Abstract
In this study, a Box-Behnken Design (BBD) has been used to optimize the recovery of bioactive compounds from jabuticaba (Myrciaria cauliflora) by-products through microwave-assisted extraction (MAE). Thus, the Box-Behnken (BBD) experimental design was followed by a response surface methodology (RSM) that would allow investigating the influence of the four independent variables (temperature, solvent composition, pH, and sample-solvent ratio) that have been considered. The results revealed that temperature and solvent composition (%MeOH) were the most significant factors regarding the yields of anthocyanins obtained from the studied plant matrix. The established optimized conditions and 10-min-extraction time resulted in yields of 9.70 ± 0.28 mg g−1 of total anthocyanins. The method exhibited good repeatability and intermediate precision, with RSD variations lower than 5% for both parameters. The developed process was also able to extract and identify anthocyanins in commercial samples (jabuticaba pulp and jam). The results obtained from the optimized MAE method suggest that this technique is not only efficient for the recovery of anthocyanins from jabuticaba by-products, but it is also beneficial for a circular economy approach.
1. Introduction
Jabuticaba (Myrciaria cauliflora (Mart.) O. Berg) is a plant grown and processed in the South and Southeast of Brazil. It produces rounded fruits that contain an edible pulp with a pleasant taste, one to four seeds, and purple skin [1]. It is a Brazilian native fruit which is widely commercialized both for fresh consumption and for the industrial production of various consumer products (e.g., ice creams, jellies, teas, liqueurs, and extracts, among others) [2]. However, approximately 50% of the jabuticaba fruit’s weight consists of skin and seeds, which are generally disposed of as waste [3].
The correct disposal of these by-products from the food industry is an unavoidable and challenging requirement in terms of environmental protection [4]. Therefore, new methods and technologies must be implemented to process agro-industrial by-products into value-added products that can be redirected into a circular economy system [5].
Jabuticaba fruit peel is generally disposed of as waste. However, it has considerable potential because of its high content of some bioactive compounds responsible for its purple color. They also confer this high fruit antioxidant, anti-inflammatory, antibacterial, antimutagenic, and antiproliferative properties [6]. According to the reports in the literature on the use of jabuticaba extracts, they can support a green strategy to improve the oxidative stability of meat products [7]. They can be encapsulated together with propolis to be used as an antioxidant additive and natural colorant [8], and also proven their capacity to boost insulin sensitivity in mice [9], among other applications. These biocompounds from jabuticaba peel can also be used for food enrichment or as a natural dye with technological purposes based on their therapeutical properties for the treatment of diabetes, neurological issues, or the prevention of cardiovascular disorders [6]. There are products derived from jabuticaba currently exported from Brazil to other countries. However, the demand for pharmaceutical products enriched with bioactive compounds to replace synthetic ones has increased recently. Technological routes for valuing jabuticaba by-products can contribute to the establishment of new technological routes for other by-products rich in products with greater added value that contribute to health.
The extraction of bioactive compounds from plant matrices can be carried out through a variety of methods, where the most traditional ones are based on mechanical agitation, high temperatures, or organic reagents that may cause the hydrolysis and oxidation of the compounds of interest [7]. On the other hand, other more modern extraction technologies, such as ultrasound [8], pressurized liquids [9,10], or even green solvents [2], have been gaining ground as preferred extraction methods. Microwaves, in particular, present some advantages, such as shorter extraction times, less solvent consumption, or a more efficient extraction of a broader range of bioactive compounds [11]. This method is based on the application of microwaves generated by electromagnetic fields in the range 300 MHz to 300 GHz [12]. Microwaves cause temperature and pressure increments in specific areas of the matrix, facilitating a rapid migration of the bioactive compounds from the plant material into the extraction solvent [13].
Considering all of the above said, the aim of this study is to develop an optimized extraction method for the bioactive compounds present in jabuticaba peel. A Box–Behnken design has been used to determine the effect of the different variables and their interactions on the extraction of the compounds of interest. Thus, this study intends to provide objective data on the application of an improved extraction method that allows more efficient management of jabuticaba by-products in the context of a circular economy.
2. Materials and Methods
2.1. Raw Materials
The jabuticaba peels for the experiments were provided by The Flask Company (Campinas, SP, Brazil). The samples were dried out at 60 °C for 48 h. A food processor was used to grind the dried jabuticaba peels. The samples were placed in a freezer and kept at −20 °C until further use.
2.2. Solvents and Reagents
HPLC-grade methanol (Panreac Quimica, S.L.U., Castellar del Valles, Spain) was mixed with Milli-Q water (MilliPore system, Bedford, MA, USA) to produce the solvents to be used for the extractions. The mobile phase for the chromatographic determinations was composed of HPLC-grade acetonitrile (Panreac Quimica, S.L.U., Castellar del Valles, Spain), Milli-Q water, and glacial acetic acid (Panreac Quimica, S.L.U., Castellar del Valles, Spain). It was used to reduce peak duplication and raise solvent acidity. The standards used for the liquid chromatographies (cyanidin 3-O-glucoside and delphinidin 3-O-glucoside) were provided by Sigma Aldrich (St. Louis, MO, USA).
2.3. Microwave-Assisted Equipment
A MARS 240/50 microwave (OneTouch Technology, CEM Corporation, Matthews, NC, USA) was used for the microwave-assisted extractions. An eight-position carousel was installed on the apparatus with the extraction chambers. Additionally, a temperature sensor and two magnetrons were installed in each chamber (one to apply microwave power to the samples and the other to monitor constant temperature over the extraction time). For the entire experiment, a power of 800 W was set.
2.4. Determining the Study Range to Be Considered for Each Factor
A series of preliminary tests were carried out in order to determine the appropriate study range to be tested for methanol percentage and temperature. Methanol percentage was tested from 0 to 100%, and temperature ranged from 25 to 150 °C. The extractions were duplicated (n = 2) using 200 mg samples and 20 mL solvent. The extraction parameters were as follows: 5 min heating ramp, 10 min extraction time, 800 W power, and no agitation. Then, the extracts were centrifuged (1702× g, 5 min), and the resulting supernatants were placed in volumetric flasks and made up to 25 mL. The extracts were then filtered through 0.22 μm membranes (Nylon Membrane Filter, FILTER-LAB, Barcelona, Spain) and quantified by means of an ultra-high-performance liquid chromatography system coupled to a L-2420U UV-Vis detector (UHPLC-UV-Vis) (Section 2.9). The data obtained were employed to generate a plot where the anthocyanins were expressed as mg per g of sample.
2.5. Optimizing the MAE According to a Box-Behnken Design
A surface-response Box–Behnken experiment design (BBD) was used to optimize the extraction conditions based on four (4) factors: temperature, methanol percentage, pH, and solvent-sample ratio. Specific data have been included in Table 1. The experimental design was based on three (3) levels: high (+1), intermediate (0), and low (−1), without any axial points. A total of 27 assays were performed in duplicate (n = 2). The extraction temperatures (X1) were 50, 75, and 100 °C. The percentages of methanol in Milli-Q water (X2) were 10, 35, and 60% (v v−1). The extraction pH (X3) varied between 2, 5, and 8. Finally, the sample-solvent ratios used (X4) were 4, 8, and 12 (mg mL−1).

Table 1.
Box-Behnken design for the MAE of compounds from jabuticaba peels.
The response variable was expressed as mg of anthocyanins per mg of jabuticaba peel (mg g−1), calculated according to the method described in Section 2.9. A second-order polynomial equation (Equation (1)) that included all the studied parameters was used to calculate the value of the response variable resulting from each extraction.
where Y is the response, β0 is the ordinate, X1 is the extraction temperature, X2 is the percentage of MetOH in the solvent, X3 is the pH of the solvent, and X4 is the sample-solvent ratio. Finally, βi, βij, and βii represent the linear, cross-product, and quadratic coefficients, respectively. The software application Statistica® (version 10.0, StatSoft Inc., Tulsa, OK, USA) was employed for the analysis of the Box–Behnken design data. To find statistically significant factors and the interactions between variables were subjected to variance analysis (ANOVA). Tukey’s test (p ≤ 0.05) was used to determine the significant differences. A mathematical model was generated, giving information on the optimal values of the significant variables, an analysis of variance, and the estimated effect of the variables on the final response and the response surface graphs. With the optimized conditions found, tests were carried out with commercial jabuticaba products (jelly and pulp). In addition to quantifying the anthocyanins by UHPLC-UV-Vis, the antioxidant activity was also determined by DPPH radical according to the method described by Miliauskas et al. [14], and the results were expressed as µM trolox equivalents (TE) per g of sample.
2.6. Optimum Extraction Time
In order to determine the optimal time for the extraction of anthocyanins, different times (2 to 30 min) were investigated under the optimal MAE conditions that had been determined by means of the Box Behnken design. The analysis was carried out in triplicate.
2.7. Repeatability and Intermediate Precision
Following the optimal conditions obtained for the MAE method, repeatability, and intermediate precision tests were performed to determine its suitability. Eight extractions were performed on the same day to evaluate its repeatability. Another eight extractions were performed on each of two consecutive days (a total of 24 extractions) to determine the intermediate precision of the method. The variation coefficients were the statistical parameters to denote the developed method’s applicability and/or accuracy.
2.8. Identification of Anthocyanin by UHPLC-Q-ToF-MS
To identify the anthocyanins present in jabuticaba, ultra-high performance liquid chromatography coupled to a quadrupole-time-of-flight mass spectrometer (UHPLC-Q-ToF-MS) (Xevo G2 QToF, Waters Corp., Milford, MA, USA) was used. Specifically, the identification was carried out following the method previously published by our research group [14]. The software application MassLynx (Waters Corp., Milford, MA, USA) was used to control the equipment and process the mass spectra.
2.9. Anthocyanin Quantification by UHPLC-UV-Vis
After the anthocyanins in the jabuticaba peel extracts had been identified, they were separated and quantified using ultra-high-performance liquid chromatography (UHPLC) with the equipment available to our research group (Elite HPLC LaChrom Ultra System, Hitachi, Tokyo, Japan) coupled to an L-2420U UV-Vis detector, an L-2200U autosampler, an L-2300 column oven, and two L-2160 U pumps. The analysis procedure and conditions were the same as those described in one of our previous works [11]. Two major anthocyanins were separated and analyzed: cyanidin 3-O-glucoside (C3G) and delphinidin 3-O-glucoside (D3G). The concentrations of C3G (y = 181,419.52x + 2846.43, R2 = 0.9997) and D3G (y = 175,703.24x + 3742.16, R2 = 0.9996) were calculated based on the calibration curves generated by each standard. The resulting data were expressed as the sum of the two anthocyanins in mg per g of jabuticaba peels.
3. Results and Discussion
3.1. Identification and Separation of Anthocyanins
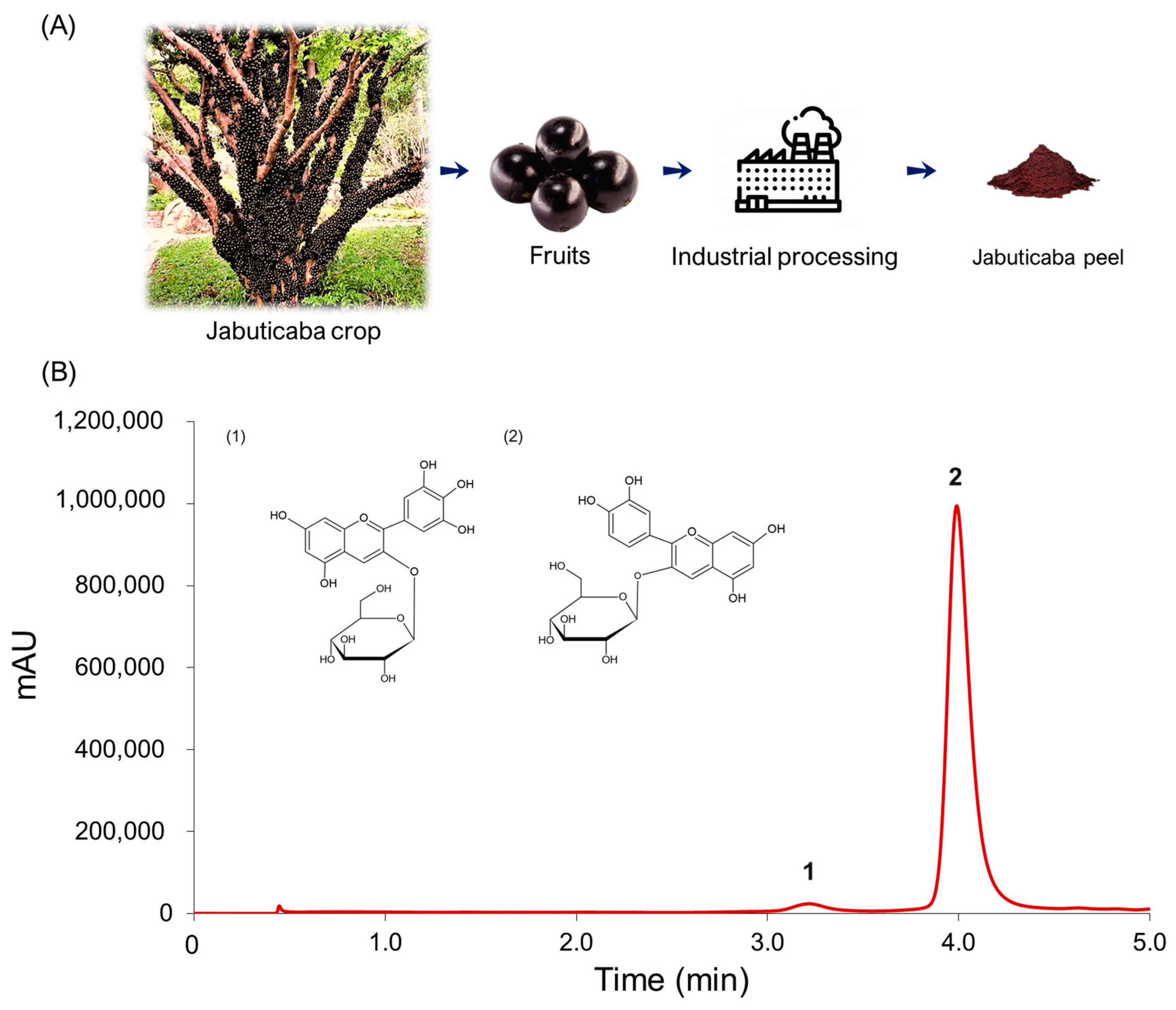
Once the mass chromatogram had been obtained, the anthocyanins present in the extract could be identified according to their retention times and molecular weight following the existing bibliography. Two major anthocyanins could be identified by UHPLC-Q-ToF-MS in the extracts: delphinidin 3-O-glucoside (D3G) and cyanidin 3-O-glucoside (C3G). The molecular ions [M+] presented the following m/z ratio: 465 (D3G) and 449 (C3G). Once the anthocyanins had been identified, they were separated by UHPLC-UV-Vis. Figure 1 shows the chromatogram (λ = 520 nm) of the anthocyanins that had been identified and separated. C3G was the major compound at 96.65%, and D3G represented just 3.35% of the anthocyanins.
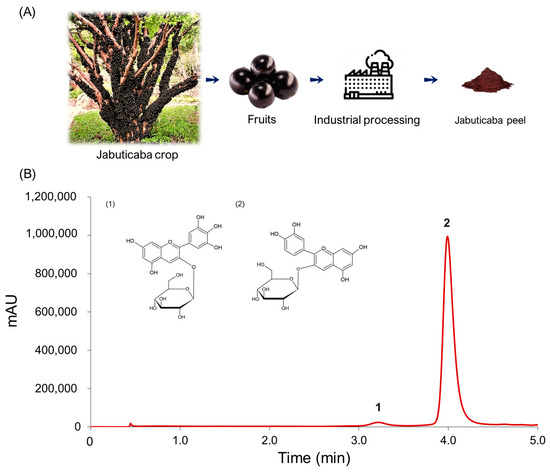
Figure 1.
Recovery of the anthocyanins from jabuticaba by-products. (A) Jabuticaba peel as industrial processing residue; (B) Chromatogram of the anthocyanins identified in jabuticaba peel extracts by UHPLC-UV-Vis (λ = 520 nm). 1. D3G; 2. C3G.
3.2. Determining the Study Range to Be Considered for Each Factor
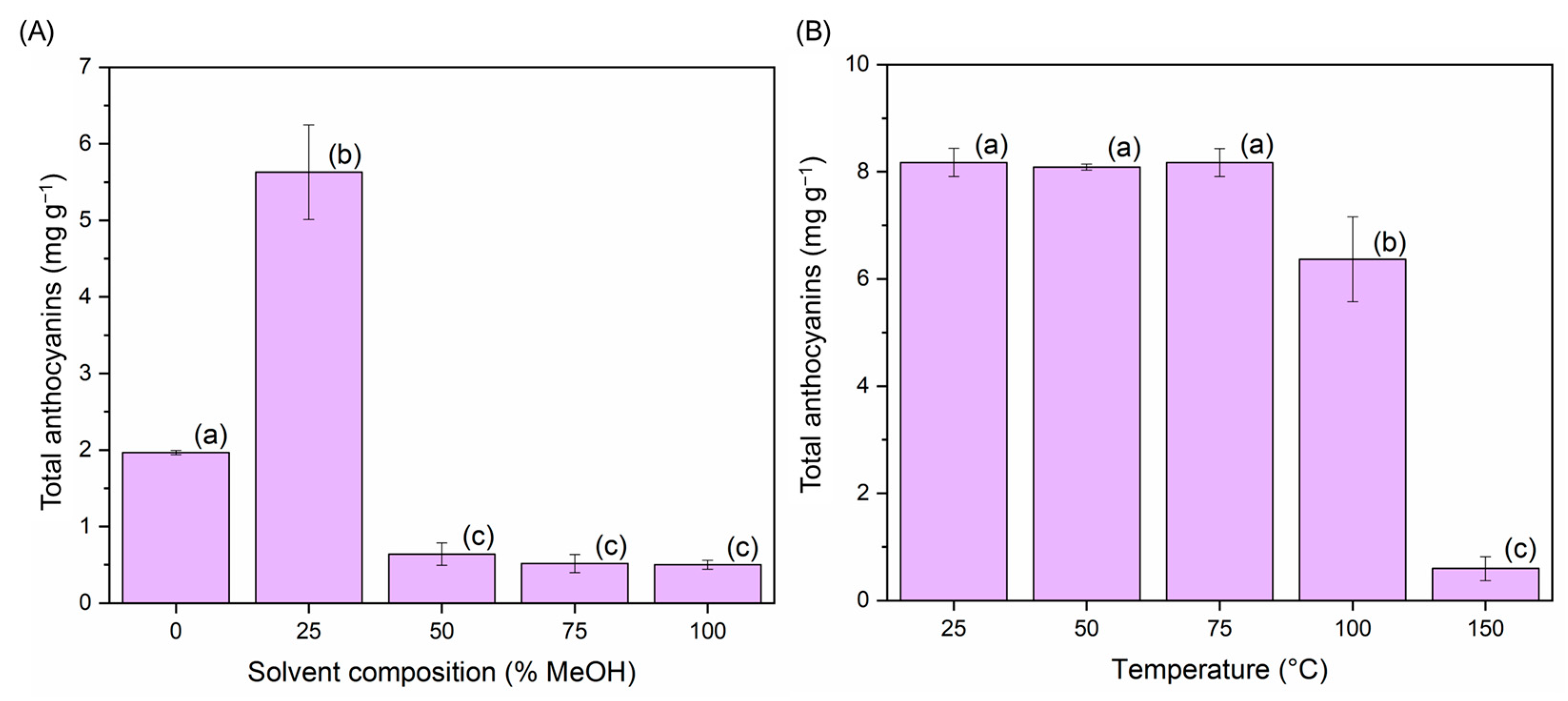
A univariate study on the percentage of methanol in the water of the extraction solvent and on the temperature was performed to determine the Box-Behnken design operating range. For this, several initial extractions were carried out in triplicate, where only the percentage of methanol in the extraction solvent (0, 25, 50, 75, and 100%) was modified. The remaining parameters were set at the following constant values: temperature: 50 °C; extraction pH 5; sample-solvent ratio: 8 mg mL−1. Similarly, to delimit the temperature range for the Box-Behnken design, several tests were carried out in triplicate at different operating temperatures (25, 50, 75, 100, and 150 °C), while the rest of the parameters remained at the following invariable values: extraction solvent at 25% MeOH in water; extraction pH: 5; sample-solvent ratio: 8 mg mL−1. The response in either case represented the total amount of anthocyanins extracted (mg anthocyanins/g sample) (Figure 2). Determinations performed by UHPLC-UV-Vis as presented in Section 2.9.
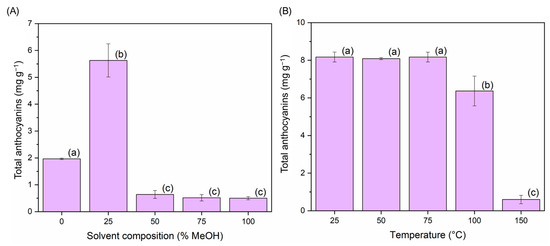
Figure 2.
Anthocyanin yields (mg anthocyanins g−1 sample) (n = 3): (A) different methanol percentages in the extraction solvent (water: methanol) and (B) different temperatures. Different letters in each bar indicate significant differences by Tukey’s test at p ≤ 0.05.
Figure 2A shows that when higher percentages of methanol (>25%) were used, the recovery of compounds went down. This is mainly due to a change in the polarity of the extraction solvent. Figure 2B shows that when the extraction temperature reaches over 100 °C, a considerable reduction in the amount of anthocyanins extracted takes place. This is due to the thermal degradation of these compounds and represents a substantial loss of the bioactive properties of the extracts [15].
Given these results and the experience of our research group in the extraction of anthocyanins by MAE, the range of methanol for the Box–Behnken experimental design (BBD) was established at 10–60% (v v−1), and the temperature at 50–100 °C.
3.3. MAE Optimization
The BBD was chosen as the approach to ascertain the impact of the MAE factors involved in the method that had been developed to extract biocompounds from jabuticaba peels. A total of four factors were selected to be assessed, and a total of 27 experiments (Table 1) were performed at random for this purpose. Table 2 shows the actual and predicted anthocyanin extraction yields. MAE-18 achieved the greatest recovery of total anthocyanins, followed by MAE-27 and MAE-11. It can also be observed that higher temperatures caused the degradation of the anthocyanins, as in MAE-2 (100 °C), where a lower recovery of anthocyanins was accomplished. However, a certain amount of heating of the extractive medium is necessary to facilitate the process, as higher temperatures favor the solubility of the biocompounds by breaking their chemical bonds, which results in higher mass transfer rates [16,17]. A correct temperature selection according to the type of biocompounds to be extracted is essential [9,18]. Anthocyanins extracted from plant sources, such as jabuticaba peels in this study, can be used either as a natural dye or for their antioxidant properties in food, cosmetics or nutraceuticals.

Table 2.
Anthocyanin extraction yields according to the BBD experimental design.
The results were analyzed by BBD-RSM at 95% confidence. Table 3 presents the values obtained from the statistical analysis. The quadratic interaction of temperature and solvent composition was confirmed to be significant (p-value < 0.05) concerning the extraction yields obtained by MAE.

Table 3.
Analysis of variance of the polynomic model adjusted to the extraction of total anthocyanins from jabuticaba peel.
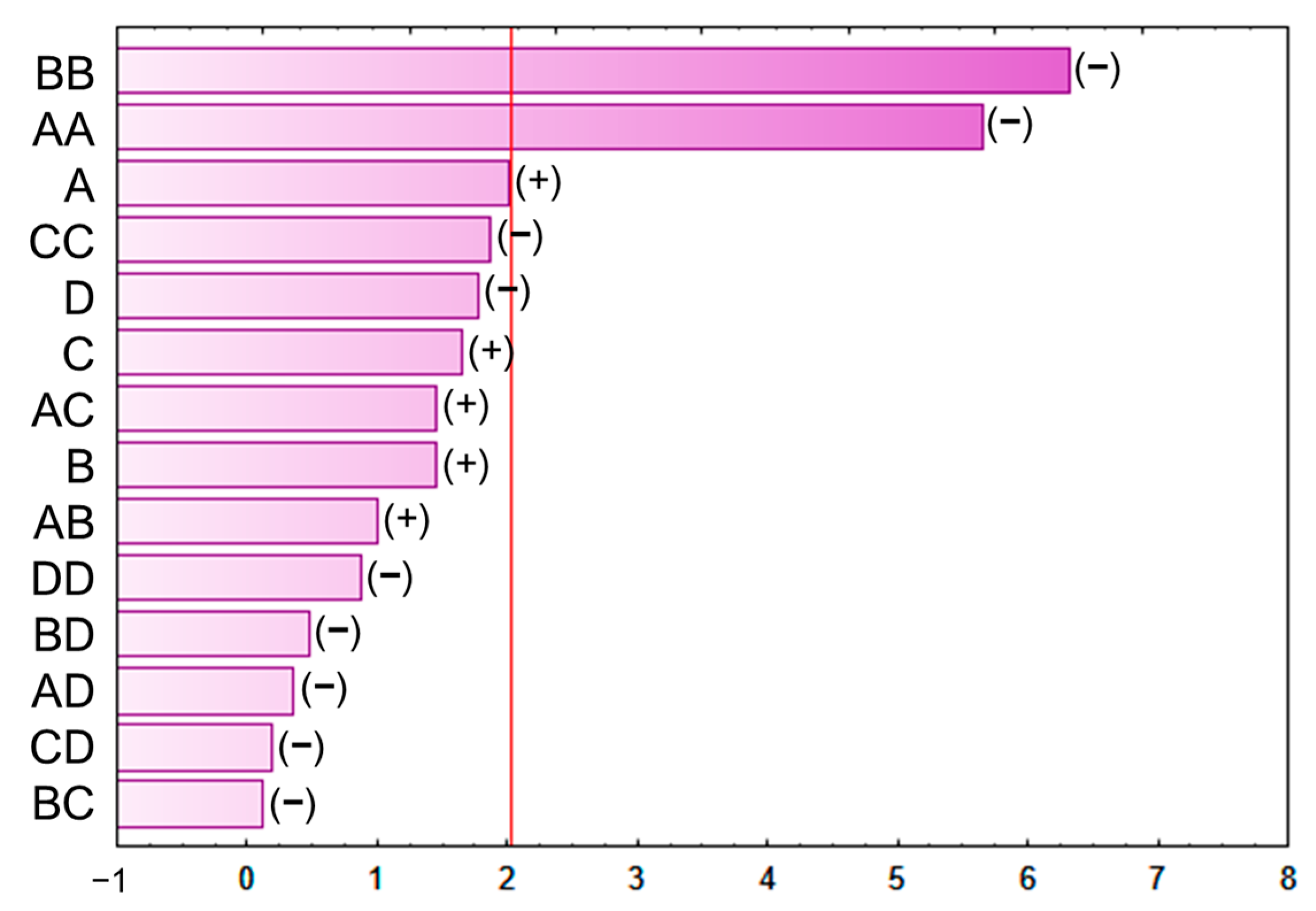
The results from the BBD-RSM were also graphically represented using a Pareto chart (Figure 3) for easier identification of the linear and quadratic interactions of the extraction variables as well as the interactions between them. The evaluated factors were considered statistically significant only when they were located on the right side of the line, which indicates p-value 0.05. Those with positive effects are those whose greater value results in higher extraction yields, and those with negative effects are the ones with the opposite behavior, i.e., the higher their value, the lower the extraction yield [19]. Thus, the quadratic interaction of either variable, temperature, or solvent composition was the interaction that exhibited the most statistically significant positive effects.
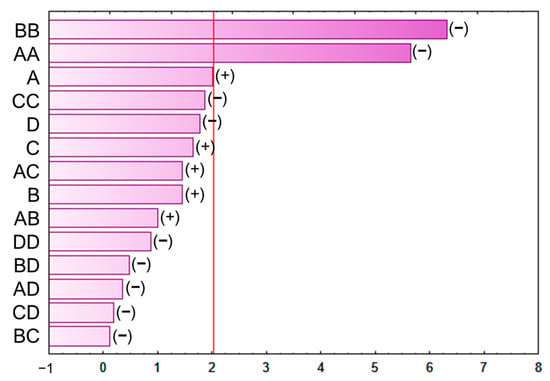
Figure 3.
Pareto chart of the BBD–RSM used to optimize the extraction procedure.
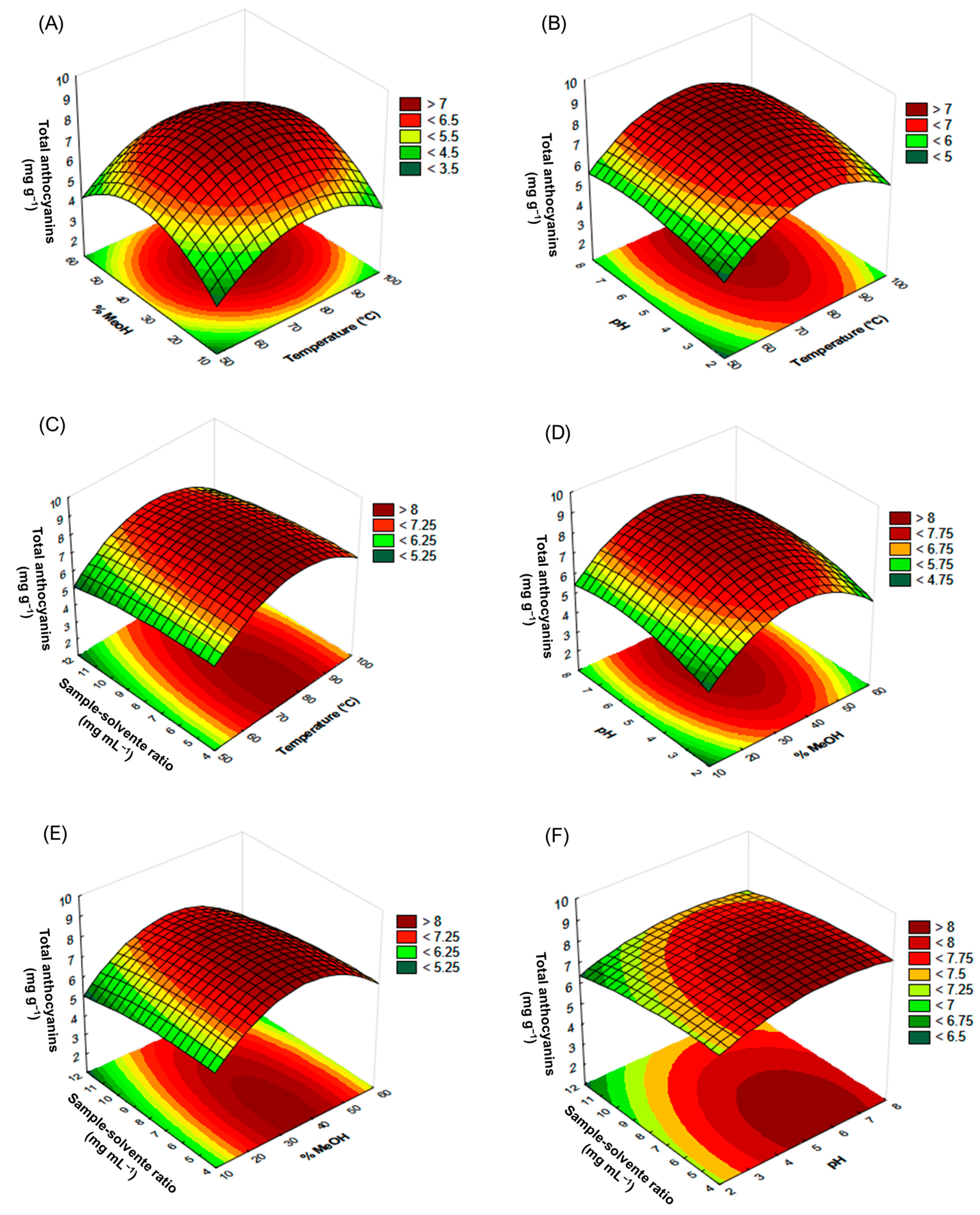
Three-dimensional response surface plots were elaborated for a more detailed analysis (Figure 4). It could be observed from the graph rounded shape that in the case of solvent composition ratio and temperature (Figure 4A), the greatest yields were obtained when the intermediate values of the experimental design were applied. The effect of pH on the yields was also more significant when the mid values considered for the study were used, which can be seen in Figure 4B–D. When the effect of the sample-solvent ratio is examined, it can be seen that when interacting with the pH (Figure 4F), lower ratios result in greater yields; however, when observing the interaction of the sample-solvent ratio with the temperature or with the solvent composition (Figure 4C–E), varying sample-solvent ratios did not affect the final yield. Therefore, the critical values that would result in the best possible optimization of the MAE of anthocyanins from jabuticaba peels have been determined. Equation 2 is presented only with the statistically significant variables (p-value < 0.05) of the second-order polynomial equation (Equation (1)), which allow for predicting the response variable.
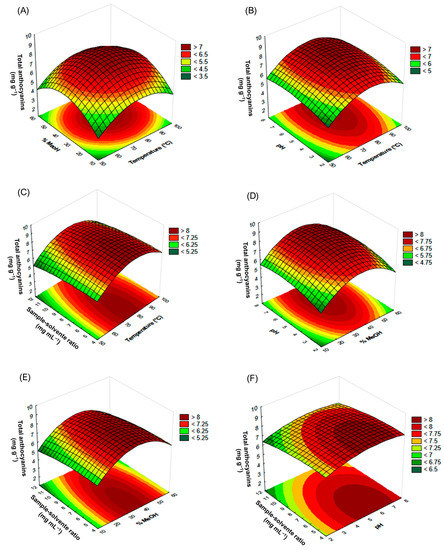
Figure 4.
Response surface graph of the anthocyanin extracted by MAE from jabuticaba peels. (A) Temperature × %MeOH interaction, (B) temperature × pH interaction, (C) temperature × sample-solvent ratio interaction, (D) %MeOH × pH interaction, (E) %MeOH × sample-solvent ratio interaction, and (F) pH × sample-solvent ratio interaction.
The optimal values established for the extraction of anthocyanins by means of microwave-assisted extraction were: Temperature: 81 °C; solvent composition: 38% MeOH in water; pH: 6 and sample-solvent ratio 4.7 mg mL−1. Once the optimum conditions had been determined, a quadruplicate extraction was carried out under those conditions, with total anthocyanin yields of 9.70 ± 0.28 mg g−1, which represents a performance increment concerning the extractions carried out following the Box-Behnken design. Thus, there was a 13.58% increase in anthocyanin recovery compared to the best conditions according to the experimental design (MAE-18). Moreover, the yields obtained in this study have been greater than those reported by other research studies where other extraction methods had been used [8,20,21]. We could, therefore, conclude that the variables in the extraction procedure had been truly optimized to produce maximum yields through MAE.
3.4. Optimal Extraction Time
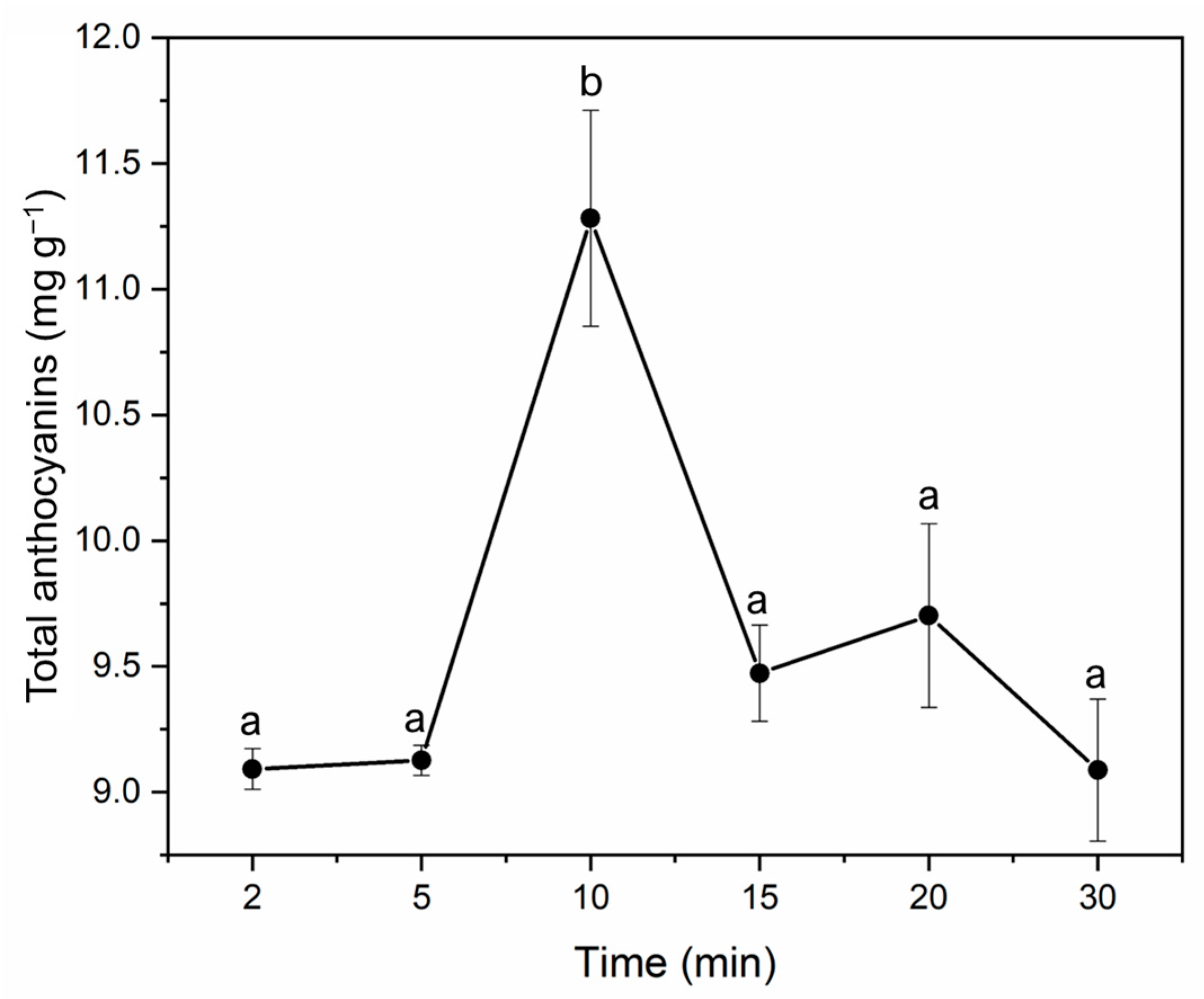
Different periods were tested in order to determine the ideal extraction time (2, 5, 10, 15, 20, and 30 min). The rest of the variables remained at the established optimum values. The tests were conducted in quadruplicate, and the amount of anthocyanins in each extract was quantified. The average total anthocyanins obtained from each extraction time have been displayed in Figure 5. It can be seen that 10 min was confirmed to generate the greatest recoveries (11.24 mg total anthocyanins g−1 of the sample). Similar extraction yields of anthocyanins from jabuticaba have been reported by other researchers [10,22]. Longer extraction times proved to have a detrimental effect on the anthocyanins because of their prolonged exposure to high temperatures [9].
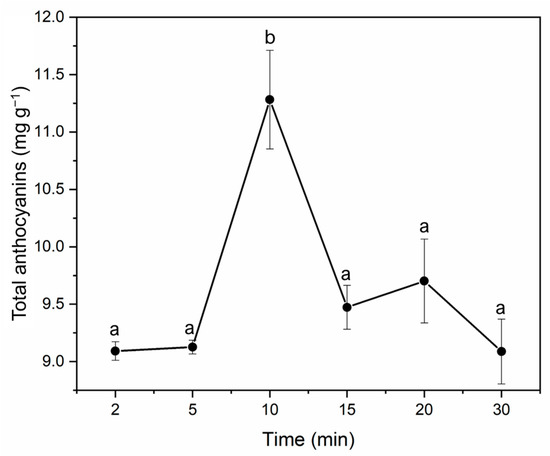
Figure 5.
Amount of anthocyanins (mg total anthocyanins g−1 of sample) extracted from jabuticaba peels by MAE using different times. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
3.5. Repeatability and Intermediate Precision
The repeatability and intermediate precision of the extraction method were assessed. Eight extractions per day were carried out on three consecutive days (a total of 24 extractions) and under the established optimal conditions. The relative standard deviation of the extraction method was determined as 1.46% for repeatability, while the RSD for its intermediate precision was measured at 3.78%. Since both coefficients are below the generally accepted 5% variation limit, the extraction process can be regarded as repeatable and with adequate intermediate precision [23].
3.6. Aplication to Jabuticaba Commercially Available Products
The optimum anthocyanin extraction conditions established for the MAE were applied to jabuticaba commercial products, specifically to jabuticaba pulp and jam. In addition, these results were compared against those obtained under the established optimum conditions for the extraction of jabuticaba peel samples in our study. All the extractions were carried out in triplicate and anthocyanins were quantified by UHPLC-UV-Vis and antioxidant activity by DPPH radical. The results can be seen in Table 4.

Table 4.
Total anthocyanins (mg g−1) and antioxidant activity (µM TE g−1) of the different commercial samples of jabuticaba.
The total anthocyanins extraction yields obtained from pulp and jam were much lower than those previously obtained from the jabuticaba peel samples used for our research. It was, nevertheless, confirmed that in terms of antioxidant activity, the jabuticaba peel samples used for our study and the commercial jabuticaba pulp presented some similarities. On the other hand, according to our expectations and based on its composition, with a much lower anthocyanin content and its nearly white color, the jabuticaba jam was proven to hold considerably lesser antioxidant activity and total anthocyanins. These result may be explained by a series or factors such as growing conditions, processing or storage, all of which may have a negative effect on the integrity of the biocompounds present in this plant since anthocyanins are biomolecules already known for their temperature sensitivity and they can also be easily altered by specific handling procedures [24,25]. The poor recoveries obtained from the fruit jam could be attributable to its formulation, which includes a series of foreign ingredients, other than the fruit pulp, that constitutes about 60% m/m of the samples [8]. It is also a product that involves a larger number of processes than those required to obtain just the fruit pulp. Similar results of antioxidant activity have been found between the pulp and the peel, demonstrating that not only anthocyanins provide antioxidant capacity to the extracts but also other components present in jabuticaba, such as phenolic compounds [26]. Although the extracts obtained from the commercial samples determined a smaller amount of anthocyanins concerning that found in our research extractions, the developed MAE method was confirmed to be suitable for performing the extraction of those biocompounds. One of the main method disadvantages, when applied on an industrial scale, is the high cost of the equipment and the time required for cooling, however, these are easily overcome when considering the high market value of anthocyanins.
4. Conclusions
MAE has been proven to be an efficient method for the recovery of value-added products from jabuticaba peels. This would allow the valorization of these by-products from fruit processing. The anthocyanins extracted by the developed method can then be evaluated for their capacity as a natural binder or antioxidant capacity. The optimization of the MAE process to obtain the greatest possible yields revealed that temperature and solvent composition (%MeOH) are the most influential variables in the extraction process. The optimal conditions for the MAE method were established: 81 °C temperature; 30% MeOH, pH 6, 4.7 mg mL−1 sample-solvent ratio, and 10 min extraction time. The method was assessed for intermediate precision and repeatability, with RSDs below 5% registered for both features. Finally, the developed method has been applied to different commercial jabuticaba products. It was concluded that microwave-assisted extraction is a technology with a promising potential for the recovery of bioactive compounds from jabuticaba by-products and, therefore can positively contribute to a practicable circular economy structure.
Author Contributions
Conceptualization, T.L.C.T.B., C.C. and G.F.B.; methodology, C.C. and G.F.B.; validation, C.C., G.F.B. and M.A.R.; formal analysis, L.E.N.C. and T.F.-C.; investigation, T.L.C.T.B., C.C. and G.F.B.; resources, M.P., G.F.B., M.A.R. and T.F.-C.; data curation, L.E.N.C. and G.F.B., writing-original draft preparation, T.L.C.T.B. and L.E.N.C.; writing-review and editing, C.C., G.F.B., M.A.R. and T.F.-C.; visualization, G.F.B.; supervision, C.C. and G.F.B.; project administration, G.F.B., M.A.R. and T.F.-C.; funding acquisition, M.P., M.A.R. and T.F.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Brazilian Science and Research Foundation (CNPq, Brazil) (productivity grants 302451/2021-8 and 302610/2021-9); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) (Finance code 001); São Paulo Research Foundation (FAPESP, Brazil) (grant numbers 2018/14582-5 for M.A.R.; 2018/14938-4 for T.F.C.; 2020/16248-5 and 2022/02039-0 for T.L.C.T.B.; and 2021/04096-9 for L.E.N.C.). This work has also been supported by the project “EQC2018-005135-P” (Equipment for liquid chromatography using mass spectrometry and ion chromatography), of the State Subprogram of Re-search Infrastructures and Technical Scientific Equipment.
Institutional Review Board Statement
No applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Acknowledgments
The authors are grateful to the “Instituto de Investigación Vitivinícola y Agroalimentaria” (IVAGRO) for providing the necessary facilities to carry out the research. A special acknowledgment is extended to the Mass Spectrometry Division from the Central Research Services for Science and Technology (SC-ICYT) of the University of Cadiz for the collaboration throughout the analysis of the samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donadio, L.C. Jabuticaba (Myrciaria Jaboticaba (Vell.) Berg); Funep: Jaboticabal, Brazil, 2000; ISBN 85-87632-10-8. [Google Scholar]
- Gadioli Tarone, A.; Keven Silva, E.; Dias de Freitas Queiroz Barros, H.; Baú Betim Cazarin, C.; Roberto Marostica Junior, M. High-Intensity Ultrasound-Assisted Recovery of Anthocyanins from Jabuticaba by-Products Using Green Solvents: Effects of Ultrasound Intensity and Solvent Composition on the Extraction of Phenolic Compounds. Food Res. Int. 2021, 140, 110048. [Google Scholar] [CrossRef]
- Morales, P.; Barros, L.; Dias, M.I.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Ramirez Asquieri, E.; Berrios, J.D.J. Non-Fermented and Fermented Jabuticaba (Myrciaria Cauliflora Mart.) Pomaces as Valuable Sources of Functional Ingredients. Food Chem. 2016, 208, 220–227. [Google Scholar] [CrossRef]
- Yousefloo, A.; Babazadeh, R. Designing an Integrated Municipal Solid Waste Management Network: A Case Study. J. Clean. Prod. 2020, 244, 118824. [Google Scholar] [CrossRef]
- Gianico, A.; Gallipoli, A.; Gazzola, G.; Pastore, C.; Tonanzi, B.; Braguglia, C.M. A Novel Cascade Biorefinery Approach to Transform Food Waste into Valuable Chemicals and Biogas through Thermal Pretreatment Integration. Bioresour. Technol. 2021, 338, 125517. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Pereira, C.; Calhelha, R.C.; José Alves, M.; Abreu, R.M.V.; Barros, L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Jabuticaba Residues (Myrciaria Jaboticaba (Vell.) Berg) Are Rich Sources of Valuable Compounds with Bioactive Properties. Food Chem. 2020, 309, 125735. [Google Scholar] [CrossRef]
- Nunes Mattos, G.; Pessanha de Araújo Santiago, M.C.; Sampaio Doria Chaves, A.C.; Rosenthal, A.; Valeriano Tonon, R.; Correa Cabral, L.M. Anthocyanin Extraction from Jaboticaba Skin (Myrciaria Cauliflora Berg.) Using Conventional and Non-Conventional Methods. Foods 2022, 11, 885. [Google Scholar] [CrossRef]
- Fernández-Barbero, G.; Pinedo, C.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; García-Barroso, C. Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Jabuticaba (Myrciaria Cauliflora) Fruit through a Box-Behnken Experimental Design. Food Sci. Technol. 2019, 39, 1018–1029. [Google Scholar] [CrossRef]
- Barroso, T.; Sganzerla, W.; Rosa, R.; Castro, L.; Maciel-Silva, F.; Rostagno, M.; Forster-Carneiro, T. Semi-Continuous Flow-through Hydrothermal Pretreatment for the Recovery of Bioproducts from Jabuticaba (Myrciaria Cauliflora) Agro-Industrial by-Product. Food Res. Int. 2022, 158, 111547. [Google Scholar] [CrossRef] [PubMed]
- Barroso, T.L.C.T.; da Rosa, R.G.; Sganzerla, W.G.; Castro, L.E.N.; Maciel-Silva, F.W.; Rostagno, M.A.; Forster-Carneiro, T. Hydrothermal Pretreatment Based on Semi-Continuous Flow-through Sequential Reactors for the Recovery of Bioproducts from Jabuticaba (Myrciaria Cauliflora) Peel. J. Supercrit. Fluids 2022, 191, 105766. [Google Scholar] [CrossRef]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Extraction of Antioxidant Compounds from Onion Bulb (Allium Cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods. Antioxidants 2022, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Abdullah; Khan, F.; Niaz, K. Introduction to Natural Products Analysis. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–15. [Google Scholar]
- Zhou, H.-Y.; Liu, C.-Z. Microwave-Assisted Extraction of Solanesol from Tobacco Leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Park, J.-S.; Han, J.-M.; Surendhiran, D.; Chun, B.-S. Physicochemical and Biofunctional Properties of Sargassum Thunbergii Extracts Obtained from Subcritical Water Extraction and Conventional Solvent Extraction. J. Supercrit. Fluids 2022, 182, 105535. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Subcritical Water Extraction of Flavanones from Defatted Orange Peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of Microwave-Assisted Extraction for the Recovery of Bioactive Compounds from the Chilean Superfruit (Aristotelia Chilensis (Mol.) Stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Carrera, C.; Palma, M.; Barbero, G.F.; Aliaño-González, M.J. Ultrasound-Assisted Extraction of Betalains from Opuntia Fruit Pulp of Different Color Varieties. Agronomy 2022, 12, 2604. [Google Scholar] [CrossRef]
- Rodrigues, S.; Fernandes, F.A.N.; de Brito, E.S.; Sousa, A.D.; Narain, N. Ultrasound Extraction of Phenolics and Anthocyanins from Jabuticaba Peel. Ind. Crops Prod. 2015, 69, 400–407. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Fonteles, T.V.; Rodrigues, S.; de Brito, E.S.; Tiwari, B.K. Ultrasound-Assisted Extraction of Anthocyanins and Phenolics from Jabuticaba (Myrciaria Cauliflora) Peel: Kinetics and Mathematical Modeling. J. Food Sci. Technol. 2020, 57, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhou, W. Monte Carlo Modelling of Non-Isothermal Degradation of Two Cyanidin-Based Anthocyanins in Aqueous System at High Temperatures and Its Impact on Antioxidant Capacities. Food Chem. 2014, 148, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Bradley, R.L.; Edberg, S.; Krueger, D.A.; Lopez-Avila, V.; MacNeil, J.D. Peer-Verified Methods Advisory Committee. J. AOAC Int. 1996, 79, 324. [Google Scholar] [CrossRef]
- Muche, B.M.; Speers, R.A.; Rupasinghe, H.P.V. Storage Temperature Impacts on Anthocyanins Degradation, Color Changes and Haze Development in Juice of “Merlot” and “Ruby” Grapes (Vitis Vinifera). Front. Nutr. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, B.K.; Aaby, K.; Skrede, G. Effect of Temperature on Stability of Anthocyanins, Ascorbic Acid and Color in Strawberry and Raspberry Jams. Food Chem. 2020, 316, 126297. [Google Scholar] [CrossRef] [PubMed]
- Nkurunziza, D.; Pendleton, P.; Sivagnanam, S.P.; Park, J.-S.; Chun, B.S. Subcritical Water Enhances Hydrolytic Conversions of Isoflavones and Recovery of Phenolic Antioxidants from Soybean Byproducts (Okara). J. Ind. Eng. Chem. 2019, 80, 696–703. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).