Host Susceptibility of CIMMYT’s International Spring Wheat Lines to Crown and Root Rot Caused by Fusarium culmorum and F. pseudograminearum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germplasm Selection
2.2. Inoculum Preparation
2.3. Growth Room Experiment
2.4. Greenhouse
2.5. Field Conditions
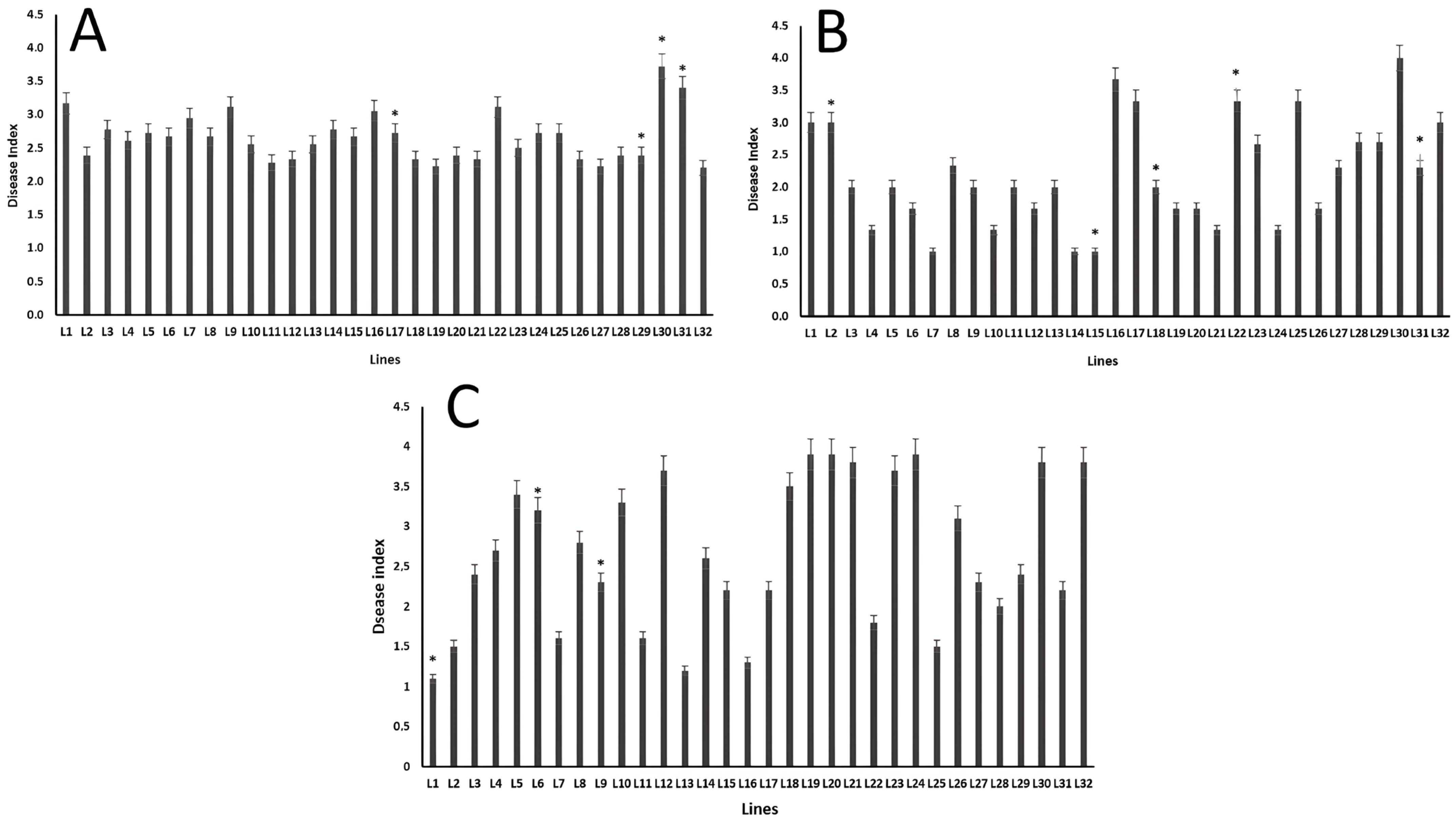
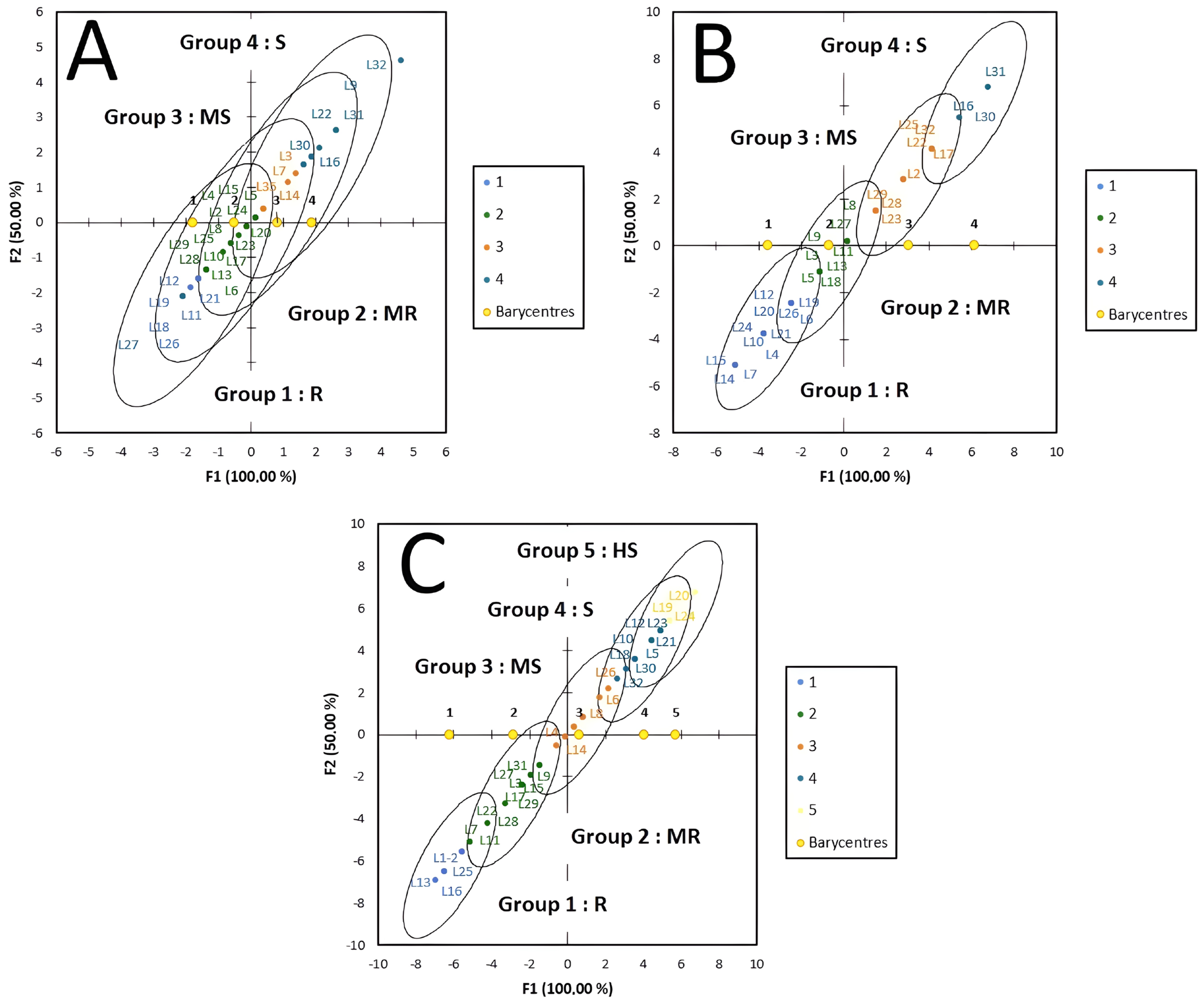
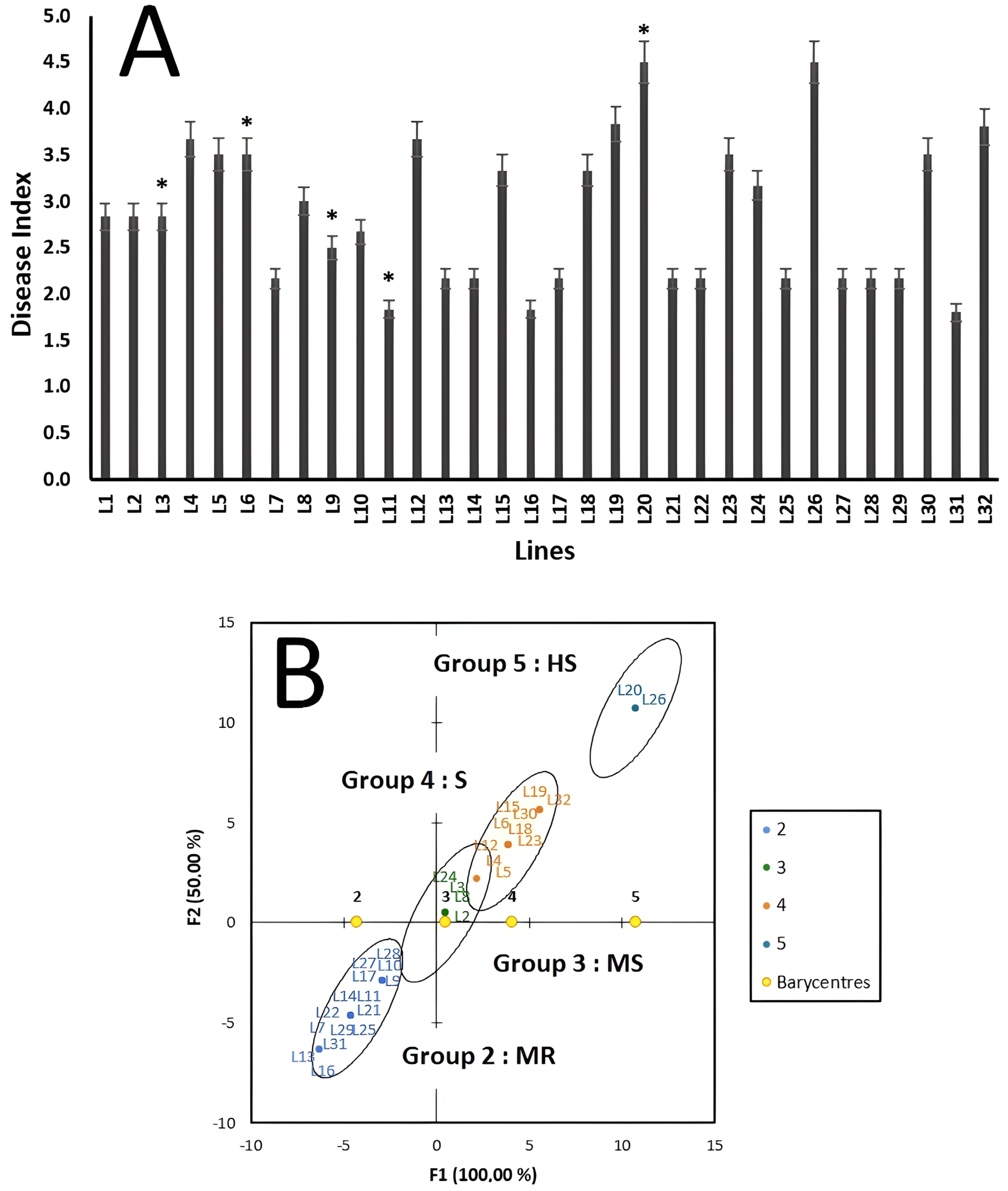
2.6. Disease Assessment and Data Analysis
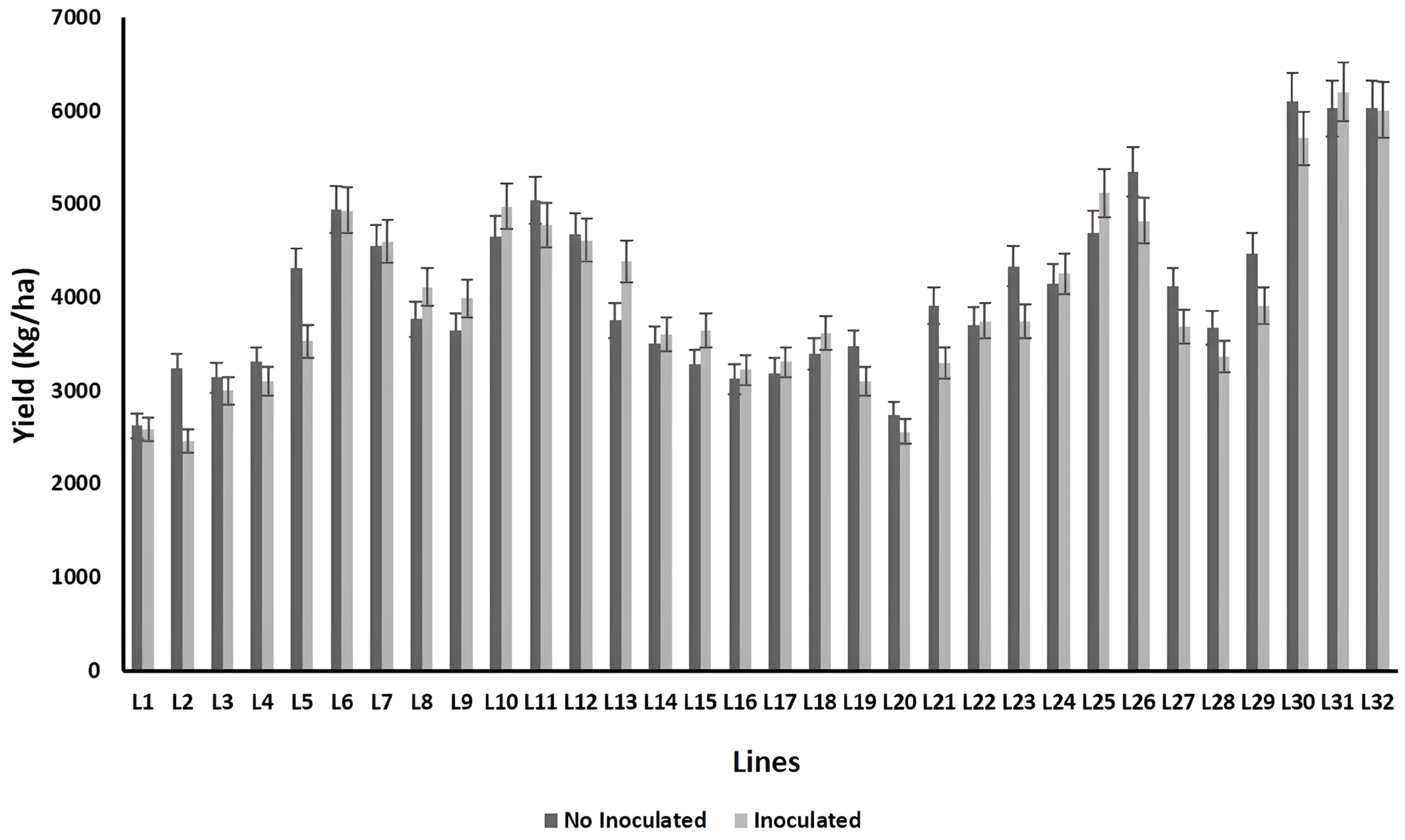
2.7. Yield Performance
2.8. Statistical Analysis
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowden, R.L.; Hunger, R.M.; Morrill, W.L.; Murray, T.D.; Smiley, R.W. Compendium of Wheat Diseases and Pests; Bockus, W.W., Ed.; APS Press: Saint Paul, MN, USA, 2010; Volume 3. [Google Scholar] [CrossRef]
- Breiman, A.; Graur, D. Wheat evolution. Isr. J. Plant Sci. 1995, 43, 85–98. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization Statistical Database. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 February 2022).
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision. Available online: https://ageconsearch.umn.edu/record/288998/ (accessed on 18 November 2021).
- Adhikari, S.; Kumari, J.; Jacob, S.R.; Prasad, P.; Gangwar, O.P.; Lata, C.; Kumar, S. Landraces-potential treasure for sustainable wheat improvement. Genet. Resour. Crop Evol. 2022, 69, 499–523. [Google Scholar] [CrossRef]
- Braun, H.J.; Sãulescu, N.N. Breeding winter and facultative wheat. Cent. Asia 2002, 9, 5. [Google Scholar]
- Bentley, A.; Donovan, J.; Sonder, K.; Voss, R.; Rutsaert, P.; Poole, N.; Kamoun, S.; Saunders, D.G.O.; Hodson, D.; Hughes, D.P.; et al. Another Food Crisis? The Ukraine Conflict, Global Wheat Supply and Food Security (Version V1). Zenodo 2022. Available online: https://zenodo.org/record/6380085#.Y3y1zH1ByUk (accessed on 15 July 2021).
- Chakraborty, S.; Liu, C.J.; Mitter, V.; Scott, J.B.; Akinsanmi, O.A.; Ali, S.; Dill-Macky, R.; Nicol, J.; Backhouse, D.; Simpfendorfer, S. Pathogen population structure and epidemiology are keys to wheat crown rot and Fusarium Head Blight management. Australas. Plant Pathol. 2006, 35, 643–655. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A. Pathogenicity of fungi associated with wheat crown rot complex in Oregon and Washington. Plant Dis. 2005, 89, 949–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunali, B.; Nicol, J.M.; Hodson, D.; Uckun, Z.; Buyuk, O.; Erdurmus, E.; Hekimhan, H.; Aktas, H.; Aydın Akbudak, M.; Bagci, S.A. Root and crown rot fungi associated with spring, facultative, and winter wheat in Türkiye. Plant Dis. 2008, 98, 1299–1306. [Google Scholar] [CrossRef] [Green Version]
- Özer, G.; Paulitz, T.C.; Imren, M.; Alkan, M.; Muminjanov, H.; Dababat, A.A. Identity and pathogenicity of fungi associated with crown and root rot of dryland winter wheat in Azerbaijan. Plant Dis. 2020, 104, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Bozoğlu, T.; Derviş, S.; Imren, M.; Amer, M.; Özdemir, F.; Paulitz, T.C.; Morgounov, A.; Dababat, A.A.; Özer, G. Fungal pathogens associated with crown and root rot of wheat in central, eastern, and southeastern Kazakhstan. J. Fungi 2022, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Cook, R.J.; Veseth, R.J. Wheat Health Management; American Phytopathological Society: St. Paul, MN, USA, 1991. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Chrpova, J.; Sip, V.; Matejova, E.; Sykorova, S. Progression of deoxynivalenol concentrations in spikes and kernels of winter wheat cultivars after inoculation with Fusarium culmorum. Czech. J. Genet. Plant Breed. 2006, 42, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.V.; Vasanthi, S. Mycotoxin food safety risk in developing countries. In Food Safety in Food Security and Food Trade; (No. 569-2016-39053); International Food Policy Research Institute: Washington, DC, USA, 2003. [Google Scholar]
- Sneller, C.; Guttieri, M.; Paul, P.; Costa, J.; Jackwood, R. Variation for resistance to kernel infection and toxin accumulation in winter wheat infected with F. graminearum. Phytopathology 2012, 102, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, L.W.; Backhouse, D.; Summerell, B.A.; Swan, L.J. Crown rot of wheat. In Fusarium—Paul E. Nelson Memorial Symposium; Summerell, J.F., Leslie, D., Backhouse, W.L., Bryden, L.W.B., Eds.; American Phytopathological Society Press: St. Paul. MN, USA, 2001; pp. 271–295. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.S.; Trevathan, L.E. Identity and pathogenicity of fungi associated with root and crown rot of soft red winter wheat grown on the upper coastal plain land resource area of Mississippi. J. Phytopatol. 2000, 148, 77–85. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Smiley, R.W.; Cook, R.J. Insights into the prevalence and management of soilborne cereal pathogens under direct seeding in the Pacific Northwest, USA. Can. J. Plant Pathol. 2002, 24, 416–428. [Google Scholar] [CrossRef]
- Backhouse, D.; Abubakar, A.A.; Burgess, L.W.; Dennisc, J.I.; Hollaway, G.J.; Wildermuth, G.B.; Henry, F.J. Survey of Fusarium species associated with crown rot of wheat and barley in eastern Australia. Australas. Plant Pathol. 2004, 33, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Smiley, R.W.; Patterson, L.M. Pathogenic fungi associated with Fusarium foot rot of winter wheat in the semiarid Pacific Northwest. Plant Dis. 1996, 80, 944–949. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Mitter, V.; Simpfendorfer, S.; Backhouse, D.; Chakraborty, S. Identity and pathogenicity of Fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust. J. Agric. Res. 2004, 55, 97–107. [Google Scholar] [CrossRef]
- Bozoğlu, T.; Özer, G.; Imren, M.; Paulitz, T.C.; Dababat, A.A. First report of crown rot caused by Fusarium redolens on wheat in Kazakhstan. Plant Dis. 2021, 105, 3302. [Google Scholar] [CrossRef]
- Özer, G.; İmren, M.; Bayraktar, H.; Paulitz, T.C.; Muminjanov, H.; Dababat, A.A. First report of Fusarium hostae causing crown rot on wheat in Azerbaijan. Plant Dis. 2019, 103, 3278. [Google Scholar] [CrossRef]
- Özer, G.; Erper, I.; İmren, M.; Bozoglu, T.; Ozdemir, F.; Dababat, A.A. First report of crown rot caused by Fusarium algeriense on wheat in Kyrgyzstan. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Gebremariam Shikur, E.; Sharma-Poudyal, D.; Paulitz, T.C.; Erginbas-Orakci, G.; Karakaya, A.; Dababat, A.A. Identity and pathogenicity of Fusarium species associated with crown rot on wheat (Triticum spp.) in Türkiye. Eur. J. Plant Pathol. 2018, 150, 387–399. [Google Scholar] [CrossRef]
- Xu, F.; Yang, G.; Wang, J.; Song, Y.; Liu, L.; Zhao, K.; Li, Y.; Han, Z. Spatial distribution of root and crown rot fungi associated with winter wheat in the North China Plain and its relationship with climate variables. Front. Microbiol. 2018, 9, 1054. [Google Scholar] [CrossRef]
- Mishra, P.K.; Tewari, J.P.; Clear, R.M.; Turkington, T.K. Genetic diversity and recombination within populations of Fusarium pseudograminearum from western Canada. Int. Microbiol. 2006, 9, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, T.; Xu, X.; Parry, D. Association of Fusarium species in the wheat stem root complex. Eur. J. Plant Pathol. 2003, 109, 769–774. [Google Scholar] [CrossRef]
- Dyer, A.T.; Johnston, R.H.; Hogg, A.C.; Johnston, J.A. Comparison of pathogenicity of the Fusarium crown rot (FCR) complex (F. culmorum, F. pseudograminearum and F. graminearum) on hard red spring and durum wheat. Eur. J. Plant Pathol. 2009, 125, 387–395. [Google Scholar] [CrossRef]
- Alkan, M.; Göre, M.E.; Bayraktar, H.; Özer, G. Genetic variation of Fusarium spp. isolates associated with root and crown rot of winter wheat using retrotransposon-based iPBS assays. Int. J. Agric. Wildlife Sci. 2014, 5, 250–259. [Google Scholar] [CrossRef]
- Chakraborty, S.; Obanor, F.; Westecott, R.; Abeywickrama, K. Wheat crown rot pathogens Fusarium graminearum and F. pseudograminearum lack specialization. Phytopathology 2010, 100, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Chekali, S.; Gargouri, S.; Paulitz, T.; Nicol, J.M.; Rezgui, M.; Nasraoui, B. Effects of Fusarium culmorum and water stress on durum wheat in Tunisia. Crop Prot. 2011, 30, 718–725. [Google Scholar] [CrossRef]
- Hekimhan, H.; Bagci, S.A.; Nicol, J.; Arisoy, R.Z.; Taner, S. Dryland root rot: A major threat to winter cereal production under sub-optimal growing conditions. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 27 September–1 October 2004. [Google Scholar]
- Bagci, S.A.; Hekimhan, H.; Mergoum, M.; Aktas, H.; Taner, S.; Tulukcu, E.; Ekiz, H. Effects of Foot and Root Rot Pathogens on Yields of Some Cereal Genotypes and Determination of Resistance Sources. In Proceedings of the 4th Field Crops Congress, Tekirdağ, Turkey, 17–21 September 2001; pp. 115–120. [Google Scholar]
- Aktas, H.; Kinaci, E.; Yildirim, A.F.; Sayin, L.; Kural, A. Determination of the effects of root and foot rot pathogens on yield components in cereals which are problems in Konya province and solutions. In Proceedings of the Cereal Symposium, Konya, Türkiye, 8–11 June 1999; Ekiz, H., Ed.; Ministry of Agriculture and Rural Affairs, Bahri Dağdaș International Winter Cereals Research Center: Konya, Turkey, 1999; pp. 392–403. [Google Scholar]
- Swan, L.J.; Backhouse, D.; Burgess, L.W. Surface soil moisture and stubble management practice effects on the progress of infection of wheat by Fusarium pseudograminearum. Aust. J. Exp. Agric. 2000, 40, 693–698. [Google Scholar] [CrossRef]
- Wildermuth, G.B.; Thomas, G.A.; Radford, B.J.; McNamara, R.B.; Kelly, A. Crown rot and common root rot in wheat grown under different tillage and stubble treatments in southern Queensland, Australia. Soil Tillage Res. 1997, 44, 211–224. [Google Scholar] [CrossRef]
- Evans, M.L.; Hollaway, G.J.; Dennis, J.I.; Correll, R.; Wallwork, H. Crop sequence as a tool for managing populations of Fusarium pseudograminearum and F. culmorum in south-eastern Australia. Australas. Plant Pathol. 2010, 39, 376–382. [Google Scholar] [CrossRef]
- Simpfendorfer, S. Seed-Borne Fusarium Threatens Crown Rot Control Strategies—Tamworth 2011. Northern Grains Region Trial Results Autumn 2013 Research & Extension—Independent Research for Industry Editors: Loretta Serafin, Steven Simpfendorfer, Matthew Gardner and Guy McMullen NSW Department of Primary Industries. 2013, pp. 137–139. Available online: https://eprints.usq.edu.au/25153/1/Moore_etal_PV.pdf (accessed on 16 November 2017).
- Akgül, D.S.; Erkilic, A. Effect of wheat cultivars, fertilizers, and fungicides on Fusarium foot rot disease of wheat. Turk. J. Agric. For. 2016, 40, 101–108. [Google Scholar] [CrossRef]
- Moya-Elizondo, E.A.; Rew, L.J.; Jacobsen, B.J.; Hogg, A.C.; Dyer, A.T. Distribution and prevalence of Fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 2011, 95, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariyar, S.R.; Dababat, A.A.; Nicol, J.M.; Erginbas-Orakci, G.; Goll, M.B.; Watrin, C.; Sikora, R. Fungicide seed treatment and host resistance for the management of wheat crown rot caused by Fusarium culmorum. Basic Res. J. Agric. Sci. Rev. 2014, 3, 116–121. [Google Scholar]
- Sohail, Q.; Erginbas-Orakci, G.; Ozdemir, F.; Jighly, A.; Dreisigacker, S.; Bektas, H.; Dababat, A.A. Genome-Wide Association Study of Root-Lesion Nematodes Pratylenchus Species and Crown Rot Fusarium culmorum in Bread Wheat. Life 2022, 12, 372. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: Recent progress and future prospects. Mol. Plant Pathol. 2018, 19, 1547–1562. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, F.; Koc, N.K.; Paulitz, T.; Nicol, J.M.; Schroeder, K.L.; Poole, G. Determination of fusarium crown rot resistance in wheat to Fusarium culmorum and Fusarium pseudogramineaum using real time PCR. Crop Prot. 2020, 135, 105204. [Google Scholar] [CrossRef]
- Mitter, V.; Zhang, M.C.; Liu, C.J.; Ghosh, R.; Ghosh, M.; Chakraborty, S.A. high-throughput glasshouse bioassay to detect crown rot resistance in wheat germplasm. Plant Pathol. 2006, 55, 433–441. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Chakraborty, S.; Manners, J.M.; Kazan, K. A simple method for the assessment of crown rot disease severity in wheat seedlings inoculated with Fusarium pseudograminearum. J Phytopathol. 2008, 156, 751–754. [Google Scholar] [CrossRef]
- Yang, X.M.; J Yang, X.; Ma, J.; Li, H.; Ma, H.; Yao, J.; Liu, C. Different genes can be responsible for crown rot resistance at different developmental stages of wheat and barley. Eur. J. Plant Pathol. 2010, 128, 495–502. [Google Scholar] [CrossRef]
- Liu, C.; Ogbonnaya, F.C. Resistance to Fusarium crown rot in wheat and barley: A review. Plant Breed. 2015, 134, 365–372. [Google Scholar] [CrossRef]
- Bovill, W.D.; Horne, M.; Herde, D.; Davis, M.; Wildermuth, G.B.; Sutherland, M.W. Pyramiding QTL increases seedling resistance to crown rot (Fusarium pseudograminearum) of wheat (Triticum aestivum). Theor. Appl. Genet. 2010, 1211, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, N.L.; Sutherland, M.W. Histopathological assessment of wheat seedling tissues infected by Fusarium pseudograminearum. Plant Pathol. 2013, 623, 679–687. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.A.; Klink, H. Composition and predominance of Fusarium species causing Fusarium head blight in winter wheat grain depending on cultivar susceptibility and meteorological factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Spanic, V.; Zdunic, Z.; Drezner, G.; Sarkanj, B. The pressure of Fusarium disease and its relation with mycotoxins in the wheat grain and malt. Toxins 2019, 11, 198. [Google Scholar] [CrossRef] [Green Version]
- Collard, B.C.Y.; Grams, R.A.; Bovill, W.D.; Percy, C.D.; Jolley, R.; Lehmensiek, A.; Sutherland, M.W. Development of molecular markers for crown rot resistance in wheat: Mapping of QTLs for seedling resistance in a ‘2-49′בJanz’population. Plant Breed. 2005, 124, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
| Locality | Average Annual Temperature | Annual Precipitation | Humidity |
|---|---|---|---|
| Eskişehir | 12.7 °C | 200–390 mm | 67.1% |
| Yozgat | 13.3 °C | 450–570 mm | 54.6% |
| Konya | 23.6 °C | 124–300 mm | 57.9% |
| Ent | CNAME | CID |
|---|---|---|
| 1 | PRL/2*PASTOR//WAXWING*2/KRONSTAD F2004/4/PBW343*2/KUKUNA//KRONSTAD F2004/3/PBW343*2/KUKUNA | 546,349 |
| 2 | DANPHE/2*BAJ #1 | 546,357 |
| 3 | PICAFLOR #1/5/FRET2/KUKUNA//FRET2/3/YANAC/4/FRET2/KIRITATI | 553,138 |
| 4 | DANPHE/3/PBW343*2/KUKUNA//PBW343*2/KUKUNA | 553,204 |
| 5 | FRANCOLIN #1/BAJ #1 | 553,377 |
| 6 | SOKOLL/3/PASTOR//HXL7573/2*BAU*2/4/PASTOR//MILAN/KAUZ/3/BAV92 | 554,318 |
| 7 | TUKURU//BAV92/RAYON/6/NG8201/KAUZ/4/SHA7//PRL/ VEE#6/3/FASAN/5/MILAN/KAUZ*2/7/KINGBIRD #1 | 559,533 |
| 8 | WHEAR/KUKUNA/3/C80.1/3*BATAVIA//2*WBLL1*2/6/ WBLL1*2/4/YACO/PBW65/3/KAUZ*2/TRAP//KAUZ/5/KACHU #1 | 559,752 |
| 9 | PBW343*2/KUKUNA//PBW343*2/KUKUNA/3/2*BAJ #1 | 559,939 |
| 10 | PAURAQ/4/HUW234+LR34/PRINIA//PBW343*2/KUKUNA/3/ ROLF07 | 545,670 |
| 11 | ATTILA*2/PBW65/5/PRL/2*PASTOR/4/CHOIX/STAR/3/HE1/3*CNO79//2*SERI/6/PFUNYE #1 | 546,353 |
| 12 | WHEAR/KUKUNA/3/C80.1/3*BATAVIA//2*WBLL1/8/VEE#8// JUP/BJY/3/F3.71/TRM/4/BCN/5/KAUZ/6/MILAN/KAUZ/7/ SKAUZ/PARUS//PARUS/9/KACHU | 546,418 |
| 13 | KACHU*2/BECARD | 546,469 |
| 14 | BABAX/LR42//BABAX/3/ER2000/4/2*MUNAL | 549,129 |
| 15 | ROBIN,KEN | 448,396 |
| 16 | BABAX/LR42//BABAX*2/4/SNI/TRAP#1/3/KAUZ*2/TRAP// KAUZ*2/5/MUNAL #1 | 546,537 |
| 17 | MERCATO/4/FRAME//MILAN/KAUZ/3/PASTOR/5/WHEAR/ SOKOLL | 548,932 |
| 18 | KENYA SUNBIRD/2*KACHU | 541,193 |
| 19 | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/NAVJ07 | 549,534 |
| 20 | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/HUW234+LR34/ PRINIA//PBW343*2/KUKUNA/3/ROLF07 | 549,549 |
| 21 | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/MASSIV/PPR47.89C | 549,913 |
| 22 | W15.92/4/PASTOR//HXL7573/2*BAU/3/WBLL1/5/SOKOLL/3/ PASTOR//HXL7573/2*BAU | 552,587 |
| 23 | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/GLADIUS | 552,597 |
| 24 | W15.92/4/PASTOR//HXL7573/2*BAU/3/WBLL1*2/5/WHEAR/ SOKOLL | 554,181 |
| 25 | TRCH/SRTU//KACHU*2/3/PVN | 559,568 |
| 26 | SOKOLL/3/PASTOR//HXL7573/2*BAU/4/2*PASTOR//HXL7573/2*BAU/3/SOKOLL/WBLL1 | 560,562 |
| 27 | 249 (Check-CR-Mr) | |
| 28 | Altay (Check-CR-Mr) | |
| 29 | Yelken (Check-CR-Mr) | |
| 30 | Kızıltan (Check-CR-S) | |
| 31 | Gerek (Check-CR-S) | |
| 32 | Kutluk (Check-CR-S) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, F. Host Susceptibility of CIMMYT’s International Spring Wheat Lines to Crown and Root Rot Caused by Fusarium culmorum and F. pseudograminearum. Agronomy 2022, 12, 3038. https://doi.org/10.3390/agronomy12123038
Özdemir F. Host Susceptibility of CIMMYT’s International Spring Wheat Lines to Crown and Root Rot Caused by Fusarium culmorum and F. pseudograminearum. Agronomy. 2022; 12(12):3038. https://doi.org/10.3390/agronomy12123038
Chicago/Turabian StyleÖzdemir, Fatih. 2022. "Host Susceptibility of CIMMYT’s International Spring Wheat Lines to Crown and Root Rot Caused by Fusarium culmorum and F. pseudograminearum" Agronomy 12, no. 12: 3038. https://doi.org/10.3390/agronomy12123038
APA StyleÖzdemir, F. (2022). Host Susceptibility of CIMMYT’s International Spring Wheat Lines to Crown and Root Rot Caused by Fusarium culmorum and F. pseudograminearum. Agronomy, 12(12), 3038. https://doi.org/10.3390/agronomy12123038






