Abstract
French tamarisk, Tamarix gallica L. (family Tamaricaceae) is a deciduous tree that, like other halophytes, grows in a wide variety of saline habitats thanks to its powerful phenolics-based antioxidant system. Given that antioxidant properties are usually linked to the presence of compounds with antifungal properties, in the work presented herein the antimicrobial activity of T. gallica bark extract was investigated against four phytopathogenic species of genus Fusarium. According to the results of gas chromatography–mass spectroscopy, the phytochemical profile of the aqueous ammonia extract included 1-(2,4,6-trihydroxyphenyl)-2-pentanone; 3,5-dimethoxy-4-hydroxycinnam aldehyde; trans-squalene; 4-hydroxy-3,5-dimethoxy-benzaldehyde; dihydro-3-methylene-2,5-furandione; 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone; and 4-hydroxy-3,5-dimethoxy-benzoic acid as main constituents. Concerning in vitro antifungal activity, EC90 effective concentrations in the 335–928 μg·mL−1 range were obtained against F. acuminatum, F. culmorum, F. equiseti, and F. graminearum, remarkably lower than those of two conventional fungicides (viz. mancozeb and fosetyl-Al). The antifungal activity of the extract was tested further in wheat and maize grain protection bioassays, confirming that the treatment effectively controlled F. graminearum at a concentration of 375 µg·mL−1. Given this promising activity, T. gallica bark extracts may be susceptible to valorization as a natural and sustainable biorational for Fusarium spp. control.
1. Introduction
Halophytes can complete their life cycles in highly salinized habitats without having significant detrimental effects on their growth or development. However, they account for, approximately, just 1% of all terrestrial plants, and the majority of them have neither ornamental nor economic value, which restricts their growth and use. It is therefore essential to look for (and make use of) beneficial halophytes in the development of moderately and severely salinized areas, which are vulnerable to desertification and ecological fragility owing to their lack of cover vegetation [1].
More than 60 species of halophytic plants are included in the genus Tamarix (family Tamaricaceae), popularly known as ‘tamarisk’ and ‘salt cedar’, which can be cultivated practically everywhere in the globe, improving the environment while also bringing economic advantages [2] (except in humid environments, in which they behave as invasive plants, impeding the development of other native species). Native to hot and arid areas, tamarisk species may also be found in temperate climates [3]. These plants are distinguished by having needle-shaped leaves covered with salt secreted by salt glands, which play a key function in ionic balance regulation and in osmotic and turgor pressure maintenance under high salinity [4]. Studies on various Tamarix species have revealed a number of phytochemicals, the most significant of which are polyphenolic substances such as tannins, phenolic acids, and flavonoids, which are related to their main pharmacological properties, summarized in the review by Bahramsoltani et al. [5].
Tamarix gallica L. is a deciduous halophyte tree or shrub with a long lifespan, native to coastal and arid environments. It can withstand a variety of environmental stresses, including salt, high temperatures, and drought whilst growing up to 4 m. Its hermaphrodite flowers are small, five-petaled, white to pink, and flower throughout the spring and summer; they grow in long, drooping, narrow clusters that are up to three inches long. Seeds are small and black, with a sessile tuft of hygroscopic unicellular hairs attached to one end. Tamarix gallica has a smooth, reddish-brown bark that becomes furrowed and ridged with age [6].
As noted above, halophytes have a powerful antioxidant system based on certain phenolic compounds, terpenoids (carotenoids and essential oils), and vitamins, which are crucial to plants’ normal growth, development, and defense against damage and infection [7]. In addition, these compounds have a wide spectrum of medicinal properties, such as anti-inflammatory, anti-allergic, antithrombotic, cardioprotective, and vasodilator effects, hepatoprotective and chemopreventive properties, and promising behavior as antioxidant and antimicrobial agents [8].
In the case of T. gallica, a total phenol content of 334.19 ± 8.47 mg GAE/g DW (and a flavonoids content of 159.73 ± 6.28 mg CE/g DW) was reported for a leaf methanolic extract [9], higher than that obtained for a methanolic extract of shoots (with a total phenol content of 200 mg GAE/g DW) [10]. The flower phenolic fingerprint of T. gallica includes seven phenolic acids (chlorogenic, trans-cinnamic, p-coumaric, gallic, sinapic, syringic, and vanillic acid) and six flavonoids (amentoflavone, apigenin, (+)-catechin, flavone, isoquercetin, and quercetin). As for the leaves, in addition to the phenolics identified in flowers, rosmarinic and ferulic acids were identified by Ksouri et al. [7]. Aside from these chemicals, Boulaaba et al. [11] reported the presence of the flavonoid kaempferol in flower extracts, and the existence of six compounds in the leaf extract, including quercetin 3-O-glucuronide. In turn, Said et al. [10] identified the phenolics naringin and caffeic acid in the leaf extract. The above phytochemicals, it has been suggested, account for the antibacterial activity of T. gallica against Micrococcus luteus (Schroeter) Cohn and its antifungal activity (especially against Candida glabrata (H.W. Anderson) S.A. Mey. and Yarrow and Candida albicans (C.P. Robin) Berkhout) [11].
Concerning the opportunities for the valorization of T. gallica extracts, their application as biorationals for crop protection may be particularly interesting. Among staple food crops, wheat and maize are especially important in terms of their contribution to food security [12]. However, cereal production is threatened by climate change and plant disease epidemics [13]. For instance, Fusarium head blight (FHB) severely reduces grain production quality and quantity in cereal crops including wheat, maize, and barley [14]. More than sixteen species, including Fusarium graminearum Schwabe (the major FHB pathogen), Fusarium culmorum (Wm.G. Sm.) Sacc., Fusarium pseudograminearum O’Donnell and T. Aoki, Fusarium avenaceum (Fr.) Sacc., Fusarium equiseti (Corda) Sacc., and Fusarium poae (Peck) Wollenw., are part of the FHB species complex. All produce mycotoxins, low molecular weight toxic secondary metabolites of high thermal stability and bioaccumulation capacity, which are potentially harmful to both human and animal health [15].
Although unpredictable, Fusarium outbreaks have increased in frequency in northern and central Europe as F. graminearum has invaded areas formerly dominated by the presence of F. culmorum [16]. Fungicide applications are regarded as a crucial and often utilized method for managing FHB. Factors such as the active molecule applied, timing, manner, rate of administration, cereal variety, and the presence of Fusarium species and pathogenic races affect the efficacy of the treatments and mycotoxin reduction [17]. Triazoles (i.e., tebuconazole, metconazole, and prothioconazole), carbendazim, strobilurins (i.e., azoxystrobin), and their combinations are frequently used to control FHB [18]. In particular, azoxystrobin alone should be avoided, given that it may enhance the production of the deoxynivalenol toxin [19]. Alternatives to synthetic fungicides are being sought to reduce the accumulation of pesticide residues in food and the environment.
In this context, with the aim of searching for alternatives to the application of fungicides, taking into consideration Article 14 of Directive 2009/128/EC, this work covers the use of gas chromatography–mass spectrometry (GC−MS) to characterize the phytochemicals found in T. gallica bark aqueous ammonia extract, as well as the evaluation of its antifungal activity for the control of Fusarium spp. The effectiveness of this extract was first tested in vitro against F. acuminatum, F. culmorum, F. equiseti, and F. graminearum, and further tested for grain protection at storage against F. graminearum. The reported findings may be useful for the sustainable postharvest protection of wheat and maize grains, promoting their storability and food safety.
2. Material and Methods
2.1. Reagents
Ammonium hydroxide solution (CAS No. 1336-21-6, 50% v/v aq. soln.) was supplied by Alfa Aesar (Ward Hill, MA, USA). Acetic acid (CAS No. 64-19-7, purum, 80% in H2O); squalene (CAS No. 111-02-4, analytical standard); 1-(2,4,6-trihydroxyphenyl)-2-pentanone (CAS No. 443678-79-3); syringaldehyde (CAS No. 134-96-3); sinapinaldehyde (CAS No. 4206-58-0); and Tween® 20 (CAS No. 9005-64-5) were purchased from Sigma Aldrich Química S.A. (Madrid, Spain). Becton, Dickinson, and Company (Franklin Lakes, NJ, USA) supplied the potato dextrose broth (PDB) and potato dextrose agar (PDA).
The Plant Health and Certification Service of the Government of Aragon provided the commercial fungicides used for comparison purposes, namely Vondozeb® (mancozeb 75%; reg. no. 18632; UPL Iberia) and Fesil® (fosetyl-Al 80%, reg. no. 18795; Bayer).
2.2. Fungal Isolates
Fungal isolates of F. acuminatum (42/63/2022) and F. graminearum (CRD 002/99) were supplied by the Regional Diagnostic Center of Aldearrubia (Junta de Castilla y León); F. equiseti (MYC-1403) was obtained from the Centre for Agri-Food Research and Technology of Aragon (CITA); and F. culmorum (CECT 20493) was acquired from the Spanish Type Culture Collection (CECT; Valencia, Spain).
2.3. Plant Material and Extraction Procedure
To attain the dissolution of polyphenols and other bioactive compounds of interest contained in T. gallica bark, an aqueous ammonia extraction medium was chosen, given its ability to remove acetyl groups from xylan polymers, reduce cellulose crystallinity, selectively breakdown and remove lignin from substrates, and increase porosity while releasing low amounts of sugar degradation compounds. Aqueous ammonia pretreatment is also affordable, non-corrosive, non-polluting, safe to use, and recyclable [20]. This choice is supported by other recent work involving bark extracts [21,22,23].
The extract was prepared from a composite sample of the bark of ten specimens of T. gallica from the Paseo de San Pedro, in Llanes (Asturias, Spain; 43°25’30.9″ N 4°45’31.2″ W), collected in May 2021 (Figure 1). The bark samples were thoroughly mixed, dried, and ground into a fine powder to facilitate the extraction process. The preparation of the bark extract followed the procedure previously reported in reference [22]. The bark powder sample (previously digested in aqueous ammonia solution for 2 h) was sonicated for 10 min, with a 2 min pause after every 2.5 min of sonication, using a model UIP1000hdT probe-type ultrasonicator from Hielscher Ultrasonics (Teltow, Germany). The sample was then allowed to stand for 24 h, and acetic acid was used to bring the pH to neutral. Finally, the solution was centrifuged for 15 min at 9000 rpm, and the supernatant was filtered using Whatman No. 1 paper.

Figure 1.
(a) Tamarisk of the Paseo de San Pedro, in Llanes (Asturias, northern Spain), (b) trunk of a T. gallica specimen, (c) detail of T. gallica bark.
2.4. Extract Characterization
The infrared vibrational spectrum was recorded using a Nicolet iS50 Fourier-transform infrared spectrometer from Thermo Scientific (Waltham, MA, USA) with an in-built diamond attenuated total reflection (ATR) system. The spectrum was acquired with a resolution of 1 cm−1 spanning the 400–4000 cm−1 range, using the interferograms produced by co-adding 64 scans.
The aqueous ammonia extract of T. gallica bark was studied by gas chromatography–mass spectrometry (GC–MS) at the University of Alicante’s Research Support Services (STI), with an Agilent Technologies (Santa Clara, CA, USA) model 7890A gas chromatograph connected to a model 5975C quadrupole mass spectrometer. The operating conditions were as follows: 280 °C injector temperature; splitless mode; 1 µL injection volume; 60 °C initial temperature for 2 min, followed by a ramp of 10 °C per min up to a final temperature of 300 °C, kept for 15 min. An Agilent Technologies HP-5MS UI chromatographic column (30 m in length, 0.250 mm diameter, and 0.25 µm film) was used for the separation of the compounds. The mass spectrometer settings were as follows: 230 °C electron impact source temperature; 150 °C quadrupole temperature; 70 eV ionization energy. For calibration, test mixture 2 for apolar capillary columns according to Grob (Supelco 86501) and PFTBA tuning standards supplied by Sigma Aldrich Química S.A. (Madrid, Spain) were utilized. For chemical identification, mass spectra and retention times were compared to those of reference compounds and the National Institute of Standards and Technology database.
2.5. In Vitro Antifungal Activity Evaluation
The antimicrobial activity of the treatments was evaluated using the agar dilution method (or ‘poisoned food method’), in accordance with EUCAST standard antifungal susceptibility testing protocols [24]. To obtain concentrations in the 62.5−1500 μg·mL−1 range, aliquots of stock solution were mixed into a pouring PDA medium. Mycelial disks (Ø = 5 mm) from the margins of 1-week-old PDA cultures of the Fusarium spp. tested were transferred to PDA plates prepared with the aforementioned concentrations (three plates per treatment and concentration, with two duplicates). Incubation was conducted at 25 °C in the dark for one week. As a control, pure PDA media was used. Growth inhibition was calculated as ((dc − dt)/dc) × 100, where dc and dt represent the mean diameters of the control and treated colonies, respectively. Determination of EC50 and EC90 values (50% and 90% maximal effective concentration, respectively) was carried out using PROBIT analysis in IBM SPSS Statistics v.25 (IBM, Armonk, NY, USA).
2.6. Preparation of Conidial Suspension of F. graminearum
A conidial suspension of F. graminearum was produced using the approach reported by Buzón-Durán et al. [25], with slight changes. Conidia of F. graminearum were harvested from 1-week-old PDB cultures (200 mL broth maintained in the dark at 25 °C and 140 rpm in an orbital stirrer incubator). To eliminate hyphal fragments, the suspension was filtered through two layers of sterile muslin. A hemocytometer (Weber Scientific International Ltd., Teddington, Middlesex, UK) was used for spore concentration determination, and the final concentration was adjusted to 1 × 106 spores (conidia)·mL−1.
2.7. Stored Wheat and Maize Grain Protection Assays
The effect of T. gallica bark extract on the protection of stored wheat and maize grains against F. graminearum was determined according to Perczak et al. [26], with slight modifications. Soft winter wheat variety cv. ‘Rimbaud’ grains (Agrusa; Mollerussa, Lérida, Spain) and maize cv. ‘P0937′ grains (DuPont Pioneer; Johnston, IA, USA), supplied by Piensos y Cereales Isabelio Sánchez-García (El Tejado de Béjar, Salamanca, Spain), were used in the experiments. Grains were surface sterilized by immersion in sodium hypochlorite 3% for 2 min and then rinsed with sterile milli-Q water three times, before being dried at room temperature in a laminar flow hood on sterile absorbent paper. Grain treatments (50 g of wheat or maize grains per treatment) were conducted by immersion in 100 mL of T. gallica extract (at a concentration equivalent to the MIC obtained in the in vitro experiments, adding 0.2% Tween® 20) at room temperature, under agitation, for 15 min. In the positive and negative controls, distilled water with 0.2% Tween® 20 was used. After drying for 30 min, at room temperature in a laminar flow hood, the grains were inoculated with the conidial suspension (prepared as described in the previous subsection). The samples were then incubated in a dark chamber at 25 °C for 28 days. Each treatment was repeated three times.
2.8. In Vitro Germination Assays
The effect of T. gallica bark extract on the germination of wheat and maize grains was assessed according to International Seed Testing Association (ISTA) standards [27]. The procedure was similar to the one indicated for the stored grain protection assays, using 20 maize grains and 50 wheat grains per treatment and replicate. Each treatment was repeated three times, and, for each treatment, three replicates of wheat or maize grains were placed in glass plates, using the between-paper method, and maintained under constant humid conditions. Germination was evaluated after four and six days for wheat and maize, respectively, with grains deemed germinated if they produced a well-developed seedling.
2.9. Statistics
Provided that the homogeneity and homoscedasticity requirements were met, according to the Shapiro–Wilk and Levene tests, the results of the in vitro mycelium growth inhibition experiments were analyzed using a one-way analysis of variance, followed by Tukey test for the post hoc comparison of means at p < 0.05.
3. Results
3.1. Bark Vibrational Characterization
Table 1 provides a summary of the primary infrared absorption bands found in the bark of T. gallica, which are consistent with the presence of functional groups such as polyphenols, alkaloids, organic acid esters, and other phytoconstituents. The main bands of the leaf vibrational spectrum [28] are also indicated for comparison purposes.

Table 1.
Main bands in the infrared spectra of T. gallica bark and leaves.
3.2. Bark Extract Constituents
Among the twenty-five compounds identified in the aqueous ammonia extract by GC−MS (Table 2), the nine most abundant (percentages > 3.5%) were: 1-(2,4,6-trihydroxyphenyl)-2-pentanone (11.8%); sinapinaldehyde or 3,5-dimethoxy-4-hydroxycinnamaldehyde (10%); trans-squalene or supraene (9.9%); syringaldehyde or 4-hydroxy-3,5-dimethoxy-benzaldehyde (8.1%); dihydro-3-methylene-2,5-furandione (7.5%); 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone (7.2%); 4-hydroxy-3,5-dimethoxy-benzoic acid (6.6%); 2,6-dimethoxy-phenol (4%); and hexadecanoic acid, methyl ester (3.7%). Figure 2 depicts the chemical structures of the main phytochemicals found in T. gallica bark extract.

Table 2.
Major phytochemical compounds identified in the aqueous ammonia extract of T. gallica bark by GC−MS.
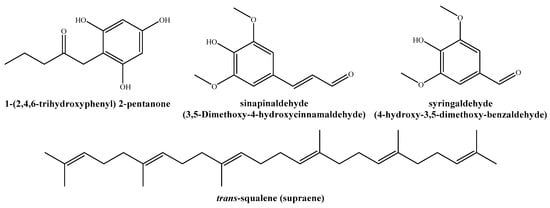
Figure 2.
Main phytochemicals identified in the aqueous ammonia extract of T. gallica bark.
If the aforementioned compounds are grouped into categories, the extract of T. gallica bark consists of phenolic compounds (50%), triterpenes (12%), flavonoids (10%), alkaloids (10%), and fatty acid methyl esters (5%).
3.3. Extract Antifungal Activity
3.3.1. In Vitro Activity
The results of in vitro anti-Fusarium activity tests of T. gallica bark extract and its main phytochemical constituents are depicted in Figure 3 and Figures S1–S4. Tamarix gallica bark aqueous ammonia extract suppressed Fusarium spp. growth at concentrations ranging from 375 to 1000 µg·mL−1, depending on the Fusarium species, and showed the highest efficacy against F. graminearum (MIC = 375 µg·mL−1). Regarding its four main phytoconstituents, 1-(2,4,6-trihydroxyphenyl)-2-pentanone featured the highest antifungal activity, with inhibition values in the 250–375 µg·mL−1 range, better than those obtained for sinapinaldehyde (in the 500–750 µg·mL−1 range) and for trans-squalene and syringaldehyde (ranging from 375 to 750 µg·mL−1). To facilitate the comparison of their efficacies, effective concentration values are summarized in Table 3.
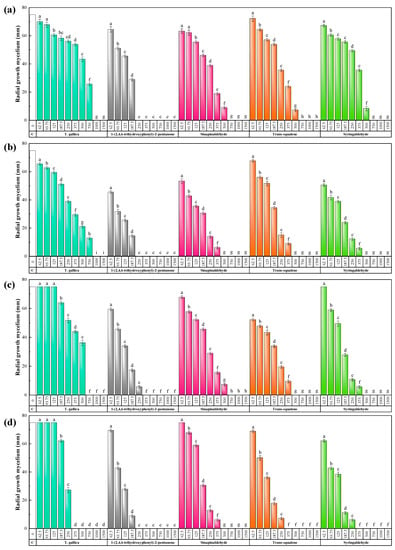
Figure 3.
Inhibition of the radial growth of the mycelium of (a) F. acuminatum, (b) F. culmorum, (c) F. equiseti, and (d) F. graminearum in the in vitro tests performed in PDA medium incorporating different concentrations (in the 62.5–1500 µg·mL−1 range) of T. gallica bark extract or of its main phytochemical constituents (viz., 1-(2,4,6-trihydroxyphenyl)-2-pentanone, sinapinaldehyde, trans-squalene, and syringaldehyde). The efficacies of concentrations labeled with the same letters are not statistically different at p < 0.05. Error bars represent standard deviations.

Table 3.
Effective concentrations (expressed in µg·mL−1) against F. acuminatum, F. culmorum, F. equiseti, and F. graminearum of T. gallica bark aqueous ammonia extract and four of its main constituents.
For comparison purposes, two conventional synthetic fungicides were also tested against the aforementioned four Fusarium taxa. Results are summarized in Table 4. At the recommended dose (i.e., 1500 µg·mL−1), dithiocarbamate (mancozeb) resulted in complete suppression of the mycelial growth of F. acuminatum, but it required ten times the recommended dose (15,000 µg·mL−1) to completely inhibit F. culmorum, F. equiseti, and F. graminearum. The organophosphorus fungicide (fosetyl-Al) completely inhibited the growth of F. culmorum and F. graminearum at the recommended dose (i.e., 2000 µg·mL−1), but required a higher concentration (i.e., 20,000 µg·mL−1) to achieve complete inhibition of F. acuminatum. It is worth noting that, at the latter concentration, only 64.4% of the mycelial growth of F. equiseti was inhibited, thus indicating that a concentration higher than 20,000 μg·mL−1 would be required for complete inhibition.

Table 4.
Radial growth of the mycelium of F. acuminatum, F. culmorum, F. equiseti, and F. graminearum in the in vitro assays performed on a PDA medium with different concentrations of two commercial synthetic fungicides, namely a tenth of the recommended dose (Rd/10), the recommended dose (Rd), and ten times the recommended dose (Rd × 10).
3.3.2. Protection of Wheat and Maize Grains
To assess the effectiveness of the T. gallica bark extract for the postharvest protection of wheat and maize grains, promoting their storability and food safety, ex situ tests were conducted against F. graminearum. After 28 days of incubation, in wheat and maize grain samples artificially infected with this pathogen, no mycelial development was observed in the grains treated with T. gallica bark extract, while the positive control grains (inoculated and treated only with distilled water) showed clear fungal colonization (Figure 4). Therefore, the treatment showed a clear protective effect on both wheat and maize stored grains exposed to F. graminearum at a concentration of 375 µg·mL−1 (i.e., the MIC value determined in the in vitro tests).

Figure 4.
Effect of the application of T. gallica bark extract on the growth of F. graminearum: (a) negative control wheat grains, (b) positive control wheat grains, (c) wheat grains inoculated with F. graminearum and treated with T. gallica bark extract at a dose of 375 µg·mL−1, (d) negative control maize grains, (e) positive control maize grains, (f) maize grains inoculated with F. graminearum and treated with T. gallica bark extract at a dose of 375 µg·mL−1. Only one replicate per treatment is shown.
3.4. Germination Assays
Regarding germination tests (Figure 5), no significant differences were observed between the negative control (grains treated with distilled water; not shown), with a 99−100% germination rate, and the grains treated with T. gallica bark extract at 375 µg·mL−1, with germination percentages of 98 and 96% for wheat and maize grains, respectively. This finding suggests that the application of T. gallica bark extract would not be phytotoxic to wheat and maize grains. The germination percentage of the positive control (i.e., artificially inoculated grains with no treatment) was notably lower, with germination rates of 78 and 88% for wheat and maize, respectively, but it clearly improved in the case of inoculated and treated grains (89 and 95.5% germination rate, respectively).

Figure 5.
Germination tests: (a) wheat grains treated with T. gallica bark extract at a dose of 375 µg·mL−1; (b) positive control wheat grains (inoculated with F. graminearum and treated with distilled water); (c) wheat grains inoculated with F. graminearum and treated with T. gallica bark extract at a dose of 375 µg·mL−1; (d) maize grains treated with T. gallica bark extract at a dose of 375 µg·mL−1; (e) positive control maize grains (inoculated with F. graminearum and treated with distilled water); and (f) maize grains inoculated with F. graminearum and treated with T. gallica bark extract at a dose of 375 µg·mL−1. Only one replicate per treatment is shown.
4. Discussion
4.1. On the Phytochemical Composition and Mode of Action
The high phenolics content is in agreement with that reported in flowers by Boulaaba et al. [29] (135.3 mg GAE/g DW) and would explain the high antioxidant activity observed by Nisar et al. [30] and by Lefahal et al. [31].
Concerning the antifungal mechanism of the main compound categories identified in the extract (viz. phenolic, flavonoids, and organic acids), according to a recent study on Tamarix aphylla (L.) Karst. extracts by Al-Otibi et al. [32], their activity should be ascribed to their ability to induce hyper acidification via proton donation at the plasma membrane interface and intracellular cytosolic acidification, disrupting ATP synthesis [33]. Makarewicz et al. [34] hypothesized that the hydrophobic phenolic compounds initially bind to the plasma membrane, cell wall, and lipopolysaccharide–water interface of the cell without penetration. Their stacking on the plasma membrane would affect membrane fluidity, resulting in destabilization and partial disruption, which would allow the phenolic compounds to enter the cytosol. Their toxicity mechanism against microorganisms would also include enzyme inhibition and nonspecific interactions with proteins. On the other hand, the flavonoid antifungal activity has been attributed to their ability to complex with extracellular and soluble proteins and cell walls [35].
In a more detailed analysis, the activity of the extract should be referred to the most representative phytochemicals (or to synergies between some of them), as discussed below.
1-(2,4,6-trihydroxyphenyl)-2-pentanone is a phenolic compound previously reported, for instance, in Elaphoglossum spathulatum (Bory) T. Moore methanol extract [36], in Polygala javana DC ethanolic extract [37], in pyroligneous acid obtained from slow pyrolysis from palm kernel shell [38], in wood extractives of Populus tomentosa Carrière [39], and in Aquilaria malaccensis Lam. ethanolic extract [40]. The latter was shown to have antibacterial activity against Acinetobacter baumannii Bouvet and Grimont 1986 and Klebsiella pneumoniae (Schroeter 1886) Trevisan 1887.
3,5-dimethoxy-4-hydroxycinnamaldehyde (or sinapinaldehyde) is a low molecular weight phenolic acid intermediate in the formation of lignin. Sinapinaldehyde has previously been found, in lower proportions than those reported for the aqueous ammonia extract of T. gallica bark, in the aerial parts of the halophyte Cladium mariscus L. (Pohl.) [41], in the leaves of Strelitzia nicolai Regel and Koch [42], and in raw materials such as in the wood of Populus lasiocarpa Oliv. and P. tomentosa (0.35 and 0.34%, respectively) [39], in the heartwood of Fraxinus excelsior L. and Fraxinus americana L. [43], in the fibers of Senra incana Cav. and Cocos nucifera L., and in the seeds of Coix lacryma-jobi L. [44]. Shreaz et al. [45] examined several cinnamaldehydes, including sinapaldehyde, finding that it was an effective anticandidal agent against several azole-sensitive and azole-resistant clinical isolates, with MIC values in the 100−200 μg·mL−1 range. Its antifungal activity was related to the inhibition of plasma membrane-ATPase (PM-ATPase), the lowering of intracellular pH, and the depletion of NADPH, together with damage caused to membranes and cell walls. Its limited toxicity together with its broad spectrum of activity suggested that sinapaldehyde could be developed as an antifungal.
Squalene is a lipophilic triterpene, a natural precursor of ergosterol, crucial in the plasmatic membrane of fungi [46]. It has previously been identified in Acalypha indica L., Ammannia baccifera L., Abrus precatorius L., Abutilon indicum L., Cuscuta reflexa Roxb. [47], Cucurbita maxima Duchesne [48], Jasminum grandiflorum L. [49], and Leucas aspera (Willd.) Link [50], and—more recently—by our research group in the bark of Quercus ilex subsp. ballota (Desf.) Samp., with a content of 13% [21], slightly higher than that obtained in the bark of T. gallica (9.9%). It has been demonstrated that squalene has antifungal properties against Candida spp. [51]. Intracellular accumulation of squalene is known to disrupt fungal cell membranes, possibly via the formation of squalene vesicles that weaken fungal cells by removing critical membrane lipid components [52]. Terbinafine and other antifungal drugs’ mode of action is based on inhibiting squalene peroxidase, resulting in squalene accumulation [53]. Currently, research on squalene monooxygenase and epoxidase enzymes is a promising area for the development of new antifungal drugs [54,55]. Reports on the antifungal action of trans-squalene for other supraene-rich natural products have been summarized in [21].
Syringaldehyde or 3,5-dimethoxy-4-hydroxybenzaldehyde is a phenolic aldehyde found in a wide range of plants, according to the comprehensive summary by Wu et al. [56]. It possesses significant broad-spectrum antimicrobial activity, being highly effective against bacteria such as Bacillus subtilis (Ehrenberg) Cohn, K. pneumonia, Staphylococcus aureus Rosenbach, and Pseudomonas aeruginosa (Schroeter) Migula, and against the formation of Aspergillus spp. biofilms [57,58].
Given the activity demonstrated in the in vitro tests by the four phytochemicals discussed above (Figure 3), and taking into consideration that their antimicrobial activity is further supported by the findings of other research groups, the antifungal activity of the extract should be mainly ascribed to these compounds. Nonetheless, contributions from other constituents present in the extract in lower amounts and the existence of synergistic behaviors cannot be ruled out.
4.2. Antimicrobial Activity Comparison
4.2.1. Comparison with Other Tamarix gallica Extracts
The high content of polyphenols (including quercetin, kaempferol, coumarin, and rhamnocitin, among others) reported for other T. gallica organs, primarily flowers, would be responsible for their biological capacity against multidrug-resistant clinical infections (S. aureus, Micrococcus luteus, Escherichia coli (Migula) Castellani and Chalmers, Pseudomonas spp., Klebsiella spp., Enterococcus faecalis (Andrewes and Horder 1906) Schleifer and Kilpper-Balz 1984, Bacillus spp., Listeria monocytogenes (Murray et al.) Pirie, and Candida spp.) as shown in Table S1. However, it is worth noting that the concentrations assayed in [7,11], ranging from 100 to 300 mg·mL−1, were two to three orders of magnitude higher than those assayed herein and that complete inhibition was not attained in most cases.
4.2.2. Comparison with other Tamaricaceae Family Bark Extracts
A literature survey for other species of the Tamaricaceae family with established antimicrobial activity was conducted to compare the results.
The antimicrobial activity of T. aphylla bark is the one that has received the most attention in the literature. Bibi et al. [59] studied its antifungal activity against Aspergillus flavus Link, Aspergillus fumigatus Fresen., Aspergillus niger Tiegh., Fusarium oxysporum Schltdl., Penicillium notatum Westling, and Saccharomyces cerevisiae Desm. Extracts in different solvents were assayed (viz. methanol, ethanol, chloroform, distilled water, and acetone), finding that the chloroform extract was the most effective, inhibiting the growth of F. oxysporum by 97.68%, A. flavus by 88.48%, A. fumigatus by 91.46 %, and P. notatum by 87.46% at a concentration of 2000 µg·mL−1. Iqbal et al. [60] investigated the efficacy of a fixed oil against bacteria and fungi. Its maximum effectiveness was obtained against B. subtilis (MIC = 125 µg·mL−1), C. glabrata (MIC = 400 µg·mL−1), and E. coli (MIC = 500 µg·mL−1); additionally, it showed moderate activity against C. albicans, S. aureus, Shigella flexneri Castellani and Chalmers, and Trichphyton longifusus (Flórián and Galgoczy) Ajello, with MIC values in the 1000−2000 µg·mL−1 range. Finally, it showed low efficacy against Salmonella typhi (Schroeter) Warren and Scott. (MIC = 3000 µg·mL−1) and Fusarium solani (Mart.) Sacc. (MIC = 4000 µg·mL−1); there was no inhibitory effect against P. aeruginosa, Microsporum canis E. Bodin ex Guég., or A. flavus. Its antimicrobial activity was related to the presence of capric acid and lauric acid in high amounts [61].
On the other hand, Ren et al. [62] evaluated a Tamarix ramosissima Ledeb. bark ethanolic extract against some foodborne pathogens, finding a moderate-low bactericidal effect against S. aureus, L. monocytogenes, Bacillus cereus Frankland and Frankland, and Shigella flexneri Castellani and Chalmers, with MIC values of 5000 µg·mL−1; and a lower activity against E. coli (MIC = 10,000 µg·mL−1), P. aeruginosa and S. typhi (MIC > 10,000 µg·mL−1). However, it showed no activity against the four fungi tested: Penicillium expansum Link, A. niger, Acremonium strictum (Gams) Summerbell, and Penicillium citrinum Thom. Mikaeili et al. [63] assessed an aqueous decoction of T. ramosissima bark against Trichophyton verrucosum Bodin and Epidermophyton floccosum (Harz) Langeron and Miloch., reporting inhibition zone values of 18.3 and 23.3 mm, respectively, at a concentration of 500,000 µg·mL−1.
Although comparisons of the activities reported above for other tamarisk species extracts with those reported in this work for T. gallica should be taken with care (given that the activity is solvent- and fungal isolate-dependent), if inhibitory values against Fusarium spp. are analyzed, it may be inferred that T. aphylla would have lower effectiveness than T. gallica (with inhibition values higher than 2000 µg·mL−1 against F. oxysporum and F. solani, vs. 375−1000 µg·mL−1 for T. gallica against F. acuminatum, F. culmorum, F. equiseti, and F. graminearum).
4.2.3. Comparison with Conventional Fungicides
Several fungicides, including those in the benzimidazole group (carbendazim, benomyl), azoles (hexaconazole, prochloraz, propiconazole, tebuconazole, and triadimenol), and dithiocarbamates (mancozeb) are useful for the control of FHB. The basic technique for managing FHB involves the use of azoles, which block the ergosterol production pathway and decrease mycotoxin concentration and FHB symptoms [64]. Strobirulins (azoxystrobin), on the other hand, limit FHB by blocking electron transport in the mitochondrial respiratory chain, reducing aerobic energy production and inhibiting fungal growth [65]. None of them, however, has led to total FHB control [14]. The severity of the disease, the crop’s level of natural resistance, and the spraying method play a significant role in the effectiveness.
In this work, two conventional fungicides were tested against the four Fusarium isolates for reference purposes. As shown in Table 4, their effectiveness was substantially lower than that of the T. gallica bark extract (Table 3): while full inhibition was attained for the natural product at concentrations in the 375−1000 µg·mL−1 range, doses of 1500 and 15,000 µg·mL−1 were needed in the case of mancozeb, and fosetyl-Al concentrations in the 2000−20,000 µg·mL−1 range were required to control three of the Fusarium taxa (provided that complete inhibition of F. acuminatum was not reached even at the highest dose of this last chemical).
4.3. Limitations of the Study
According to Tokarev et al. [66], who tested four fungicides in vitro (viz. pyraclostrobin, thiram, fludioxonil, and a combination of imazalil+metalaxyl+tebuconazole) against ten strains of Fusarium spp., the sensitivity of F. acuminatum, F. graminearum, F. semitectum, F. culmorum, F. sporotrichioides, and F. equiseti strains to fungicides was higher than that of strains belonging to F. oxysporum, F. solani, F. verticillioides, and F. proliferatum. Hence, further tests on the effectiveness of the bark extract against these later taxa would be needed before moving to field trials.
Another important point would be related to the presence of mycotoxins in the treated grains. There is growing evidence that fungicides may not be as effective at reducing the generation of toxins because, in some circumstances, they may act as stressors that trigger the biosynthesis of toxins. Certain Fusarium species can produce mycotoxins when exposed to sublethal levels of some fungicides: for instance, application of sublethal doses of tebuconazole induced fumonisin expression in Fusarium verticillioides (Sacc.) Nirenberg and Fusarium proliferatum (Matsush.) Nirenberg ex Gerlach and Nirenberg [67], and trichothecenes in Fusarium langsethiae Torp and Nirenberg, as did low doses of prochloraz [68]. However, in F. graminearum the application of low concentrations of tebuconazole did not lead to a significant increase in trichothecenes, whereas the application of propiconazole did [69]. Additional research is needed to determine the influence of T. gallica bark extracts at different doses on mycotoxin production.
5. Conclusions
Gas chromatography–mass spectroscopy characterization of Tamarix gallica bark aqueous ammonia extract allowed for the identification of 1-(2,4,6-trihydroxyphenyl)-2-pentanone; 3,5-dimethoxy-4-hydroxycinnam aldehyde; trans-squalene; 4-hydroxy-3,5-dimethoxy-benzaldehyde; dihydro-3-methylene-2,5-furandione; and 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone as the main constituents. In vitro mycelial growth inhibition tests showed that the extract and the aforementioned four phytochemicals displayed high activity against four Fusarium taxa responsible for the so-called Fusarium head blight (FHB) in cereals, resulting in complete inhibition at concentrations ranging from 375 to 1000 µg·mL−1 in the case of the extract, and in the 250–750 µg·mL−1 range for its constituents. These inhibitory concentration values were lower than those required when using mancozeb and fosetyl-Al synthetic fungicides, tested for comparison purposes. Further ex situ bioassays on wheat and maize grains artificially infected with F. graminearum confirmed the effectiveness of the bark extract against this pathogen, attaining full protection of wheat and maize grains at a concentration equal to the MIC determined in the in vitro tests (375 µg·mL−1), with no symptoms of phytotoxicity based on germination tests. These findings support the potential of this halophyte as a valuable source of natural bioactive compounds and pave the way for the valorization of its bark to obtain high added-value products, such as biorationals for cereal protection against FHB.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy13020496/s1, Figure S1: Growth inhibition of F. acuminatum for T. gallica bark, 1-(2,4,6-trihydroxyphenyl)-2-pentanone, sinapinaldehyde, trans-squalene, and syringaldehyde. Figure S2: Growth inhibition of F. culmorum for T. gallica bark, 1-(2,4,6-trihydroxyphenyl)-2-pentanone, sinapinaldehyde, trans-squalene, and syringaldehyde. Figure S3: Growth inhibition of F. equiseti for T. gallica bark, 1-(2,4,6-trihydroxyphenyl)-2-pentanone, sinapinaldehyde, trans-squalene, and syringaldehyde. Figure S4: Growth inhibition of F. graminearum for T. gallica bark, 1-(2,4,6-trihydroxyphenyl)-2-pentanone, sinapinaldehyde, trans-squalene, and syringaldehyde. Table S1. Antimicrobial activity of T. gallica leaf and flower extracts reported in the literature.
Author Contributions
Conceptualization, V.G.-G. and A.C.-G.; methodology, V.G.-G. and A.C.-G.; validation, J.C.-G.; formal analysis, E.S.-H., J.C.-G. and P.M.-R.; investigation, E.S.-H., V.G.-G., A.C.-G., J.C.-G., J.M.-G. and P.M.-R.; resources, J.M.-G.; writing—original draft preparation, E.S.-H., V.G.-G., A.C.-G., J.C.-G., J.M.-G. and P.M.-R.; writing—review and editing, E.S.-H., V.G.-G. and P.M.-R.; visualization, E.S.-H.; supervision, P.M.-R.; project administration, A.C.-G. and J.M.-G.; funding acquisition, J.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union through Horizon Program (HORIZON-CL6-2022-FARM2FORK-01) under project ‘Agro-ecological strategies for resilient farming in West Africa (CIRAWA)’, with project ID 101084398, and by the Junta de Castilla y León under project VA258P18, with FEDER co-funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their relevance to an ongoing Ph.D. thesis.
Acknowledgments
The authors would like to acknowledge Piensos y Cereales Isabelio Sánchez-García for providing the wheat and maize seeds for the bioassays. The authors would also like to acknowledge Pilar Blasco and Pablo Candela from the Technical Research Services of the University of Alicante for conducting the GC–MS analysis. José Luis Palomo Gómez and Jaime Alonso Herrero from the Aldearrubia Regional Diagnostic Center (Junta de Castilla y León) are gratefully acknowledged for providing the F. acuminatum isolate used in the study. Melissa Boyd is gratefully acknowledged for improving the use of English in the manuscript and her diligent proofreading of this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Duan, Q.; Zhu, Z.; Wang, B.; Chen, M. Recent progress on the salt tolerance mechanisms and application of tamarisk. Int. J. Mol. Sci. 2022, 23, 3325. [Google Scholar] [CrossRef]
- Han, Z.; Yin, W.; Zhang, J.; Niu, S.; Ren, L. Active anti-erosion protection strategy in tamarisk (Tamarix aphylla). Sci. Rep. 2013, 3, 3429. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, L.; Pan, B. Biological and ecological characteristics of Tamarix L. and its effect on the ecological environment. Sci. China Ser. D Earth Sci. 2002, 45, 18–22. [Google Scholar] [CrossRef]
- Dawalibi, V.; Monteverdi, M.; Moscatello, S.; Battistelli, A.; Valentini, R. Effect of salt and drought on growth, physiological and biochemical responses of two Tamarix species. Iforest Biogeosci. For. 2015, 8, 772–779. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 112245. [Google Scholar] [CrossRef]
- Elamin, M. A review of biological and pharmacological activities from the aerial part of tamarisk. Int. J. Pharm. Res. Allied Sci. 2016, 5, 22–36. [Google Scholar]
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Bakrouf, A.; Magné, C.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009, 47, 2083–2091. [Google Scholar] [CrossRef]
- Lee, J.M.; Yim, M.-J.; Lee, D.-S.; Lee, M.S.; Park, Y.G.; Jeon, J.H.; Choi, G. Comparison of biological activities of Korean halophytes. Nat. Prod. Sci. 2018, 24, 247–252. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as medicinal plants against human infectious diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Said, S.; Noureddine, G.; Eddine, L.S.; Abdelmadjid, G.; Djamel, B.; Tliba, A. Phenolic content, HPLC analysis and antioxidant activity extract from Tamarix gallica and Tamarix articulata growing in Southeast of Algeria. Res. J. Pharm. Technol. 2018, 11, 3826–3832. [Google Scholar] [CrossRef]
- Boulaaba, M.; Snoussi, M.; Saada, M.; Mkadmini, K.; Smaoui, A.; Abdelly, C.; Ksouri, R. Antimicrobial activities and phytochemical analysis of Tamarix gallica extracts. Ind. Crops Prod. 2015, 76, 1114–1122. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Gurung, S.; Hansen, J.M.; Bonman, J.M.; Gironella, A.I.N.; Adhikari, T.B. Multiple disease resistance to four leaf spot diseases in winter wheat accessions from the USDA National Small Grains Collection. Crop Sci. 2012, 52, 1640–1650. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Leslie, J.F.; Moretti, A.; Mesterházy, Á.; Ameye, M.; Audenaert, K.; Singh, P.K.; Richard-Forget, F.; Chulze, S.N.; Ponte, E.M.D.; Chala, A.; et al. Key global actions for mycotoxin management in wheat and other small grains. Toxins 2021, 13, 725. [Google Scholar] [CrossRef]
- Feksa, H.R.; Do Couto, H.T.Z.; Garozi, R.; De Almeida, J.L.; Gardiano, C.G.; Tessmann, D.J. Pre- and postinfection application of strobilurin-triazole premixes and single fungicides for control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Crop Prot. 2019, 117, 128–134. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Q.; Zhang, G.; Wu, J.; Zhu, F.; Yang, H.; Zhuang, Y. Carbendazim-resistance of Gibberella zeae associated with fusarium head blight and its management in Jiangsu Province, China. Crop Prot. 2019, 124, 104866. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J. Statistical optimization of aqueous ammonia pretreatment and enzymatic hydrolysis of corn cob powder for enhancing sugars production. Biochem. Eng. J. 2021, 174, 108106. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Balduque-Gil, J.; Barriuso-Vargas, J.J.; Casanova-Gascón, J.; González-García, V.; Cuchí-Oterino, J.A.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Holm Oak (Quercus ilex subsp. ballota (Desf.) Samp.) Bark Aqueous Ammonia Extract for the Control of Invasive Forest Pathogens. Int. J. Mol. Sci. 2022, 23, 11882. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Martín-Ramos, P.; Martín-Gil, J.; Santiago-Aliste, A.; Hernández-Navarro, S.; Oliveira, R.; González-García, V. Bark extract of Uncaria tomentosa L. for the control of strawberry phytopathogens. Horticulturae 2022, 8, 672. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Casanova-Gascón, J.; Barriuso-Vargas, J.J.; Balduque-Gil, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Valorization of Quercus suber L. bark as a source of phytochemicals with antimicrobial activity against apple tree diseases. Plants 2022, 11, 3415. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W.; EUCAST-AFST. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid conjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K.; Waśkiewicz, A. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens 2020, 9, 23. [Google Scholar] [CrossRef]
- Olesen, M.; Duijn, B.v.; Boelt, B. Introduction of New Methods: Spectral Imaging. Seed Test. Int. 2014, 147, 10–13. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Tamarix gallica leaf extract mediated novel route for green synthesis of CuO nanoparticles and their application for N-arylation of nitrogen-containing heterocycles under ligand-free conditions. RSC Adv. 2015, 5, 40628–40635. [Google Scholar] [CrossRef]
- Boulaaba, M.; Tsolmon, S.; Ksouri, R.; Han, J.; Kawada, K.; Smaoui, A.; Abdelly, C.; Isoda, H. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology 2013, 65, 927–936. [Google Scholar] [CrossRef]
- Nisar, J.; Ali Shah, S.M.; Ayaz, S.; Akram, M.; Rashid, A.; Mustafa, I.; Nisar, Z. In vitro comparative evaluation of Tamarix gallica extracts for antioxidant and antidiabetic activity. Exp. Biol. Med. 2022, 15353702221139208. [Google Scholar] [CrossRef]
- Lefahal, M.; Makhloufi, E.-H.; Trifa, W.; Ayad, R.; El Hattab, M.; Benahmed, M.; Keskin, M.; Akkal, S. The cosmetic potential of the medicinal halophyte Tamarix gallica L. (Tamaricaceae) growing in the eastern part of Algeria: Photoprotective and antioxidant activities. Comb. Chem. High Throughput Screen. 2021, 24, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Al-Otibi, F.; Moria, G.A.; Alharbi, R.I.; Yassin, M.T.; Al-Askar, A.A. The antifungal properties of Tamarix aphylla extract against some plant pathogenic fungi. Microorganisms 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in Middle Eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Socolsky, C.; Salvatore, A.; Asakawa, Y.; Bardón, A. Bioactive new bitter-tasting p-hydroxystyrene glycoside and other constituents from the fern Elaphoglossum spathulatum. Arkivoc 2003, 10, 347–355. [Google Scholar] [CrossRef]
- Alagammal, M.; Tresina, P.; Mohan, V. GC-MS determination of bioactive components of Polygala javana DC. Int. J. Curr. Pharm. Res. 2012, 4, 42–44. [Google Scholar]
- Rabiu, Z.; Hamzah, M.A.A.M.; Hasham, R.; Zakaria, Z.A. Characterization and antiinflammatory properties of fractionated pyroligneous acid from palm kernel shell. Environ. Sci. Pollut. Res. 2021, 28, 40535–40543. [Google Scholar] [CrossRef]
- Peng, W.; Li, D.; Zhang, M.; Ge, S.; Mo, B.; Li, S.; Ohkoshi, M. Characteristics of antibacterial molecular activities in poplar wood extractives. Saudi J. Biol. Sci. 2017, 24, 399–404. [Google Scholar] [CrossRef]
- Kamal, K.M.; Hashim, Y.Z.H.Y.; Sani, M.S.A.; Rahim, N.A.; Maifiah, M.H.M.; Jihadi, N.I.M. Combination of polymyxin B and Aquilaria malaccensis extract enhanced the killing and inhibited the growth of Acinetobacter baumannii and Klebsiella pneumoniae. Malays. J. Microbiol. 2021, 18, 27–36. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Custódio, L.; Mecha, D.; Zengin, G.; Cziáky, Z.; Sotkó, G.; Pereira, C.G. Nutritional and phyto-therapeutic value of the halophyte Cladium mariscus L. (Pohl.): A special focus on seeds. Plants 2022, 11, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.; Saad, A.; Ahmed, W.; Refahy, L.; Nasr, S. HPLC-DAD-ESI-MS/MS characterization of bioactive secondary metabolites from Strelitzia nicolai leaf extracts and their antioxidant and anticancer activities in vitro. Pharmacogn. Res. 2018, 10, 368–378. [Google Scholar] [CrossRef]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, A.M.; Hernández, T.; Estrella, I.; Pinto, E. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. J. Mass Spectrom. 2012, 47, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Ralph, J.; Marques, G.; Gutiérrez, A.; Martínez, Á.T.; del Río, J.C. Structural characterization of lignin isolated from coconut (Cocos nucifera) coir fibers. J. Agric. Food. Chem. 2013, 61, 2434–2445. [Google Scholar] [CrossRef]
- Shreaz, S.; Bhatia, R.; Khan, N.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Influences of cinnamic aldehydes on H+ extrusion activity and ultrastructure of Candida. J. Med. Microbiol. 2013, 62, 232–240. [Google Scholar] [CrossRef]
- Zare, B.; Sepehrizadeh, Z.; Faramarzi, M.A.; Soltany-Rezaee-Rad, M.; Rezaie, S.; Shahverdi, A.R. Antifungal activity of biogenic tellurium nanoparticles against Candida albicans and its effects on squalene monooxygenase gene expression. Biotechnol. Appl. Biochem. 2014, 61, 395–400. [Google Scholar] [CrossRef]
- Suman, T.Y.; Elumalai, D.; Kaleena, P.K.; Rajasree, S.R.R. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind. Crops Prod. 2013, 47, 239–245. [Google Scholar] [CrossRef]
- Stevenson, D.G.; Eller, F.J.; Wang, L.; Jane, J.-L.; Wang, T.; Inglett, G.E. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J. Agric. Food. Chem. 2007, 55, 4005–4013. [Google Scholar] [CrossRef]
- Wei, F.H.; Chen, F.L.; Tan, X.M. Gas chromatographic-mass spectrometric analysis of essential oil of Jasminum officinale L var grandiflorum flower. Trop. J. Pharm. Res. 2015, 14, 149–152. [Google Scholar] [CrossRef]
- Anandan, A.; Eswaran, R.; Doss, A.; Sangeetha, G.; Anand, S. Chemical compounds investigation of Lucas aspera leaves-a potential folklore medicinal plant. Asian J. Pharm. Clin. Res. 2012, 5, 86–88. [Google Scholar]
- Masuda, A.; Akiyama, S.-I.; Kuwano, M.; Ikekawa, N. Potentiation of antifungal effect of amphotericin B by squalene, an intermediate for sterol biosynthesis. J. Antibiot. 1982, 35, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E. Mechanisms of action of systemic antifungal agents. J. Am. Acad. Dermatol. 1993, 28, S28–S34. [Google Scholar] [CrossRef]
- Belter, A.; Skupinska, M.; Giel-Pietraszuk, M.; Grabarkiewicz, T.; Rychlewski, L.; Barciszewski, J. Squalene monooxygenase–a target for hypercholesterolemic therapy. Biol. Chem. 2011, 392, 1053–1075. [Google Scholar] [CrossRef]
- Nowosielski, M.; Hoffmann, M.; Wyrwicz, L.S.; Stepniak, P.; Plewczynski, D.M.; Lazniewski, M.; Ginalski, K.; Rychlewski, L. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J. Chem. Inf. Model. 2011, 51, 455–462. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Y.-S.; Lin, K.; Huang, X.; Chen, Y.-j.; Lai, D.; Kang, N.; Huang, L.; Weng, C.-F. A narrative review: The pharmaceutical evolution of phenolic syringaldehyde. Biomed. Pharmacother. 2022, 153, 113339. [Google Scholar] [CrossRef] [PubMed]
- Fillat, A.; Gallardo, O.; Vidal, T.; Pastor, F.; Díaz, P.; Roncero, M. Enzymatic grafting of natural phenols to flax fibres: Development of antimicrobial properties. Carbohydr. Polym. 2012, 87, 146–152. [Google Scholar] [CrossRef]
- Musthafa, S.A.; Dabdoub, W.; Sadiq, M.; Munuswamy-Ramanujam, G. Syringaldehyde isolated from Abutilon indicum Linn. leaves exhibits broad spectrum anti-microbial activity. Mater. Today Proc. 2022, 50, 335–339. [Google Scholar] [CrossRef]
- Bibi, S.; Afzal, M.; Aziz, N.; Din, B.; Khan, M.; Khan, A.; Komal, H. Antifungal activity of Tamarix aphylla (L.) Karst. stem-bark extract against some pathogenic fungi. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 541–545. [Google Scholar]
- Iqbal, A.; Begum, N.; Rabbi, F.; Akhtar, N.; Khan, W.M.; Shah, Z. In-vitro antimicrobial, antioxidant and enzyme inhibitory activities of fixed oil extracted from stem bark of Tamarix aphylla. Pharm. Chem. J. 2022, 56, 1116–1122. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.l.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, hispidulin, and cirsimaritin identified in Tamarix ramosissima barks from southern Xinjiang and their antioxidant and antimicrobial activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef]
- Mikaeili, A.; Karimi, I.; Modarresi, M.; Shahbazi, A.; Jalilian, N. Antimycotic activity of aqueous extract of Tamarix ramosissima Ledeb. bark on in vitro and in vivo guinea pig model of dermatophytosis. Int. J. Life Sci. Pharma Res. 2018, 8, 8–15. [Google Scholar]
- Paul, P.A.; McMullen, M.P.; Hershman, D.E.; Madden, L.V. Meta-analysis of the effects of triazole-based fungicides on wheat yield and test weight as influenced by Fusarium head blight intensity. Phytopathology 2010, 100, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Cendoya, E.; Nichea, M.J.; Monge, M.d.P.; Zachetti, V.G.L.; Chiacchiera, S.M.; Ramirez, M.L. Effect of fungicides commonly used for Fusarium head blight management on growth and fumonisin production by Fusarium proliferatum. Rev. Argent. Microbiol. 2021, 53, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, Y.; Orina, A.; Gavrilova, O.; Gagkaeva, T.; Glupov, V. The effect of fungicides on growth of Fusarium fungi in vitro. BIO Web Conf. 2020, 18, 00022. [Google Scholar] [CrossRef]
- Marín, P.; de Ory, A.; Cruz, A.; Magan, N.; González-Jaén, M.T. Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Valle-Algarra, F.M.; Mateo, R.; Jiménez, M.; Magan, N. Effect of fenpropimorph, prochloraz and tebuconazole on growth and production of T-2 and HT-2 toxins by Fusarium langsethiae in oat-based medium. Int. J. Food Microbiol. 2011, 151, 289–298. [Google Scholar] [CrossRef]
- Kulik, T.; Lojko, M.; Jestoi, M.; Perkowski, J. Sublethal concentrations of azoles induce tri transcript levels and trichothecene production in Fusarium graminearum. FEMS Microbiol. Lett. 2012, 335, 58–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).