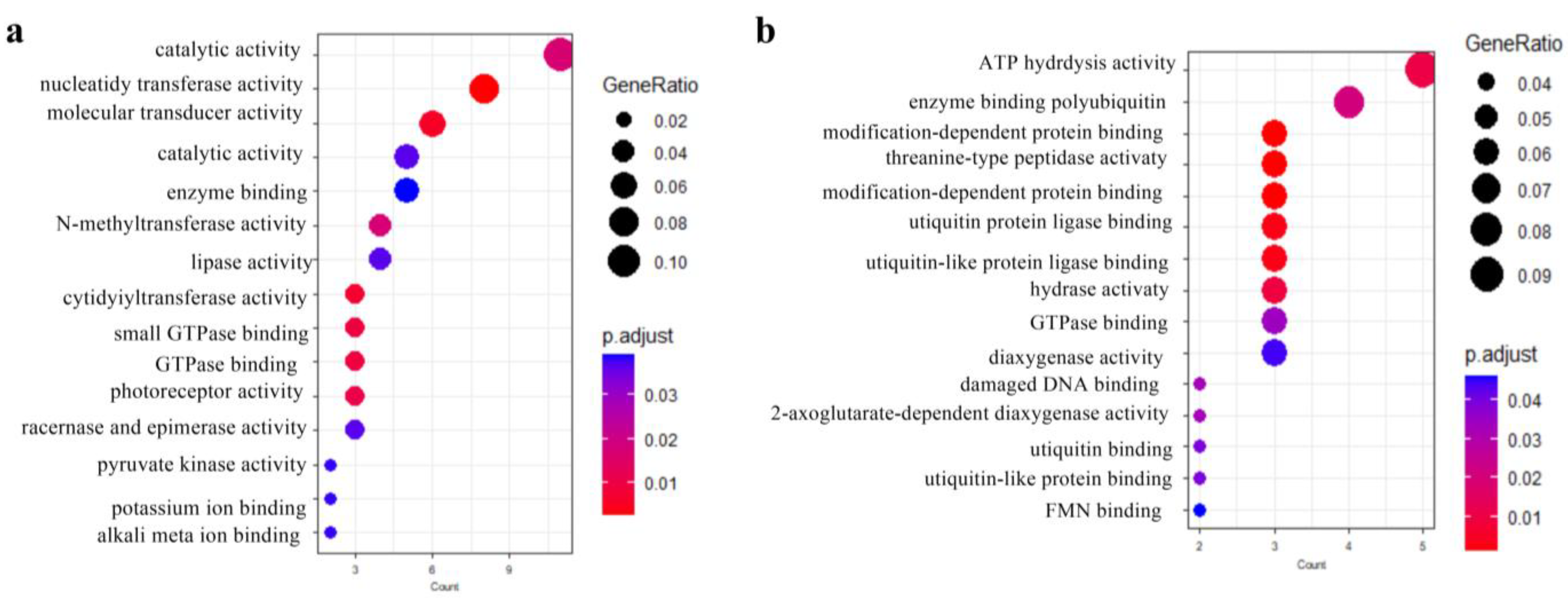
3.4. Functional Enrichment and Network Analysis of Candidate Genes
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.; Wang, J.; Liao, S.; Zhang, Y.; Lu, X.; Guo, X.; Zhao, C. Study on the micro-phenotype of different types of maize kernels based on Micro-CT. Smart Agric. 2021, 3, 16–28. [Google Scholar]
- Rousseau, D.; Widiez, T.; Di Tommaso, S.; Rositi, H.; Adrien, J.; Maire, E.; Langer, M.; Olivier, C.; Peyrin, F.; Rogowsky, P.M. Fast virtual histology using X-ray in-line phase tomography: Application to the 3D anatomy of maize developing seeds. Plant Methods 2015, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Ma, Z.; Song, R. Maize endosperm development. J. Integr. Plant Biol. 2021, 63, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Guelpa, A.; du Plessis, A.; Kidd, M.; Manley, M. Non-destructive Estimation of Maize (Zea mays L.) Kernel Hardness by Means of an X-ray Micro-computed Tomography (μCT) Density Calibration. Food Bioprocess Technol. 2015, 8, 1419–1429. [Google Scholar] [CrossRef]
- Yin, X.; Ming, B.; Hou, J.; Ming, B.; Zhang, Y.; Guo, X.; Gao, S.; Wang, K.; Hou, P.; Li, S. Effects of various grain positions of ear on the internal structural parameters of maize grain using X-ray μCT. Trans. Chin. Soc. Agric. Eng. 2021, 37, 8–14. [Google Scholar]
- Hou, J.; Zhang, Y.; Jin, X.; Dong, P.; Guo, Y.; Wang, K.; Fan, Y.; Li, S. Structural parameters for X-ray micro-computed tomography (μCT) and their relationship with the breakage rate of maize varieties. Plant Methods 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Qu, J.; Feng, W.; Zhang, X.; Xu, S.-T.; Xue, J.-Q. Dissecting the genetic architecture of maize kernel size based on genome-wide association study. Acta Agron. Sin. 2022, 1, 203–214. [Google Scholar]
- Li, X.; Wang, M.; Zhang, R.; Fang, H.; Fu, X.; Yang, X.; Li, J. Genetic architecture of embryo size and related traits in maize. Crop. J. 2021, 10, 204–215. [Google Scholar] [CrossRef]
- Cui, F.; Li, X.; Lu, S.; Zhang, X.; Qi, Y. Genome-wide Association Study of Kernel Colors in Maize. Mol. Plant Breed. 2022, 10, 1–15. [Google Scholar]
- Zhou, H.; Riche, A.B.; Hawkesford, M.J.; Whalley, W.R.; Atkinson, B.S.; Sturrock, C.J. Determination of wheat spike and spikelet architecture and grain traits using X-ray Computed Tomography imaging. Plant Methods 2021, 17, 26. [Google Scholar] [CrossRef]
- Jiang, Z.; Tu, H.; Bai, B.; Yang, C.; Zhao, B.; Guo, Z.; Liu, Q.; Zhao, H.; Yang, W.; Xiong, L.; et al. Combining UAV-RGB high-throughput field phenotyping and genome-wide association study to reveal genetic variation of rice germplasms in dynamic response to drought stress. New Phytol. 2021, 232, 440–455. [Google Scholar] [CrossRef]
- Yang, X.; Gao, S.; Xu, S.; Zhang, Z.; Prasanna, B.M.; Li, L.; Li, J.; Yan, J. Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol. Breed. 2010, 28, 511–526. [Google Scholar] [CrossRef]
- Liu, X.; Huang, M.; Fan, B.; Buckler, E.; Zhang, Z. Iterative Usage of Fixed and Random Effect Models for Powerful and Efficient Genome-Wide Association Studies. PLoS Genet. 2016, 12, e1005767. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. GAPIT Version 3: Boosting Power and Accuracy for Genomic Association and Prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Zhang, T.; Lv, W.; Zhang, H.; Ma, L.; Li, P.; Ge, L.; Li, G. Genome-wide analysis of the basic Helix-Loop-Helix (bHLH) transcription factor family in maize. BMC Plant Biol. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Delseny, M.; Bies-Etheve, N.; Carles, C.; Hull, G.; Vicient, C.M.; Raynal, M.; Grellet, F.; Aspart, L. Late Embryogenesis Abundant (LEA) protein gene regulation during Arabidopsis seed maturation. J. Plant Physiol. 2001, 158, 419–427. [Google Scholar] [CrossRef]
- Guo, M.; Rupe, M.A.; Dieter, J.A.; Zou, J.; Spielbauer, D.; Duncan, K.E.; Howard, R.J.; Hou, Z.; Simmons, C.R. Cell Number Regulator1 Affects Plant and Organ Size in Maize: Implications for Crop Yield Enhancement and Heterosis. Plant Cell 2010, 22, 1057–1073. [Google Scholar] [CrossRef]
- Gallie, D.R.; Young, T.E. The ethylene biosynthetic and perception machinery is differentially expressed during endosperm and embryo development in maize. Mol. Genet. Genom. 2004, 271, 267–281. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, W.; Fu, R.; Zhang, Y. Genome-wide characterization of 2-oxoglutarate and Fe(II)-dependent dioxygenase family genes in tomato during growth cycle and their roles in metabolism. BMC Genom. 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Von Lanken, C.; Yang, J.; Lawrence, C.B.; Hunt, A.G. The yeast polyadenylate-binding protein (PAB1) gene acts as a disease lesion mimic gene when expressed in plants Enhanced Reader. Plant Mol. Biol. 2000, 42, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Yang, Y.; Xu, J.; Li, X.; Leng, Y.; Dai, L.; Huang, L.; Shao, G.; Ren, D.; Hu, J.; et al. EARLY SENESCENCE1 Encodes a SCAR-LIKE PROTEIN2 That Affects Water Loss in Rice. Plant Physiol. 2015, 169, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Q.; Wang, C. Isolation and Functional Characterization of ZmDBP2 Encoding a Dehydration-Responsive Element-Binding Protein in Zea mays. Plant Mol. Biol. Report. 2011, 29, 60–68. [Google Scholar] [CrossRef]
- Tenhaken, R.; Doerks, T.; Bork, P. DCD–a novel plant specific domain in proteins involved in development and programmed cell death. BMC Bioinform. 2005, 6, 169. [Google Scholar] [CrossRef]
- Menninger, J.R.; Walker, C.; Tan, P.F. Studies on the Metabolic Role of Peptidyl-tRNA Hydrolase. Mol. Genet. Genom. 1973, 121, 307–324. [Google Scholar] [CrossRef]
| Type | Traits | Unit | Description |
|---|---|---|---|
| Kernel | K_Length | mm | Kernel length |
| K_Width | mm | Kernel width | |
| K_Thick | mm | Kernel thickness | |
| K_Volume | mm3 | Kernel volume | |
| K_Area | mm2 | Kernel surface area | |
| K_SSA | mm2/mm3 | Specific surface area | |
| K_Sph | / | Kernel sphericity | |
| Embryo | EM_Volume | mm3 | embryo volume |
| EM_Ratio | / | EM_Volume/K_Volume | |
| R_EM2EN | / | EM_Volume/EN_Volume | |
| Endosperm | EN_Volume | mm3 | Endosperm volume |
| EN_S_Volume | mm3 | Silty endosperm volume | |
| EN_H_Volume | mm3 | Horny endosperm volume | |
| R_S2H | / | EN_S_Volume/EN_H_Volume | |
| EN_Ratio | / | EN_Volume/K_Volume | |
| Cavity | C_Volume | mm3 | Cavity volume |
| C_P_V | mm3 | Subcutaneous cavity | |
| C_EM_V | mm3 | Embryo cavity | |
| C_EN_V | mm3 | endosperm cavity | |
| C_Ratio | / | C_Volume / K_Volume |
| Traits | Range | Mean | Standard Deviation | Skewness | Kurtosis | Coefficient of Variation cv/% |
|---|---|---|---|---|---|---|
| K_Length | 5.93–12.16 | 9.78 | 0.98 | −0.08 | 0.11 | 10.02 |
| K_Width | 3.73–10.44 | 8.23 | 0.80 | −0.50 | 2.77 | 9.7 |
| K_Thick | 3.21–7.64 | 5.28 | 0.71 | 0.33 | −0.11 | 13.4 |
| K_Volume | 31.06–319.95 | 200.08 | 41.45 | 0.07 | 0.30 | 20.7 |
| K_Area | 59.07–636.33 | 259.26 | 65.71 | 1.80 | 6.16 | 25.3 |
| K_SSA | 0.97–2.65 | 1.32 | 0.27 | 1.92 | 3.65 | 20.5 |
| K_Sph | 0.21–0.69 | 0.42 | 0.09 | 0.26 | −0.26 | 21.4 |
| EM_Volume | 3.80–67.45 | 25.30 | 7.27 | 1.31 | 4.84 | 28.7 |
| EM_Ratio | 0.09–0.25 | 0.12 | 0.03 | 1.83 | 4.44 | 25.0 |
| R_EM2EN | 0.11–0.37 | 0.17 | 0.05 | 2.03 | 5.23 | 29.4 |
| EN_Volume | 22.05–239.08 | 150.13 | 32.68 | 0.06 | 0.20 | 21.7 |
| EN_S_Volume | 0.76–182.68 | 46.23 | 27.56 | 1.55 | 3.42 | 59.6 |
| EN_H_Volume | 21.29–173.96 | 103.91 | 27.81 | −0.25 | −0.10 | 26.8 |
| R_S2H | 0.17–28.74 | 3.48 | 2.85 | 3.55 | 22.37 | 81.9 |
| EN_Ratio | 0.63–0.83 | 0.75 | 0.03 | −1.02 | 1.70 | 4.0 |
| C_Volume | 0.36–16.12 | 3.52 | 2.34 | 2.01 | 6.56 | 66.5 |
| C_P_V | 0.02–13.49 | 1.95 | 1.78 | 2.36 | 8.38 | 91.3 |
| C_EM_V | 0.05–3.63 | 0.98 | 0.58 | 1.41 | 2.96 | 59.2 |
| C_EN_V | 0.00–11.81 | 1.01 | 1.63 | 3.05 | 12.05 | 163 |
| C_Ratio | 0.002–0.08 | 0.017 | 0.011 | 2.13 | 7.48 | 64.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wang, J.; Zhang, Y.; Lu, X.; Du, J.; Guo, X. CT-Based Phenotyping and Genome-Wide Association Analysis of the Internal Structure and Components of Maize Kernels. Agronomy 2023, 13, 1078. https://doi.org/10.3390/agronomy13041078
Li D, Wang J, Zhang Y, Lu X, Du J, Guo X. CT-Based Phenotyping and Genome-Wide Association Analysis of the Internal Structure and Components of Maize Kernels. Agronomy. 2023; 13(4):1078. https://doi.org/10.3390/agronomy13041078
Chicago/Turabian StyleLi, Dazhuang, Jinglu Wang, Ying Zhang, Xianju Lu, Jianjun Du, and Xinyu Guo. 2023. "CT-Based Phenotyping and Genome-Wide Association Analysis of the Internal Structure and Components of Maize Kernels" Agronomy 13, no. 4: 1078. https://doi.org/10.3390/agronomy13041078
APA StyleLi, D., Wang, J., Zhang, Y., Lu, X., Du, J., & Guo, X. (2023). CT-Based Phenotyping and Genome-Wide Association Analysis of the Internal Structure and Components of Maize Kernels. Agronomy, 13(4), 1078. https://doi.org/10.3390/agronomy13041078






