Effect of Water Regime, Nitrogen Level, and Biostimulant Application on the Water and Nitrogen Use Efficiency of Wild Rocket [Diplotaxis tenuifolia (L.) DC]
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Experimental Site
2.2. Climatic Trend
2.3. Treatments, Experimental Design, and Crop Management
2.4. Yield, above Ground, and Root Biomass
2.5. Total Nitrogen of above Ground Biomass and Roots
2.6. Nitrogen Uptake and Nitrogen Use Efficiency
- (1)
- The apparent recovery efficiency (RE);
- (2)
- Internal utilization efficiency (IE);
- (3)
- The partial productivity factor (PFPn) of the applied N;
- (4)
- The agronomic efficiency (AE) of application;
- (5)
- Physiological efficiency (PE).
2.7. Water Use Efficiency
2.8. Statistical Analysis
3. Results
3.1. Nitrogen Uptake and Nitrogen Use Efficiency
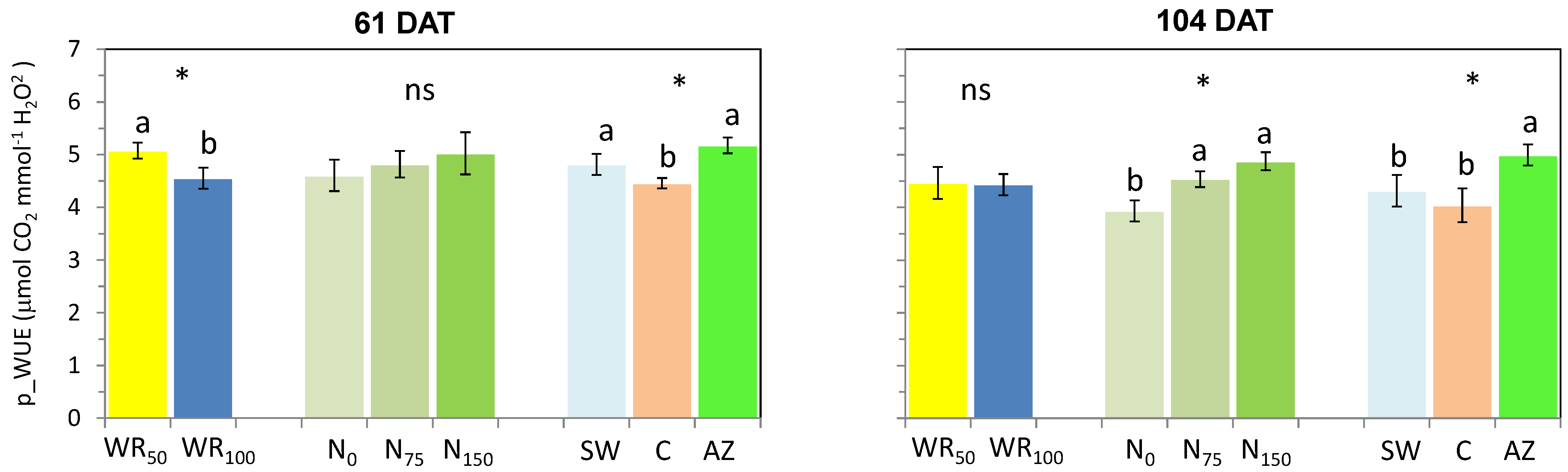
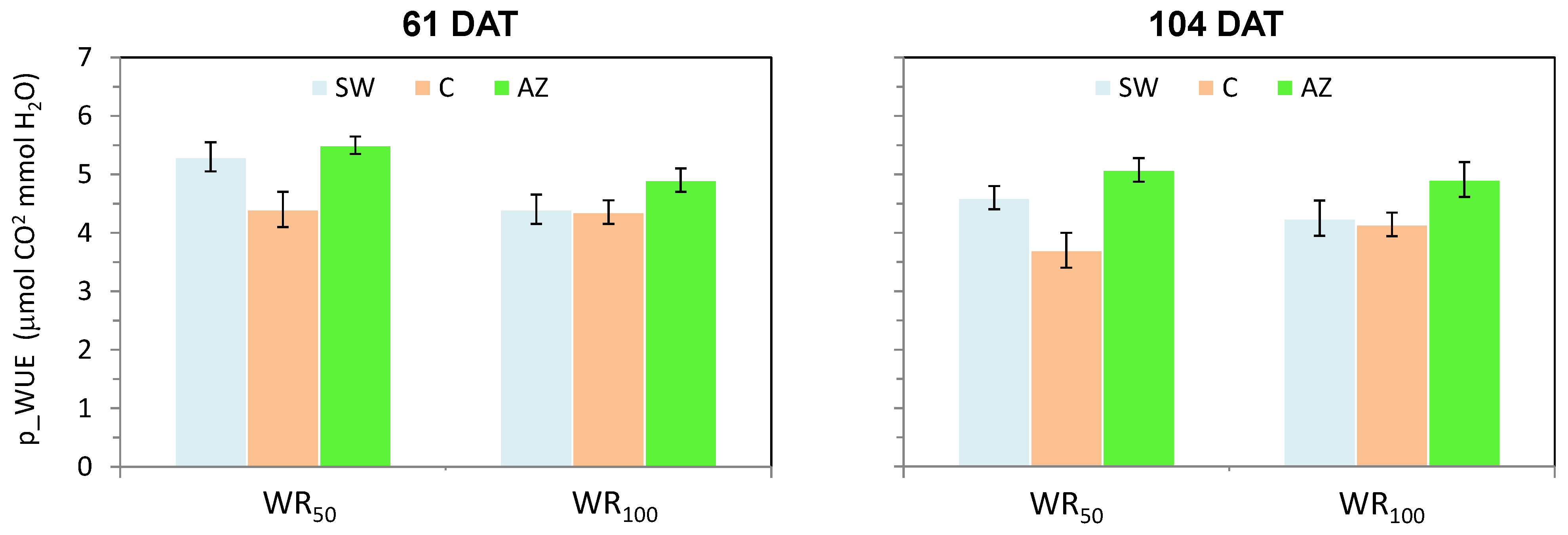
3.2. Water Use Efficiency
4. Discussion
4.1. Nitrogen Uptake and Nitrogen Use Efficiency
4.2. Water Use Efficiency
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laik, R.; Kumara, B.H.; Pramanick, B.; Singh, S.K.; Nidhi; Alhomrani, M.; Gaber, A.; Hossain, A. Labile Soil Organic Matter Pools Are Influenced by 45 Years of Applied Farmyard Manure and Mineral Nitrogen in the Wheat—Pearl Millet Cropping System in the Sub-Tropical Condition. Agronomy 2021, 11, 2190. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Grange, E.; Mannino, G.; van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea, C.M. A biostimulant seed treatment improved heat stress tolerance during cucumber seed germination by acting on the antioxidant system and glyoxylate cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-priming with seaweed extracts enhances yield of salt-stressed tomato plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Ghosh, A.; Zodape, S.T. Foliar Nutrient Management through Kappaphycus and Gracilaria Saps in Rice-Potato-Green Gram Crop Sequence. J. Sci. Ind. Res. 2014, 73, 613–617. [Google Scholar]
- Cantore, V.; Lechkar, O.; Karabulut, E.; Sellami, M.H.; Albrizio, R.; Boari, F.; Stellacci, A.M.; Todorovic, M. Combined effect of deficit irrigation and strobilurin application on yield, fruit quality and water use efficiency of “cherry” tomato (Solanum lycopersicum L.). Agric. Water Manage. 2016, 167, 53–61. [Google Scholar] [CrossRef]
- Boari, F.; Cantore, V.; Di Venere, D.; Sergio, L.; Candido, V.; Schiattone, M.I. Pyraclostrobin can mitigate salinity stress in tomato crop. Agric. Water Manage. 2019, 222, 254–264. [Google Scholar] [CrossRef]
- Candido, V.; Boari, F.; Cantore, V.; Castronuovo, D.; Di Venere, D.; Perniola, M.; Sergio, L.; Viggiani, R.; Schiattone, M.I. Interactive effect of nitrogen and Azoxystrobin on yield, quality, nitrogen and water use efficiency of wild rocket in Southern Italy. Agronomy 2020, 10, 849. [Google Scholar] [CrossRef]
- Schiattone, M.I.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Di Venere, D.; Perniola, M.; Renna, M.; Sergio, L.; Candido, V. Effects of nitrogen, Azoxystrobin and a biostimulant based on brown algae and yeast on wild rocket features at harvest and during storage. Agronomy 2021, 11, 2326. [Google Scholar] [CrossRef]
- Candido, V.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Sergio, L.; Todorovic, M.; Schiattone, M.I. Interactive effect of water regime, nitrogen rate and biostimulant application on physiological and biochemical traits of wild rocket. Agric. Water Manage. 2023, 277, 108075. [Google Scholar] [CrossRef]
- Schiattone, M.I.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Di Venere, D.; Perniola, M.; Sergio, L.; Todorovic, M.; Candido, V. Effect of water regime, nitrogen level and biostimulants application on yield and quality traits of wild rocket [Diplotaxis tenuifolia (L.) DC.]. Agric. Water Manage. 2023, 277, 108078. [Google Scholar] [CrossRef]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef]
- La Bella, S.; Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Sabatino, L. Impact of Ecklonia maxima seaweed extract and Mo foliar treatments on biofortification, spinach yield, quality and NUE. Plants 2021, 10, 1139. [Google Scholar] [CrossRef]
- Łangowski, Ł.; Goñi, O.; Ikuyinminu, E.; Feeney, E.; O’Connell, S. Investigation of the direct effect of a precision Ascophyllum nodosum biostimulant on nitrogen use efficiency in wheat seedlings. Plant Physiol. Biochem. 2022, 179, 44–57. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Yong, J.W.H. Harnessing synergistic biostimulatory processes: A plausible approach for enhanced crop growth and resilience in organic farming. Biology 2022, 11, 41. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. PPB 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Turěcová, V.; Strnad, M.; van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Seaweed carbohydrates. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 57–95. ISBN 978-1-119-35725-4. [Google Scholar]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweed. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 31–55. ISBN 978-1-119-35725-4. [Google Scholar]
- MacKinnon, S.L.; Hiltz, D.; Ugarte, R.; Craft, C.A. Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J. Appl. Phycol. 2010, 22, 489–494. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signalling in Arabidopsis: How to obtain insights into a complex signalling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Amaro, A.C.E.; Baron, D.; Ono, E.O.; Rodrigues, J.D. Physiological effects of strobilurin and carboxamides on plants: An overview. Acta Physiol. Plant. 2020, 42, 4. [Google Scholar] [CrossRef]
- Liang, S.; Xu, X.; Lu, Z. Effect of Azoxystrobin fungicide on the physiological and biochemical indices and ginsenoside contents of ginseng leaves. J. Ginseng Res. 2018, 42, 175–182. [Google Scholar] [CrossRef]
- Lopes, A.; Schumacher, P.V.; Martínez, A.; Netto, A.; Chalfun-Junior, A. Insights into the positive effect of Pyraclostrobin on sugarcane productivity. Agronomy 2018, 8, 122. [Google Scholar] [CrossRef]
- Boari, F.; Todorovic, M.; Albrizio, R.; Sellami, M.H.; Schiattone, M.I.; Cantore, V. Le strobilurine su pomodoro migliorano le rese e l’uso dell’acqua. L’Inftore Agrario 2017, 73, 42–45. [Google Scholar]
- Giuliani, M.M.; Carucci, F.; Nardella, E.; Francavilla, M.; Ricciardi, L.; Lotti, C.; Gatta, G. Combined effects of deficit irrigation and strobilurin application on gas exchange, yield and water use efficiency in tomato (Solanum lycopersicum L.). Sci. Hortic. 2018, 233, 149–158. [Google Scholar] [CrossRef]
- Van Dingenen, J.; Antoniou, C.; Filippou, P.; Pollier, J.; Gonzalez, N.; Dhondt, S.; Goossens, A.; Fotopoulos, V.; Inzé, D. Strobilurins as growth-promoting compounds: How stroby regulates Arabidopsis leaf growth. Plant Cell Environ. 2017, 40, 1748–1760. [Google Scholar] [CrossRef]
- Amaro, A.C.E.; Ramos, A.R.P.; Macedo, A.C.; Ono, E.O.; Rodrigues, J.D. Effects of the fungicides Azoxystrobin, Pyraclostrobin and Boscalid on the physiology of japanese cucumber. Sci. Hortic. 2018, 228, 66–75. [Google Scholar] [CrossRef]
- Joshi, J.; Sharma, S.; Guruprasad, K.N. Foliar application of Pyraclostrobin fungicide enhances the growth, rhizobial-nodule formation and nitrogenase activity in soybean (Var. JS-335). Pestic. Biochem. Physiol. 2014, 114, 61–66. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Pre-harvest nitrogen and Azoxystrobin application enhances postharvest shelf-life in butterhead lettuce. Postharvest Biol. Technol. 2013, 85, 67–76. [Google Scholar] [CrossRef]
- Conversa, G.; Bonasia, A.; Lazzizera, C.; Elia, A. Pre-harvest nitrogen and Azoxystrobin application enhances raw product quality and post-harvest shelf-life of baby spinach (Spinacia oleracea L.). J. Sci. Food Agric. 2014, 94, 3263–3272. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.; De Pascale, S.; Cozzolino, E.; Pannico, A.; Giordano, M.; Teliban, G.; Cuciniello, A.; Rouphael, Y. Production, leaf quality and antioxidants of perennial wall rocket as affected by crop cycle and mulching type. Agronomy 2019, 9, 194. [Google Scholar] [CrossRef]
- Schiattone, M.I.; Candido, V.; Cantore, V.; Montesano, F.F.; Boari, F. Water use and crop performance of two wild rocket genotypes under salinity conditions. Agric. Water Manage. 2017, 194, 214–221. [Google Scholar] [CrossRef]
- Caruso, G.; Parrella, G.; Giorgini, M.; Nicoletti, R. Crop systems, quality and protection of Diplotaxis tenuifolia. Agriculture 2018, 8, 55. [Google Scholar] [CrossRef]
- Schiattone, M.I. Interventi Agronomici per Migliorare la Produzione, la qualità e l’efficienza d’uso dell’acqua e dell’azoto della ruchetta [Diplotaxis tenuiflia (L.) DC.]. Ph.D. Tesis, Università degli Studi della Basilicata, Potenza, Italy, 2018; p. 191. [Google Scholar]
- Di Venere, D.; Calabrese, N.; Linsalata, V.; Cardinali, A.; Bianco, V.V. Influence of sowing time on phenolic composition of rocket. Acta Hort. 2000, 533, 343–350. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Elementi, S.; Neri, R. Exploring New Potential Health-Promoting Vegetables: Glucosinolates and sensory attributes of rocket salads and related Diplotaxis and Eruca species. J. Sci. Food Agric. 2009, 89, 713–722. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Rincón-Cervera, M.A.; González-Fernández, M.J.; Guil-Guerrero, J.L. Phytochemical composition and antitumor activities of new salad greens: Rucola (Diplotaxis tenuifolia) and corn salad (Valerianella locusta). Plant Foods Hum. Nutr. 2016, 71, 197–203. [Google Scholar] [CrossRef] [PubMed]
- USDA. Keys to Soil Taxonomy, 10th ed.; Soil Survey Staff; United States Department of Agriculture NRCS, US Government Printing Office: Washington, DC, USA, 2006; p. 332.
- Ghaemi, A.A.; Rafiee, M.R. Evapotranspiration and yield of eggplant under salinity and water deficit: A comparison between greenhouse and outdoor cultivation. Mod. Appl. Sci. 2016, 10, 8–18. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements. In FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; p. 300. [Google Scholar]
- Dobermann, A. Nitrogen Use Efficiency—State of the Art. In Agronomy & Horticulture—Faculty Publications; University of Nebraska—Lincoln: Lincoln, NE, USA, 2005; Volume 316, pp. 1–16. [Google Scholar]
- Santamaria, P.; Elia, A.; Serio, F. Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes. J. Plant Nutr. 2002, 25, 245–258. [Google Scholar] [CrossRef]
- Karam, F.; Mounzer, O.; Sarkis, F.; Lahoud, R. Yield and nitrogen recovery of lettuce under different irrigation regimes. J. Appl. Hort. 2002, 4, 70–76. [Google Scholar] [CrossRef]
- Zhang, J.; Sha, Z.; Zhang, Y.; Bei, Z.; Cao, L. The effects of different water and nitrogen levels on yield, water and nitrogen utilization efficiencies of spinach (Spinacia oleracea L.). Can. J. Plant Sci. 2015, 95, 671–679. [Google Scholar] [CrossRef]
- Ceylan, S.; Mordogan, N.; Cakigi, H.; Yoldas, F. Effects of different nitrogen levels on the yield and nitrogen accumulation in the rocket. Asian J. Plant Sci. 2002, 1, 482–483. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Stavridou, E. Deep root growth and nitrogen uptake by rocket (Diplotaxis tenuifolia L.) as affected by nitrogen fertilizer, plant density and leaf harvesting on a coarse sandy soil. Soil Use Manage. 2017, 33, 62–71. [Google Scholar] [CrossRef]
- Albrizio, R.; Todorovic, M.; Matic, T.; Stellacci, A. Comparing the interactive effects of water and nitrogen on durum wheat and barley grown in a Mediterranean environment. Field Crops Res. 2010, 115, 179–190. [Google Scholar] [CrossRef]
- Benincasa, P.; Guiducci, M.; Tei, F. The nitrogen use efficiency: Meaning and sources of variation—Case studies on three vegetable crops in central Italy. HortTechnology 2011, 21, 266–273. [Google Scholar] [CrossRef]
- Ronga, D.; Parisi, M.; Pentangelo, A.; Mori, M.; Di Mola, I. Effects of nitrogen management on biomass production and dry matter distribution of processing tomato cropped in Southern Italy. Agronomy 2019, 9, 855. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen use and uptake efficiency and crop performance of baby spinach (Spinacia oleracea L.) and lamb’s lettuce (Valerianella locusta L.) grown under variable sub-optimal N regimes combined with plant-based biostimulant application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; Nocerino, S.; Sifola, M.I.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Can seaweed extract improve yield and quality of brewing barley subjected to different levels of nitrogen fertilization? Agronomy 2021, 11, 2481. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant application under different nitrogen fertilization levels: Assessment of yield, leaf quality, and nitrogen metabolism of tunnel-grown lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Aujla, M.S.; Thind, H.S.; Buttar, G. Fruit yield and water use efficiency of eggplant (Solanum melongema L.) as influenced by different quantities of nitrogen and water applied through drip and furrow irrigation. Sci. Hortic. 2007, 112, 142–148. [Google Scholar] [CrossRef]
- Zotarelli, L.; Dukes, M.D.; Scholberg, J.M.S.; Muñoz-Carpena, R.; Icerman, J. Tomato nitrogen accumulation and fertilizer use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agric. Water Manage. 2009, 96, 1247–1258. [Google Scholar] [CrossRef]
- Song, X.-Z.; Zhao, C.-X.; Wang, X.-L.; Li, J. Study of nitrate leaching and nitrogen fate under intensive vegetable production pattern in Northern China. Comptes Rendus Biol. 2009, 332, 385–392. [Google Scholar] [CrossRef]
- Bryson, R.J.; Leandro, L.; Jones, D.R. The physiological effects of Kresoxim-methyl on wheat leaf greenness and the implications for crop yield. In In The BCPC Conference: Pests and Diseases, Proceedings of An International Conference Held at the Brighton Hilton Metropole Hotel, Brighton, UK, 13–16 November 2000; British Crop Protection Council: Farnham, UK, 2000; Volume 2, pp. 739–746. [Google Scholar]
- Goñi, O.; Łangowski, Ł.; Feeney, E.; Quille, P.; O’Connell, S. Reducing nitrogen input in barley crops while maintaining yields using an engineered biostimulant derived from Ascophyllum nodosum to enhance nitrogen use efficiency. Front. Plant Sci. 2021, 12, 664682. [Google Scholar]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Quille, P.; Claffey, A.; Feeney, E.; Kacprzyk, J.; Ng, C.K.-Y.; O’Connell, S. The effect of an engineered biostimulant derived from Ascophyllum nodosum on grass yield under a reduced nitrogen regime in an agronomic setting. Agronomy 2022, 12, 463. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant- and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Gooding, M.J.; Gregory, P.J.; Ford, K.E.; Pepler, S. Fungicide and cultivar affect post-anthesis patterns of nitrogen uptake, remobilization and utilization efficiency in wheat. J. Agric. Sci. 2005, 143, 503–518. [Google Scholar] [CrossRef]
- Gooding, M.; Gregory, P.; Ford, K.E.; Ruske, R.E. Recovery of nitrogen from different sources following applications to winter wheat at and after anthesis. Field Crops Res. 2007, 100, 143–154. [Google Scholar] [CrossRef]
- Köehle, H.; Grossmann, K.; Jabs, T.; Gerhard, M.; Kaiser, W.; Glaab, J.; Conrath, U.; Seehaus, K.; Herms, S. Physiological effects of the strobilurin fungicide F 500 on plants. In Modern Fungicides and Antifungal Compounds III; Lyr, H., Russell, P.E., Dehne, H.W., Sisler, H.D., Eds.; Intercept: Andover, UK, 2002; pp. 61–74. [Google Scholar]
- Carucci, F.; Gatta, G.; Gagliardi, A.; Vita, P.D.; Giuliani, M.M. Strobilurin effects on nitrogen use efficiency for the yield and protein in durum wheat grown under rainfed Mediterranean conditions. Agronomy 2020, 10, 1508. [Google Scholar] [CrossRef]
- Jamieson, P.D.; Semenov, M.A. Modelling nitrogen uptake and redistribution in wheat. Field Crops Res. 2000, 68, 21–29. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Kanungo, M.; Joshi, J. Impact of Pyraclostrobin (F-500) on crop plants. Plant Sci. Today 2014, 1, 174–178. [Google Scholar] [CrossRef]
- Glaab, J.; Kaiser, W.M. Increased nitrate reductase activity in leaf tissue after application of the fungicide Kresoxim-methyl. Planta 1999, 207, 442–448. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Wu, F.Z.; Bao, W.K.; Li, F.L.; Wu, N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica 2008, 46, 40–48. [Google Scholar] [CrossRef]
- Blunden, G.; Currie, M.; Mathe, J.; Hohmann, J.; Critchley, A.T. Variations in betaine yields from marine algal species commonly used in the preparation of seaweed extracts used in agriculture. Phycology 2009, 76, 14. [Google Scholar]
- Neily, W.; Shishkov, L.; Tse, T.; Titus, D. Acadian LSC Helps reduce salinity stress in pepper seedlings- cv. California Wonder. PGRSA Newsl 2008, 1, 14. [Google Scholar]
- do Rosário Rosa, V.; Farias Dos Santos, A.L.; Alves da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-induced drought tolerance in grapevine is associated with physiological and biochemical changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Cantore, V.; Boari, F.; Rubino, P. Consumi idrici ed efficienza d’uso dell’acqua della ruchetta selvatica (Diplotaxis tenuifolia L. DC.). [Water consumption and water use efficiency of wild rocket (Diplotaxis tenuifolia L. DC.)]. In Proceedings of the VI Giornate Scientifiche SOI, Spoleto, Italy, 22–25 April 2002; pp. 529–530. [Google Scholar]
- Ueno, O.; Bang, S.W.; Wada, Y.; Kondo, A.; Ishihara, K.; Kaneko, Y.; Matsuzawa, Y. Structural and biochemical dissection of photorespiration in hybrids differing in genome constitution between Diplotaxis tenuifolia (C3-C4) and radish (C3). Plant Physiol. 2003, 132, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, S.B.; Milroy, S.P. Crop water use and water use efficiency on irrigated cotton farms in Australia. Agric. Water Manage. 2003, 61, 179–194. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 1998; p. 540. [Google Scholar]
- Zhang, X.; Chen, S.; Sun, H.; Pei, D.; Wang, Y. Dry matter, harvest index, grain yield and water use efficiency as affected by water supply in winter wheat. Irrig. Sci. 2008, 27, 1–10. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Gatta, G.; Nardella, E.; Tarantino, E. Water saving strategies assessment on processing tomato cultivated in Mediterranean Region. Ital. J. Agron. 2016, 11, 69–76. [Google Scholar] [CrossRef]
- Lovelli, S.; Potenza, G.; Castronuovo, D.; Perniola, M.; Candido, V. Yield, quality and water use efficiency of processing tomatoes produced under different irrigation regimes in Mediterranean environment. Ital. J. Agron. 2017, 12, 17–24. [Google Scholar] [CrossRef]
- Rahimi, A.; Sayadi, F.; Dashti, H.; Tajabadi pour, A. Effects of water and nitrogen supply on growth, water-use efficiency and mucilage yield of isabgol (Plantago ovata Forsk). J. Soil Sci. Plant Nutr. 2013, 13, 341–354. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Nardella, E.; Gatta, G.; De Caro, A.; Quitadamo, M. Processing tomato cultivated under water deficit conditions: The effect of Azoxystrobin. Acta Hortic. 2011, 914, 287–294. [Google Scholar] [CrossRef]
- Gilardi, G.; Demarchi, S.; Gullino, M.L.; Garibaldi, A. Management of leaf spot of wild rocket using fungicides, resistance inducers and a biocontrol agent, under greenhouse conditions. Crop Prot. 2015, 71, 39–44. [Google Scholar] [CrossRef]
| Parameters | Units | Values |
|---|---|---|
| Particle-size analysis | ||
| Total sand (2 > ø > 0.02 mm) | (g 100 g−1) | 20.6 |
| Silt (0.02 > ø > 0.002 mm) | (g 100 g−1) | 48.8 |
| Clay (ø < 0.002 mm) | (g 100 g−1) | 30.6 |
| Chemical properties | ||
| Total nitrogen (Kjeldahl method) | (g kg−1) | 1.16 |
| Available phosphorus (Olsen method) | (mg kg−1) | 37.2 |
| Exchangeable potassium (ammonium acetate method) | (mg kg−1) | 345.4 |
| Organic matter (Walkley Black method) | (g kg−1) | 19.2 |
| Total limestone (Dietrich-Fruhling method) | (g kg−1) | 22.4 |
| Active limestone | (g kg−1) | 10.3 |
| ECe (2:1) | (dS m−1) | 0.35 |
| ESP | (%) | 1.3 |
| pH (pH in H2O) | - | 7.5 |
| Hydrological properties | ||
| Field capacity | (cm3 cm−3) | 37.1 |
| Wilting point (−1.5 MPa) | (cm3 cm−3) | 23.2 |
| Bulk density | (kg dm−3) | 1.23 |
| Treatments | 1st Crop Cycle | 2nd Crop Cycle | ||||||
|---|---|---|---|---|---|---|---|---|
| N_AGB | N_r | Nup_AGB | Nup_r | N_AGB | N_r | Nup_AGB | Nup_r | |
| (%) | (%) | (g m-2) | (g m-2) | (%) | (%) | (g m-2) | (g m-2) | |
| Water regimes (WRs) | ns | ns | * | * | ns | ns | * | * |
| WR50 | 4.3 | 3.3 | 3.8 | 1.8 | 4.0 | 3.3 | 2.6 | 1.5 |
| WR100 | 4.6 | 3.7 | 5.2 | 2.3 | 4.4 | 3.7 | 4.1 | 1.9 |
| Nitrogen levels (NLs) | * | * | ** | * | * | * | ** | * |
| N0 | 3.9 b | 2.9 b | 2.7 c | 1.5 b | 3.5 b | 3.0 b | 1.2 c | 1.2 b |
| N75 | 4.4 ab | 3.5 ab | 4.6 b | 2.0 ab | 4.3 ab | 3.5 ab | 3.5 b | 1.9 a |
| N150 | 5.0 a | 4.1 a | 6.2 a | 2.8 a | 4.8 a | 4.0 a | 5.4 a | 2.0 a |
| Biostimulants (BSs) | ns | ns | ns | ns | ns | ns | ns | ns |
| C | 4.4 | 3.4 | 4.3 | 1.9 | 4.0 | 3.4 | 3.1 | 1.6 |
| AZ | 4.4 | 3.5 | 4.8 | 2.2 | 4.3 | 3.6 | 3.6 | 1.7 |
| SW | 4.5 | 3.5 | 4.4 | 2.1 | 4.3 | 3.6 | 3.3 | 1.8 |
| All interactions | ns | ns | ns | ns | ns | ns | ns | ns |
| Treatments | RE | IE | PFPn | AE | PE |
|---|---|---|---|---|---|
| (g Nup g-1 N) | (g FW g−1 Nup) | (g FW g−1 N) | (g FW g−1 N) | (g FW g−1 Nup) | |
| 1st Crop Cycle | |||||
| Water regimes (WRs) | * | ns | ** | ** | ns |
| WR50 | 0.39 | 188.8 | 151.5 | 66.7 | 166.8 |
| WR100 | 0.55 | 184.8 | 205.2 | 94.4 | 172.6 |
| Nitrogen levels (NLs) | ns | * | ** | * | * |
| N0 | - | 193.7 a | - | - | - |
| N75 | 0.49 | 189.3 a | 218.3 | 88.0 | 177.8 |
| N150 | 0.45 | 177.4 b | 138.3 | 73.1 | 161.6 |
| Biostimulants (BSs) | * | * | * | * | * |
| C | 0.43 b | 178.3 b | 166.5 b | 69.3 c | 162.9 c |
| AZ | 0.42 b | 203.0 a | 185.1 a | 81.9 b | 168.8 b |
| SW | 0.55a | 179.1 b | 183.3 a | 90.5 a | 177.4 a |
| All interactions | ns | ns | ns | ns | ns |
| 2nd Crop Cycle | |||||
| Water regimes (WRs) | * | ns | ** | ** | ns |
| WR50 | 0.45 | 187.3 | 126.3 | 81.9 | 181.6 |
| WR100 | 0.82 | 192.4 | 201.4 | 146.5 | 180.4 |
| Nitrogen levels (NLs) | ns | * | ** | * | ns |
| N0 | - | 198.4 a | - | - | - |
| N75 | 0.66 | 186.2 b | 184.1 | 118.0 | 180.8 |
| N150 | 0.61 | 185.0 b | 143.6 | 110.4 | 181.3 |
| Biostimulants (BSs) | * | ns | * | * | * |
| C | 0.59 b | 188.7 | 155.7 b | 109.5 b | 170.0 b |
| AZ | 0.64 ab | 191.1 | 170.7 a | 113.6 ab | 186.6 a |
| SW | 0.66 a | 189.9 | 165.2 a | 119.5 a | 186.4 a |
| All interactions | ns | ns | ns | ns | ns |
| Treatments | 1st Crop Cycle | 2nd Crop Cycle | ||||
|---|---|---|---|---|---|---|
| Y_WUE | B_WUE | IY_WUE | Y_WUE | B_WUE | IY_WUE | |
| Water regimes (WRs) | ns | ns | ** | * | ns | * |
| WR50 | 12.3 | 1.5 | 17.1 | 9.6 | 1.2 | 11.4 |
| WR100 | 11.4 | 1.3 | 11.6 | 8.3 | 0.9 | 8.9 |
| Nitrogen levels (NLs) | ** | * | ** | ** | * | ** |
| N0 | 7.4 c | 1.0 c | 9.0 c | 3.4 c | 0.5 c | 3.9 c |
| N75 | 12.4 b | 1.5 b | 15.1 b | 9.2 b | 1.2 b | 10.4 b |
| N150 | 15.7 a | 1.8 a | 18.9 a | 14.3 a | 1.6 a | 16.1 a |
| Biostimulants (BSs) | * | * | * | * | * | * |
| C | 10.8 b | 1.3 b | 13.0 b | 8.3 b | 1.0 b | 9.3 b |
| AZ | 12.7 a | 1.4 ab | 15.5 a | 9.8 a | 1.2 a | 11.1 a |
| SW | 12.1 a | 1.5 a | 14.6 a | 8.9 b | 1.1 ab | 10.0 ab |
| All interactions | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candido, V.; Cantore, V.; Castronuovo, D.; Denora, M.; Schiattone, M.I.; Sergio, L.; Todorovic, M.; Boari, F. Effect of Water Regime, Nitrogen Level, and Biostimulant Application on the Water and Nitrogen Use Efficiency of Wild Rocket [Diplotaxis tenuifolia (L.) DC]. Agronomy 2023, 13, 507. https://doi.org/10.3390/agronomy13020507
Candido V, Cantore V, Castronuovo D, Denora M, Schiattone MI, Sergio L, Todorovic M, Boari F. Effect of Water Regime, Nitrogen Level, and Biostimulant Application on the Water and Nitrogen Use Efficiency of Wild Rocket [Diplotaxis tenuifolia (L.) DC]. Agronomy. 2023; 13(2):507. https://doi.org/10.3390/agronomy13020507
Chicago/Turabian StyleCandido, Vincenzo, Vito Cantore, Donato Castronuovo, Michele Denora, Maria Immacolata Schiattone, Lucrezia Sergio, Mladen Todorovic, and Francesca Boari. 2023. "Effect of Water Regime, Nitrogen Level, and Biostimulant Application on the Water and Nitrogen Use Efficiency of Wild Rocket [Diplotaxis tenuifolia (L.) DC]" Agronomy 13, no. 2: 507. https://doi.org/10.3390/agronomy13020507
APA StyleCandido, V., Cantore, V., Castronuovo, D., Denora, M., Schiattone, M. I., Sergio, L., Todorovic, M., & Boari, F. (2023). Effect of Water Regime, Nitrogen Level, and Biostimulant Application on the Water and Nitrogen Use Efficiency of Wild Rocket [Diplotaxis tenuifolia (L.) DC]. Agronomy, 13(2), 507. https://doi.org/10.3390/agronomy13020507












