Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Growing Conditions and Treatments
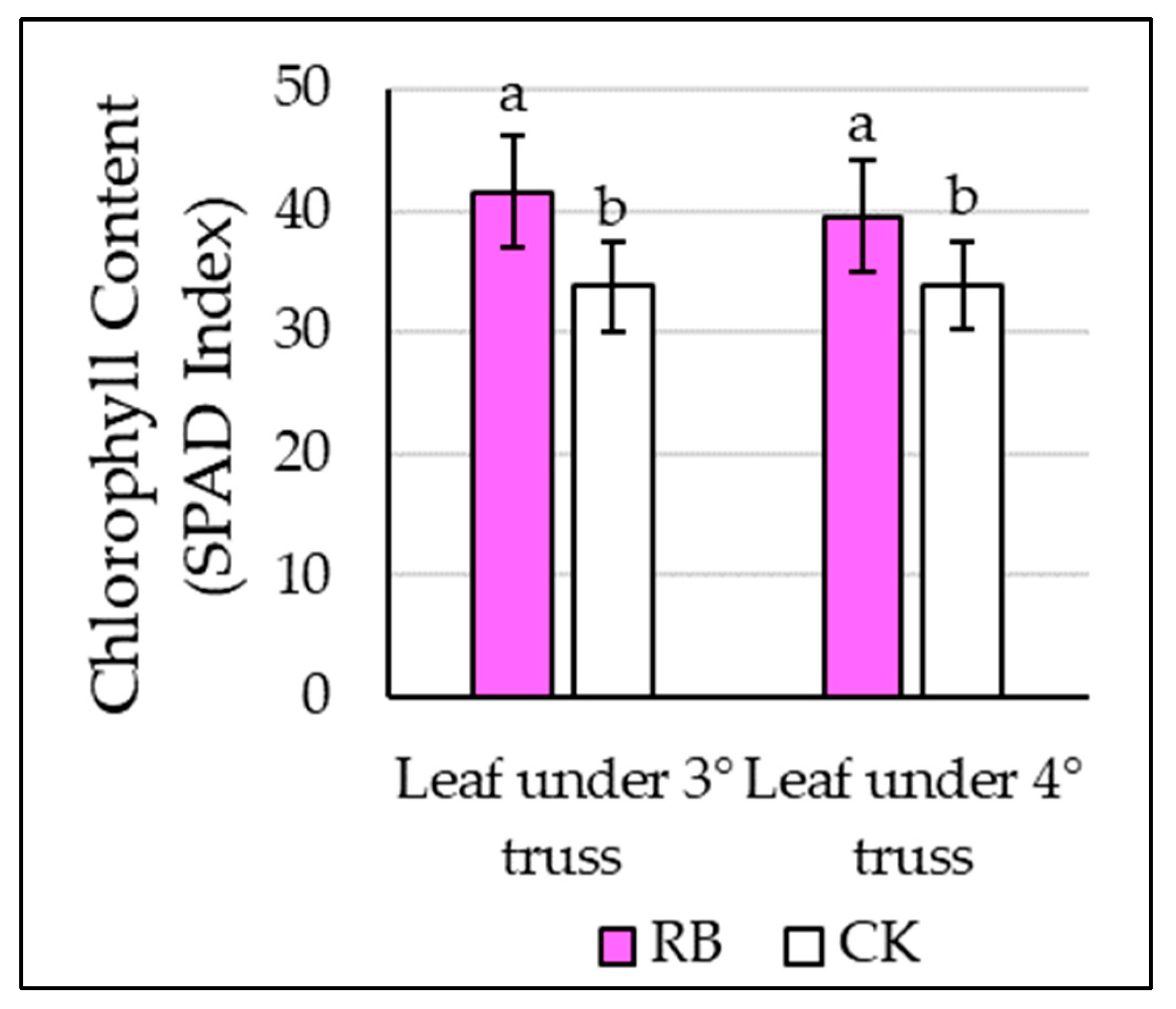
2.2. Plant Vegetative, Physiological, and Biochemical Measurements
2.3. Fruit Development and Yield
2.4. Fruit Quality
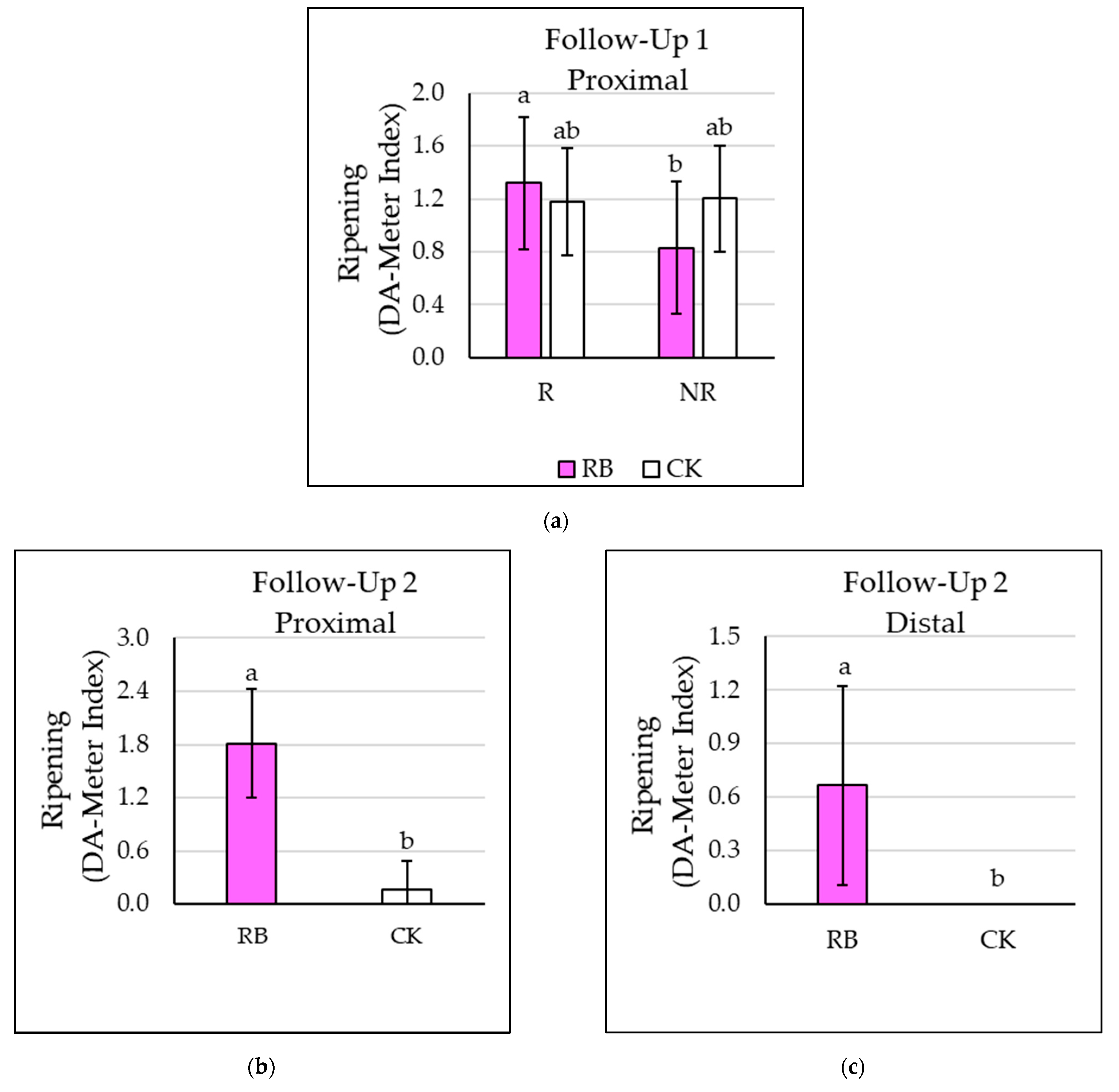
2.4.1. Non-Destructive Measurements
2.4.2. Destructive Measurements
2.5. Biochemical Analysis
Lycopene and β-carotene content
2.6. Energy Cost Assessment
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heuvelink, E.; Okello, R.C. Developmental processes. In Tomatoes; Heuvelink, E., Ed.; CABI: Wallingford, UK, 2018; Volume 27, pp. 59–88. [Google Scholar] [CrossRef]
- Jo, W.J.; Shin, J.H. Effect of Leaf-Area Management on Tomato Plant Growth in Greenhouses. Hortic. Environ. Biotechnol. 2020, 61, 981–988. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.L.; Fernie, A.R. An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, R.K.; Bishnoi, C. Assimilate partitioning and distribution in fruit crops: A review. J. Pharmacogn. Phytochem. 2017, 6, 479–484. [Google Scholar] [CrossRef]
- Ronga, D.; Zaccardelli, M.; Lovelli, S.; Perrone, D.; Francia, E.; Milc, J.; Ulrici, A.; Pecchioni, N. Biomass production and dry matter partitioning of processing tomato under organic vs conventional cropping systems in a Mediterranean environment. Sci. Hortic. 2017, 224, 163–170. [Google Scholar] [CrossRef]
- Aslani, L.; Gholami, M.; Mobli, M.; Sabzalian, M.R. The influence of altered sink-source balance on the plant growth and yield of greenhouse tomato. Physiol. Mol. Biol. Plants 2020, 26, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Bihmidine, S.; Hunter III, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Forney, C.; Bondada, B.; Leng, F.; Xie, Z.S. The molecular regulation of carbon sink strength in grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 11, 606918. [Google Scholar] [CrossRef]
- Hachmann, T.L.; Echer, M.d.M.; Dalastra, G.M.; Vasconcelos, E.S.; Guimarães, V.F. Tomato cultivation under different spacings and different levels of defoliation of basal leaves. Bragantia 2014, 73, 399–406. [Google Scholar] [CrossRef]
- Appolloni, E.; Orsini, F.; Pennisi, G.; Gabarrell Durany, X.; Paucek, I.; Gianquinto, G. Supplemental LED lighting effectively enhances the yield and quality of greenhouse truss tomato production: Results of a meta-analysis. Front. Plant Sci. 2021, 12, 596927. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Garnier, E.; Shipley, B.; Roumet, C.; Laurent, G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct. Ecol. 2001, 15, 688–695. [Google Scholar] [CrossRef]
- Calone, R.; Cellini, A.; Manfrini, L.; Lambertini, C.; Gioacchini, P.; Simoni, A.; Barbanti, L. The C4 Atriplex halimus vs. The C3 Atriplex hortensis: Similarities and Differences in the Salinity Stress Response. Agronomy 2021, 11, 1967. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.P.; Marcelis, L.F. Quantifying the source–sink balance and carbohydrate content in three tomato cultivars. Front. Plant Sci. 2015, 6, 416. [Google Scholar] [CrossRef] [PubMed]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F. Supplementary LED interlighting improves yield and precocity of greenhouse tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Lopez Camelo, A.; Gomez, P. Modelling postharvest colour changes in long shelf life tomatoes. In XXV International Horticultural Congress; Acta Horticulturae: Brussel, Belgium, 1998; p. 9. [Google Scholar]
- Anthon, G.; Barrett, D.M. Standardization of a rapid spectrophotometric method for lycopene analysis. Acta Hortic. 2006, 758, 111–128. [Google Scholar] [CrossRef]
- EUROSTAT. Available online: https://ec.europa.eu/eurostat/en/ (accessed on 14 December 2022).
- Li, L.; Ljung, K.; Breton, G.; Schmitz, R.J.; Pruneda-Paz, J.; Cowing-Zitron, C.; Cole, B.J.; Ivans, L.J.; Pedmale, U.V.; Jung, H.S. Linking photoreceptor excitation to changes in plant architecture. Genes Dev. 2012, 26, 785–790. [Google Scholar] [CrossRef]
- Dierck, R.; Dhooghe, E.; Van Huylenbroeck, J.; Van Der Straeten, D.; De Keyser, E. Light quality regulates plant architecture in different genotypes of Chrysanthemum morifolium Ramat. Sci. Hortic. 2017, 218, 177–186. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Fan, X.X.; Xu, Z.G.; Liu, X.Y.; Tang, C.M.; Wang, L.W.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Drozdova, I.S.; Bondar, V.V.; Mokronosov, A.T. Blue, red and blue plus red light control of chlorophyll content and CO2 gas exchange in barley leaves: Quantitative description of the effects of light quality and fluence rate. Physiol. Plant. 1992, 85, 632–638. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Balducci, L.; Fierravanti, A.; Rossi, S.; Delzon, S.; De Grandpré, L.; Kneeshaw, D.D.; Deslauriers, A. The paradox of defoliation: Declining tree water status with increasing soil water content. Agric. For. Meteorol. 2020, 290, 108025. [Google Scholar] [CrossRef]
- Glanz-Idan, N.; Wolf, S. Upregulation of photosynthesis in mineral nutrition-deficient tomato plants by reduced source-to-sink ratio. Plant. Signal. Behav. 2020, 15, 1712543. [Google Scholar] [CrossRef] [PubMed]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I.A. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Appolloni, E.; Paucek, I.; Pennisi, G.; Stringari, G.; Gabarrell Durany, X.; Orsini, F.; Gianquinto, G. Supplemental LED Lighting Improves Fruit Growth and Yield of Tomato Grown under the Sub-Optimal Lighting Condition of a Building Integrated Rooftop Greenhouse (i-RTG). Horticulturae 2022, 8, 771. [Google Scholar] [CrossRef]
- Pinheiro, J.; Alegria, C.; Abreu, M.; Gonçalves, E.M.; Silva, C.L. Kinetics of changes in the physical quality parameters of fresh tomato fruits (Solanum lycopersicum, cv.‘Zinac’) during storage. J. Food Eng. 2013, 114, 338–345. [Google Scholar] [CrossRef]
- Alba, R.; Cordonnier-Pratt, M.M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant. Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef]
- Nájera, C.; Guil-Guerrero, J.L.; Enríquez, L.J.; Álvaro, J.E.; Urrestarazu, M. LED-enhanced dietary and organoleptic qualities in postharvest tomato fruit. Postharvest Biol. Technol. 2018, 145, 151–156. [Google Scholar] [CrossRef]
- Dannehl, D.; Schwend, T.; Veit, D.; Schmidt, U. Increase of yield, lycopene, and lutein content in tomatoes grown under continuous PAR spectrum LED lighting. Front. Plant Sci. 2021, 12, 611236. [Google Scholar] [CrossRef]
- Casierra-Posada, F.; Torres, I.D.; Blanke, M.M. Fruit quality and yield in partially defoliated strawberry plants in the tropical highlands. Gesunde Pflanz. 2013, 65, 107–112. [Google Scholar] [CrossRef]
- EC (2022). Available online: https://agriculture.ec.europa.eu/data-and-analysis/markets/overviews/market-observatories/fruit-and-vegetables/tomatoes-statistics_en (accessed on 14 December 2022).
- Niu, Y.; Lyu, H.; Liu, X.; Zhang, M.; Li, H. Effects of supplemental lighting duration and matrix moisture on net photosynthetic rate of tomato plants under solar greenhouse in winter. Comput. Electron. Agric. 2022, 198, 107102. [Google Scholar] [CrossRef]
- Paskhin, M.O.; Yanykin, D.V.; Gudkov, S.V. Current Approaches to Light Conversion for Controlled Environment Agricultural Applications: A Review. Horticulturae 2022, 8, 885. [Google Scholar] [CrossRef]

| Element | Unit | Nutrient Solution |
|---|---|---|
| N-NO3 | mmol L−1 | 14.0 |
| N-NH4 | mmol L−1 | 1.00 |
| P | mmol L−1 | 1.00 |
| K | mmol L−1 | 8.00 |
| Ca | mmol L−1 | 4.00 |
| Mg | mmol L−1 | 1.50 |
| Na | mmol L−1 | 0.00 |
| S-SO4 | mmol L−1 | 2.50 |
| Cl | μmol L−1 | 0.00 |
| Fe | μmol L−1 | 15.0 |
| B | μmol L−1 | 20.0 |
| Cu | μmol L−1 | 1.00 |
| Zn | μmol L−1 | 5.00 |
| Mn | μmol L−1 | 10.0 |
| Mo | μmol L−1 | 1.00 |
| A (µmol m−2 s−1) | ΦPSII | qP | ||
|---|---|---|---|---|
| Light | RB | 6.7 ± 1.5 a | 0.4 ± 0.1 a | 0.5 ± 0.1 a |
| CK | 5.1 ± 1.7 b | 0.3 ± 0.1 b | 0.4 ± 0.2 b | |
| Defoliation | R | 6.4 ± 1.7 a | 0.4 ± 0.1 a | 0.5 ± 0.1 a |
| NR | 5.4 ± 1.7 a | 0.3 ± 0.1 b | 0.4 ± 0.1 b |
| Fruit Volume Follow-Up 1 (cm3) | Fruit Volume Follow-Up 2 (cm3) | ||||
|---|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | ||
| Light | RB | 605.3 ± 236.3 a | 652.5 ± 259.2 a | 428.4 ± 207.6 a | 262.2 ± 146.5 a |
| CK | 650.7 ± 208.8 a | 493.1 ± 217.8 b | 200.4 ± 162.8 b | 119.6 ± 93.0 b | |
| Defoliation | R | 697.8 ± 228.4 a | 543.8 ± 275.0 a | 289.8 ± 178.6 a | 228.7 ± 139.6 a |
| NR | 562.0 ± 200.9 b | 595.0 ± 249.5 a | 391.6 ± 243.5 a | 212.7 ± 152.8 a | |
| Total Yield (g plant−1) | Red Fruit Yield (g plant−1) | Green Fruit Yield (g plant−1) | |
|---|---|---|---|
| RB | 3261.3 ± 515 a | 1980.2 ± 479 a | 624.0 ± 249 a |
| CK | 1495.7 ± 305 b | 127.8 ± 193 b | 843.8 ± 260 a |
| Variation (%) | +118 | +1449 | −26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appolloni, E.; Paucek, I.; Pennisi, G.; Manfrini, L.; Gabarrell, X.; Gianquinto, G.; Orsini, F. Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity. Agronomy 2023, 13, 671. https://doi.org/10.3390/agronomy13030671
Appolloni E, Paucek I, Pennisi G, Manfrini L, Gabarrell X, Gianquinto G, Orsini F. Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity. Agronomy. 2023; 13(3):671. https://doi.org/10.3390/agronomy13030671
Chicago/Turabian StyleAppolloni, Elisa, Ivan Paucek, Giuseppina Pennisi, Luigi Manfrini, Xavier Gabarrell, Giorgio Gianquinto, and Francesco Orsini. 2023. "Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity" Agronomy 13, no. 3: 671. https://doi.org/10.3390/agronomy13030671
APA StyleAppolloni, E., Paucek, I., Pennisi, G., Manfrini, L., Gabarrell, X., Gianquinto, G., & Orsini, F. (2023). Winter Greenhouse Tomato Cultivation: Matching Leaf Pruning and Supplementary Lighting for Improved Yield and Precocity. Agronomy, 13(3), 671. https://doi.org/10.3390/agronomy13030671








