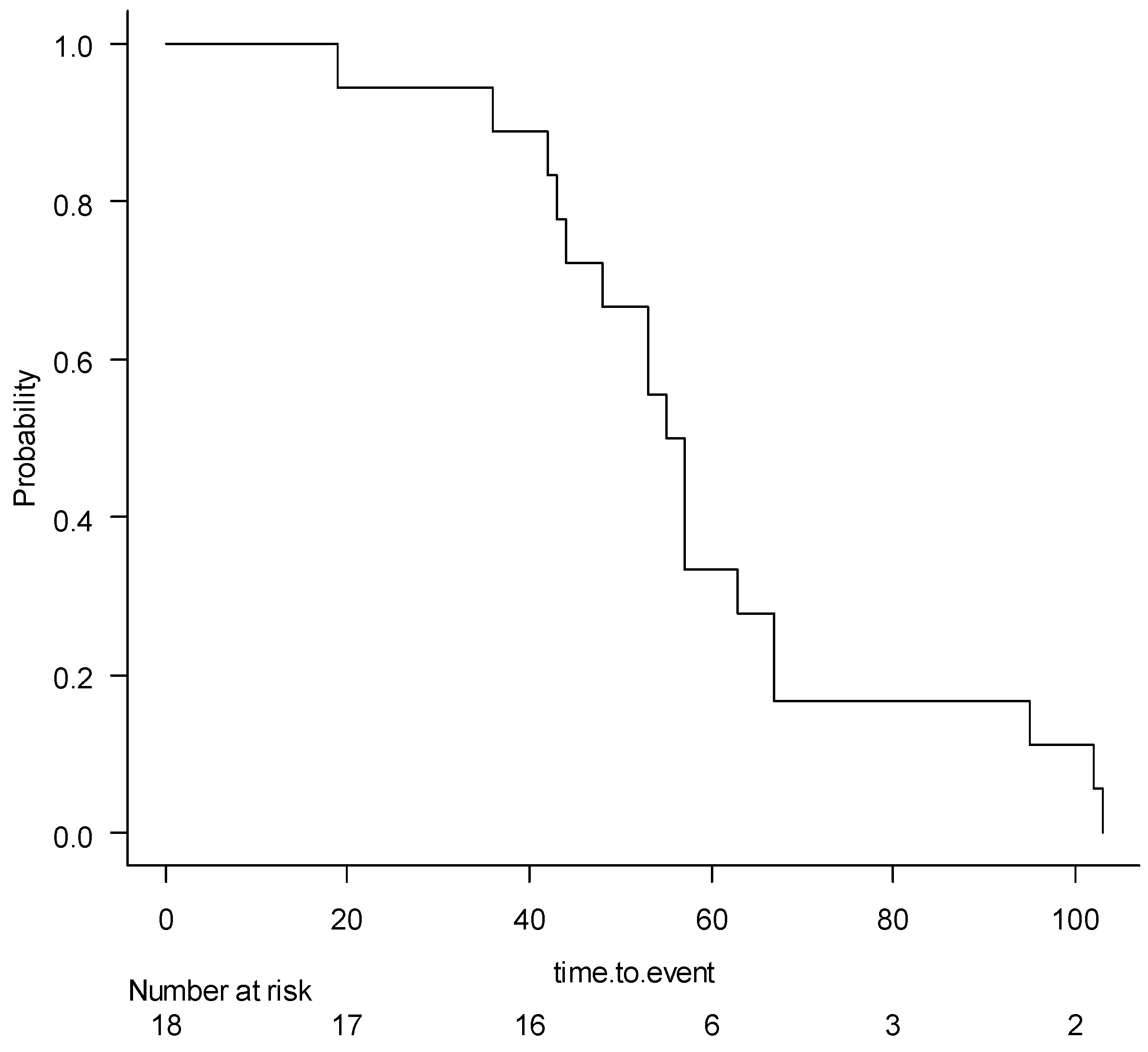Simple Summary
Winter cereal silages are becoming a crucial component of dairy nutrition for Mediterranean countries, partially replacing maize silage even in high-producing cows. Concerns regarding the definition of their final quality and the potential for aerobic deterioration, leading to loss of dry matter, feed intake, and milk yield, need to be investigated. We evaluate the effect of different ensiling conditions, including the use of inoculants and freshly harvested plant composition, on the final silage quality, and the most relevant risk factors associated with the silage’s aerobic deterioration. The dry matter at harvest affected the entire final silage quality, and acetic acid varies between the control (pure water) and the hetero-/homofermentative. The aerobic stability for all samples was 59.2 ± 23.6 h without statistical differences within the thesis, ensuring a sufficient time for a convenient silo unload rate.
Abstract
Winter cereal silages can suffer from an inadequate fermentative path which can lead to aerobic instability. We evaluate the pre-ensiled conditions influencing the final fermentative quality and its aerobic stability. We studied the use of hetero/homofermentative inoculants on two early-harvest wheat samples (312 and 348 g/kg of dry matter—DM levels) undergoing three ensiling delays. The fermentative profiles were evaluated during the first ten d of ensiling, at 60 d and after 7 and 14 d of aerobic exposure. Aerobic stability was recorded during fourteen d after the silo opening. Significant (p < 0.05) differences of the final fermentative profile were related mainly to DM levels at harvest, while the use of the inoculant affected only the acetic acid significantly. Finally, the sealing delay did not significantly affect the silage fermentative profile. The overall aerobic stability was 59.2 ± 23.6 h, and cumulative temperatures were lower than 438 for seven d of aerobic exposure and higher than 1526 for 14 d. Although the homofermentative inoculants reduced the counts of yeasts in the final silage for the earlier harvested wheat, the other samples showed yeasts counts of ~4.9 Log10 colony-forming units/g, with the presence of spoilage fungi (Pichia and Geotricum).
1. Introduction
Winter cereal crops (WCC) are becoming more frequent in dairy feeding due to their ability to replace maize silage and support milk yield above 41 kg/d when included in 10% of the diet dry matter (DM) [1], and the use of barley in replacing maize silage was demonstrated to preserve milk and cheese quality and yield [2]. Moreover, cover crops are used as self-produced forages and can prevent soil erosion [1], efficiently use fall-applied manure and reduce nitrate leaching [3]. According to the Italian National Statistic Institute (ISTAT), in 2020 the North Italy district produced more than 81% of the Italian milk yield (1.33 × 109 kg) [4], which increased by 125% in the period 2006 to 2019. North Italy’s dairy feeding system is primarily based on maize silage or maize grain [2], and in the period 2006 to 2019 the arable land sown for maize silage increased by 146% (299,663 ha in 2019) compared to barley silage, which increased by over 186% (3933 ha in 2019) [4].
Future projections for the Mediterranean climate show a decrease in rainfall precipitation exceeding −25% in the warm season [5] and, consequently, lower water availability for agriculture. In particular, the Veneto Region (north-east of Italy) in October 2021–June 2022 showed −41% of rainfall compared to the average of the preceding 20 years [6]. An indicator that links the yields to water consumption is the Water Footprint, whose estimation is raised to contrast the effects of climatic variables [7]. In this context, among the WCC, wheat can reduce evaporation losses, preserve soil water during the irrigated period, and increase the system’s sustainability [8], therefore, assuming greater importance in the future dairy context. However, due to the increased temperature, a late harvest affects wheat forage by reducing yield, DM digestibility, leaf/stem ratio and N concentration [9]. Conversely, an early harvest could protect the wheat forage from the high temperatures of late spring and the lack of water availability.
Silage fermentation quality is evaluated through pH levels and various compounds, such as lactic, acetic, propionic and butyric acids, alcohols and esters [10]. To simplify the quality evaluation, various indexes have been considered to summarize the overall fermentative quality of silage, such as the Flieg-Zimmer score (FZs) [11], the Vanbelle and Bertin score [12,13], the German agricultural society (DLG) score [14,15], the homolactic index [16,17], the standardised quality score (SQS) [18], and the fermentative quality index (FQI) score [19]. The composition of the fresh ensiled plant, including the DM, crude protein (CP), fibre, N-free extract and water-soluble carbohydrates (WSC) content, affects the final quality of the silage [10,20,21]. Additionally, various factors can decrease the aerobic stability of silages, providing a chance for opportunistic microorganisms such as yeast (Saccharomyces, Candida, Cryptococcus, and Pichia), moulds, bacilli, and acetic acid bacteria to become active and generate heat while consuming nutrients from the silage, which can cause spoilage [11,22,23]. Under certain conditions, inoculation with bacteria may be recommended to improve silage quality and aerobic stability [10,22,24,25,26], affecting the increment in DM Intake (DMI), milk fat and CP concentrations [27], milk yield and diet digestibility [28]. However, the stability of silages against aerobic deterioration varies dramatically with the use of homofermentative (HOM) lactic acid bacteria (LAB) or heterofermentative (HET) LAB [29].
The pre-ensiled composition can be assessed to compute a prognostic risk score for the aerobic stability of maize silage [30,31]. Additionally, research has shown that post-silage quality can be partially linked to fresh maize’s pre-silage characteristics, even using machine learning techniques under different harvesting and ensiling conditions [20]. Furthermore, using portable Near-Infrared Spectroscopy (NIRS) instruments permits on-farm rapid, ecologic and cheap analysis of forages [32,33,34]. Although NIRS may not be more precise and accurate than chemical analysis, it is considered a reliable and repeatable tool in agriculture research [35,36,37,38,39,40]. The NIRS’s success is related to its rapidity and cost-effectiveness in sample analysis [41]. Furthermore, the elimination of the drying procedure for sample preparation helps to prevent the loss of volatile compounds [42]. Additionally, NIRS recently became commercially available for the harvesting machine, as reported by the Deutsche Landwirtschafts-Gesellschaft—the German Agricultural Society [43].
This study aimed to examine the impact of pre-ensiling traits on the quality of silage, DM loss, and aerobic stability of early-harvested wheat ensiled at two different levels of DM at harvest, under varying conditions and with different bacterial inoculations. The focus was placed on southern Europe’s weather due to limited knowledge of the impact of varying ensiling conditions on WCC and wheat.
2. Materials and Methods
2.1. Experimental Design
The WCC (wheat) was sown on two commercial farms (Farm A and Farm B) located in the Veneto Region (North-East of Italy) in the autumn of 2020, with light clay soil and water rainfall of 647.0 mm and 9.2 °C on average. The sown density was 400 seeds/m2. Harvests occurred on June 3 and 8, 2021, and wheat was ensiled at 312 (low dry matter = DML) and 348 g/kg (high dry matter = DMH) of the DM for Farms A and B, respectively. Freshly harvested wheat forages (FWH) from DML and DMH were inoculated with a heterofermentative Lentilactobacillus buchneri CCM 1819 (HET, KWS LACTOSTABILITY, AGRAVIS Raiffeisen AG, Munster, Germany) at a concentration of 2 × 105 colony-forming units (CFU)/g of FHW or with a mixture of homofermentative LAB (HOM) at a concentration of 3 × 105 CFU/g of FHW. The HOM was composed of Lactiplantibacillus plantarum NCIMB 30083–1k207736 (1.61 × 105 CFU/g), L. plantarum NCIMB 30084–1k207737 (0.31 × 105 CFU/g), Peditococcus pentosaceus DSM 23688–1k1010 (1.61 × 104 CFU/g), P. pentosaceus DSM 23689–1k1019 (1.61 × 104 CFU/g) and Enterococcus faecium 22502–1k20602 (0.96 × 105 CFU/g). The control group C was inoculated with the same volume of pure water. The inoculation was conducted in a large sterile container, allowing adequate mixing. The HET, HOM and C were ensiled (at d zero, T0) after 0 (D0), 6 (D6), or 20 (D20) h of air exposure. In summary, the trial consisted of two DM at harvest (DML and DMH) × three tested inoculants (C, HET, HOM) × three sealing delays (D0, D6, D20) = 18 combinations.
2.2. Silage Preparation and Conservation
FWH samples were prepared and inoculated for ensiling in duplicate (repetitions one and two; n = 18 combinations × 2 repetitions = 36), and per each duplicate about 1 kg of the sample was rapidly vacuum-packed and refrigerated (4 °C) until it was analysed (within the same day) with a pre-calibrated NIRS instrument (FOSS DS 2500, Foss Analytical, Hileroed, Denmark). Samples were ensiled in a 20-L truncated conic plastic bucket. The buckets were shielded using a 150 µm SealPlus Film permeable to oxygen at the rate of 48 cm3/m2/24 h at 23 °C and 65% RH (SealPlus by Gamma Srl, Mondovi, CN, Italy), sealed with robust tape and 10 kg of gravel used as a compressor. The sealed buckets (n = 18 combinations × two repetitions = 36) were stored for 60 d (T60). Moreover, in order to evaluate the ensiling pattern, FWH samples for HET, HOM, and C from repetition one were ensiled in a 5-L cylindric metal bucket, sealed with their hardcover tap and stored for 2, 3, 6, and 10 d (T2, T3, T6, and T10, respectively; n = 18 combinations × 4 opening time = 72 buckets). All buckets were kept in a dark room at a stable temperature of approximately 23 ± 2 °C before opening and submitting the sample to reference methods for the analysis. All samples from 5-L buckets on the opening days were evaluated to not to exceed 2 °C above room temperature, and samples were analysed for pH and ammonia. On d T60, the 20-L silages were opened and a 15 cm thick layer of silage was removed to discharge the eventually spoiled silage and was not considered for further quality analysis, and 1 kg of silage was promptly submitted to NIRS analysis. The remaining portion was loosed into a 20-L opened and squared polystyrene pan (495 × 295 × 140 mm in dimension). After 7 (T67) and 14 d (T74) from T60, sample weights were recorded and 1 kg of silage was promptly submitted to NIRS analysis. To ensile the samples into 20-L and 5-L, a 1-ton hydraulic press (141 kg/cm2) was used.
A data logger was positioned 7 cm under the silage surface of 20-L buckets of the repetition one of all samples and, at T60, repositioned 5 cm under the silage surface of the 20-L polystyrene pan, recording the temperature every 30 min with a precision of 0.1 °C (Elitech USB Temperature Datalogger RC-5, London, UK) throughout the period of the trial. A single data logger was used to record the room temperature throughout the entire period. The cumulative temperature (TCUM, °C) was calculated as the hourly accumulated temperature rise 2 °C above the ambient temperature over 7–14 d of air exposure [44].
2.3. Proximate Composition, Fermentative Analysis and Physical Characteristics of Pre- and Post-Ensiled Wheat
The estimated chemical traits were: DM (g/kg), ash, CP, ether extract (EE), alfa-amylase neutral detergent fiber (aNDF), acid detergent fiber (ADF), lignin (sulfuric acid, sa), WSC, starch, pH (pure value), ammonia/total N (ammonia, % of total N), lactic, acetic, propionic and butyric acid, and ethanol. Where not otherwise defined, data were expressed as g/kg of the DM. The calibrations performances were reported in Supplementary Materials Table S1, and the NIRS analyses were performed in triplicate before the average, using a FOSS NIRSystem 5000 scanning monochromator (FOSS NIRSystem, Silver Pring, MD, USA) and following previous studies [36,45]. The reference methods used to calibrate the NIRS instrument for proximate composition were detailed and described in previous studies [18,19] and further reported together with those for the fermentative profile. The DM and ash were determined according to the #934.01 and #942.05 [46], and the #2001.11 [47], #2003.05 [46], and #996.11 [48] AOAC methods were used for CP, EE, and starch, respectively. The aNDF and ADF were determined using an AnkomFiber Analyser (Ankom Technology Corporation, Fairport, NY, USA). The aNDF was performed with sodium sulphite, heat-stable alpha-amylase, F57 bags with 25 µm pore size and included residual ash [49,50]; non-sequential ADF was evaluated according to Vogel et al. 1999 [51] and lignin (sa) in sulfuric acid [52]. Lactic, acetic, propionic, butyric acids and ethanol were extracted in an acid solution (sulphuric acid 0.6 N) and analysed using high-performance liquid chromatography [53]. The ammonia was analysed according to Megazyme’s assay procedure [54] and pH as proposed by Martillotti et al. [55].
Density was calculated as the ratio of the amount of pre-ensiled FWH, corrected for the DM content, to the volume of the 20-L bucket and was expressed as kg/m3. The porosity (Φ) was calculated according to the formula proposed by Richard et al. (2004) [56], as reported in Equation (1):
where ρwb, bulk density wet basis (g/cm3); DM, dry matter; ρw, water density (1 g/cm3); OM, organic matter; ρom, organic matter density (1.6 g/cm3); ρash, ash density (2.5 g/cm3). The DM density (DMd, kg/m3) was calculated as the amount of DM (kg) per 1 m3. The DM loss (DMloss) was calculated as reported in Equation (2) [31]:
Φ = 1 − ρwb × {[(1 − DM)/ρw] + [(DM × OM)/ρom] + [(DM × (1 − OM))/ρash]}
DMloss = (DM of pre-ensiled − (DM of post − ensiled))/(DM of pre − ensiled)) × 100
Two silage quality indexes, the FZs [11] and the FQI score [19], were calculated.
2.4. Microbiological Analysis
Samples for microbiological analysis were collected in triplicate at three different times (T0, T6, and T60) for each of the combinations of the two DM levels at harvest (DML and DMH) and for the three inoculants (C, HET, and HOM), but only for delay 0 h (D0) (n = three replicates × three sampling times × two DM at harvest × three inoculants × one sealing delay = 54).
For the microbiological analyses, a 20 g sample was homogenised in a sterile bag with 180 mL of Maximum Recovery Diluent (MRD 8 g of NaCl/L, 1 g of bacteriological peptone/L) and for 2 min. Each microbial target was analysed by spreading methods in a Petri dish after the appropriate decimal dilutions on MRD. The total viable counts (TVC) were enumerated on Plate Count Agar (Biokar Diagnostics, ZAC de Ther, Allonne, Beauvais Cedex, France) after incubation at 30 °C for 72 h. LAB count was performed on MRSA agar supplemented with 0.0125 g/L bromocresol green (De Man, Rogosa and Sharpe agar, Biokar Diagnostics) incubated in anaerobic conditions for 48–72 h at 30 °C. Oxytetracycline Glucose Yeast Extract Agar (OGYE Oxoid) was used for yeast and mould counts (25 °C, 3–5 d). Results were defined as Log10 CFU/g of silage with a Limit of Detection (LOD) of the spread plate method of 100 CFU/g. A representative number of colonies per plate (3–5) was collected according to their morphology and colour from MRSA and OGYE in order to perform a biomolecular identification of LAB, mould and yeasts by the sequence of 16S rRNA (bacteria), ITS1 and ITS2 (ITS, Internal Transcribed Spacers) of the ribosomal DNA (Fasolato et al., 2016).
2.5. Statistical Analysis
The normal distribution for continuous variables was assessed using the Shapiro–Wilk test and visual inspection. Pre-ensiled traits for the harvested wheat were submitted to an ANOVA considering the effects of DM at harvest, density and porosity. FZs, FQI, DMloss, post-ensiled DM, and fermentative traits were submitted to an ANOVA considering the effects of DM at harvest, the use of inoculants, the sealing delay, and their interactions. A linear model (lm) with a multivariable Akaike’s information criterion in the backwards (lm-AIC-backward) was estimated for the pre- ensiled WCC chemical traits to predict the FQI and DMloss. As regards the hours before the occurrence of aerobic instability, they were considered only for samples in which the event occurred. Post hoc pairwise comparisons were run between factors’ levels using Bonferroni’s correction. The assumptions of the linearity of the model were graphically tested on the residuals.
The event for the survival analysis was defined as the aerobic instability and the outcome as the time, expressed in hours, to the first event of aerobic stability failure (aerobic deterioration), defined as the silage exceeding the room temperature of 2 °C [31]. Observations were censored at 336 h (14 d). The silage outcome was evaluated using a Kaplan–Meier curve and log-rank test, and Bonferroni’s adjustment was adopted for the pairwise comparisons. A Cox proportional hazard regression tested the effect on the event for each covariate separately (univariate approach) for pre- and post-ensiled composition. Further, a Cox proportional hazard regression for a multivariable Akaike’s information criterion in the backwards (Cox-AIC-backward) model was estimated for the pre- and post-ensiled WCC chemical traits to estimate the aerobic stability. The proportional hazard assumption was evaluated using a visual approach (Schoenfeld, Martingala, beta, and score residuals) and Grambsh and Therneau test [57,58]. To perform these statistical analyses R version 4.0.2 (22 June 2020, The R Foundation, Vienna, Austria) was used, besides the Rcmdrpackage version 2.6–2 [59,60] and RStudio Version 1.2.1578 (Posit, PBC, Boston, MA, USA). The statistical significance was set at a p-value lower than 0.05.
For data from the microbiological analysis, a multivariate statistical analysis was adopted by the non-parametric combination (NPC) test conducted with the free software NPC Test R10 [61]. The partial tests (partial p-values) were estimated per each single microbial variable (TVC; LAB; Mould; Yeast), and the Global p-value was defined by the non-parametric combination procedure and the Tippet combining function on the overall microbial profile [62]. The DM levels and inoculant (C; HOM; HET) were tested as main factors in a separate manner. Moreover, DM was defined as a stratification block according to the NPC Test’s C-sample procedure for dependent variables to evaluate the differences between inoculants in each stratum of DM.
3. Results
The normality of the data was warranted for all variables after a visual inspection of the distribution. Descriptive statistics for the values of the chemical and physical properties of pre-ensiled WCC for DML and DMH, and the ANOVA for the effects of the DM at harvest, are reported in Table 1.

Table 1.
The mean, the standard deviation (sd), the median, and the first to third interquartile range (IQR) for pre-ensiled chemical and physical composition of the low dry matter (DML) and high dry matter (DMH) of the winter cereal crop (WCC) harvests.
3.1. Effects of the DM at Harvest, Inoculation and Sealing Delay on the Final Silage Quality (T60)
The main effects of the DM at harvest, inoculation and sealing delay and their interactions on the final silage quality at 60 d of ensiling is reported in Table 2, Table 3, Table 4 and Table 5. The DML resulted in more intense fermentative profiles compared to DMH, but no significant difference was highlighted for the DM loss at T60. The use of inoculants showed significant differences only for acetic acid and propionic acid and, tendentially, a better FQI for the homofermentative inoculant. Surprisingly, the acetic acid was reported as higher in the homofermentative use than the heterofermentative inoculant. The DM loss at T60 was significantly lower for the sealing delay at D6 compared to D0 (Table 4), and there was a higher DM loss value (13.9%) at D0 of the DMH, compared to D20 of DML (7.82%, p = 0.033).

Table 2.
The main effects of the DM at harvest on the post-ensiled quality at 60 d of ensiling (T60).

Table 3.
The main effects of the use of inoculants on the final silage quality at 60 d of ensiling (T60).

Table 4.
The main effects of the sealing delays on the post-ensiled quality at 60 d of ensiling (T60).

Table 5.
The main effects of the interactions of the DM at harvest, the use of inoculants, and the sealing delays on the final silage quality at 60 d of ensiling (T60).
At the lm-AIC-backward, the FQI was predicted with an Adjusted R2 = 0.20 (p = 0.038, AIC = 145, Vif < 5.21), as in Equation (3):
FQI = −242 + 0.62 × CP + 1.81 × EE −0.16 × starch + 0.49 × density + 241 × porosity
At the lm-AIC-backward, the DMloss (%) was predicted with an Adjusted R2 = 0.16 (p = 0.022, AIC = 81.3, Vif = 2.95), as in Equation (4):
DMloss = −9.31 + 0.12 × DM − 0.06 × ADF
3.2. Effects of the DM at Harvest, Inoculation and Sealing Delay on the First Ten d for pH and Ammonia
The effects of DM at harvest and inoculation on the pH and ammonia pattern for T2, T3, T6, and T10 are reported in Figure 1a–d. The patterns of pH and ammonia for T2, T3, T6, and T10 on delaying the silo sealing show non-significant differences (p > 0.05).
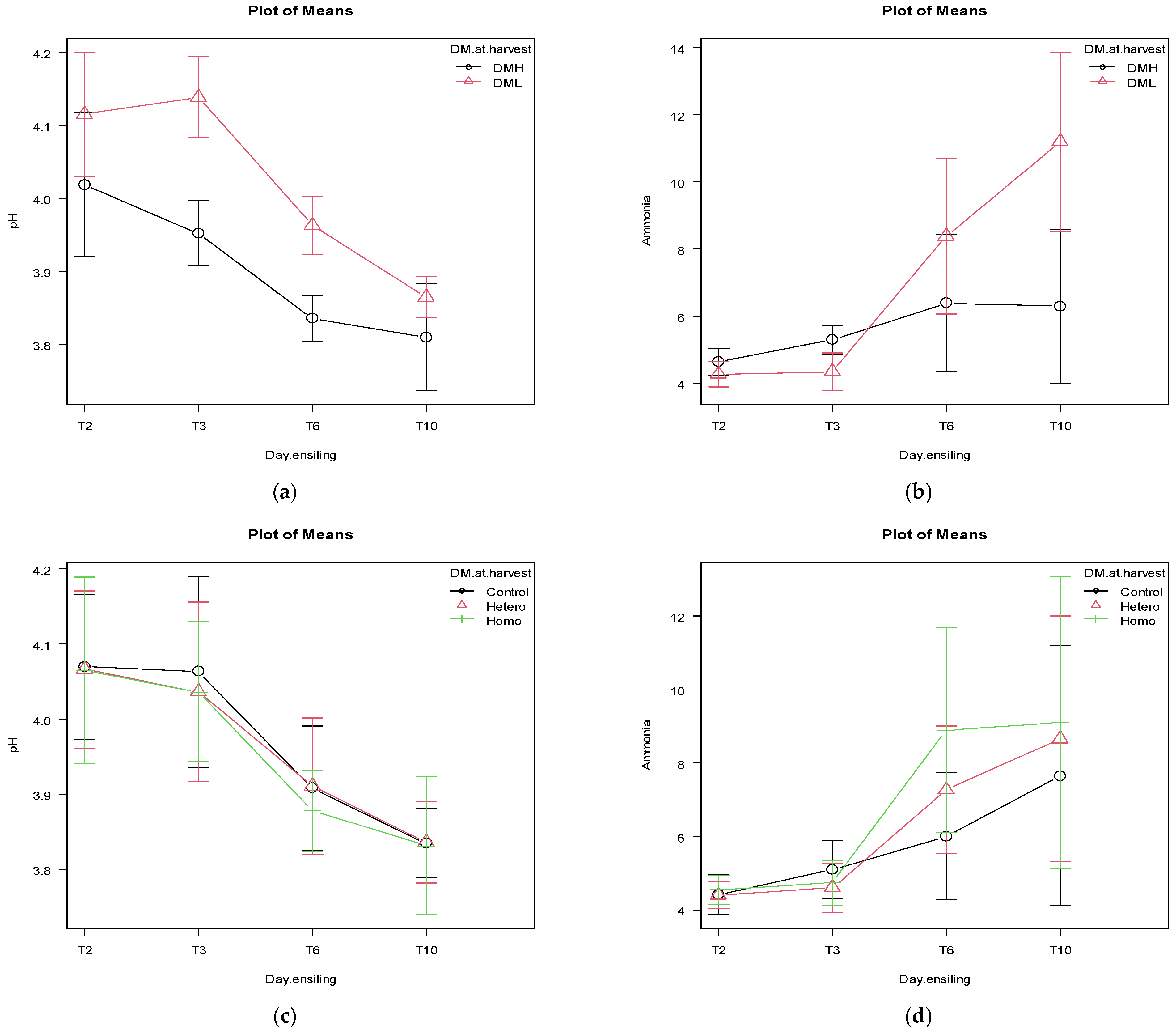
Figure 1.
The effects of dry matter (DM) at harvest (DML = 31.2% DM, DMH = 34.8% DM) and days after ensiling (2 -T2, 3 -T3, 6 -T6, and 10 d -T10) on the pH (p = 0.06) and ammonia patterns (p < 0.0001), panels (a) and (b), respectively. The effects of inoculation (control = pure water, hetero = heterofermentative, homo = homofermentative) at harvest and days after ensiling (2 -T2, 3 -T3-, 6 -T6-, and 10 d -T10) on the pH (p = 0.99) and ammonia patterns (p = 0.82), panels (c) and (d), respectively. Bars represents the 95% confidence intervals.
The effects of DM at harvest, inoculation and sealing delay for pH and ammonia patterns within T2, T3, T6, and T10 are reported in Tables S2–S4, respectively. The pH differs significantly for the effects of DM at harvest at T2, T3, and T6, while only in T2 for the sealing delay. The ammonia significantly or tendentially differs in T2, T3, T6 and T10 for the effect of the DM at harvest.
3.3. Silage Quality on T67 and T74
The main effects of the DM at harvest, inoculation and sealing delay and their interactions on the final silage quality at seven d of aerobic exposure (T67) are reported in Tables S5–S8. The main effects of the DM at harvest, inoculation and sealing delay and their interactions on the final silage quality at fourteen d of aerobic exposure (T74) are reported in Tables S9–S12.
3.4. Risk Analysis for Aerobic Stability
The TCUM at T67 (7 d of aerobic exposition) was 294 and 401 °C for DML and DMH, respectively (p = 0.369); 433, 238 and 370 °C for C, HET and HOM, respectively (p = 0.398); and 438, 238 and 365 °C for D0, D6 and D20, respectively (p = 0.387). Moreover, the TCUM at T74 was 2008 and 1777 °C for DML and DMH, respectively (p = 0.557); 1923, 1979 and 1775 °C for C, HET and HOM, respectively (p = 0.905); and 2152, 1526 and 2000 °C for D0, D6 and D20, respectively (p = 0.410).
The Kaplan–Meier curve for the aerobic instability occurrence in the harvested wheat ensiled at different conditions is reported in Figure 2. The median survival rate was 56 h. The Kaplan–Meier curves for the main effects of DM at harvest, the use of inoculants, and the sealing delays are reported in Figures S1–S3, and had no significant effects. The Kaplan-Meir curves for the effect of the use of inoculants, stratified for the DML and DMH and sealing delays 0 h, 6 h, and 20 h, were non-significant and are reported in Figure S4A,B, and Figure S5A–C, respectively.
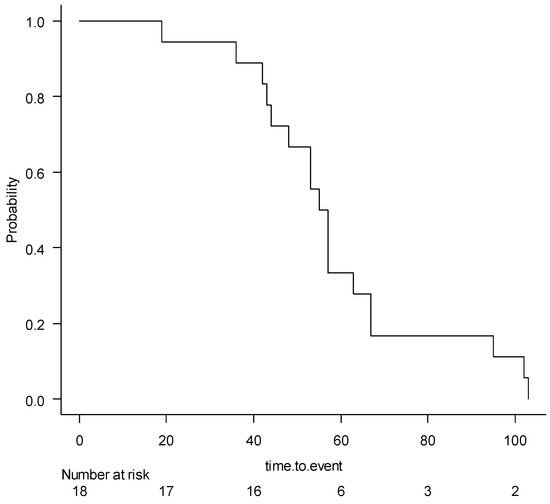
Figure 2.
The Kaplan–Meier survival curve for aerobic instability occurrence of the wheat samples undergoing different ensiling conditions.
All silage showed aerobic deterioration before the censure (336 h), and the mean for aerobic stability was 59.2 ± 23.6 h.
Table 6 reports the HRs values for the univariable Cox models for the pre- and post-ensiled traits and the categorical variables (factors). The Somer’s Dxy concordance index was greater than 0.6 for all variables except for CP (0.50), lignin (sa) (0.54), WSC (0.52), starch (0.49) and density (0.55), for the pre-ensiled traits, and DM (0.54), ash (0.59), CP (0.57), EE (0.52), pH (0.53), ammonia (0.56), propionic acid (0.45), butyric acid (0.59), FZs (0.51) and FQI (0.53), for the post-ensiled traits, and the use of inoculants (0.56). The Schoenfeld residuals and the test were not statistically significant for each; therefore, we can assume the proportional hazards assumption for all variables.

Table 6.
Univariable Hazard Ratio (HR), distribution and average composition of wheat samples.
Table 7 reports the coefficients and HRs for the selected pre-ensiled traits from the Cox-AIC-backward model predicting the event (Somer’s Dxy concordance index = 0.89; AIC = 65.7); however, the Schoenfeld residuals were evaluated, and the test is not statistically significant for each of the covariates, but not in the global test (p < 0.0001). Therefore, we cannot assume the proportional hazards assumption.

Table 7.
The Hazard Ratio (HR) for the selected pre-ensiled traits from the Cox-AIC-backward model.
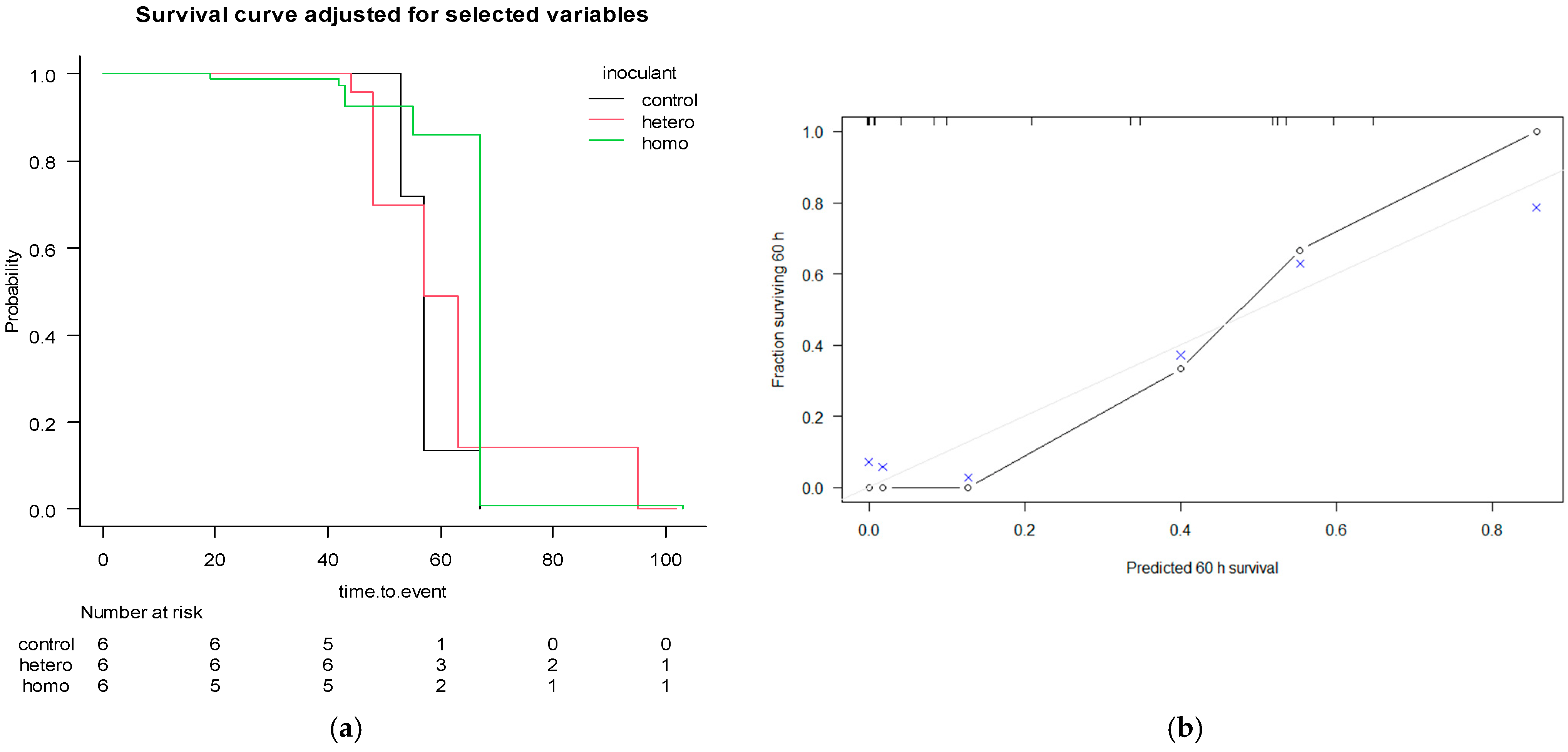
The survival curves for the AIC-Cox multivariable model are represented in Figure 3.
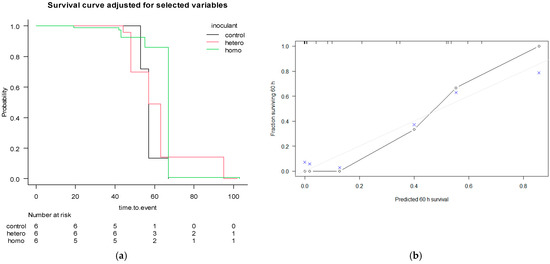
Figure 3.
Kaplan–Meier adjusted for AIC-Cox multivariable model with covariates crude protein (CP), aNDF, ADF, lignin (sa), starch, water-soluble carbohydrates (WSC), density and porosity (a), stratified for the effect of the use of inoculants (control = pure water; hetero = heterofermentative; homo = homofermentative), and its calibration performances (b), with bootstrapping and resampling 500 times, 3 subjects per group. The grey line represents the ideal fit, the “o” represents the theoretical and “×” represents the resampling optimisation.
3.5. Microbiological Quality of Silage at the Final Fermentation Time (T60)
The microbiological features analysed after 60 d of fermentation (T60) are reported in Figure S6. The boxplots showed that TVC and LAB are over the 7.1 Log10 CFU/g in most of the studied theses. In DML, the LAB counts of the HOM thesis presented a significant variability (min 5–max 8.2 Log10 CFU/g). However, no statistical differences were highlighted by the NPC test. The partial p-values generally suggested no significant effects of DM or inoculants; significant global-p-values were observed for the inoculum stratified per DM (Global p-value = 0.033). The global p-values considered all the microbial features as a signature, suggesting a different ratio between targets (TVC; LAB; mould; yeasts), primarily due to the different inocula. Moreover, for DML, the mould and the yeast were not enumerated in the HOM thesis (<LOD) (Figure S6), while in HET and C the yeasts overcome the 5 (min 5–max 7.6) Log CFU/g (partial p-values 0.035). The moulds count median was usually lower than 4.7 Log CFU/g, with 60% of the samples lower than the LOD. Figure S7 shows that the genus Pica was the most frequent in the identified yeasts, while in the moulds the most frequent taxon was Geotrichum.
4. Discussion
4.1. Effects of the DM at Harvest, Inoculation and Sealing Delay on the Final Silage Quality (T60)
In this study, the FHW was ensiled at two DM levels at harvest and inoculated with hetero-/homofermentative inoculants compared to a control (pure water). Moreover, these conditions were tested for a progressive delay in silo sealing. As expected, the earlier harvest (DML = 312 g/kg of DM) compared to a later one (DMH = 348 g/kg of DM) showed a higher CP and WSC value and lower aNDF content. These findings are compatible with those reported by Ronga et al. [9], except for the aNDF, which is reported to decrease with the later harvest. Additionally, the starch was lower in the DMH, and the differences are probably due to the diverse harvested fields. The lignin (sa) content was similar for DML and DMH, and its level is related to the undigested aNDF (uNDF) by the relation UNDF 2.4 × lignin (sa) [63]. Raffrenato [64,65] suggests that several factors, such as genetics, the agronomic conditions, and the physiological stage of the plant at harvest, seem to determine the link between the lignin (sa) content in the cell wall and its effects on the extent of digestion at the same lignin (sa) content. Therefore, it appears that the ratio uNDF 240 h/lignin (sa) is proper among forage groups and within groups, and can be evaluated through the use of a power function of the type y = axb, with y representing the ratio and x being lignin (sa) (on aNDFom basis) [64]. Here, the lignin (sa) did not significantly differ between the DML and DMH groups, but the lignin (sa) on the aNDF basis was 8.33 and 7.50 for DML and DMH, respectively (p = 0.027). These values are markedly higher than those reported by Raffrenato [64] for very young, early vegetative grasses (from 2.0 to 3.0), or even more mature grasses (higher than 5.0). Applying the suggested formula [64] for the mature grass (y = axb; a = 13.851, b = 0.621), the predicted ratio (%) lignin (sa) to uNDF 240 h was 3.75 and 4.00 for DML and DMH, respectively (p = 0.021), while the uNDF 240 h (%) was 11.7 and 10.6 for DML and DMH, respectively (p = 0.237).
These findings do not agree with those from Ronga et al. [9], who found that uNDF 240 h increases in later harvest and suggested that lignin (sa) and the harvest time influence the uNDF 240 h, even if the magnitude of values are comparable. Moreover, increasing one unit of NDF digestibility is related to a 0.17 kg increase in DM intake and a 0.25 kg increase in 4% fat-corrected milk [66]. This effect probably happens because the higher levels of lignin (sa), correlated with uNDF 240 h content of the lignified crops, remain in the rumen for more time due to their slower rate of digestion, decreasing the DMI and, in turn, the animal performance [67]. However, the inclusion of 10% (DM-based) wheat silage (replacing maize silage) in the diet of lactating dairy cows may support milk yield (MY) above 41 kg/d without affecting the DMI, but decreasing the MY compared to the use of maize silage alone [1]. That was probably due to the low starch content of wheat (10.0 g/kg) harvested at the boot stage compared to maize silage (345 g/kg). Conversely, in our study, the starch content of post-ensiled wheat (T60) was 61.0 and 76.2 for DML and DMH, respectively. The post-ensiled quality at T60 was, in general, well preserved. The ammonia content and pH were lower than those reported in the literature [68,69,70], but higher compared to a sorghum silo [44], while lactic and acetic acids were higher [69,70]. Lb also produces 1,2-propanediol during the metabolism of lactic acid to acetic acid [71]. Further, inoculation with Lb may affect propionic acid’s concentration due to microorganisms capable of converting 1,2-propanediol to propionic acid [72]. Our findings are inconsistent with the cited bibliography, but Kleinschmit and Kung [72] supposed that there could be a crop-specific effect. Even though we find lower WSC values in pre-ensiled wheat compared to the literature [68,69], the higher water contents at harvest for fresh forage used in our study probably allow the microorganisms a better fermentative substrate [73]. However, the fermentative traits are similar to those reported by Tabacco et al. [44], evaluated in experimental conditions similar to ours, and higher than those reported for sorghum ensiled for 93 d. In our study, differences are reported for all fermentative parameters except pH with increasing stages of maturity, and the quality (FQI and FZs) tends to decrease with later harvests. The DM loss did not differ for harvesting maturity, and values are approximately four to five times higher than those reported for maize silage and sorghum silages harvested in north Italy at 352 and 404 g/kg in DM, respectively [44], approximatively double with respect to the values reported by Kleinschmit and Kung [72] or even more [74]. However, compared to the experiment from Tabacco et al. [11], in our trials the mini-silos were probably more permeable to air, and the packing density was approximately 34% lower, which may allow for more intense respiratory activity.
The use of inoculants did not affect the fermentative and quality traits, including the DM loss, except for the acetic and propionic acid, which did not show biologically evaluable differences. This finding is probably due to the prevalence of the Lb in all samples and the presence of Geotrichum, which may catalyse the lactic acid. However, the silo sealing delays did not show significant differences for all fermentative and quality traits. Nevertheless, the DM losses were slightly lower for six h of sealing delay. The LAB converts WSC into organic acids, mainly lactic acid, resulting in forage acidification by a rapid pH decline. During the anaerobic conservation of the silage, the lactic acid is converted gradually to acetic acid by hetero-lactic bacteria, such as Lb [74]. Ensiling wheat at 301 g/kg of DM using inoculants (Lactiplantibacillus plantarum) results in a lower pH than untreated, and pH values decrease faster [75] even at DM contents higher than 654 g/kg for grains silage [76]. In these circumstances, the multiplication of the indigenous LAB could be limited to higher DM content at harvest. Therefore, exogenous inoculants could supplement to overcome this effect and result in significantly faster and improved ensiling [77]; however, different maize and wheat silage behaviours are reported [73,77], probably due to moisture and water activity differences. In our study, the DM of the post-ensiled (T60) silage was relatively low (<300 g/kg) for the three tested inoculants (control, hetero-/homofermentative); consequently, it is possible that the endogenous bacteria multiply enough to warrantee a rapid and efficient fermentative pattern, causing the inoculation to no longer be necessary even with delayed silos sealing. Moreover, in our experiment, no statistical differences at T60 within the thesis were observed (Figure S6). However, the LAB levels in all the theses were similar to those reported in maize silage supplemented with Lb after 90 d of fermentation [78].
4.2. Effects of the DM at Harvest, Inoculation and Sealing Delay on the First Ten Days for pH and Ammonia
Nevertheless, the ensiling first ten days were characterised by a rapid pH decrease and ammonia increase, with differences in the patterns for DML and DMH, but an equal final point at T10 and T60 for pH. In contrast, ammonia showed similar patterns for DML and DMH but a different final point for ammonia, which showed a higher value for DMH in T10 and T60 (Table S1 and Figure S1). Regarding the use of inoculants or sealing delays, the patterns of the first ten days did not show differences among treatments (Table S2 and S3). However, our findings showed pH values markedly lower than those reported for FWH collected at 456 g/kg of DM, or whole-crop barley harvested at 453 g/kg [77,79]; furthermore, they found differences in patterns and the final value of pH for the untreated or Lp-inoculated silages. These findings suggest that our study’s initial DM content of FWH was adequate to warrant satisfactory ensiling patterns and final pH values.
4.3. Silage Quality on T67 and T74
The aerobic stability of silage after silo opening could lead to a significant problem for farm profitability and feed quality. Exposition to air (i.e., due to the feed-out rate) involves aerobic microbiological activity to a different extent, up to considerable (more than 20%) DM loss [80]. In addition, well-fermented silage from homolactic inoculants may spoil faster because it results in a greater level of WSC and lactic acid used as a growth substrate for yeast and moulds [81,82]. Conversely, the heterofermentative silages fermented by Lb could improve the stability of silages via the anaerobic degradation of lactic acid to acetic acid [72]. Lb is commonly suggested to partially prevent aerobic deterioration at the farm level [83]. However, it is also associated with increased DM losses during anaerobic conservation of the silage and lower losses during the first 14th d of air exposure in maize and sorghum silages [44]. The role of the air exposure during the feed-out phase to the spoiling process of the mass is outstanding due to the activity of the lactate assimilating yeast, which metabolises the WSC and fermentative end-products into carbon dioxide and water, increasing the temperature and the pH and decreasing the silage quality and even digestibility [11]. In addition, when aerobic activity begins, the temperature rises rapidly and may reach more than 20 °C above room temperature. This means that the accumulated temperature can be more than 500 °C daily. This evidence occurs due to air infiltration in the mass, which could reach up to 1–2 m from the silage surface [84]. In the present study, the threshold of the 14th d was chosen concordantly with the average silage time in the peripheral areas of farm bunker silos at risk of air exposure when a feed-out rate of 0.7 to 1.4 m/wk is adopted [85]. Tobacco [44] found that 1000 °C of accumulated hourly differences between silage and air temperature are associated with a 10% loss in estimated milk yield potential. In our study, the TCUM was lower than 438 °C for seven d of aerobic exposure and higher than 1526 °C for 14 d, without any statistical differences among DM at harvest, inoculants or sealing delays. However, in T74 all samples exceeded the threshold of 1000 °C, suggesting a loss of estimated milk yield potential for peripheral areas of the silo.
A previous study [44] demonstrated that the inoculation with Lb in sorghum silage might maintain the potential of milk yield of harvest forage up to 7 d of air exposure. In contrast, untreated or Lp-inoculated silages consistently decrease milk yield potential, related to mould development exceeding the 4 log cfu/g of silage. Moreover, when mould exceeds 8 log CFU/g of silage, the milk yield potential is halved and this threshold corresponds to the average values of mould count for visible moulded maize silages [86]. Here, the mould counts were close to the 4.7 Log CFU/g, especially in hetero and control samples. However, no significant differences were found because most samples showed minimal contamination. Moreover, a higher level of fungi, primarily yeasts, is usually associated with low aerobic stability [10]. In addition, the DML showed a lot of yeasts very close to the worst thresholds proposed for the silage stability (≥6 Log threshold) [10], with the dominance of the spoilage generable to metabolise lactate as Pica or Geotrichum [10,87,88].
After seven d of aerobic exposure, pH and ammonia increased but the increment of pH was more evident for DML, while the ammonia did not show differences between DM levels. The pH patterns are supported by a decrement of lactic and acetic acid, with greater values falling for DML. These findings are similar to those found in the literature [79]. However, the use of inoculants did not show the difference in T67 silage fermentative profiles, quality traits and DM loss. Finally, all differences between the DM at harvest, the use of the inoculant and the sealing delay levels disappeared at T74, probably due to an intense aerobic instability in the first 100 h (Figure 1) as a consequence of the intense activity of mould and yeast.
4.4. Risk Analysis for Aerobic Stability
The aerobic stability of the silage is a complex pathway related to several factors. First, the crop at harvest should have very low counts of yeast and moulds. This target can be reached by rapid field wilting or immediate harvests of grass and legume forages [89]. Maize and whole crops cereals increase the risk of mould for warm and wet weather in the pre-harvest or by increased maturity at the harvest due to changes in the epiphytic microbial community [90,91,92]. To an extent the pre-ensiled composition, such as the high buffering capacity, leads to aerobic stability and is most likely to impair efficient fermentation with minimal loss of DM and nutritional value during storage [90]. However, further investigation may contribute to underlining the role of fresh crop composition in aerobic stability; it was noted that late crop maturity and plant senescence are associated with decreased aerobic stability of the silage [90].
To the authors’ best knowledge, limited data are available to evaluate the risks for aerobic instability onset related to the factors of pre-ensiled composition. Previous studies were conducted for maize silage [30,31] but not wheat silage. In maize silage, among pre-ensiled traits, CP, EE, aNDF, lignin (sa) and WSC [30] or ADF and lignin (sa) [31] were associated with the risk of aerobic instability. However, the late maturity at harvest (DM = 337 g/kg) was associated with a greater risk of aerobic instability (HR = 95, 95% CI = 2.43–20.7) compared to early harvest (DM = 283 g/kg) [31]. However, differences in aerobic stability and plant maturity (i.e., sorghum compared to maize) were noted for different crops [90], and such differences could partially explain the inconsistent results of the present study compared with those with maize. Finally, in the present study, we did not find any association among pre-ensiled, post-ensiled and factors with the risk of aerobic instability, except for the ash (pre-ensiled) and the acetic acid (post-ensiled), which both resulted in a preservative function for the onset (Table 7). The Kaplan–Meier curves confirm these findings for the DM at harvest (Figure S1), the use of inoculants (Figure S2), and the sealing delays (Figure S3). Stratifying for the DM at harvest or for the sealing delay, the Kaplan–Meier curves for using the inoculants did not differ (Figures S4 and S5). All these findings suggest that the pre-ensiling conditions ensured that a silage’s quality is favourable to ensure the aerobic stability of the product, regardless of the use of the inoculants. Nevertheless, previous studies resumed in a meta-analysis [72] report the times of exposure to air before spoiling as 25 h for untreated silage (LB0), 35 h for silage treated with Lb at ≤100,000 cfu/g of fresh forage (LB1), and 503 h for silage treated with Lb at >100,000 cfu/g of fresh forage (LB2). However, cited authors reported a yeast count of 4.18 ± 0.48, 3.10 ± 0.60, and 1.88 ± 0.46 Log10 CFU/g for LB0 LB1 and LB2 in maize silage, respectively. Noticeably, the previously cited review reports an ensiling period of 60–180 d. The ensiling duration of more than 90 d is reported to have greater improvements (compared to control) in aerobic stability and yeast reduction compared to <60 d of ensiling [93]. Kleinschmit and Kung [72] reported nearly undetectable yeasts in small grains (0.96, 0.56, and 0.56 Log10 cfu/g for LB0, LB1, and LB2, respectively) and 206, 226, and 245 h of aerobic stability for LB0, LB1, and LB2, respectively. The latter values are considerably higher than those in our study for the time of aerobic stability. Our findings can be due to several reasons. First, in our study, the ensiling period was 60 d, while a prolonged period could lead to a more stable aerobic sample. In our study, for the early harvest silage (DML), the HOM effectively reduced spoilage microorganisms such as mould and yeast at T60.
However, recently, authors [94] concluded that delaying (0 compared to 24 h) ensiling maize silage at 268 ensiled at g/kg of DM negatively affects the efficiency of fermentation, as reflected by increased DM losses, and the presence of air in the very early phases of the ensiling process may reduce the aerobic stability. Moreover, de Melo et al. [95], evaluated a blend of Lentilactobacillus buchneri and Lactiplantibacillus plantarum coupled with a short (0, 90, 150, or 210 min between chopping and sealing) delay in five replicates to evaluate the fermentation, aerobic stability, and chemical composition of maize silages harvested at 293 g/kg of DM. They found that, in maize silage, air exposure before sealing increases fermentation losses and reduces the quality of silages and, conversely, the use of inoculants increases the silage quality and the aerobic stability (80.45 and 213 h for control and inoculated, respectively) and reduces the DM loss (7.33 and 5.60% for control and inoculated, respectively). Nevertheless, the same authors report that, more generally, the influence of the sealing delay on aerobic stability is recognised to be conflicting in the literature.
4.5. Microbiological Quality of Silage at the Final Fermentation Time (T60)
No differences were observed in yeasts counts according to DM levels (DML 5.1 vs. 4.8 DMH log10 CFU/g). However, the percentages of samples <LOD were 30% and 44%, respectively. Moreover, yeast loads (except for the HOM × DML) were 2 Log10 CFU/g higher than Kleinschmit and Kung’s values [72]. These contaminations may have affected the time of aerobic stability. In corn silage, increasing the levels of LB-inoculant also showed a reduction in yeast contamination (from 4.18 to 1.88 Log CFU/g) [72]. Moreover, also studying the conditions able to improve the dominance of LB in winter cereal crops could be considered a challenge for further investigation.
Finally, at T60, the rates of lactic acid:acetic acid were 2.64, 2.59, and 2.55 for C, HET, and HOM, which were lower than the desirable ratio of 3:1, indicating a dominant homolactic fermentation, and farther from 5.3:1 reported for LB0 of small grain silage, while being higher than 0.8:1 and 0.6:1 reported for LB1 and LB2, respectively [72]. Baah et al. [79] reported a ratio lactic:acetic acids of 8:1 for HOM treated and 1:4 for the control, in barley silage, with a lactic acid content of 48.26 and 81.17, and 13.85 and 9.81 g/kg in control and HOM treated samples, respectively. These findings mean that, in our study, there was no prevalent homolactic fermentation in C and HOM nor an intense heterolactic fermentation in HET. Moreover, our study’s few hours of aerobic stability (hours of stability) are consistent with the acetic acid content in HET, which resulted in half of those reported in the meta-analysis [72].
The authors are aware that the present study’s limitations may affect the reported results. Kroschewski et al. [96] gave indications on how to minimize the effect of sampling location on silage variables due to different forage compositions and the choice of replications, which are necessary to warrant the significance and relevance of the results. They suggested considering the individual treatment variance for the correct choice of sample size. Therefore, in our study, a lack of significance of the results can be due to the sample numerosity, primarily referring to the low number of repetitions for individual treatment. Secondly, even if the use of a narrow DM range at early harvest was in the scope of the study, this fact does not allow the comparison of a full range of DM at harvest. Moreover, the results may appear limited to the geographical context of northern Italy. However, the Veneto region is one of the leading regions in Italy for the dairy sector and has a prominent position in the European dairy industry. Nevertheless, more in-depth studies will be carried out in the future, considering a more comprehensive DM range at harvest. Finally, a further evaluation of the microbiological aspects related to the harvested WCC, which were not among the main objective of this study, is warranted.
5. Conclusions
Unlike maize, limited data are available in the literature for winter cereal crops, wheat in particular. In our study, wheat harvested at 312 and 348 g/kg DM, even with different pre-ensiled compositions, showed non-different results concerning silage quality, DM loss, and aerobic stability. At the studied DM at harvest, the use of HET or HOM did not differ compared to the control, and the sealing delays at 6 or 20 h did not show relevant differences compared to the 0 h delay. Even though the aerobic stability was lower compared to values reported in the literature, values are suitable for the aerobic exposure of ordinary feed-out rate (24–48 h). These results suggest that the used DM ranges at harvest could be good options for farmers to obtain good quality and stable wheat silage, regardless of ensiling conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13020508/s1, Figure S1: The Kaplan–Meier survival curve for the wheat harvested at different dry matter (DM, g/kg), p = 0.132. DML: low dry matter at harvest = 312 g/kg; DMH: high dry matter at harvest = 348 g/kg; Figure S2: The Kaplan–Meier survival curve for the samples underwent different inoculations, p = 0.746. Control: pure water; hetero: heterofermentative; homo: homofermentative; Figure S3: The Kaplan–Meier survival curve for the samples underwent delays at sealing (h), p = 0.104; 0 h: zero h of dealing; 6 h: six h of dealing; 20 h: 20 h of dealing; Figure S4: The Kaplan–Meier survival curve for the samples underwent different inoculations stratified for the dry matter (DM, g/kg) at harvest. Control: pure water; hetero: heterofermentative; homo: homofermentative. DML: low dry matter at harvest = 312 g/kg; DMH: high dry matter at harvest = 348 g/kg. Panel A DML, p = 0.672; panel B DMH, p = 0.157; Figure S5: The Kaplan–Meier survival curve for the samples underwent different inoculations stratified for the delays at sealing (hours). Control: pure water; hetero: heterofermentative; homo: homofermentative; 0 h: zero h of dealing; 6 h: six h of dealing; 20 h: 20 h of dealing. Panel A: 0 h, p = 0.138; panel B: 6 h, p = 0.741; panel C: 20 h, p = 0.741; Figure S6: The microbiological features of samples were analysed after 60 d of fermentation. The box plot represents the minimum, first quartile, median, third quartile, and maximum. TVC = total viable count; LAB = lactic acid bacteria; Figure S7: Molecular identification of the yeasts and mould strains collected after 60 d of fermentation; Table S1: Calibration performances for winter cereal crop (WCC) fresh whole plant and silage for a FOSS NIRSystem 5000 scanning monochromator; Table S2: The main effect of the DM at harvest on the pH and ammonia patterns at d 2 (T2), 3 (T3), 6 (T6), and 10 (T10) from the day of sealing the mini-silos; Table S3: The main effect of the use of inoculants (control, heterofermentative, and homofermentative) on the pH and ammonia patterns at d 2 (T2), 3 (T3), 6 (T6), and 10 (T10) from the day of sealing the mini-silos; Table S4: The main effect of 0 (0 h), 6 (6 h), and 20 h (20 h) of sealing delaying on the pH and ammonia patterns at d 2 (T2), 3 (T3), 6 (T6), and 10 (T10) from the day of sealing the mini-silos; Table S5: The main effect of the DM at harvest on the silage quality after seven d (T67) of aerobic exposure; Table S6: The main effect of the use of inoculants on the silage quality after seven d (T67) of aerobic exposure; Table S7: The main effect of sealing delays in the silage quality after seven d (T67) of aerobic exposure; Table S8: The interactions of the DM at harvest, the use of inoculants, and the sealing delays on the silage quality after seven d (T67) of aerobic exposure; Table S9: The main effect of the DM at harvest on the silage quality after 14 d (T74) of aerobic exposure; Table S10: The main effect of the use of inoculants on the silage quality after 14 d (T74) of aerobic exposure; Table S11: The main effect of the sealing delays on the silage quality after s14 d (T74) of aerobic exposure; Table S12: The interactions of the DM at harvest, the use of inoculants, and the sealing delays on the silage quality after 14 d of aerobic exposure.
Author Contributions
Conceptualization, L.S. and I.A.; methodology, L.S., L.M., S.C. and I.A; formal analysis, L.S; investigation, L.S, L.M. and S.C; resources, LM; data curation, L.S.; writing—original draft preparation, L.S.; writing—review and editing, L.S., L.M., S.C. and G.M.; visualization, L.S.; supervision, I.A.; project administration, L.S.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was financially supported by KWS Italia S.P.A.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors greatly appreciate Elena Valleriani, Mattia Zago, Giacomo Bison, and Luana Mellia for their support in collecting data during the survey procedure.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Harper, M.T.; Oh, J.; Giallongo, F.; Roth, G.W.; Hristov, A.N. Inclusion of Wheat and Triticale Silage in the Diet of Lactating Dairy Cows. J. Dairy Sci. 2017, 100, 6151–6163. [Google Scholar] [CrossRef]
- Migliorati, L.; Boselli, L.; Pirlo, G.; Moschini, M.; Masoero, F. Corn Silage Replacement with Barley Silage in Dairy Cows’ Diet Does Not Change Milk Quality, Cheese Quality and Yield. J. Sci. Food Agric. 2017, 97, 3396–3401. [Google Scholar] [CrossRef] [PubMed]
- Carey, P.L.; Cameron, K.C.; Di, H.J.; Edwards, G.R.; Chapman, D.F. Sowing a Winter Catch Crop Can Reduce Nitrate Leaching Losses from Winter-Applied Urine under Simulated Forage Grazing: A Lysimeter Study. Soil Use Manag. 2016, 32, 329–337. [Google Scholar] [CrossRef]
- Istituto Nazionale Di Statistica. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_LATTE (accessed on 9 May 2022).
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Dipartimento Regionale per la Sicurezza del Territorio. Rapporto Sulla Risorsa Idrica in Veneto; Dipartimento Regionale per la Sicurezza del Territorio: Padova, Italy, 2022. [Google Scholar]
- Garofalo, P.; Ventrella, D.; Kersebaum, K.C.; Gobin, A.; Trnka, M.; Giglio, L.; Dubrovský, M.; Castellini, M. Water Footprint of Winter Wheat under Climate Change: Trends and Uncertainties Associated to the Ensemble of Crop Models. Sci. Total Environ. 2019, 658, 1186–1208. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ayuso, M.; Quemada, M.; Vanclooster, M.; Ruiz-Ramos, M.; Rodriguez, A.; Gabriel, J.L. Assessing Cover Crop Management under Actual and Climate Change Conditions. Sci. Total Environ. 2018, 621, 1330–1341. [Google Scholar] [CrossRef]
- Ronga, D.; Prà, A.D.; Immovilli, A.; Ruozzi, F.; Davolio, R.; Pacchioli, M.T. Effects of Harvest Time on the Yield and Quality of Winter Wheat Hay Produced in Northern Italy. Agronomy 2020, 10, 917. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Woolford, M.K.K. The Detrimental Effects of Air on Silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Bolsen, K.K.; Lin, C.J. History of Silage. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; pp. 1–30. [Google Scholar] [CrossRef]
- Vanbelle, M.; Bertin, G. L’ensilage, Aspects Biologiques Nouveaux; Sanofi Santé Animale: Paris, France, 1985. [Google Scholar]
- Gallo, A.; Giuberti, G.; Bruschi, S.; Fortunati, P.; Masoero, F. Use of Principal Factor Analysis to Generate a Corn Silage Fermentative Quality Index to Rank Well- or Poorly Preserved Forages. J. Sci. Food Agric. 2016, 96, 1686–1696. [Google Scholar] [CrossRef]
- Kaiser, E.; Weiß, K.; Nussbaum, H.; Kalzendorf, C.; Pahlow, G.; Schenkel, H.; Schwarz, F.J.; Spiekers, H.; Staudacher, W.; Thaysen, J. Grobfutterbewertung Teil B—DLG-Schlüssel Zur Beurteilung Der Gärqualität von Grünfuttersilagen Auf Basis Der Chemischen Untersuchung. Available online: https://www.lksh.de/fileadmin/PDFs/Landwirtschaft/Futter-_und_Substratkonservierung/FuKo_DLG_Grobfutterbewertung_B.pdf (accessed on 5 September 2022).
- Xiccato, G.; Cinetto, M.; Carazzolo, A.; Cossu, M.E. The Effect of Silo Type and Dry Matter Content on the Maize Silage Fermentation Process and Ensiling Loss. Anim. Feed Sci. Technol. 1994, 49, 311–323. [Google Scholar] [CrossRef]
- Xiccato, G.; Trocino, A.; Carazzolo, A. Ensiling and Nutritive Value of Kenaf (Hibiscus Cannabinus). Anim. Feed Sci. Technol. 1998, 71, 229–240. [Google Scholar] [CrossRef]
- Segato, S.; Marchesini, G.; Serva, L.; Contiero, B.; Magrin, L.; Andrighetto, I. Assessment of Fermentative Quality of Ensiled High-Moisture Maize Grains by a Multivariate Modelling Approach. Agronomy 2022, 12, 429. [Google Scholar] [CrossRef]
- Andrighetto, I.; Serva, L.; Gazziero, M.; Tenti, S.; Mirisola, M.; Garbin, E.; Contiero, B.; Grandis, D.; Marchesini, G. Proposal and Validation of New Indexes to Evaluate Maize Silage Fermentative Quality in Lab-Scale Ensiling Conditions through the Use of a Receiver Operating Characteristic Analysis. Anim. Feed Sci. Technol. 2018, 242, 31–40. [Google Scholar] [CrossRef]
- Serva, L.; Marchesini, G.; Chinello, M.; Contiero, B.; Tenti, S.; Mirisola, M.; Grandis, D.; Andrighetto, I. Use of Near-Infrared Spectroscopy and Multivariate Approach for Estimating Silage Fermentation Quality from Freshly Harvested Maize. Ital. J. Anim. Sci. 2021, 20, 859–871. [Google Scholar] [CrossRef]
- Archibald, J.G.; Kuzmeski, J.W.; Russell, S. Grass Silage Quality as Affected by Crop Composition and by Additives. J. Dairy Sci. 1960, 43, 1648–1653. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L. The Effect of Lactobacillus buchneri, Lactobacillus plantarum, or a Chemical Preservative on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Brüning, D.; Gerlach, K.; Weiß, K.; Südekum, K.H. Effect of Compaction, Delayed Sealing and Aerobic Exposure on Maize Silage Quality and on Formation of Volatile Organic Compounds. Grass Forage Sci. 2018, 73, 53–66. [Google Scholar] [CrossRef]
- Jia, T.; Wang, B.; Yu, Z.; Wu, Z. The Effects of Stage of Maturity and Lactic Acid Bacteria Inoculants on the Ensiling Characteristics, Aerobic Stability and in Vitro Digestibility of Whole-Crop Oat Silages. Grassl. Sci. 2021, 67, 55–62. [Google Scholar] [CrossRef]
- Addah, W.; Baah, J.; Groenewegen, P.; Okine, E.K.; McAllister, T.A. Comparison of the Fermentation Characteristics, Aerobic Stability and Nutritive Value of Barley and Corn Silages Ensiled with or without a Mixed Bacterial Inoculant. Can. J. Anim. Sci. 2011, 91, 133–146. [Google Scholar] [CrossRef]
- Muck, R.E. A Lactic Acid Bacterial Strain to Improve Aerobic Stability of Silages; US Dairy Forage Research Center: Madison, WI, USA, 1996; pp. 42–43. [Google Scholar]
- Daniel, J.L.P.P.; Queiroz, O.C.M.M.; Arriola, K.G.; Daetz, R.; Basso, F.; Romero, J.J.; Adesogan, A.T. Effects of Homolactic Bacterial Inoculant on the Performance of Lactating Dairy Cows. J. Dairy Sci. 2018, 101, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.M.; Vyas, D.; et al. Meta-Analysis of Effects of Inoculation with Homofermentative and Facultative Heterofermentative Lactic Acid Bacteria on Silage Fermentation, Aerobic Stability, and the Performance of Dairy Cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Tabacco, E.; Piano, S.; Cavallarin, L.; Bernardes, T.F.; Borreani, G. Clostridia Spore Formation during Aerobic Deterioration of Maize and Sorghum Silages as Influenced by Lactobacillus buchneri and Lactobacillus plantarum Inoculants. J. Appl. Microbiol. 2009, 107, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Serva, L.; Magrin, L.; Marchesini, G.; Andrighetto, I. Short Communication: Prognostic Values of a Multiparametric Risk Score in Maize Silage Undergoing Different Ensiling Conditions. Agronomy 2022, 12, 774. [Google Scholar] [CrossRef]
- Serva, L.; Andrighetto, I.; Marchesini, G.; Contiero, B.; Grandis, D.; Magrin, L. Prognostic Capacity Assessment of a Multiparameter Risk Score for Aerobic Stability of Maize Silage Undergoing Heterofermentative Inoculation (Lactobacillus buchneri) in Variable Ensiling Conditions. Anim. Feed Sci. Technol. 2021, 281, 115116. [Google Scholar] [CrossRef]
- Berzaghi, P.; Serva, L.; Piombino, M.; Mirisola, M.; Benozzo, F. Prediction Performances of Portable near Infrared Instruments for at Farm Forage Analysis. Ital. J. Anim. Sci. 2005, 4, 145–147. [Google Scholar] [CrossRef]
- Marchesini, G.; Serva, L.; Garbin, E.; Mirisola, M.; Andrighetto, I. Near-Infrared Calibration Transfer for Undried Whole Maize Plant between Laboratory and on-Site Spectrometers. Ital. J. Anim. Sci. 2017, 17, 66–72. [Google Scholar] [CrossRef]
- Evangelista, C.; Basiricò, L.; Bernabucci, U. An Overview on the Use of near Infrared Spectroscopy (Nirs) on Farms for the Management of Dairy Cows. Agriculture 2021, 11, 296. [Google Scholar] [CrossRef]
- Harris, P.A.; Nelson, S.; Carslake, H.B.; Argo, C.M.G.; Wolf, R.; Fabri, F.B.; Brolsma, K.M.; van Oostrum, M.J.; Ellis, A.D. Comparison of NIRS and Wet Chemistry Methods for the Nutritional Analysis of Haylages for Horses. J. Equine Vet. Sci. 2018, 71, 13–20. [Google Scholar] [CrossRef]
- Marchesini, G.; Serva, L.; Chinello, M.; Gazziero, M.; Tenti, S.; Mirisola, M.; Garbin, E.; Contiero, B.; Grandis, D.; Andrighetto, I. Effect of Maturity Stage at Harvest on the Ensilability of Maize Hybrids in the Early and Late FAO Classes, Grown in Areas Differing in Yield Potential. Grass Forage Sci. 2019, 74, 415–426. [Google Scholar] [CrossRef]
- Abrams, S.M.; Shenk, J.S.; Harpster, H.W. Potential of Near Infrared Reflectance Spectroscopy for Analysis of Silage Composition. J. Dairy Sci. 1988, 71, 1955–1959. [Google Scholar] [CrossRef]
- Park, R.S.; Agnew, R.E.; Gordon, F.J.; Steen, R.W.J.J. The Use of near Infrared Reflectance Spectroscopy (NIRS) on Undried Samples of Grass Silage to Predict Chemical Composition and Digestibility Parameters. Anim. Feed Sci. Technol. 1998, 72, 155–167. [Google Scholar] [CrossRef]
- Reeves, J.B.; Blosser, T.H.; Colenbrander, V.F. Near Infrared Reflectance Spectroscopy for Analyzing Undried Silage. J. Dairy Sci. 1989, 72, 79–88. [Google Scholar] [CrossRef]
- Sørensen, L.K. Prediction of Fermentation Parameters in Grass and Corn Silage by near Infrared Spectroscopy. J. Dairy Sci. 2004, 87, 3826–3835. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, S.H.; Choi, K.C.; Lim, Y.C.; Kim, J.H.; Lee, K.W.; Choi, G.J. Prediction of the Chemical Composition and Fermentation Parameters of Winter Rye Silages by Near Infrared Spectroscopy. J. Korean Soc. Grassl. Forage Sci. 2014, 34, 209–213. [Google Scholar] [CrossRef]
- Liu, X.; Han, L.J. Evaluation of Near-Infrared Reflectance Spectroscopy (NIRS) for Predicting Chemical Composition of Straw Silage. J. Anim. Feed Sci. 2006, 15, 329–336. [Google Scholar] [CrossRef]
- Schuchmann, G.H. DLG Test Report 7020. Available online: https://pruefberichte.dlg.org/filestorage/7020_e.pdf (accessed on 19 July 2022).
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry Matter and Nutritional Losses during Aerobic Deterioration of Corn and Sorghum Silages as Influenced by Different Lactic Acid Bacteria Inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef]
- Segato, S.; Marchesini, G.; Serva, L.; Magrin, L.; Contiero, B.; Andrighetto, I.; Serva, L. A Machine Learning-Based Assessment of Maize Silage Dry Matter Losses by Net-Bags Buried in Farm Bunker Silos. Agriculture 2022, 12, 785. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 2nd Revision, 17th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemist: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Ferreira, G.; Mertens, D.R. Measuring Detergent Fibre and Insoluble Protein in Corn Silage Using Crucibles or Filter Bags. Anim. Feed Sci. Technol. 2007, 133, 335–340. [Google Scholar] [CrossRef]
- Schlau, N.; Mertens, D.R.; Taysom, K.; Taysom, D. Technical Note: Effects of Filter Bags on Neutral Detergent Fiber Recovery and Fiber Digestion in Vitro. J. Dairy Sci. 2021, 104, 1846–1854. [Google Scholar] [CrossRef]
- Vogel, K.P.; Pedersen, J.F.; Masterson, S.D.; Toy, J.J. Evaluation of a Filter Bag System for NDF, ADF, and IVDMD Forage Analysis. Crop Sci. 1999, 39, 276–279. [Google Scholar] [CrossRef]
- Ankom Determining Acid Detergent Lignin in Beakers. Available online: http//www.ankom.com/media/documents/Method_8_Lignin_in_beakers_3_13_13.pdf (accessed on 9 March 2014).
- Martillotti, F.; Puppo, S. Liquid Chromatographic Determination of Organic Acids in Silages and Rumen Fluids. Ann. dell’Istituto Sper. Zootec. 1985, 18, 1–10. [Google Scholar]
- Urea/Ammonia Assay Procedure. UREA/AMMONIA (Rapid). Available online: https://secure.megazyme.com/files/Booklet/K-URAMR_DATA.pdf (accessed on 5 July 2022).
- Martillotti, F.; Anotngiovanni, M.; Rizzi, L.; Santi, E.; Bittante, G. Metodi Di Analisi per La Valutazione Degli Alimenti D’impiego Zootecnico; CNR IPRA; Quaderni Metodologici: Milano, Italy, 1987. [Google Scholar]
- Richard, T.L.; Veeken, A.H.M.; de Wilde, V.; Hamelers, H.V.M. Air-Filled Porosity and Permeability Relationships during Solid-State Fermentation. Biotechnol. Prog. 2004, 20, 1372–1381. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Abeysekera, W.W.M.M.; Sooriyarachchi, M.R. Use of Schoenfeld’s Global Test to Test Proportional Hazards Assumption in the Cox Proportional Hazards Model: An Application to Clinical Study. J. Natl. Sci. Found. Sri Lanka 2009, 37, 41–51. [Google Scholar] [CrossRef]
- Downie, T. Using the R Commander: A Point-and-Click Interface for R. J. Stat. Softw. 2016, 75, 1–4. [Google Scholar] [CrossRef]
- Fox, J. The R Commander: A Basic-Statistics Graphical User Interface to R. J. Stat. Softw. 2005, 14, 1–42. [Google Scholar] [CrossRef]
- Pesarin, F.; Salmaso, L. Permutation Tests for Complex Data: Theory, Applications and Software. Available online: http://www.wiley.com/legacy/wileychi/pesarin/material.html (accessed on 30 November 2022).
- Fasolato, L.; Carraro, L.; Facco, P.; Cardazzo, B.; Balzan, S.; Taticchi, A.; Andreani, N.A.; Montemurro, F.; Martino, M.E.; Di Lecce, G.; et al. Agricultural By-Products with Bioactive Effects: A Multivariate Approach to Evaluate Microbial and Physicochemical Changes in a Fresh Pork Sausage Enriched with Phenolic Compounds from Olive Vegetation Water. Int. J. Food Microbiol. 2016, 228, 34–43. [Google Scholar] [CrossRef]
- Chandler, J.A.; Jewell, W.J.; Gossett, J.M.; Van Soest, P.J.; Robertson, J.B. Predicting Methane Fermentation Biodegradability. Biotechnol. Bioeng. Symp. 1980, 10, 93–107. [Google Scholar]
- Raffrenato, E.; Ross, D.A.; Van Amburgh, M.E. Development of an in Vitro Method to Determine Rumen Undigested ANDFom for Use in Feed Evaluation. J. Dairy Sci. 2018, 101, 9888–9900. [Google Scholar] [CrossRef]
- Raffrenato, E.; Fievisohn, R.; Cotanch, K.W.; Grant, R.J.; Chase, L.E.; Van Amburgh, M.E. Effect of Lignin Linkages with Other Plant Cell Wall Components on in Vitro and in Vivo Neutral Detergent Fiber Digestibility and Rate of Digestion of Grass Forages. J. Dairy Sci. 2017, 100, 8119–8131. [Google Scholar] [CrossRef]
- Oba, M.; Allen, M.S. Evaluation of the Importance of the Digestibility of Neutral Detergent Fiber from Forage: Effects on Dry Matter Intake and Milk Yield of Dairy Cows. J. Dairy Sci. 1999, 82, 589–596. [Google Scholar] [CrossRef]
- Allen, M.S. Effects of Diet on Short-Term Regulation of Feed Intake by Lactating Dairy Cattle. J. Dairy Sci. 2000, 83, 1598–1624. [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhang, T.F.; Chen, X.Z.; Li, G.D.; Zhang, J.G. Effects of Maturity Stages on the Nutritive Composition and Silage Quality of Whole Crop Wheat. Asian-Australas. J. Anim. Sci. 2012, 25, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Randby, T.; Nadeau, E.; Karlsson, L.; Johansen, A. Effect of Maturity Stage at Harvest and Kernel Processing of Whole Crop Wheat Silage on Digestibility by Dairy Cows. Anim. Feed Sci. Technol. 2019, 253, 141–152. [Google Scholar] [CrossRef]
- Walsh, K.; O’Kiely, P.; Moloney, A.P.; Boland, T.M. Intake, Performance and Carcass Characteristics of Beef Cattle Offered Diets Based on Whole-Crop Wheat or Forage Maize Relative to Grass Silage or Ad Libitum Concentrates. Livest. Sci. 2008, 116, 223–236. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, E.J.; Gottschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic Conversion of Lactic Acid to Acetic Acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L. A Meta-Analysis of the Effects of Lactobacillus buchneri on the Fermentation and Aerobic Stability of Corn and Grass and Small-Grain Silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef] [PubMed]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Sela, S. Microbiome Dynamics during Ensiling of Corn with and without Lactobacillus plantarum Inoculant. Appl. Microbiol. Biotechnol. 2018, 102, 4025–4037. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Chen, Y. Effects of Storage Period on the Composition of Whole Crop Wheat and Corn Silages. Anim. Feed Sci. Technol. 2013, 185, 196–200. [Google Scholar] [CrossRef]
- Chen, Y.; Weinberg, Z.G. The Effect of Relocation of Whole-Crop Wheat and Corn Silages on Their Quality. J. Dairy Sci. 2014, 97, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Hackl, W.; Korn, U.; Zeyner, A.; Souffrant, W.B.; Pieper, B. Effect of Ensiling Triticale, Barley and Wheat Grains at Different Moisture Content and Addition of Lactobacillus plantarum (DSMZ 8866 and 8862) on Fermentation Characteristics and Nutrient Digestibility in Pigs. Anim. Feed Sci. Technol. 2011, 164, 96–105. [Google Scholar] [CrossRef]
- Keshri, J.; Chen, Y.; Pinto, R.; Kroupitski, Y.; Weinberg, Z.G.; Saldinger, S.S. Bacterial Dynamics of Wheat Silage. Front. Microbiol. 2019, 10, 1532. [Google Scholar] [CrossRef] [PubMed]
- Agarussi, M.C.N.; Pereira, O.G.; da Silva, L.D.; da Silva, V.P.; de Paula, R.A.; Fonesca e Silva, F.; Ribeiro, K.G. Effect of Various Strains of Lactobacillus buchneri on the Fermentation Quality and Aerobic Stability of Corn Silage. Agriculture 2022, 12, 95. [Google Scholar] [CrossRef]
- Baah, J.; Addah, W.; Okine, E.K.; McAllister, T.A. Effects of Homolactic Bacterial Inoculant Alone or Combined with an Anionic Surfactant on Fermentation, Aerobic Stability and in Situ Ruminal Degradability of Barley Silage. Asian-Australas. J. Anim. Sci. 2011, 24, 369–378. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. Quantifying the Extent of Aerobic Deterioration in Corn Bunker and Pile Silages at a Farm Level. In Proceedings of the 15th International Silage Conference, Madison, WI, USA, 27–29 July 2009; US Dairy Forage Research Centre, USDA-ARS: Madison, WI, USA, 2009; pp. 321–322. [Google Scholar]
- Schmidt, R.J.; Kung, L. The Effects of Lactobacillus buchneri with or without a Homolactic Bacterium on the Fermentation and Aerobic Stability of Corn Silages Made at Different Locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Huisden, C.M.; Adesogan, A.T.; Kim, S.C.; Ososanya, T. Effect of Applying Molasses or Inoculants Containing Homofermentative or Heterofermentative Bacteria at Two Rates on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2009, 92, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Mari, L.J.; Schmidt, R.J.; Nussio, L.G.; Halladas, C.M.; Kung, L. Short Communication: An Evaluation of the Effectiveness of Lactobacillus buchneri 40788 to Alter Fermentation and Improve the Aerobic Stability of Corn Silage in Farm Silos. J. Dairy Sci. 2009, 92, 1174–1176. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Borreani, G.; Bernardes, T.F.; Tabacco, E. Aerobic Deterioration Influences the Fermentative, Microbiological and Nutritional Quality of Maize and Sorghum Silages on Farm in High Quality Milk and Cheese Production Chains. Rev. Bras. Zootec. 2008, 37, 68–77. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. The Relationship of Silage Temperature with the Microbiological Status of the Face of Corn Silage Bunkers. J. Dairy Sci. 2010, 93, 2620–2629. [Google Scholar] [CrossRef]
- Santos, M.C.; Golt, C.; Joerger, R.D.; Mechor, G.D.; Mourão, G.B.; Kung, L. Identification of the Major Yeasts Isolated from High Moisture Corn and Corn Silages in the United States Using Genetic and Biochemical Methods. J. Dairy Sci. 2017, 100, 1151–1160. [Google Scholar] [CrossRef]
- Spadaro, D.; Bustos-Lopez, M.P.; Gullino, M.L.; Piano, S.; Tabacco, E.; Borreani, G. Evolution of Fungal Populations in Corn Silage Conserved under Polyethylene or Biodegradable Films. J. Appl. Microbiol. 2015, 119, 510–520. [Google Scholar] [CrossRef]
- Jonsson, A. Enumeration and Confirmation of Clostridium Tyrobutyricum in Silages Using Neutral Red, D-Cycloserine, and Lactate Dehydrogenase Activity. J. Dairy Sci. 1990, 73, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Gharechahi, J.; Kharazian, Z.A.; Sarikhan, S.; Jouzani, G.S.; Aghdasi, M.; Hosseini Salekdeh, G. The Dynamics of the Bacterial Communities Developed in Maize Silage. Microb. Biotechnol. 2017, 10, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Benjamim da Silva, É.; Liu, X.; Mellinger, C.; Gressley, T.F.; Stypinski, J.D.; Moyer, N.A.; Kung, L. Effect of Dry Matter Content on the Microbial Community and on the Effectiveness of a Microbial Inoculant to Improve the Aerobic Stability of Corn Silage. J. Dairy Sci. 2022, 105, 5024–5043. [Google Scholar] [CrossRef]
- Arriola, K.G.; Oliveira, A.S.; Jiang, Y.; Kim, D.; Silva, H.M.; Kim, S.C.; Amaro, F.X.; Ogunade, I.M.; Sultana, H.; Pech Cervantes, A.A.; et al. Meta-Analysis of Effects of Inoculation with Lactobacillus buchneri, with or without Other Bacteria, on Silage Fermentation, Aerobic Stability, and Performance of Dairy Cows. J. Dairy Sci. 2021, 104, 7653–7670. [Google Scholar] [CrossRef]
- Weiß, K.; Kroschewski, B.; Auerbach, H.U. The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability. Fermentation 2022, 8, 48. [Google Scholar] [CrossRef]
- De Melo, N.N.; Carvalho-Estrada, P.d.A.; Tavares, Q.G.; de Moura Pereira, L.; Delai Vigne, G.L.; Camargo Rezende, D.M.L.; Schmidt, P. The Effects of Short-Time Delayed Sealing on Fermentation, Aerobic Stability and Chemical Composition on Maize Silages. Agronomy 2023, 13, 223. [Google Scholar] [CrossRef]
- Kroschewski, B.; Auerbach, H.; Weiss, K. Statistics and experimental design in silage research: Some comments on design and analysis of comparative silage experiments. In Proceedings of the XVIII International Silage Conference, Bonn, Germany, 24–26 July 2018; Gerlach, K., Südekum, K.-H., Eds.; University of Bonn: Bonn, Germany, 2018; pp. 554–560. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).