Impact of PGPR Formulations Combined with Exogenous IBA Levels to Enhance Root Capacity in Poinsettia Cuttings
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Experimental Materials and Propagation Procedure
2.3. Experimental Design, Data Collection, and Analysis
3. Results
3.1. Rooting Capacity of Poinsettia Cuttings
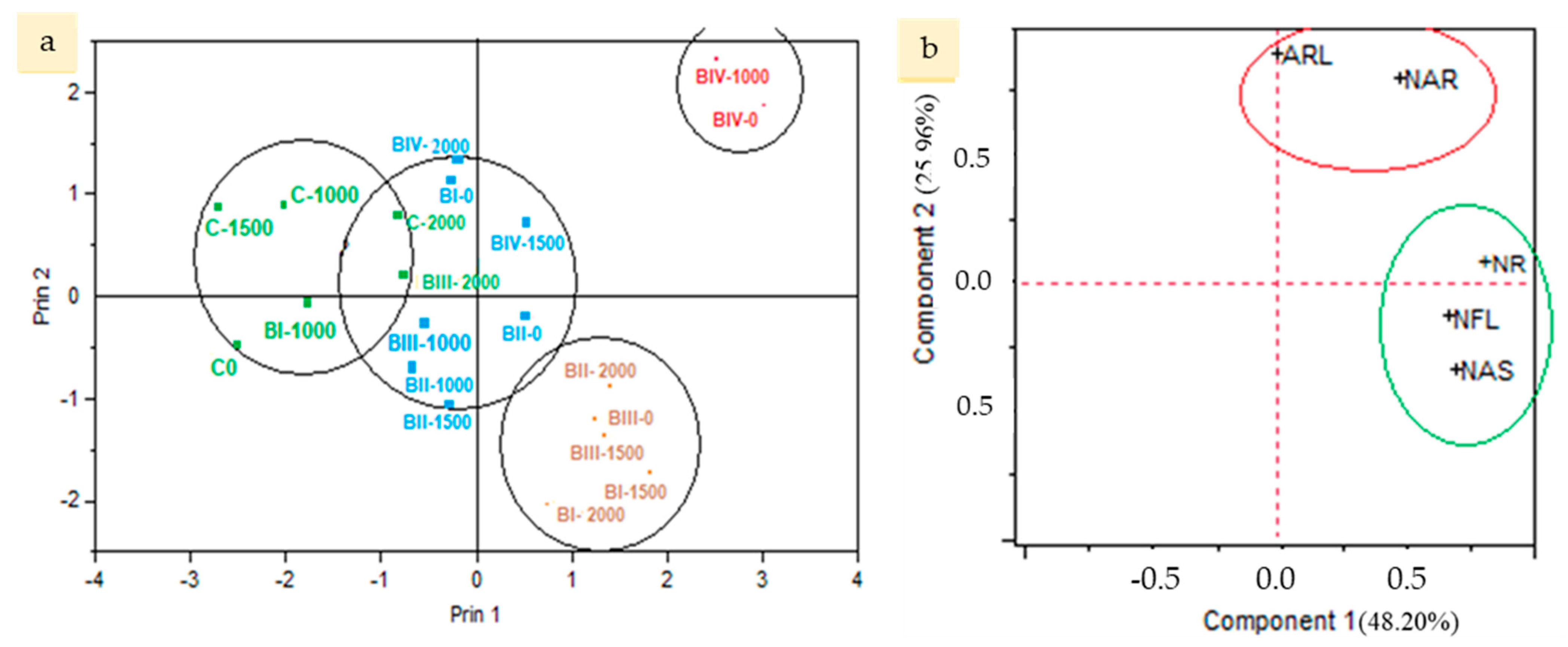
3.2. Principal Component Analysis
4. Discussion
5. Conclusions
- This study provides positive effects on the nutrition of stock mother plants with PGPR to provide sprout production by cuttings technique;
- It has been concluded that the rooting performance of poinsettia cuttings can be improved with lower IBA dose applications if the stock mother plants from which the poinsettia cuttings will be taken are nutrient-enriched with PGPR bacterial formulations and the carbohydrate content is enriched;
- It has been revealed that lower IBA dose applications can be recommended, especially for rooting cuttings taken from stock mother plants treated with the BIV bacterial formulation;
- The rooting properties of poinsettia cuttings treated with BIV bacterial formulation are adversely affected by the application of high doses of IBA;
- Through biplot and heat mapper analyses, it has been determined that BIV-0 and BIV-1000 applications are different from all other applications and C-0 applications in the rooting performance of poinsettia cuttings;
- Further studies can be carried out to determine rooting performance after the poinsettia stock mother plants were fertilized with PGPR formulations prepared with bacterial isolates tested for auxin properties.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Agricultural Statistics 2016; United States Department of Agriculture; NASS (National Agricultural Statistics Service): Washington, DC, USA, 2016.
- Hu, J.; Cai, X.; Jeong, B.R. Silicon affects root development, tissue mineral content, and expression of silicon transporter genes in poinsettia (Euphorbia pulcherrima Willd.) Cultivars. Plants 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, B.B.; Bhatt, D.; Chawla, S.L.; Patel, M.A.; Bennurmath, P. Effect of rooting hormone and media on root induction in poinsettia (Euphorbia pulcherrima Willd.). J. Ornam. Hortic. 2018, 21, 7–12. [Google Scholar] [CrossRef]
- Ramtin, A.; Khalighi, A.; Hadavi, E.; Hekmati, J. Effect of different IBA concentrations and types of cuttings on rooting and flowering Poinsettia pulcherrima L. Int. J. Agric. Sci. 2011, 1, 303–310. [Google Scholar]
- Elgimabi, M.E.N.E. Effect of season of cutting and humidity on propagation of (Ixora coccinea). Adv. Biol. Res. 2008, 2, 108–110. [Google Scholar]
- Osterc, G.; Štefančič, M.; Solar, A.; Štamper, F. Cutting propagation of chestnut (Castanea sp.): The reality or utopia? Allg. Forst-Und Jagdztg. 2009, 180, 89–93. [Google Scholar]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Zerche, S.; Kadner, R. Nitrogen-and storage-affected carbohydrate partitioning in high-light-adapted Pelargonium cuttings in relation to survival and adventitious root formation under low light. Ann. Bot. 2004, 94, 831–842. [Google Scholar] [CrossRef]
- Schwambach, J.; Fadanelli, C.; Fett-Neto, A.G. Mineral nutrition and adventitious rooting in microcuttings of Eucalyptus globulus. Tree Physiol. 2005, 25, 487–494. [Google Scholar] [CrossRef]
- Zerche, S.; Druege, U. Nitrogen content determines adventitious rooting in Euphorbia pulcherrima under adequate light independently of pre-rooting carbohydrate depletion of cuttings. Sci. Hortic. 2009, 121, 340–347. [Google Scholar] [CrossRef]
- Ruedell, C.M.; de Almeida, M.R.; Schwambach, J.; Posenato, C.F.; Fett-Neto, A.G. Pre and post-severance effects of light quality on carbohydrate dynamics and microcutting adventitious rooting of two Eucalyptus species of contrasting recalcitrance. Plant Growth Regul. 2013, 69, 235–245. [Google Scholar] [CrossRef]
- Karakurt, H.; Aslantas, R.; Ozkan, G.; Guleryuz, M. Effects of indol-3-butyric acid (IBA), plant growth promoting rhizobacteria (PGPR) and carbohydrates on rooting of hardwood cutting of MM106 Apple rootstock. Afr. J. Agric. Res. 2009, 4, 60–64. [Google Scholar]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef]
- Husen, A.; Pal, M. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f.(teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New For. 2007, 33, 309–323. [Google Scholar] [CrossRef]
- Abu-Zahra, T.R.; Hasan, M.K.; Hasan, H.S. Effect of different auxin concentration on virginia creeper (Parthenocissus quinquefolia) rooting. World Appl. Sci. J. 2012, 16, 7–10. [Google Scholar]
- Ibrahim, M.E.; Mohamed, M.A.; Khalid, K.A. Effect of plant growth regulators on the rooting of Lemon verbena cutting. J. Mater. Environ. Sci. 2015, 6, 28–33. [Google Scholar]
- Sultana, Z. Rooting Performance of Stem Cuttings of Three Ornamental Plants as Influenced by Growth Regulators. Ph.D. Thesis, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka, Bangladesh, 2006. [Google Scholar]
- Bhairavi, B.M.; Prakasha, D.P.; Kulapathi, H.; Anand, N.; Raddi, G.S.; Gollagi, S.G. Influence of Plant Growth Regulators on Rooting of Stem Cuttings in Jamun (Syzygium cumini L. Skeels). Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2997–3006. [Google Scholar] [CrossRef]
- Nordstrom, A.C.; Jacobs, F.A.; Eliasson, L. Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiol. 1991, 96, 856–861. [Google Scholar] [CrossRef]
- Štefančič, M.; Štampar, F.; Osterc, G. Influence of IAA and IBA on root development and quality of Prunus’ GiSelA 5’leafy cuttings. HortScience 2005, 40, 2052–2055. [Google Scholar] [CrossRef]
- Sochacki, D.; Chmiel, H. Effect of some factors on rooting of poinsettia (Euphorbia pulcherrima Willd.) cuttings. Zesz. Nauk. Inst. Sadow. Kwiaciarstwa 1994, 1, 77–84. [Google Scholar]
- El-Sallami, I.H.; Mahros, O.M. Effect of some growth regulators and branch portion on rootability of cutting, vegetation and flowering of poinsettia. Assiut J. Agric. Sci. 2000, 31, 71–94. [Google Scholar]
- Singh, A.K.; Singh, R. Influence of growth regulating substance on rooting of cutting of poinsettia cv. Flaming Shere. Prog. Hort. 2005, 37, 85–88. [Google Scholar]
- Runkle, E. Propagation poinsettia. Greenh. Prod. News 2007, 17, 42–44. [Google Scholar]
- Raj, M.; Kumar, R.; Lal, K.; Sirisha, L.; Chaudhary, R.; Patel, S.K. Dynamic role of plant growth promoting rhizobacteria (PGPR) in agriculture. Int. J. Chem. Stud. 2020, 8, 105–110. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Dignam, B.E.; O’Callaghan, M.; Condron, L.M.; Raaijmakers, J.M.; Kowalchuk, G.A.; Wakelin, S.A. Challenges and opportunities in harnessing soil disease suppressiveness for sustainable pasture production. Soil Biol. Biochem. 2016, 95, 100–111. [Google Scholar] [CrossRef]
- Zulfitri, A. Plant Growth Promotion by IAA-Producing Rhizobacteria in Ornamental Plant Propagation. Master’s Thesis, University of Sydney, Sydney, Australia, 2012. [Google Scholar]
- Hwang, J.; Benson, D.M. Expression of induced systemic resistance in poinsettia cuttings against Rhizoctonia stem rot by treatment of stock plants with binucleate Rhizoctonia. Biol. Control 2003, 27, 73–80. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. PGPR Amelior. Sustain. Agric. 2019, 129–157. [Google Scholar] [CrossRef]
- Kotan, R.; Sahin, F.; Ala, A. Identification and pathogenicity of bacteria isolated from pome fruits trees in eastern Anatolia region of Turkey. J. Plant Dis. Prot. 2005, 113, 8–13. [Google Scholar]
- Erman, M.; Kotan, R.; Çakmakçı, R.; Çığ, F.; Karagöz, F.; Sezen, M. Effects on growth and yield traits wheat and sugar beet of nitrogen fixer and phosphate solvent bacteria isolated from the Van Lake Basin. Turkey IV. In Proceedings of the Organic Agriculture Symposium, Erzurum, Turkey, 28 June–1 July 2010; pp. 325–329. [Google Scholar]
- Kotan, R.; Çakmakçı, R.; Şahin, F.; Karagöz, K.; Dadaşoğlu, F.; Kantar, F. Biological Control Studies for the Control of Diseases and Pests Using Bacterial Bioagents in Turkey. Turkey IV. In Proceedings of the Organic Agriculture Symposium, Erzurum, Turkey, 28 June–1 July 2010; pp. 726–738. [Google Scholar]
- Daşcı, E.; Evren, S.; Adıgüzel, M.C.; Çakmakçı, R.; Kotan, R.; Kızıloğlu, F.M.; Erat, M. Increasing resistance to water stress in sugar beet (Beta vulgaris L.) using plant growth promoting bacteria. I. In Proceedings of the International Anatolian Sugar Beet Symposium, Kayseri, Turkey, 20–22 September 2012; pp. 203–208. [Google Scholar]
- Karagöz, K.; Ateş, F.; Karagöz, H.; Kotan, R.; Çakmakçı, R. Characterization of plant growth-promoting traits of bacteria isolated from the rhizosphere of grapevine grown in alkaline and acidic soils. Eur. J. Soil Biol. 2012, 50, 144–150. [Google Scholar] [CrossRef]
- Kotan, R.; Çakir, A.; Ozer, H.; Kordali, Ş.; Çakmakci, R.; Dadasoglu, F.; Dikbaş, N.; Aydin, T.; Kazaz, C. Antibacterial effects of Origanum onites against phytopathogenic bacteria: Possible use of the extracts from protection of disease caused by some phytopathogenic bacteria. Sci. Hortic. 2014, 172, 210–220. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Yildirim, E.; Güneş, A.; Karagöz, K.; Kotan, R.; Dursun, A. Plant growth-promoting rhizobacteria improved growth, nutrient, and hormone content of cabbage (Brassica oleracea) seedlings. Turk. J. Agric. For. 2014, 38, 327–333. [Google Scholar] [CrossRef]
- Parlakova Karagöz, F.; Dursun, A.; Tekiner, N.; Kul, R.; Kotan, R. Efficacy of vermicompost and/or plant growth promoting bacteria on the plant growth and development in gladiolus. Ornam. Hortic. 2019, 25, 180–188. [Google Scholar] [CrossRef]
- Kaymak, H.; Aksoy, A.; Kotan, R. Inoculatıon wıth n-2-fıxıng plant growth promotıng rhızobacterıa to reduce nıtrogen fertılızer requırement of lettuce. Acta Sci. Pol. Hortorum Cultus 2020, 19, 23–35. [Google Scholar] [CrossRef]
- Parlakova Karagoz, F.; Dursun, A. Effects of PGPR formulations, chemical fertilizers, and their combinations on physiological traits and quality of bracts of poinsettia. J. Agric. Sci. Technol. 2020, 22, 775–787. [Google Scholar]
- Mertens, D. Plants preparation of laboratory sample. In Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC: Gaithersburg, MD, USA, 2005; pp. 1–2. [Google Scholar]
- Mertens, D. Metal in plants and pet foods. In Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC: Gaithersburg, MD, USA, 2005; pp. 3–4. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material 1. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Souza, C.D.S.; Araújo, P.C.D.; Silva, D.Y.B.D.O.; Nogueira, G.D.A.; Silva, M.J.N. Vegetative rescue of Azadirachta indica by cuttings. Rev. Árvore 2022, 46, e4632. [Google Scholar] [CrossRef]
- Aslantas, R.; Çakmakçi, R.; Sahin, F. Effect of plant growth promoting rhizobacteria on young apples trees growth and fruit yield under orchard conditions. Sci. Hortic. 2007, 111, 371–377. [Google Scholar] [CrossRef]
- Kadner, R.; Druege, U. Role of ethylene action in ethylene production and poststorage leaf senescence and survival of pelargonium cuttings. Plant Growth Regul. 2004, 43, 187–196. [Google Scholar] [CrossRef]
- Druege, U.; Baltruschat, H.; Franken, P. Piriformospora indica promotes adventitious root formation in cuttings. Sci. Hortic. 2007, 112, 422–426. [Google Scholar] [CrossRef]
- Essahibi, A.; Benhiba, L.; Fouad, M.O.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Initial nutritional status and exogenous IBA enhanced the rooting capacity of carob (Ceratonia siliqua L.) cuttings under mist system. J. Mater. Environ. Sci 2016, 7, 4144–4150. [Google Scholar]
- Tsipouridis, C.G.; Thomidis, T. Influence of natural leaf drop and nutritional status of the stock plant on rooting of peach cuttings. Hortic. Sci. 2003, 30, 108. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Vemmos, S.N.; Roussos, P.A. The role of endogenous carbohydrates and seasonal variation in rooting ability of cuttings of an easy and a hard to root olive cultivars (Olea europaea L.). Sci. Hortic. 2012, 143, 19–28. [Google Scholar] [CrossRef]
- Poupin, M.J.; Timmermann, T.; Vega, A.; Zuñiga, A.; González, B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 2013, 8, e69435. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.S.; Maurya, B.R.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Meena, R.S.; Ghosh, B.N. The needs of nutrient use efficiency for sustainable agriculture. J. Clean. Prod. 2015, 102, 562–563. [Google Scholar] [CrossRef]
- Felker, P.; Medina, D.; Soulier, C.; Velicce, G.; Velarde, M.; Gonzalez, C. A survey of environmental and biological factors (Azospirillum spp., Agrobacterium rhizogenes, Pseudomonas aurantiaca) for their influence in rooting cuttings of Prosopis alba clones. J. Arid. Environ. 2005, 61, 227–247. [Google Scholar] [CrossRef]
- Li, Q.; Saleh-Lakha, S.; Glick, B.R. The effect of native and ACC deaminase-containing Azospirillum brasilense Cd1843 on the rooting of carnation cuttings. Can. J. Microbiol. 2005, 51, 511–514. [Google Scholar] [CrossRef]
- Ribaudo, C.M.; Krumpholz, E.M.; Cassán, F.D.; Bottini, R.; Cantore, M.L.; Curá, J.A. Azospirillum sp. promotes root hair development in tomato plants through a mechanism that involves ethylene. J. Plant Growth Regul. 2006, 25, 175–185. [Google Scholar] [CrossRef]
- Kaymak, H.C.; Yarali, F.; Guvenc, I.; Donmez, M.F. The effect of inoculation with plant growth rhizobacteria (PGPR) on root formation of mint (Mentha piperita L.) cuttings. Afr. J. Biotechnol. 2008, 7, 4479–4483. [Google Scholar]
- Erturk, Y.; Ercisli, S.; Haznedar, A.; Cakmakci, R. Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol. Res. 2010, 43, 91–98. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.S.A.; El-Dsouky, M. Effect of indole-3-butyric acid (IBA) and Bacillus subtilis on rooting of Bougainvillea glabra var. sanderiana cuttings. In Proceedings of the 2010 5th Scientific Conference for Agricultural Sciences, Asyut, Egypt, 16–17 October 2010; pp. 16–17. [Google Scholar]
- Kaymak, H.C.; Irmak, M.A.; Aksoy, A.; Tekiner, N. Auxın-producıng plant growth-promotıng rhızobacterıa promote root formatıon of Epıpremnum aureum cuttıngs. JAPS J. Anim. Plant Sci. 2021, 31, 1338–1344. [Google Scholar]
- Rajan, S.A.; Radhakrishna, D. Effect of entophytic bacteria on the rooting and establishment of cuttings of hibiscus (Rosa sinensis). J. Agric. Vet. Sci. 2013, 3, 17–21. [Google Scholar]
- dos Reis Ferreıra, G.M.; de Oliveira Sılva, L.F.; Pasqual, M.; Mellonı, R.; Luz, J.M.Q.; Soares, J.D.R. Mıcrobıal bıostımulants as alternatıves for the rootıng of olıve tree cuttıngs. Biosci. J. 2022, 38, 1981–3163. [Google Scholar]
- Lyu, D.; Backer, R.; Smith, D. Plant growth-promoting rhizobacteria (PGPR) as plant biostimulants in agriculture. In Biostimulants for Sustainable Crop Production; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 197–226. [Google Scholar]
- Krisantini, S.; Johnston, M.; Williams, R.R.; Beveridge, C. Adventitious root formation in Grevillea (Proteaceae), an Australian native species. Sci. Hortic. 2006, 107, 171–175. [Google Scholar] [CrossRef]
- Bortoloso Pigatto, G.; Nunes Gomes, E.; De Cássia Tomasi, J.; Portes Ferriani, A.; Deschamps, C. Effects of indolebutyric acid, stem cutting positions and substrates on the vegetative propagation of Stevia rebaudiana Bertoni. Rev. Colomb. Cienc. Hortícolas 2018, 12, 202–211. [Google Scholar] [CrossRef]
- Blazich, F.A. Mineral nutrition and adventitious rooting. In Adventitious Root Formation in Cuttings; Davis, T.D., Haissig, B.E., Sankhla, N., Eds.; Adventitious Root Formation in Cuttings; Dioscorides Press: Portland, OR, USA, 1988; pp. 61–69. [Google Scholar]
- Bellamine, J.; Penel, C.; Greppin, H.; Gaspar, T. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul. 1998, 26, 191–194. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Rapaka, V.K.; Bessler, B.; Schreiner, M.; Druege, U. Inter- play between initial carbohydrate availability, current photosynthesis, and adventitious root formation in Pelargonium cuttings. Plant Sci. 2005, 168, 1547–1560. [Google Scholar] [CrossRef]
- Otiende, M.A.; Maimba, F.M.; Shafique, M.; Rehman, K.U.; Rehmani, M. Endogenous carbohydrate content of the cutting positions at time of severance and IBA concentration influence rooting of Rosa hybrida rootstocks. J. Environ. Agric. Sci 2020, 22, 1–9. [Google Scholar]
- Hartman, H.T.; Kester, D.E.; Davies, J.F.T.; Geneve, R.L. Plant Propagation: Principles and Practices, 8th ed.; Prentice-Hall: Boston, MA, USA, 2011; 915p. [Google Scholar]
- Lyu, D.; Backer, R.; Smith, D.L. Three plant growth-promoting rhizobacteria alter morphological development, physiology, and flower yield of Cannabis sativa L. Ind. Crops Prod. 2022, 178, 114583. [Google Scholar] [CrossRef]
- Chandramouli, H. Influence of Growth Regulators on the Rooting of Different Types of Cuttings in Bursera penicillata (DC). Master’s Thesis, University of Agricultural Sciences, Bangalore, India, 2001. [Google Scholar]
- Gaudin, V.; Vrain, T.; Jouanin, L. Bacterial genes modifying hormonal balances in plants. Plant Physiol. Biochem. 1994, 32, 11–28. [Google Scholar]
- Bredmose, N.; Kristiansen, K.; Nielsen, W. Propagation temperature, PPFD, auxin treatment, cutting size and cutting position affect root formation, axillary bud growth and shoot development in miniature rose (Rosa hybrida L.) plants and alter homogeneity. J. Hortic. Sci. Biotech. 2004, 79, 458–465. [Google Scholar] [CrossRef]
- Pacholczak, A.; Szydlo, W.; Lukaszewska, A. The effectiveness of foliar auxin application to stock plants in rooting of stem cuttings of ornamental shrubs. Propag. Ornam. Plants 2005, 5, 100–106. [Google Scholar]
- Raasch, L.D.; Bonaldo, S.M.; De Oliveira, A.A.F. Bacillus subtilis: Rooting and growth of eucalyptus mini-cuttings in the municipality of Sinop, northern of Mato Grosso state, Brazil. Biosci. J. 2013, 29, 1446–1457. [Google Scholar]
- Sezen, I.; Kaymak, H.C.; Aytatlı, B.; Dönmez, M.F.; Ercişli, S. Inoculations with plant growth promoting rhizobacteria (PGPR) stimulate adventitious root formation on semi-hardwood stem cuttings of Ficus benjamina L. Propag. Ornam. Plants 2014, 14, 152–157. [Google Scholar]
- Fracchia, F.; Mangeot-Peter, L.; Jacquot, L.; Martin, F.; Veneault-Fourrey, C.; Deveau, A. Colonization of naive roots from Populus tremula × alba involves successive waves of fungi and bacteria with different trophic abilities. Appl. Environ. Microbiol. 2021, 87, e02541-20. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Plant growth promoting rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Kalam, S.; Basu, A.; Podile, A.R. Functional and molecular characterization of plant growth promoting Bacillus isolates from tomato rhizosphere. Heliyon 2020, 6, e04734. [Google Scholar] [CrossRef] [PubMed]
- Çakmakçi, R.; Dönmez, F.; Aydın, A.; Şahin, F. Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol. Biochem. 2006, 38, 1482–1487. [Google Scholar] [CrossRef]


| Isolate No | MIS Diagnosis Result | SIM | Location (in Turkey) | Host | Nitrogen | Phosphate |
|---|---|---|---|---|---|---|
| RK-79 | Pantoea agglomerans | 0.762 | Erzurum | Apple | + | + |
| TV-12E | Paenibacillus polymyxa | 0.551 | Van | Poaceae | S+ | + |
| TV-17C | Bacillus subtilis | 0.677 | Van | Raspberry | S | W+ |
| TV-6D | Bacillus megaterium | 0.750 | Van | Poaceae | + | + |
| TV-42A * | Pseudomonas putida | 0.113 | Van | Poaceae | W+ | W+ |
| TV-91C | Bacillus megaterium | 0.474 | Van | Poaceae | + | W+ |
| TV-113C | Kluyvera cryocrescens | 0.688 | Van | Garlic | + | + |
| RK-92 | Pantoea agglomerans | 0.889 | Erzurum | Pear | + | S |
| Treatments | Bacteria Formulations (Used to Provide Plant Nutrition to Stock Mother Plants) | Contents of Treatments (Applied in Cutting Rooting) |
|---|---|---|
| Control-0 | No bacterial formulation was applied to these application groups. | Control (No bacteria + IBA application) |
| Control-1000 | 0 mg L−1 + IBA 1000 mg L−1 | |
| Control-1500 | 0 mg L−1 + IBA 1500 mg L−1 | |
| Control-2000 | 0 mg L−1 + IBA 2000 mg L−1 | |
| BI + 0 | BI: Bacteria formulation 1 (Paenibacillus polymyxa TV-12E + Pseudomonas putida TV-42A + Pantoea agglomerans RK-79) | Bacteria formulation 1 (No IBA application) |
| BI + 1000 | Bacteria formulation 1 + IBA 1000 mg L−1 | |
| BI + 1500 | Bacteria formulation 1 + IBA 1500 mg L−1 | |
| BI + 2000 | Bacteria formulation 1 + IBA 2000 mg L−1 | |
| BII + 0 | BII: Bacteria formulation 2 (Bacillus megaterium TV-91C + Pantoea agglomerans RK-92 + Bacillus subtilis TV-17C) | Bacteria formulation 2 (No IBA application) |
| BII + 1000 | Bacteria formulation 2 + IBA 1000 mg L−1 | |
| BII + 1500 | Bacteria formulation 2 + IBA 1500 mg L−1 | |
| BII + 2000 | Bacteria formulation 2 + IBA 2000 mg L−1 | |
| BIII + 0 | BIII: Bacteria formulation 3 (Bacillus megaterium TV-91C + Pantoea agglomerans RK-92 + Kluyvera cryocrescens TV-113C) | Bacteria formulation 3 (No IBA application) |
| BIII + 1000 | Bacteria formulation 3 + IBA 1000 mg L−1 | |
| BIII + 1500 | Bacteria formulation 3 + IBA 1500 mg L−1 | |
| BIII + 2000 | Bacteria formulation 3 +IBA 2000 mg L−1 | |
| BIV + 0 | BIV: Bacteria formulation 4 (Bacillus megaterium TV-91C + Pantoea agglomerans RK-79 + Bacillus megaterium TV-6D) | Bacteria formulation 4 (No IBA application) |
| BIV + 1000 | Bacteria formulation 4 + IBA 1000 mg L−1 | |
| BIV + 1500 | Bacteria formulation 4 + IBA 1500 mg L−1 | |
| BIV + 2000 | Bacteria formulation 4 + IBA 2000 mg L−1 |
| Source of Variance | Number of Rooted Cuttings (NR) | Number of Mean Roots (NAR) | Mean Root Length (ARL) (mm) | Number of Newly Formed Leaves (NFL) | Number of Mean Shoots (NAS) |
|---|---|---|---|---|---|
| Bacteria Formulation Treatments (A) | 24.31 *** | 3.36 *** | 111.05 *** | 2.55 *** | 0.48 *** |
| IBA doses (B) | 1.17 ns | 0.54 *** | 15.15 *** | 1.90 *** | 0.13 * |
| A × B | 5.74 *** | 0.93 *** | 28.23 *** | 2.78 *** | 0.32 *** |
| Bacteria Formulations | Control | BI | BII | BIII | BIV | Mean | |
|---|---|---|---|---|---|---|---|
| Mean ± SD x | |||||||
| Number of rooted cuttings (NR) | |||||||
| IBA Doses | 0 | 3.33 ± 0.58 b ** | 7.00 ± 1.00 a ** | 8.00 ± 1.00 ns | 8.33 ± 0.58 a * | 9.67 ± 0.58 a ** | 7.27 ± 2.31 NS |
| 1000 | 4.67 ± 0.58 b | 4.33 ± 0.58 b | 7.67 ± 1.53 | 8.00 ± 1.00 ab | 9.33 ± 0.58 a | 6.80 ± 2.18 | |
| 1500 | 4.67 ± 0.58 b | 8.00 ± 1.00 a | 7.00 ± 1.00 | 9.00 ± 1.00 a | 7.33 ± 0.58 b | 7.20 ± 1.66 | |
| 2000 | 6.67 ± 1.15 a | 8.33 ± 0.58 a | 8.33 ± 0.58 | 6.67 ± 0.58 b | 7.33 ± 1.15 b | 7.47 ± 1.06 | |
| Mean | 4.83 ± 1.40 C *** | 6.92 ± 1.78 B | 7.75 ± 1.06 A | 8.00 ± 1.13 A | 8.42 ± 1.31 A | ||
| Number of mean roots (NAR) | |||||||
| IBA Doses | 0 | 0.36 ± 0.16 c *** | 1.66 ± 0.12 a *** | 0.59 ± 0.09 c ** | 0.90 ± 0.15 bc ** | 2.45 ± 0.30 a *** | 1.19 ± 0.81 A *** |
| 1000 | 0.96 ± 0.09 b | 0.26 ± 0.08 c | 0.87 ± 0.04 b | 0.97 ± 0.08 b | 2.88 ± 0.12 a | 1.19 ± 0.92 A | |
| 1500 | 0.92 ± 0.05 b | 0.50 ± 0.17 b | 0.82 ± 0.09 b | 0.74 ± 0.13 c | 0.97 ± 0.10 c | 0.79 ± 0.20 B | |
| 2000 | 1.26 ± 0.08 a | 0.18 ± 0.06 c | 1.08 ± 0.09 a | 1.20 ± 0.03 a | 1.64 ± 0.50 b | 1.07 ± 0.54 A | |
| Mean | 0.88 ± 0.35 B *** | 0.65 ± 0.63 C | 0.84 ± 0.20 B | 0.95 ± 0.20 B | 1.99 ± 0.81 A | ||
| Mean root length (ARL) (mm) | |||||||
| IBA Doses | 0 | 10.66 ± 0.57 c *** | 16.33 ± 1.53 a *** | 14.67 ± 0.58 a *** | 10.67 ± 0.58 b *** | 18.67 ± 1.53 ns | 14.20 ± 3.39 A *** |
| 1000 | 17.67 ± 0.58 a | 17.00 ± 1.00 a | 9.33 ± 0.58 b | 10.33 ± 0.58 b | 18.00 ± 1.00 | 14.47 ± 4.00 A | |
| 1500 | 15.67 ± 0.58 b | 11.00 ± 1.00 b | 7.67 ± 0.58 c | 9.00 ± 1.00 c | 18.00 ± 2.65 | 12.27 ± 4.25 C | |
| 2000 | 16.33 ± 0.58 b | 8.00 ± 1.00 c | 10.33 ± 0.58 b | 14.33 ± 0.58 a | 17.00 ± 1.00 | 13.20 ± 3.67 B | |
| Mean | 15.08 ± 2.81 B *** | 13.08 ± 4.03 C | 10.50 ± 2.75 D | 11.08 ± 2.15 D | 17.92 ± 1.56 A | ||
| Bacteria Formulations | Control | BI | BII | BIII | BIV | Mean | |
|---|---|---|---|---|---|---|---|
| Mean ± SD x | |||||||
| Number of newly formed leaves (NFL) | |||||||
| IBA Doses | 0 | 0.33 ± 0.58 b * | 0.33 ± 0.58 c *** | 2.33 ± 0.58 a ** | 1.00 ± 0.00 b ** | 2.67 ± 0.58 a ** | 1.33 ± 1.11 B *** |
| 1000 | 1.00 ± 0.00 ab | 2.00 ± 0.00 b | 0.00 ± 0.00 c | 0.33 ± 0.58 c | 2.00 ± 0.00 a | 1.07 ± 0.88 B | |
| 1500 | 0.33 ± 0.58 b | 3.00 ± 0.00 a | 1.33 ± 0.58 b | 1.83 ± 0.29 a | 2.67 ± 0.58 a | 1.83 ± 1.06 A | |
| 2000 | 1.67 ± 0.58 a | 2.00 ± 0.00 b | 2.33 ± 0.58 a | 2.00 ± 0.00 a | 0.67 ± 0.58 b | 1.73 ± 0.70 A | |
| Mean | 0.83 ± 0.72 D *** | 1.83 ± 1.03 AB | 1.50 ± 1.09 BC | 1.29 ± 0.75 C | 2.00 ± 0.95 A | ||
| Number of mean shoots (NAS) | |||||||
| IBA Doses | 0 | 0.19 ± 0.02 b * | 0.70 ± 0.06 b ** | 0.50 ± 0.50 ns | 1.25 ± 0.08 a *** | 0.92 ± 0.09 a ** | 0.71 ± 0.42 A * |
| 1000 | 0.57 ± 0.24 a | 0.74 ± 0.08 b | 0.42 ± 0.30 | 0.20 ± 0.06 d | 0.78 ± 0.25 ab | 0.54 ± 0.28 B | |
| 1500 | 0.18 ± 0.01 b | 1.35 ± 0.34 a | 0.61 ± 0.09 | 0.76 ± 0.08 b | 0.60 ± 0.10 b | 0.70 ± 0.42 A | |
| 2000 | 0.39 ± 0.10 ab | 0.75 ± 0.08 b | 0.88 ± 0.08 | 0.58 ± 0.09 c | 0.16 ± 0.01 c | 0.55 ± 0.27 B | |
| Mean | 0.33 ± 0.20 C *** | 0.89 ± 0.32 A | 0.60 ± 0.31 B | 0.70 ± 0.40 B | 0.61 ± 0.32 B | ||
| Bacteria Formulation Treatments | Initial Nutrient Contents of Bract Leaf | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | mg kg−1 | ||||||||
| P | K | Ca | Mg | Fe | Mn | Zn | Cu | ||
| Control | 3.16 c *** | 2958.40 c *** | 23,406.65 c *** | 7900.27 b *** | 5044.40 ns | 205.96 b ** | 41.21 b * | 48.18 a *** | 12.05 ns |
| BI | 4.76 b | 4270.97 b | 27,176.26 ab | 10,797.56 a | 4720.05 | 233.52 a | 44.55 a | 33.99 c | 11.78 |
| BII | 4.77 b | 4337.38 b | 28,493.00 a | 10,833.58 a | 4592.27 | 234.24 a | 41.55 b | 36.83 c | 12.82 |
| BIII | 4.81 b | 4544.35 a | 28,726.76 a | 10,653.20 a | 4528.03 | 225.96 a | 44.60 a | 37.45 c | 11.62 |
| BIV | 5.73 a | 4639.67 a | 25,946.64 b | 11,155.99 a | 4798.28 | 233.57 a | 43.60 ab | 42.81 b | 12.07 |
| F | 54.157 | 208.788 | 19.190 | 27.279 | 1.963 | 6.255 | 4.756 | 21.737 | 1.076 |
| Sig. | 0.000 | 0.000 | 0.000 | 0.000 | 0.176 | 0.009 | 0.021 | 0.000 | 0.418 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parlakova Karagöz, F. Impact of PGPR Formulations Combined with Exogenous IBA Levels to Enhance Root Capacity in Poinsettia Cuttings. Agronomy 2023, 13, 878. https://doi.org/10.3390/agronomy13030878
Parlakova Karagöz F. Impact of PGPR Formulations Combined with Exogenous IBA Levels to Enhance Root Capacity in Poinsettia Cuttings. Agronomy. 2023; 13(3):878. https://doi.org/10.3390/agronomy13030878
Chicago/Turabian StyleParlakova Karagöz, Fazilet. 2023. "Impact of PGPR Formulations Combined with Exogenous IBA Levels to Enhance Root Capacity in Poinsettia Cuttings" Agronomy 13, no. 3: 878. https://doi.org/10.3390/agronomy13030878
APA StyleParlakova Karagöz, F. (2023). Impact of PGPR Formulations Combined with Exogenous IBA Levels to Enhance Root Capacity in Poinsettia Cuttings. Agronomy, 13(3), 878. https://doi.org/10.3390/agronomy13030878







