Assessing Grapevine Water Status by Integrating Vine Transpiration, Leaf Gas Exchanges, Chlorophyll Fluorescence and Sap Flow Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Setup
2.2. Vine Transpiration Measurements
2.3. Vine Water Potential
2.4. Leaf Gas Exchanges
2.5. Chlorophyll Fluorescence
2.6. Data Treatment and Statistical Analysis
3. Results
3.1. Weather Conditions
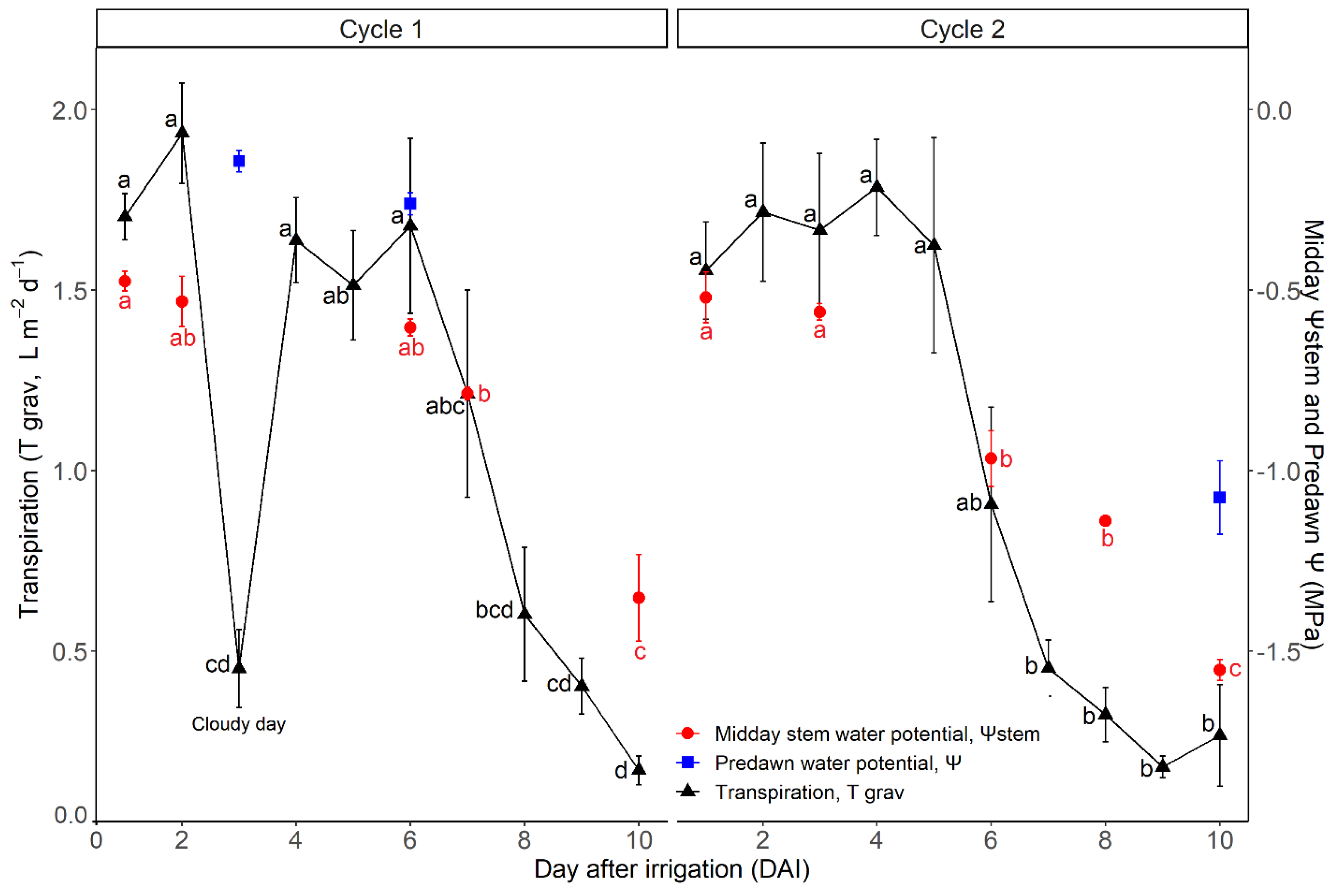
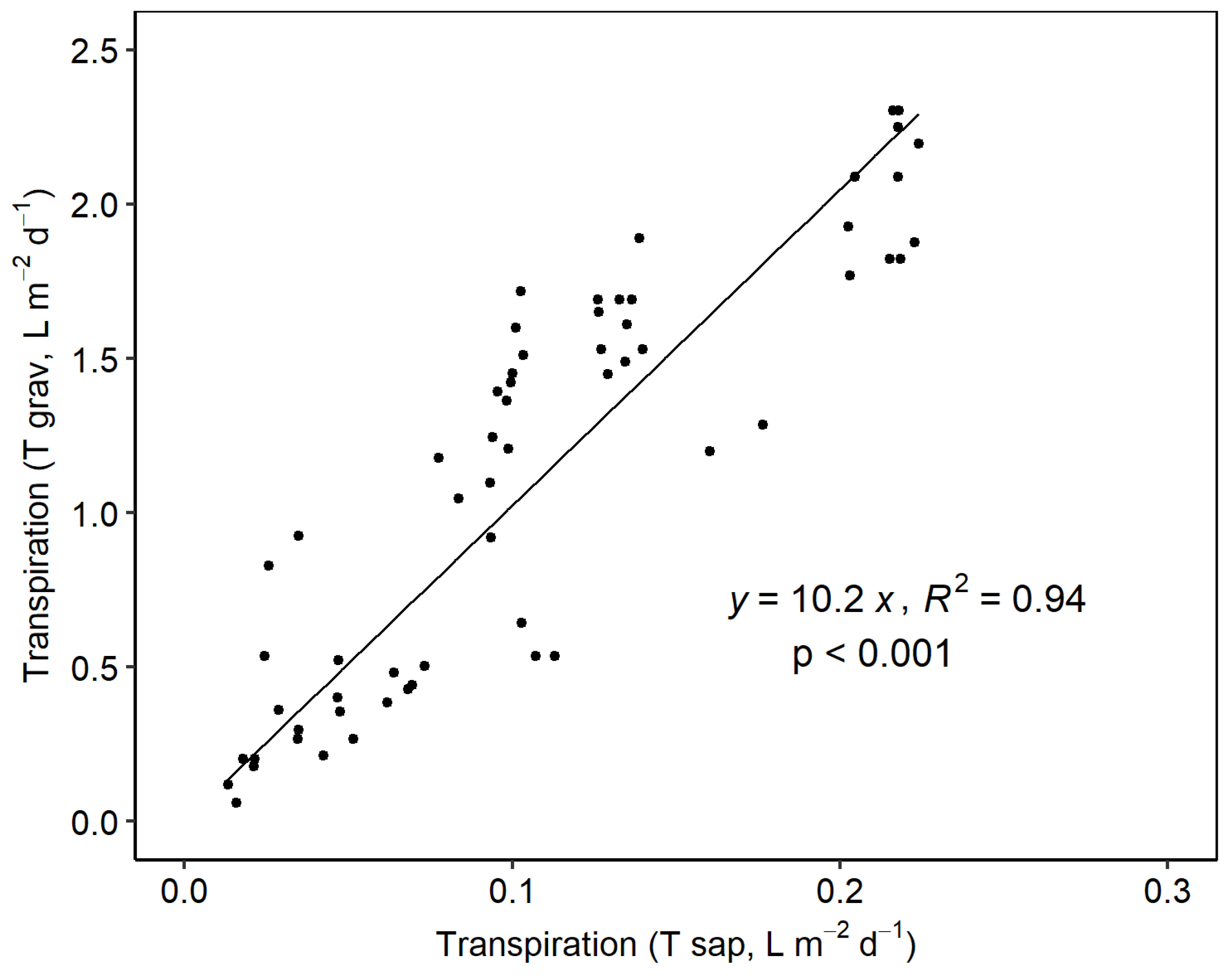
3.2. Vine Water Status and Transpiration
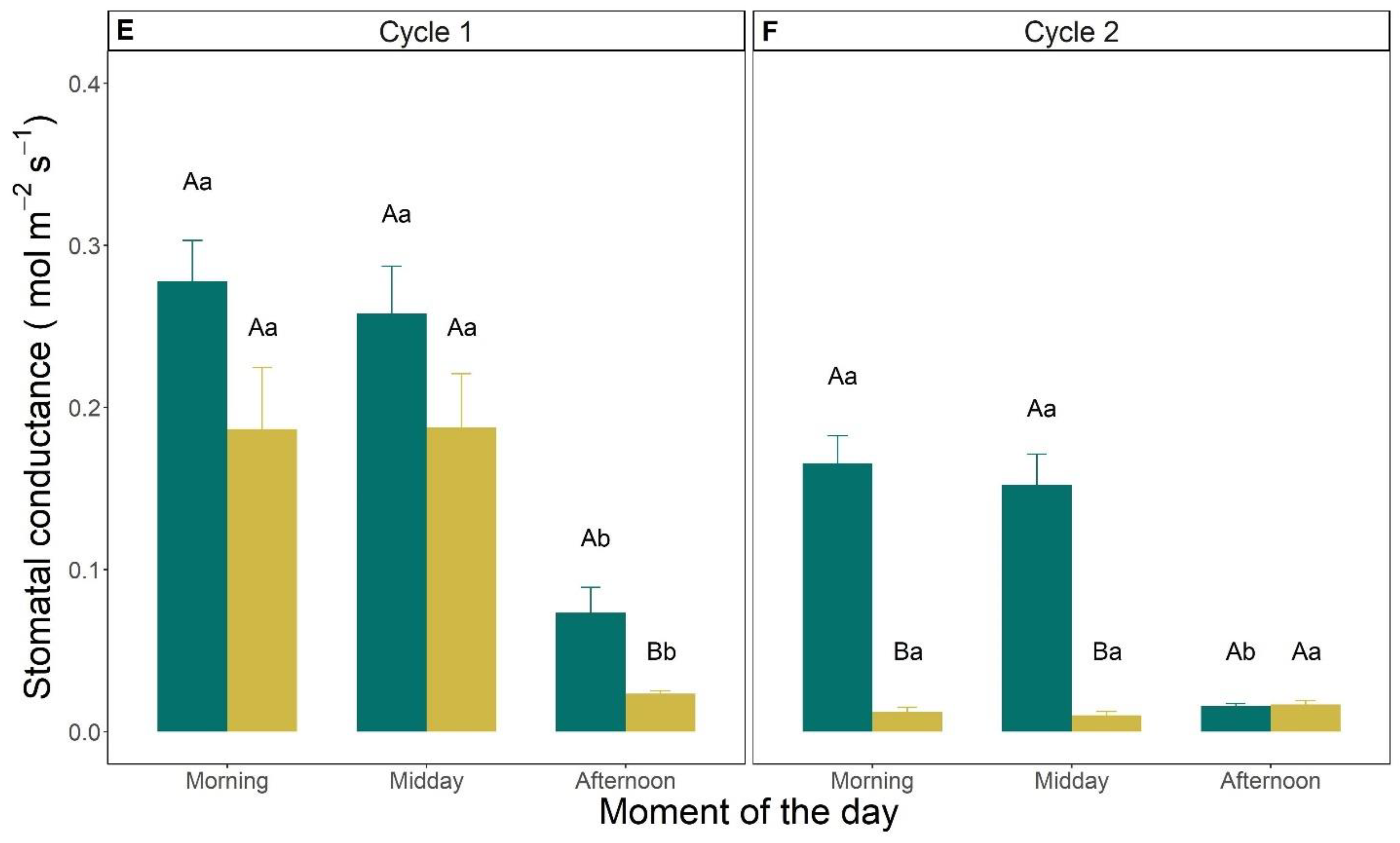
3.3. Leaf Gas Exchanges
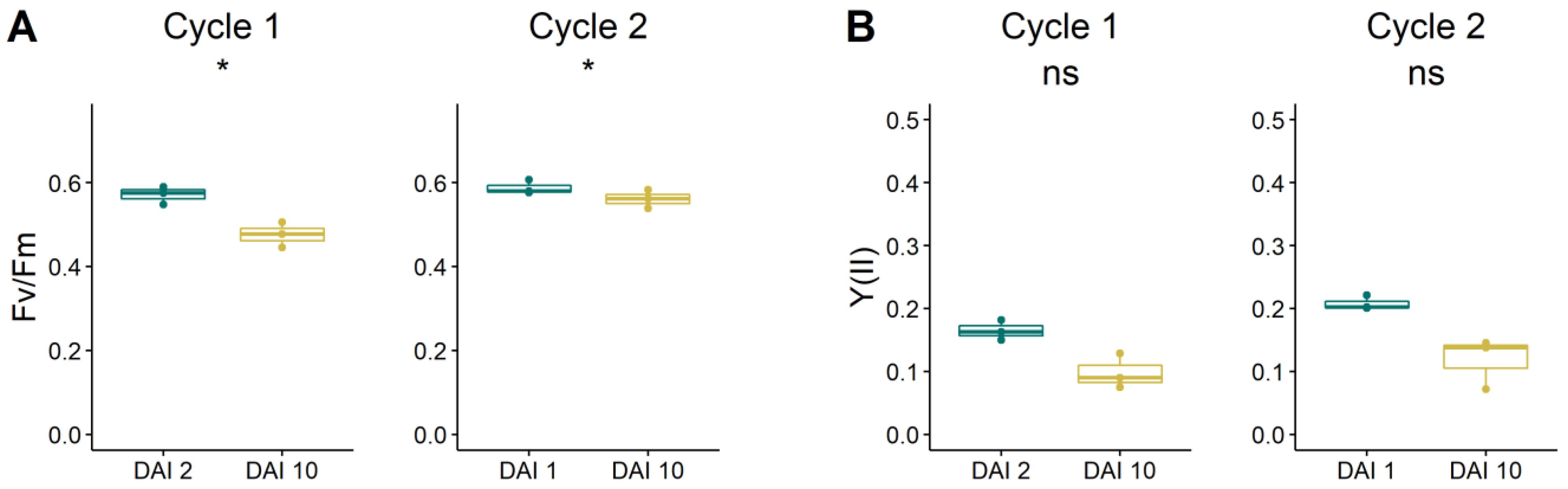
3.4. Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; Core Writing Team: Geneva, Switzerland, 2014. [Google Scholar]
- Pereira, L.S. Challenges on Water Resources Management When Searching for Sustainable Adaptation to Climate Change Focusing Agriculture. Eur. Water 2011, 34, 41–54. [Google Scholar]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Crop Yield Response to Water; United Nations FAO: Rome, Italy, 2012; p. 505. [Google Scholar]
- Geerts, S.; Raes, D. Deficit Irrigation as an On-Farm Strategy to Maximize Crop Water Productivity in Dry Areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of Pre- and Postveraison Water Deficit on Synthesis and Concentration of Skin Phenolic Compounds during Berry Growth of Vitis vinifera Cv. Shiraz. Am. Soc. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit Irrigation in Grapevine Improves Water-Use Efficiency While Controlling Vigour and Production Quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under Deficit Irrigation: Hints from Physiological and Molecular Data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Basile, B.; Marsal, J.; Mata, M.; Vallverdú, X.; Bellvert, J.; Girona, J. Phenological Sensitivity of Cabernet Sauvignon to Water Stress: Vine Physiology and Berry Composition. Am. J. Enol. Vitic. 2011, 62, 453–461. [Google Scholar] [CrossRef]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological Tools for Irrigation Scheduling in Grapevine (Vitis vinifera L.): An Open Gate to Improve Water-Use Efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Buckley, T.N. Modeling Stomatal Conductance. Plant Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, M.B. Potential Evapotranspiration. In Principles of Soil and Plant Water Relations; Academic Press: Cambridge, MA, USA, 2014; pp. 501–514. ISBN 9780124200227. [Google Scholar]
- Schultz, H.R. Differences in Hydraulic Architecture Account for Near- Isohydric and Anisohydric Behaviour of Two Field-Grown Vitis vinifera L. Cultivars during Drought. Plant Cell Environ. 2003, 23, 1393–1405. [Google Scholar] [CrossRef]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-Plant Hydraulic Conductance and Root-to-Shoot Flow of Abscisic Acid Are Independently Affected by Water Stress in Grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal and Non-Stomatal Limitations of Photosynthesis under Water Stress in Field-Grown Grapevines. Aust. J. Plant Physiol. 1999, 26, 421–433. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Modelling Photosynthetic Responses to Temperature of Grapevine (Vitis vinifera Cv. Semillon) Leaves on Vines Grown in a Hot Climate. Plant Cell Environ. 2012, 35, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmés, J.; Gulías, J.; Lefi, E.K.; Martínez-Cañellas, S.F.; Moreno, M.T.; Ribas-Carbó, M.; et al. Understanding Down-Regulation of Photosynthesis under Water Stress: Future Prospects and Searching for Physiological Tools for Irrigation Management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Choné, X.; Van Leeuwen, C.; Dubourdieu, D.; Gaudillère, J.P. Stem Water Potential Is a Sensitive Indicator of Grapevine Water Status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Wenter, A.; Zanotelli, D.; Montagnani, L.; Tagliavini, M.; Andreotti, C. Effect of Different Timings and Intensities of Water Stress on Yield and Berry Composition of Grapevine (Cv. Sauvignon Blanc) in a Mountain Environment. Sci. Hortic. 2018, 236, 137–145. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Famiani, F.; Silvestroni, O.; Zamboni, M.; Poni, S. Morpho-Structural and Physiological Response of Container-Grown Sangiovese and Montepulciano Cvv. (Vitis vinifera) to Re-Watering after a Pre-Veraison Limiting Water Deficit. Funct. Plant Biol. 2014, 41, 634–647. [Google Scholar] [CrossRef]
- Wenter, A.; Zanotelli, D.; Tagliavini, M.; Andreotti, C. Thresholds of Soil and Plant Water Availability That Affect Leaf Transpiration, Stomatal Conductance and Photosynthesis in Grapevines. Acta Hortic. 2022, 1335, 605–611. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 49–70. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Photosynthetic Response of Bottle Gourd [Lagenaria Siceraria (Molina) Standl.] to Drought Stress: Relationship between Cucurbitacins Accumulation and Drought Tolerance. Sci. Hortic. 2018, 231, 133–143. [Google Scholar] [CrossRef]
- Galat Giorgi, E.; Sadras, V.O.; Keller, M.; Perez Peña, J. Interactive Effects of High Temperature and Water Deficit on Malbec Grapevines. Aust. J. Grape Wine Res. 2019, 25, 345–356. [Google Scholar] [CrossRef]
- Díaz-Barradas, M.C.; Gallego-Fernández, J.B.; Zunzunegui, M. Plant Response to Water Stress of Native and Non-Native Oenothera Drummondii Populations. Plant Physiol. Biochem. 2020, 154, 219–228. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of Photosynthesis of C3 Plants in Response to Progressive Drought: Stomatal Conductance as a Reference Parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, D.; Lakso, A.N.; Piccioni, R.M. Transpiration of Apple Trees in a Humid Climate Using Heat Pulse Sap Flow Gauges Calibrated with Whole-Canopy Gas Exchange Chambers. Agric. For. Meteorol. 2005, 130, 85–94. [Google Scholar] [CrossRef]
- Fernández, J.E.; Green, S.R.; Caspari, H.W.; Diaz-Espejo, A.; Cuevas, M.V. The Use of Sap Flow Measurements for Scheduling Irrigation in Olive, Apple and Asian Pear Trees and in Grapevines. Plant Soil 2008, 305, 91–104. [Google Scholar] [CrossRef]
- Dragoni, D.; Lakso, A.N.; Piccioni, R.M.; Tarara, J.M. Transpiration of Grapevines in the Humid Northeastern United States. Am. J. Enol. Vitic. 2006, 57, 460–467. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Ward, E.J.; Ding, R.; Zhang, X.; Zheng, R. Evapotranspiration Components Determined by Sap Flow and Microlysimetry Techniques of a Vineyard in Northwest China: Dynamics and Influential Factors. Agric. Water Manag. 2011, 98, 1207–1214. [Google Scholar] [CrossRef]
- Wei, X.; Fu, S.; Chen, D.; Zheng, S.; Wang, T.; Bai, Y. Grapevine Sap Flow in Response to Physio-Environmental Factors under Solar Greenhouse Conditions. Water 2020, 12, 3081. [Google Scholar] [CrossRef]
- Spicer, R.; Holbrook, N.M. Within-Stem Oxygen Concentration and Sap Flow in Four Temperate Tree Species: Does Long-Lived Xylem Parenchyma Experience Hypoxia? Plant Cell Environ. 2005, 28, 192–201. [Google Scholar] [CrossRef]
- Gebauer, T.; Horna, V.; Leuschner, C. Variability in Radial Sap Flux Density Patterns and Sapwood Area among Seven Co-Occurring Temperate Broad-Leaved Tree Species. Tree Physiol. 2008, 28, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998; Volume 300, p. D05109. [Google Scholar]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. An Improved Heat Pulse Method to Measure Low and Reverse Rates of Sap Flow in Woody Plants. Tree Physiol. 2001, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Tarara, J.M.; Peña, J.E.P. Moderate Water Stress from Regulated Deficit Irrigation Decreases Transpiration Similarly to Net Carbon Exchange in Grapevine Canopies. J. Am. Soc. Hortic. Sci. 2015, 140, 413–426. [Google Scholar] [CrossRef]
- Pou, A.; Flexas, J.; Alsina, M.D.M.; Bota, J.; Carambula, C.; De Herralde, F.; Galmés, J.; Lovisolo, C.; Jiménez, M.; Ribas-Carbó, M.; et al. Adjustments of Water Use Efficiency by Stomatal Regulation during Drought and Recovery in the Drought-Adapted Vitis Hybrid Richter-110 (V. berlandieri x V. rupestris). Physiol. Plant. 2008, 134, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Delzon, S.; Domec, J.C.; Zhang, L.; Delmas, C.E.L.; Merlin, I.; Corso, D.; King, A.; Ojeda, H.; Ollat, N.; et al. Drought Will Not Leave Your Glass Empty: Low Risk of Hydraulic Failure Revealed by Long-Term Drought Observations in World’s Top Wine Regions. Sci. Adv. 2018, 4, eaao6969. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, S.; Frioni, T.; Poni, S.; Palliotti, A. Effect of Water Stress “Memory” on Plant Behavior during Subsequent Drought Stress. Environ. Exp. Bot. 2018, 150, 106–114. [Google Scholar] [CrossRef]
- Bleby, T.M.; Burgess, S.S.O.; Adams, M.A. A Validation, Comparison and Error Analysis of Two Heat-Pulse Methods for Measuring Sap Flow in Eucalyptus Marginata Saplings. Funct. Plant Biol. 2004, 31, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Spicer, R. Senescence in Secondary Xylem: Heartwood Formation as an Active Developmental Program; Elsevier Inc.: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Escalona, J.; Flexas, J.; Medrano, H. Drought Effects on Water Flow, Photosynthesis and Growth of Potted Grapevines. Vitis 2002, 41, 57–62. [Google Scholar]
- Nadezhdina, N. Sap Flow Index as an Indicator of Plant Water Status. Tree Physiol. 1999, 19, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdelkader, A.; Benyahia, F.; Bastos Campos, F.; Asensio, D.; Andreotti, C.; Tagliavini, M.; Zanotelli, D. Apple Tree Transpiration during Cycles of Progressive Drought as Assessed via Continuous Gravimetric and Xylem Sap Flux Measurements. Italus Hortus 2022, 29, 35–46. [Google Scholar] [CrossRef]
- Yamane, T.; Hamana, Y.; Nakano, M. Detection of Water-Deficit Stress from Daily Sap Flow Profiles in Peach. J. Jpn. Soc. Hortic. Sci. 2011, 80, 383–389. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Fernández, J.E.; Rodriguez-Dominguez, C.M.; Romero, R.; Diaz-Espejo, A. The Dynamics of Radial Sap Flux Density Reflects Changes in Stomatal Conductance in Response to Soil and Air Water Deficit. Agric. For. Meteorol. 2016, 218–219, 92–101. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Silvestroni, O.; Lanari, V.; D’Onofrio, C.; Matarese, F.; Bellincontro, A.; Poni, S. Physiological Parameters and Protective Energy Dissipation Mechanisms Expressed in the Leaves of Two Vitis vinifera L. Genotypes under Multiple Summer Stresses. J. Plant Physiol. 2015, 185, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.L.; Yue, X.F.; Zhao, X.F.; Zhao, H.; Fang, Y. lin Physiological, Micro-Morphological and Metabolomic Analysis of Grapevine (Vitis vinifera L.) Leaf of Plants under Water Stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, S.; Rodrigo, M.J.; Vives-Peris, V.; Gómez-Cadenas, A.; Zacarías, L.; Machado, N.; Moutinho-Pereira, J.; Dinis, L.T. Fine-Tuning of Grapevine Xanthophyll-Cycle and Energy Dissipation under Mediterranean Conditions by Kaolin Particle-Film. Sci. Hortic. 2022, 291, 110584. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of QA Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. In Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 279–319. ISBN 9781493970001. [Google Scholar]
- Klughammer, C.; Schreiber, U. Complementary PS II Quantum Yields Calculated from Simple Fluorescence Parameters Measured by PAM Fluorometry and the Saturation Pulse Method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benyahia, F.; Bastos Campos, F.; Ben Abdelkader, A.; Basile, B.; Tagliavini, M.; Andreotti, C.; Zanotelli, D. Assessing Grapevine Water Status by Integrating Vine Transpiration, Leaf Gas Exchanges, Chlorophyll Fluorescence and Sap Flow Measurements. Agronomy 2023, 13, 464. https://doi.org/10.3390/agronomy13020464
Benyahia F, Bastos Campos F, Ben Abdelkader A, Basile B, Tagliavini M, Andreotti C, Zanotelli D. Assessing Grapevine Water Status by Integrating Vine Transpiration, Leaf Gas Exchanges, Chlorophyll Fluorescence and Sap Flow Measurements. Agronomy. 2023; 13(2):464. https://doi.org/10.3390/agronomy13020464
Chicago/Turabian StyleBenyahia, Fadwa, Flávio Bastos Campos, Ahmed Ben Abdelkader, Boris Basile, Massimo Tagliavini, Carlo Andreotti, and Damiano Zanotelli. 2023. "Assessing Grapevine Water Status by Integrating Vine Transpiration, Leaf Gas Exchanges, Chlorophyll Fluorescence and Sap Flow Measurements" Agronomy 13, no. 2: 464. https://doi.org/10.3390/agronomy13020464
APA StyleBenyahia, F., Bastos Campos, F., Ben Abdelkader, A., Basile, B., Tagliavini, M., Andreotti, C., & Zanotelli, D. (2023). Assessing Grapevine Water Status by Integrating Vine Transpiration, Leaf Gas Exchanges, Chlorophyll Fluorescence and Sap Flow Measurements. Agronomy, 13(2), 464. https://doi.org/10.3390/agronomy13020464








