Promoting Strawberry (Fragaria × ananassa) Stress Resistance, Growth, and Yield Using Native Bacterial Biostimulants
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation, Characterization, and Species Identification of Potential Growth-Promoting Bacteria
2.2. Bacterial Inoculation and Test of PGP Activity In Vivo
2.3. Growth Parameters, Disease Incidence, Disease Index, and Fruit Quality
2.4. Statistical Analysis
3. Results
3.1. Functional Characterization of the Culturable Bacteriota and Selection of PGPB Candidates for In Vivo Experiments
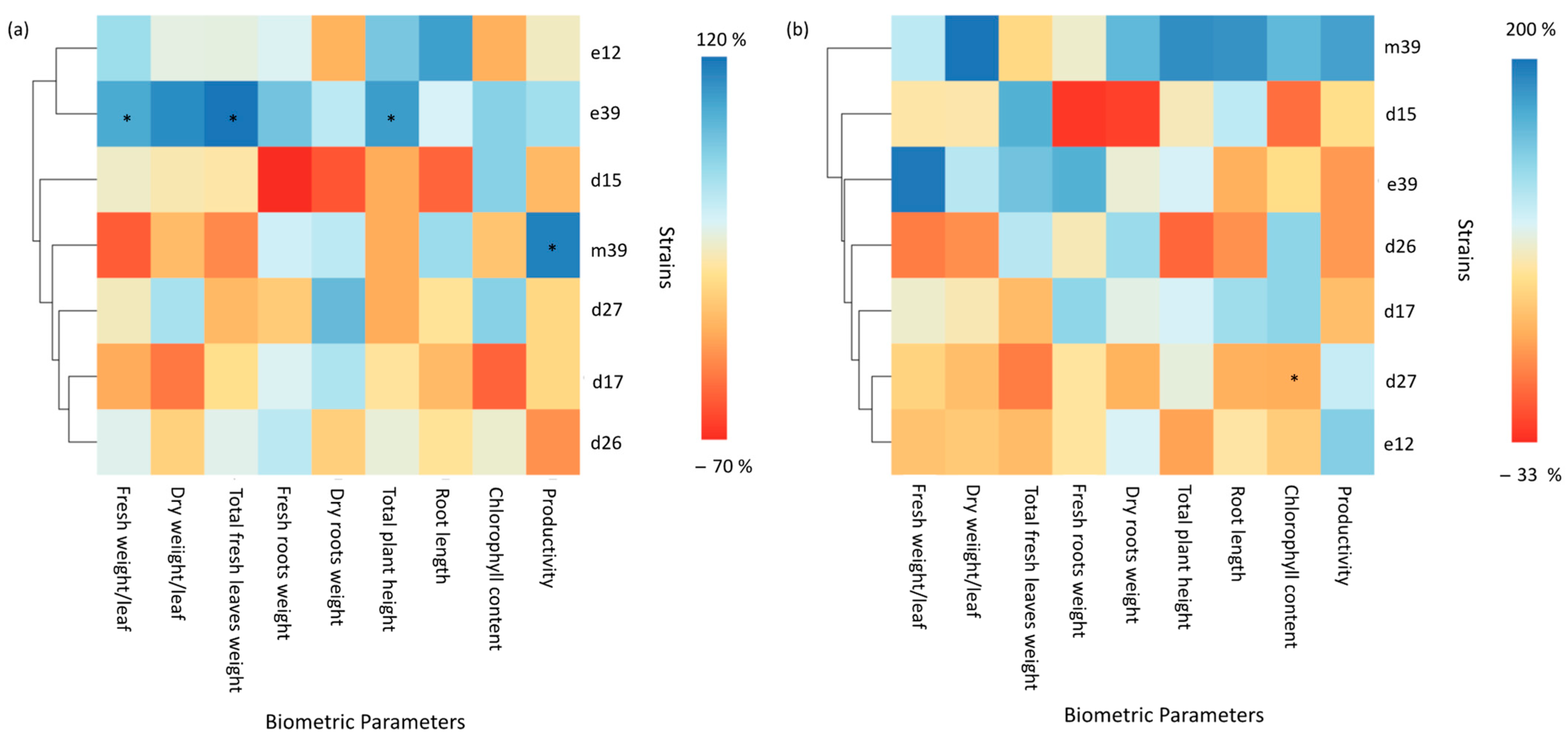
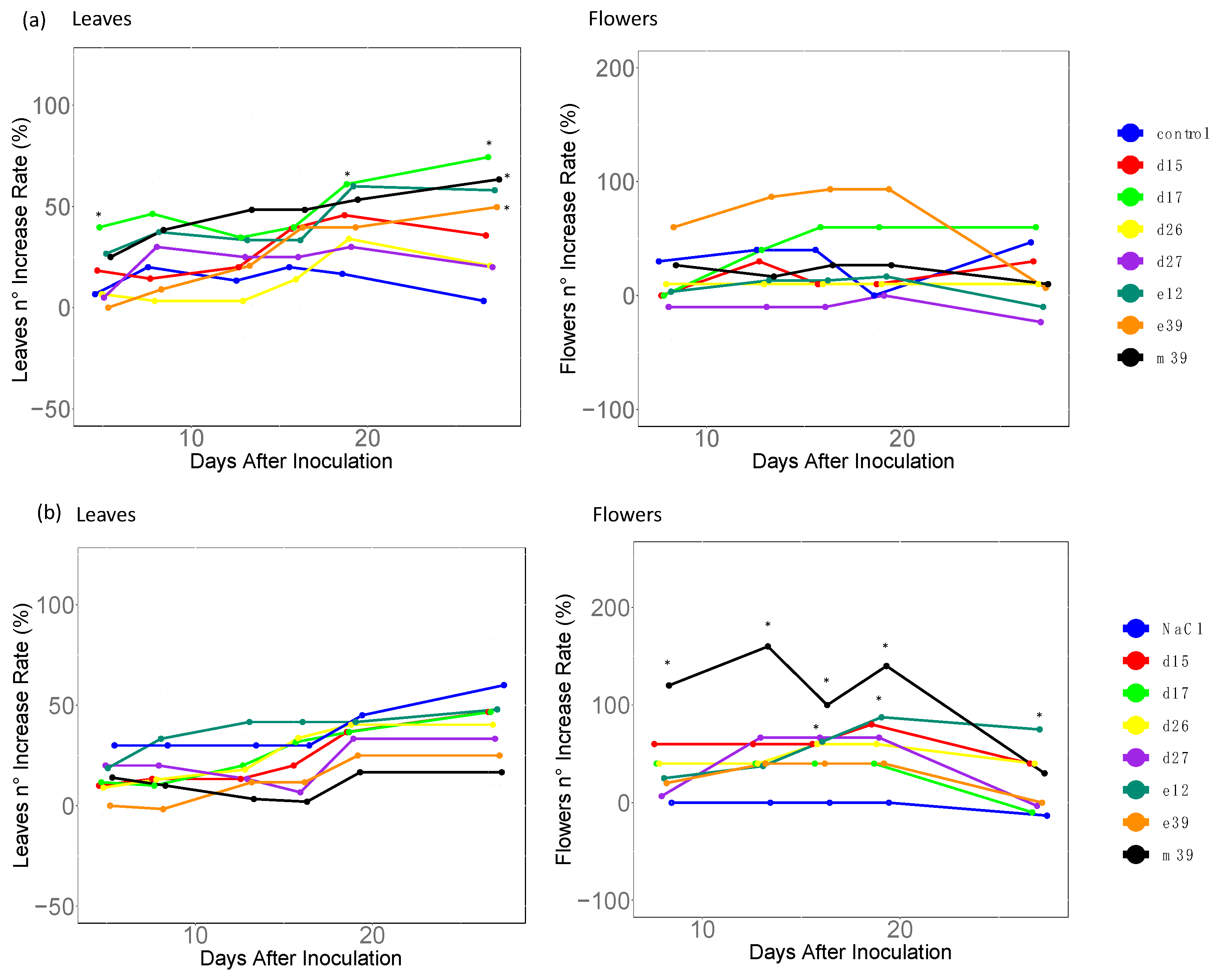
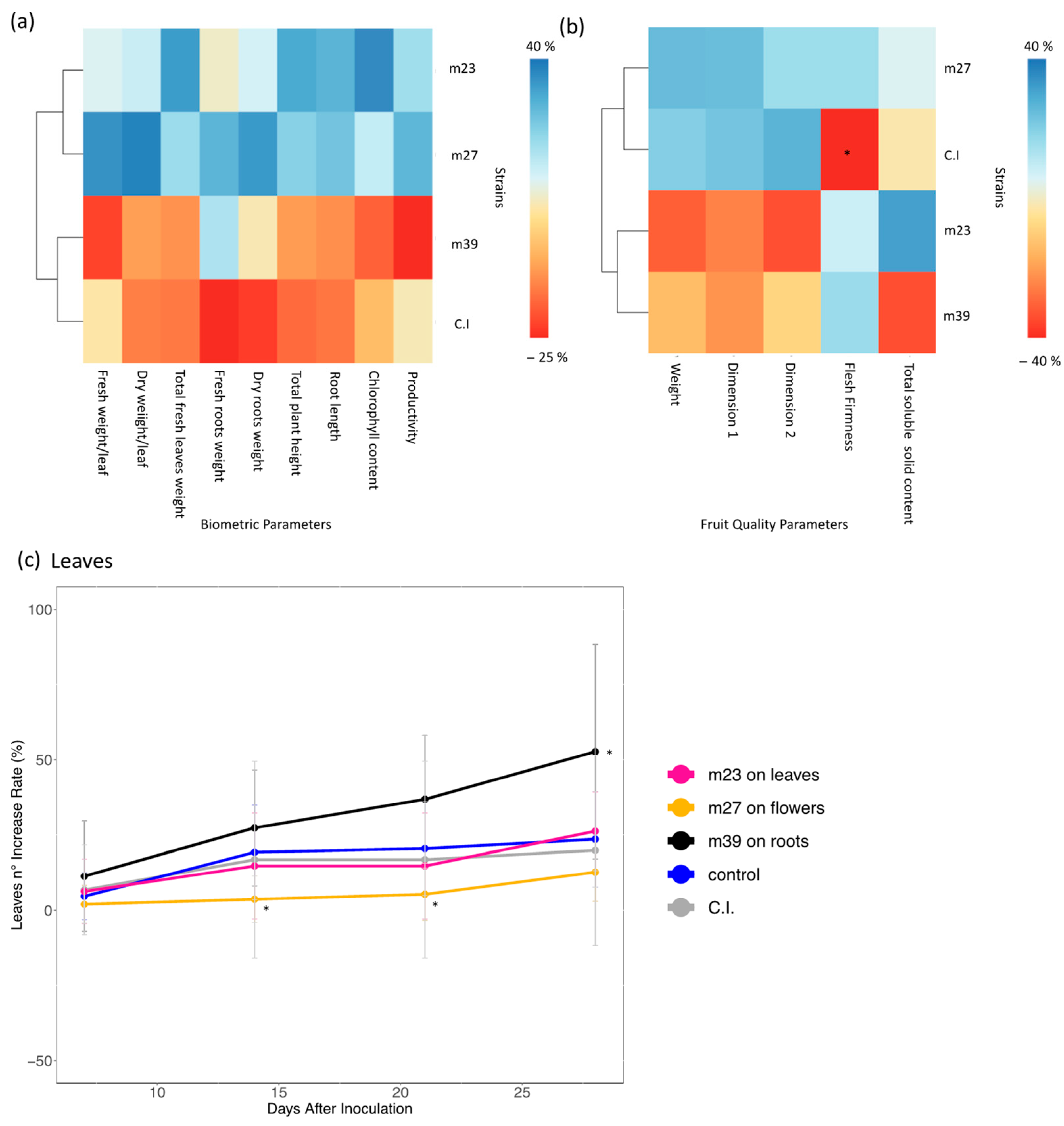
3.2. Plant Growth Promotion Activity in Saline-Stressed or Unstressed Strawberry Plants
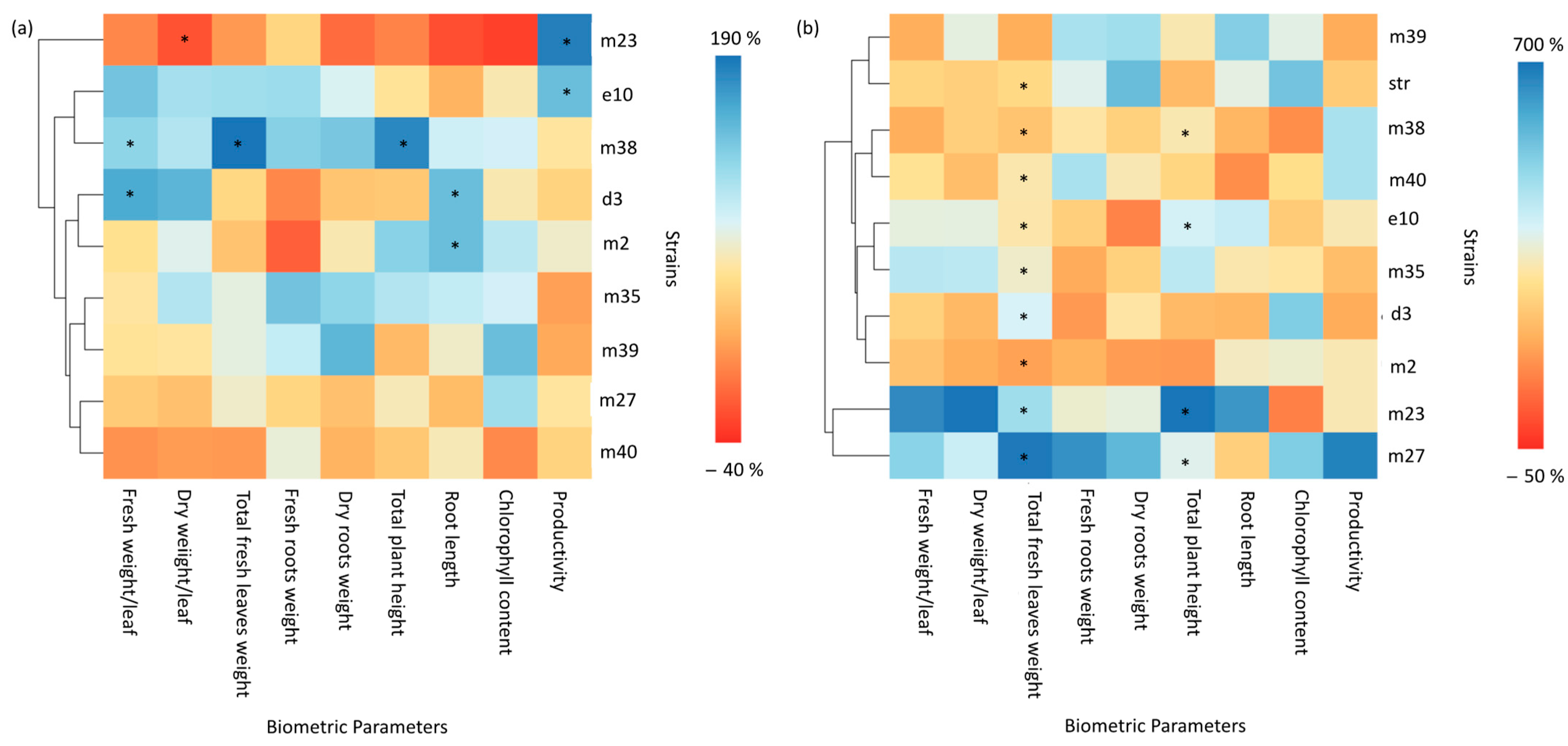
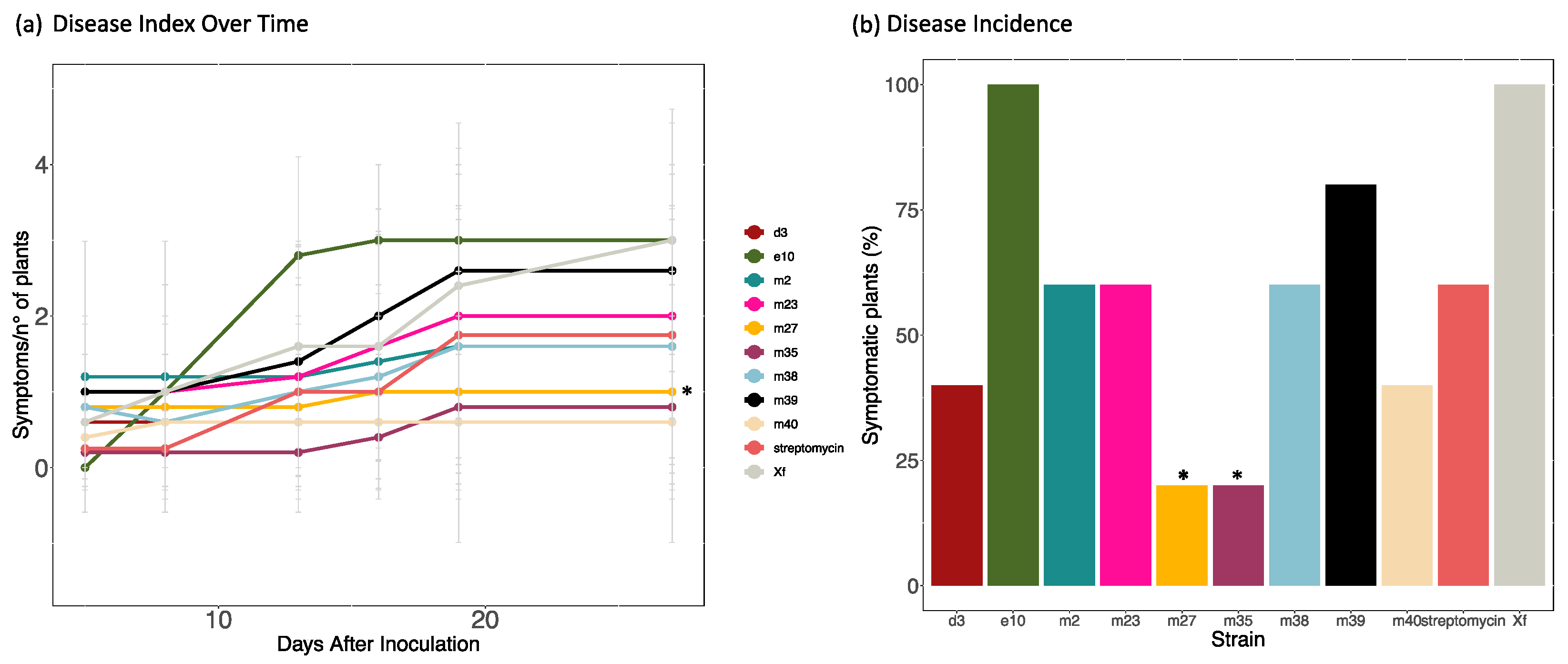
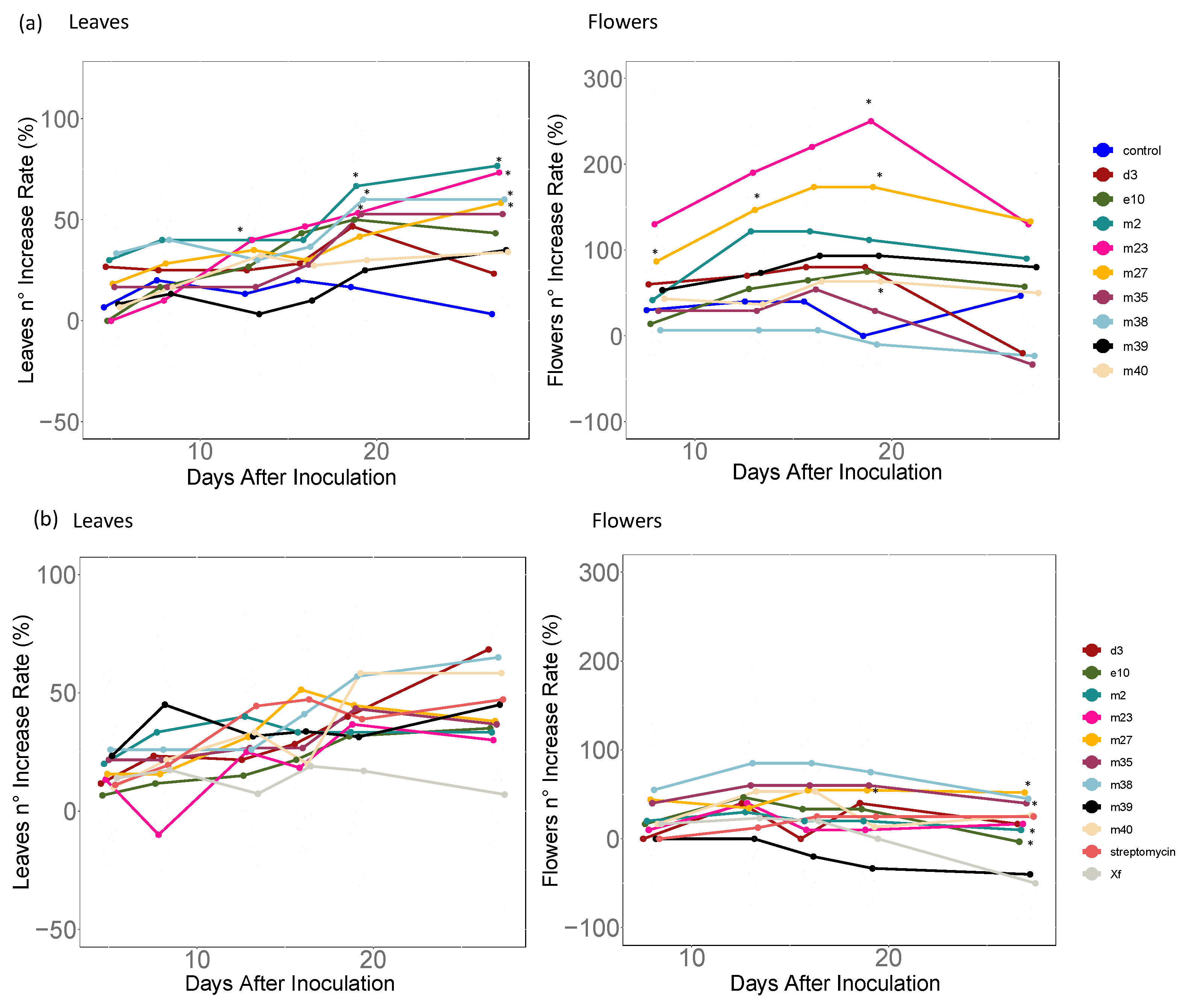
3.3. Plant Growth Promotion Activity in X. fragariae-Inoculated or healthy Strawberry Plants
3.4. Plant Growth Promotion Effect of Individually and Mixed-Inoculated Bacteria
4. Discussion
4.1. PGP Functions of Bacterial Isolates under Saline Stress
4.2. Plant Growth Promotion and Biological Control of X. fragariae
4.3. Design of Coordinated Inoculation Strategy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: www.fao.org/faostat/en (accessed on 17 January 2023).
- IndexBox EU-Strawberries-Market Analysis, Forecast, Size, Trends and Insights. Available online: www.indexbox.io/store/eu-strawberries-market-analysis-forecast-size-trends-and-insights (accessed on 22 June 2022).
- van der Wolf, J.M.; Evenhuis, A.; Kastelein, P.; Krijger, M.C.; Funke, V.Z.; van den Berg, W.; Moene, A.F. Risks for infection of strawberry plants with an aerosolized inoculum of Xanthomonas fragariae. Eur. J. Plant Pathol. 2018, 152, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of residual pesticides in fruit and vegetable waste (FVW) processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.; Wu, B.S.; MacPherson, S.; Lefsrud, M. A review of strawberry photobiology and fruit flavonoids in controlled environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi. J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Fruit yield and survival of five commercial strawberry cultivars under field cultivation and salinity stress. Sci. Hort. 2019, 243, 401–410. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, G.; Wallace, R.; Masabni, J.; Gu, M. Relative Salt Tolerance of Seven Strawberry Cultivars. Horticulturae 2015, 1, 27–43. [Google Scholar] [CrossRef]
- Attia, H.; Al-Yasi, H.; Alamer, K.; Ali, E.; Hassan, F.; Elshazly, S.; Hessini, K. Induced anti-oxidation efficiency and others by salt stress in Rosa damascena Miller. Sci. Hort. 2020, 274, 109681. [Google Scholar] [CrossRef]
- Yasir, T.A.; Khan, A.; Skalicky, M.; Wasaya, A.; Rehmani, M.I.A.; Sarwar, N.; Murbeen, K.; Aziz, M.; Hassan, M.M.; Hassan, F.A.S.; et al. Exogenous sodium nitroprusside mitigates salt stress in lentil (Lens culinaris medik.) by affecting the growth, yield, and biochemical properties. Molecules 2021, 26, 2576. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Hassan, F.; Hassan, M.M.; Brestic, M.; Sabagh, A.; et al. Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil. Agriculture 2021, 11, 310. [Google Scholar] [CrossRef]
- Kahil, A.A.; Hassan, F.A.S.; Ali, E.F. Influence of bio-fertilizers on growth, yield and anthocyanin content of Hibiscus sabdariffa L. plant under Taif region conditions. Annu. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Farneti, B.; Khomenko, I.; Muzzi, E.; Savioli, S.; Pastore, C.; Rodriguez-Estrada, M.T.; Donati, I. Does Organic Farming Increase Raspberry Quality, Aroma and Beneficial Bacterial Biodiversity? Microorganisms 2021, 9, 1617. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Spinelli, F.; Pastore, C.; Farneti, B.; Savioli, S.; Rodriguez-Estrada, M.T.; Donati, I. Contribution of fruit microbiome to raspberry volatile organic compounds emission. Postharvest Biol. Technol. 2022, 183, 111742. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marenga, E.; et al. Impact of beneficial microorganisms on strawberry growth, fruit production, nutritional quality, and volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Sessitsch, A.; Pfaffenbichler, N.; Mitter, B. Microbiome applications from lab to field: Facing complexity. Trends Plant Sci. 2019, 24, 194–198. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Banchio, E. Microbial Volatile Organic compounds produced by Bacillus amyloliquefaciens GB03 ameliorate the effects of salt stress in Mentha piperita principally through acetoin emission. J. Plant Growth Regul. 2020, 39, 764–775. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M.; Kim, Y.C. 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Leveau, J.; McSpadden Gardener, B.B.; Pierson, E.A.; Pierson, L.S., 3rd; Ryu, C.M. The multifactorial basis for plant health promotion by plant-associated bacteria. Appl. Environ. Microbiol. 2011, 77, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.F.; Wu, H.C.; Liu, Y.; Deng, J. Effect of Salt Stress on Acetoin Metabolism of an Aroma-producing Strain Bacillus subtilis. Appl. Biochem. Microbiol. 2019, 55, 506–513. [Google Scholar] [CrossRef]
- de Andrade, F.M.; de Assis Pereira, T.; Souza, T.P.; Guimarães, P.H.S.; Martins, A.D.; Schwan, R.F.; Pasqual, M.; Dória, J. Beneficial effects of inoculation of growth-promoting bacteria in strawberry. Microbiol. Res. 2019, 223–225, 120–128. [Google Scholar] [CrossRef]
- Gilbert, S.; Poulev, A.; Chrisler, W.; Acosta, K.; Orr, G.; Lebeis, S.; Lam, E. Auxin-producing bacteria from duckweeds have different colonization patterns and effects on plant morphology. Plants 2022, 11, 721. [Google Scholar] [CrossRef]
- Defez, R.; Andreozzi, A.; Bianco, C. The Overproduction of Indole-3-Acetic Acid (IAA) in endophytes upregulates nitrogen fixation in both bacterial cultures and inoculated rice plants. Microb. Ecol. 2017, 74, 441–452. [Google Scholar] [CrossRef]
- Bianco, C.; Defez, R. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 2010, 76, 4626–4632. [Google Scholar] [CrossRef]
- Bianco, C.; Defez, R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009, 60, 3097–3107. [Google Scholar] [CrossRef]
- Ayuso-Calles, M.; Flores-Félix, J.D.; Rivas, R. Overview of the Role of Rhizobacteria in Plant Salt Stress Tolerance. Agronomy 2021, 11, 1759. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting Plant–Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Santiago, C.D.; Yagi, S.; Ijima, M.; Nashimoto, T.; Sawada, M.; Ikeda, S.; Asano, K.; Orisaka, Y.; Ohwada, T. Bacterial compatibility in combined inoculations enhances the growth of potato seedlings. Microbes Environ. 2017, 32, 14–23. [Google Scholar] [CrossRef]
- Banerjee, A.; Donald, A.B.; Joshi, S.R. Native microorganisms as potent bioinoculants for plant growth promotion in shifting agriculture (Jhum) systems. J. Soil Sci. Plant Nutr. 2017, 17, 127–140. [Google Scholar] [CrossRef]
- May, A.; Coelho, L.F.; Pedrinho, A.; Batista, B.D.; Mendes, L.W.; Mendes, R.; Morandi, M.A.; Barth, G.; Viana, R.S.; Vilela, E.S.D. The use of indigenous bacterial community as inoculant for plant growth promotion in soybean cultivation. Arch. Agron. Soil Sci. 2021, 69, 135–150. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Ferrari, E.; Tanunchai, B.; Wahdan, S.F.M.; Sadubsarn, D.; Farneti, B.; Checcucci, A.; Buscot, F.; et al. Taxonomical and functional composition of strawberry microbiome is genotype-dependent. J. Adv. Res. 2022, 42, 189–204. [Google Scholar] [CrossRef]
- Bringel, F.; Castioni, A.; Olukoya, D.K.; Felis, G.E.; Torriani, S.; Dellaglio, F. Lactobacillus plantarum subsp. argentoratensis subsp. nov., isolated from vegetable matrices. Int. J. Syst. Evol. Microbiol. 2005, 55, 1629–1634. [Google Scholar] [CrossRef]
- Perpetuini, G.; Donati, I.; Cellini, A.; Orrú, L.; Giongo, L.; Farneti, B.; Spinelli, F. Genetic and functional characterization of the bacterial community on fruit of three raspberry (Rubus idaeus) cultivars. J. Berry Res. 2019, 9, 227–247. [Google Scholar] [CrossRef]
- Daranas, N.; Roselló, G.; Cabrefiga, J.; Donati, I.; Francés, J.; Badosa, E.; Spinelli, F.; Montesinos, E.; Bonaterra, A. Biological control of bacterial plant diseases with Lactobacillus plantarum strains selected for their broad-spectrum activity. Ann. Appl. Biol. 2019, 174, 92–105. [Google Scholar] [CrossRef]
- Xanthomonas fragariae; OEPP/EPPO Bulletin, PM 7/65; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 36, pp. 135–144. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.1365-2338.2006.00926.x (accessed on 17 January 2023).
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, H.; Pare, P.W. Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03). Plant Signal. Behav. 2009, 4, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S.; Esitken, A.; Cangi, R.; Sahin, F. Adventitious root formation of kiwifruit in relation to sampling date, IBA and Agrobacterium rubi inoculation. Plant Growth Regul. 2003, 41, 133–137. [Google Scholar] [CrossRef]
- Karaca, U.; Sabir, A. Sustainable mitigation of alkaline stress in grapevine rootstocks (Vitis spp.) by plant growth-promoting rhizobacteria. Erwerbs-Obstbau 2018, 60, 211–220. [Google Scholar] [CrossRef]
- Esitken, A. Use of plant growth promoting rhizobacteria in horticultural crops. In Bacteria in Agrobiology: Crop Ecosystems; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Ipek, M.; Pirlak, L.; Esitken, A.; Figen Dönmez, M.; Turan, M.; Sahin, F. Plant growth-promoting rhizobacteria (PGPR) increase yield, growth and nutrition of strawberry under high-calcareous soil conditions. J. Plant Nutr. 2014, 37, 990–1001. [Google Scholar] [CrossRef]
- Tomić, J.; Pešaković, M.; Milivojević, J.; Karaklajić-Stajić, Z. How to improve strawberry productivity, nutrients composition, and beneficial rhizosphere microflora by biofertilization and mineral fertilization? J. Plant Nutr. 2018, 41, 2009–2021. [Google Scholar] [CrossRef]
- Savini, G.; Neri, D. Strawberry architectural model. Acta Hortic. 2004, 649, 169–176. [Google Scholar] [CrossRef]
- Landi, L.; Mezzetti, B. TDZ, auxin and genotype effects on leaf organogenesis in Fragaria. Plant Cell Rep. 2006, 25, 281–288. [Google Scholar] [CrossRef]
- CABI Compendium. Rhizobium rubi (cane gall of Rubus); CABI International: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Cellini, A.; Donati, I.; Fiorentini, L.; Vandelle, E.; Polverari, A.; Venturi, V.; Buriani, G.; Vanneste, J.L.; Spinelli, F. N-Acyl Homoserine lactones and Lux Solos regulate social behaviour and virulence of Pseudomonas syringae pv. actinidiae. Microb. Ecol. 2020, 79, 383–396. [Google Scholar] [CrossRef]
- Epstein, A.H. Angular leaf spot of strawberry. Plant Dis. Rep. 1966, 50, 167. [Google Scholar]
- Bakker, P.; Pieterse, C.M.J.; Van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathol. 2007, 97, 239–243. [Google Scholar] [CrossRef]
- Wolf, A.; Fritze, A.; Hagemann, M.; Berg, G. Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int. J. Syst. Evol. Microbiol. 2002, 52, 1937–1944. [Google Scholar]
- Alavi, P.; Starcher, M.; Thallinger, G.; Zachow, C.; Müller, H.; Berg, G. Stenotrophomonas comparative genomics reveals genes and functions that differentiate beneficial and pathogenic bacteria. BMC Genom. 2014, 5, 482. [Google Scholar] [CrossRef]
- Hagemann, M.; Ribbeck-Busch, K.; Klähn, S.; Hasse, D.; Steinbruch, R.; Berg, G. The plant-associated bacterium Stenotrophomonas rhizophila expresses a new enzyme for the synthesis of the compatible solute glucosylglycerol. J. Bacteriol. 2008, 190, 5898–5906. [Google Scholar] [CrossRef]
- Pérez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernández, J.; Pelagio-Flores, R.; López-Bucio, J.; García-Juárez, P.; Macías-Rodríguez, L. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 2017, 254, 2201–2213. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Garrido-Oter, R.; Muench, P.C.; Weiman, A.; Droege, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef]
- Szymańska, S.; Sikora, M.; Hrynkiewicz, K.; Tyburski, J.; Tretyn, A.; Gołębiewski, M. Choosing source of microorganisms and processing technology for next generation beet bioinoculant. Sci. Rep. 2021, 11, 2829. [Google Scholar] [CrossRef]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Nat. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Mazzola, M.; Freilich, S. Prospects for biological soilborne disease control: Application of indigenous versus synthetic microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar] [CrossRef]
- Hillel, D.; Hatfield, J.L. Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; Volume 3. [Google Scholar]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 2011, 101, 1024–1031. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Park, J.M.; Lazarovits, G.; Bernards, M.A. Burkholderia phytofirmans-induced shoot and root growth promotion is associated with endogenous changes in plant growth hormone levels. Plant Growth Regul. 2015, 75, 199–207. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Naiman, A.D.; Latrónico, A.; de Salamone, I.E.G. Inoculation of wheat with Azospirillum brasilense and Pseudomonas fluorescens: Impact on the production and culturable rhizosphere microflora. Eur. J. Soil Biol. 2009, 45, 44–51. [Google Scholar] [CrossRef]
- Kozdrój, J.; Trevors, J.T.; Van Elsas, J.D. Influence of introduced potential biocontrol agents on maize seedling growth and bacterial community structure in the rhizosphere. Soil Biol. Biochem. 2004, 36, 1775–1784. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef]
| Strain | Species | Cultivar | Organ of Isolation | Acetoin | IAA | Siderophores | ACC-Deaminase | NH4+ | X. fragariae Inhibition | Organ of Application |
|---|---|---|---|---|---|---|---|---|---|---|
| (μM mg−1 h−1) | ||||||||||
| m39 | Agrobacterium rubi | Monterey | L | - | + | - | - | + | ++ | L/R |
| d9 | Bacillus pumilus | Darselet | F | ++ | - | - | - | ++ | - | |
| m2 | Bacillus pumilus | Monterey | L | ++ | - | - | - | + | + | L |
| d3 | Brevibacillus brevis | Darselect | L | - | - | - | - | - | ++ | L |
| e25 | Frigoribacterium sp. | Elsanta | F | + | - | - | - | + | +/- | |
| e10 | Massilia sp. | Elsanta | RS | - | - | C | - | + | + | L |
| m34 | Methylobacterium sp. | Monterey | F | - | - | - | 6.1 ± 6.9 | + | - | |
| e38 | Microbacterium sp. | Elsanta | F | - | - | - | - | - | - | |
| e15 | Pantoea agglomerans | Elsanta | F | + | - | - | - | ++ | - | |
| d15 | Pantoea agglomerans | Darselect | F | - | + | - | - | ++ | - | R |
| m8 | Pantoea agglomerans | Monterey | F | - | - | C | - | + | ++ | |
| e4 | Pseudoarthrobacter sp. | Elsanta | F | + | - | - | - | - | - | |
| m27 | Pseudomonas fluorescens | Monterey | R | + | - | C | - | + | ++ | L/F |
| d17 | Pseudomonas fluorescens | Darselect | S | - | + | C | 64.0 ± 25.6 | + | - | R |
| e12 | Pseudomonas fluorescens | Elsanta | RS | + | + | C | - | + | - | R |
| m7 | Pseudomonas fluorescens | Monterey | F | - | - | H | 135.2 ± 3.9 | + | - | |
| e39 | Pseudomonas jessenei | Darselect | RS | - | ++ | - | 33.7 ± 6.4 | + | - | R |
| d27 | Pseudomonas putida | Darselect | S | + | + | + | - | - | - | R |
| d26 | Pseudomonas siliensis | Darselect | S | - | + | + | - | - | - | R |
| e41 | Psychrobacillus sp. | Elsanta | F | - | - | - | - | + | - | |
| m42 | Psychrobacillus sp. | Monterey | F | - | - | - | - | + | - | |
| m35 | Rhizobium soli | Monterey | L | - | - | - | - | - | ++ | L |
| m38 | Rhodococcus sp. | Monterey | F | - | - | - | - | + | + | L |
| m23 | Stenotrophomonas rhizophila | Monterey | S | - | - | - | - | + | ++ | L |
| m40 | Vagococcus sp. | Monterey | L | - | + | - | - | - | + | L |
| e1 | Vagococcus sp. | Elsanta | F | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangiorgio, D.; Cellini, A.; Spinelli, F.; Donati, I. Promoting Strawberry (Fragaria × ananassa) Stress Resistance, Growth, and Yield Using Native Bacterial Biostimulants. Agronomy 2023, 13, 529. https://doi.org/10.3390/agronomy13020529
Sangiorgio D, Cellini A, Spinelli F, Donati I. Promoting Strawberry (Fragaria × ananassa) Stress Resistance, Growth, and Yield Using Native Bacterial Biostimulants. Agronomy. 2023; 13(2):529. https://doi.org/10.3390/agronomy13020529
Chicago/Turabian StyleSangiorgio, Daniela, Antonio Cellini, Francesco Spinelli, and Irene Donati. 2023. "Promoting Strawberry (Fragaria × ananassa) Stress Resistance, Growth, and Yield Using Native Bacterial Biostimulants" Agronomy 13, no. 2: 529. https://doi.org/10.3390/agronomy13020529
APA StyleSangiorgio, D., Cellini, A., Spinelli, F., & Donati, I. (2023). Promoting Strawberry (Fragaria × ananassa) Stress Resistance, Growth, and Yield Using Native Bacterial Biostimulants. Agronomy, 13(2), 529. https://doi.org/10.3390/agronomy13020529







