Harnessing the Rhizosphere Soil Microbiome of Organically Amended Soil for Plant Productivity
Abstract
:1. Introduction
2. Organic Amendments and Sustainable Agriculture
2.1. Sources of Organic Amendments and Their Impact on Soil Properties
2.2. Impact of Organic Amendments on the Structure and Diversity of Microbial Communities in the Rhizosphere
2.3. Organic Amendment and Disease Suppressive Soils
2.4. Potential Negative Effects of Organic Amendment
3. Diversity of Microbial Communities in Rhizosphere Soils of Organically Amended Soil
Survival of Rhizosphere Microbiome from OAs in Soil Conditions
4. Mechanisms Used by Microbes in Organically Amended Soil in Promoting Plant Health
4.1. Direct Mechanism
4.1.1. Synthesis of Plant Growth Hormones
4.1.2. Ability to Fix Atmospheric Nitrogen
4.1.3. Ability to Solubilize Mineral Elements, Phosphorus, and Potassium
4.1.4. Ability to Produce Siderophores
4.1.5. Lowering Plant Ethylene Levels
4.2. Indirect Mechanisms
4.2.1. Antibiosis
4.2.2. Enzyme Production
4.2.3. Induced Systemic Resistance (ISR)
4.2.4. Exopolysaccharide Production
5. Discussion
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-Sagasti, M.T.; Hernández, A.; Artetxe, U.; Garbisu, C.; Becerril, J.M. How valuable are organic amendments as tools for the phytomanagement of degraded soils? The knowns, known unknowns, and unknowns. Front. Sustain. Food Syst. 2018, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Boldt-Burisch, K.; Schillem, S.; Schneider, B.U.; Hüttl, R.F. The effect of nitrogen-modified lignite granules on mycorrhization and root and shoot growth of Secale cereale (winter rye) in a nutrient-deficient, sandy soil. Arch. Agron. Soil Sci. 2020, 68, 1117–1130. [Google Scholar] [CrossRef]
- Cerecetto, V.; Smalla, K.; Nesme, J.; Garaycochea, S.; Fresia, P.; Sørensen, S.J.; Babin, D.; Leoni, C. Reduced tillage, cover crops and organic amendments affect soil microbiota and improve soil health in Uruguayan vegetable farming systems. FEMS Microbiol. Ecol. 2021, 97, fiab023. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, S. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPPC: Geneva, Switzerland, 2019. [Google Scholar]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by-omics approaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An underground revolution: Biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Quince, C.; Macdonald, C.A.; Khachane, A.; Thomas, N.; Al-Soud, W.A.; Sørensen, S.J.; He, Z.; White, D.; Sinclair, A.; et al. Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 2014, 16, 2408–2420. [Google Scholar] [CrossRef]
- Hueso-González, P.; Muñoz-Rojas, M.; Martínez-Murillo, J.F. The role of organic amendments in drylands restoration. Curr. Opin. Environ. Sci. Health 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Rahman, G.; Rahman, M.M.; Alam, M.S.; Kamal, M.Z.; Mashuk, H.; Datta, R.; Meena, R.S. Biochar and organic amendments for sustainable soil carbon and soil health. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R., Pathan, S., Ceccherini, M., Eds.; Springer: Singapore, 2020; pp. 45–85. [Google Scholar]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Mazzoleni, S.; Abd-ElGawad, A.M. Microbiota modulation of allelopathy depends on litter chemistry: Mitigation or exacerbation? Sci. Total Environ. 2021, 776, 145942. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Di Silverio, N.; Carrino, L.; Cesarano, G.; Assaeed, A.M.; Abd-ElGawad, A.M. Decomposition and organic amendments chemistry explain contrasting effects on plant growth promotion and suppression of Rhizoctonia solani damping off. PLoS ONE 2020, 15, e0230925. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Masowa, M.; Kutu, F.; Babalola, O.; Mulidzi, A. Physico-chemical properties and phyto-toxicity assessment of cocomposted winery solid wastes with and without effective microorganism inoculation. Res. Crops 2018, 19, 549–559. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Bonanomi, G.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Mixtures of organic amendments and biochar promote beneficial soil microbiota and affect Fusarium oxysporum f. sp. lactucae, Rhizoctonia solani and Sclerotinia minor disease suppression. Plant Pathol. 2022, 71, 818–829. [Google Scholar] [CrossRef]
- Postma, J.; Schilder, M.T. Enhancement of soil suppressiveness against Rhizoctonia solani in sugar beet by organic amendments. Appl. Soil Ecol. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Kennedy, A.C.; de Luna, L.Z. Rhizosphere. In Encyclopedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Oxford, UK, 2005; pp. 399–406. [Google Scholar]
- Bano, S.; Wu, X.; Zhang, X. Towards sustainable agriculture: Rhizosphere microbiome engineering. Appl. Microbiol. Biotechnol. 2021, 105, 7141–7160. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Liu, J.; Zhou, Z.; Zhang, T.; Wang, X. Bacterial diversity as affected by application of manure in red soils of subtropical China. Biol. Fertil. Soils 2017, 53, 639–649. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Liu, Z.; Guo, Q.; Feng, Z.; Liu, Z.; Li, H.; Sun, Y.; Liu, C.; Lai, H. Long-term organic fertilization improves the productivity of kiwifruit (Actinidia chinensis Planch.) through increasing rhizosphere microbial diversity and network complexity. Appl. Soil Ecol. 2020, 147, 103426. [Google Scholar] [CrossRef]
- Lu, P.; Bainard, L.D.; Ma, B.; Liu, J. Bio-fertilizer and rotten straw amendments alter the rhizosphere bacterial community and increase oat productivity in a saline–alkaline environment. Sci. Rep. 2020, 10, 19896. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, M.M.; Minarsch, E.-M.L.; Durai Raj, A.C.; Rineau, F.; Schröder, P. Changes of soil-rhizosphere microbiota after organic amendment application in a Hordeum vulgare L. short-term greenhouse experiment. Plant Soil 2020, 455, 489–506. [Google Scholar] [CrossRef]
- Zhang, J.; Bei, S.; Li, B.; Zhang, J.; Christie, P.; Li, X. Organic fertilizer, but not heavy liming, enhances banana biomass, increases soil organic carbon and modifies soil microbiota. Appl. Soil Ecol. 2019, 136, 67–79. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parr, J.F.; Hornick, S.B. Agricultural use of organic amendments: A historical perspective. Am. J. Altern. Agric. 1992, 7, 181–189. [Google Scholar] [CrossRef]
- Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Hesham, A.E.-L.; Taher, M.A.; Fawy, K.F. The effects of different sewage sludge amendment rates on the heavy metal bioaccumulation, growth and biomass of cucumbers (Cucumis sativus L.). Environ. Sci. Pollut. Res. 2017, 24, 16371–16382. [Google Scholar] [CrossRef]
- Latare, A.; Kumar, O.; Singh, S.; Gupta, A. Direct and residual effect of sewage sludge on yield, heavy metals content and soil fertility under rice–wheat system. Ecol. Eng. 2014, 69, 17–24. [Google Scholar] [CrossRef]
- Kutu, F.R.; Masowa, M.M. Nitrogen and potassium mineralization from winery solid waste composts in sandy and sandy loam soils. Arch. Agron. Soil Sci. 2018, 64, 1094–1105. [Google Scholar] [CrossRef]
- Masowa, M.M.; Kutu, F.R.; Shange, P.L.; Mulidzi, R.; Vanassche, F.M. The effect of winery solid waste compost application on maize growth, biomass yield, and nutrient content under greenhouse conditions. Arch. Agron. Soil Sci. 2016, 62, 1082–1094. [Google Scholar] [CrossRef]
- Vida, C.; de Vicente, A.; Cazorla, F.M. The role of organic amendments to soil for crop protection: Induction of suppression of soilborne pathogens. Ann. Appl. Biol. 2020, 176, 1–15. [Google Scholar] [CrossRef]
- Arif, I.; Batool, M.; Schenk, P.M. Plant microbiome engineering: Expected benefits for improved crop growth and resilience. Trends Biotechnol. 2020, 38, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, W.; Li, G. Adoption of organic soil amendments and its impact on farm performance: Evidence from wheat farmers in China. Aust. J. Agric. Resour. Econ. 2020, 65, 367–390. [Google Scholar] [CrossRef]
- Larney, F.J.; Angers, D.A. The role of organic amendments in soil reclamation: A review. Can. J. Soil Sci. 2012, 92, 19–38. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, J.; Li, H.; La, S.; Tian, Y.; Gao, L. Biochar addition combined with daily fertigation improves overall soil quality and enhances water-fertilizer productivity of cucumber in alkaline soils of a semi-arid region. Geoderma 2020, 363, 114170. [Google Scholar] [CrossRef]
- Ni, X.; Song, W.; Zhang, H.; Yang, X.; Wang, L. Effects of mulching on soil properties and growth of tea olive (Osmanthus fragrans). PLoS ONE 2016, 11, e0158228. [Google Scholar] [CrossRef] [Green Version]
- Wesseling, J.; Stoof, C.; Ritsema, C.; Oostindie, K.; Dekker, L. The effect of soil texture and organic amendment on the hydrological behaviour of coarse-textured soils. Soil Use Manag. 2009, 25, 274–283. [Google Scholar] [CrossRef]
- Tani, E.; Abraham, E.; Chachalis, D.; Travlos, I. Molecular, genetic and agronomic approaches to utilizing pulses as cover crops and green manure into cropping systems. Int. J. Mol. Sci. 2017, 18, 1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storr, T.; Simmons, R.W.; Hannam, J.A. A UK survey of the use and management of cover crops. Ann. Appl. Biol. 2019, 174, 179–189. [Google Scholar] [CrossRef]
- Cesarano, G.; Zotti, M.; Antignani, V.; Marra, R.; Scala, F.; Bonanomi, G. Soil sickness and negative plant-soil feedback: A reappraisal of hypotheses. J. Plant Pathol. 2017, 99, 545–570. [Google Scholar]
- Mazzola, M.; Reardon, C.L.; Brown, J. Initial Pythium species composition and Brassicaceae seed meal type influence extent of Pythium-induced plant growth suppression in soil. Soil Biol. Biochem. 2012, 48, 20–27. [Google Scholar] [CrossRef]
- Nallanchakravarthula, S.; Marupakula, S.; Alström, S.; Finlay, R.D.; Mahmood, S. Changes in the root fungal microbiome of strawberry following application of residues of the biofumigant oilseed radish. Appl. Soil Ecol. 2021, 168, 104116. [Google Scholar] [CrossRef]
- Sayara, T.; Basheer-Salimia, R.; Hawamde, F.; Sánchez, A. Recycling of Organic Wastes through Composting: Process Performance and Compost Application in Agriculture. J. Agron. 2020, 10, 1838. [Google Scholar] [CrossRef]
- Jones, B.E.; Haynes, R.; Phillips, I. Effect of amendment of bauxite processing sand with organic materials on its chemical, physical and microbial properties. J. Environ. Manag. 2010, 91, 2281–2288. [Google Scholar] [CrossRef]
- Esperschütz, J.; Gattinger, A.; Mäder, P.; Schloter, M.; Fließbach, A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. FEMS Microbiol. Ecol. 2007, 61, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.; Jinger, D.; Jatav, H.S.; Banjara, T. Use of pressmud compost for improving crop productivity and soil health. Int. J. Chem. Stud. 2017, 5, 384–389. [Google Scholar]
- Vida, C.; Bonilla, N.; de Vicente, A.; Cazorla, F.M. Microbial profiling of a suppressiveness-induced agricultural soil amended with composted almond shells. Front. Microbiol. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datt, N.; Singh, D. Enzymes in relation to soil biological properties and sustainability. In Sustainable Management of Soil and Environment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 383–406. [Google Scholar]
- Aytenew, M.; Bore, G. Effects of organic amendments on soil fertility and environmental quality: A review. J. Plant Sci. 2020, 8, 112–119. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Q.; Wang, F.; Zhang, J.; Chen, Y.; Zhang, C.; Liu, G.; Zhang, H.; Ma, C.; Zhang, J. The variation in the rhizosphere microbiome of cotton with soil type, genotype and developmental stage. Sci. Rep. 2017, 7, 3940. [Google Scholar] [CrossRef] [PubMed]
- Cúcio, C.; Engelen, A.H.; Costa, R.; Muyzer, G. Rhizosphere microbiomes of European seagrasses are selected by the plant, but are not species specific. Front. Microbiol. 2016, 7, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Vivanco, J.M.; Manter, D.K. Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Appl. Soil Ecol. 2016, 107, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; He, P.; Zhou, W.; He, X. Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol. Biochem. 2015, 80, 70–78. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; Van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef] [Green Version]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A.H. Long-term fertilization rather than plant species shapes rhizosphere and bulk soil prokaryotic communities in agroecosystems. Appl. Soil Ecol. 2020, 154, 103641. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; van Bruggen, A. Mineral and Organic Fertilizers Distinctly Affect Fungal Communities in the Crop Rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- De Corato, U. Disease-suppressive compost enhances natural soil suppressiveness against soil-borne plant pathogens: A critical review. Rhizosphere 2020, 13, 100192. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Mitchell, E.A.; Adl, S.; Bonkowski, M.; Dunthorn, M.; Ekelund, F.; Fernández, L.D.; Jousset, A.; Krashevska, V.; Singer, D. Soil protists: A fertile frontier in soil biology research. FEMS Microbiol. Rev. 2018, 42, 293–323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-B.; He, J.-Z.; Geisen, S.; Han, L.-L.; Wang, J.-T.; Shen, J.-P.; Wei, W.-X.; Fang, Y.-T.; Li, P.-P.; Zhang, L.-M. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2019, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-B.; He, J.-Z.; Quan, Z.; Wu, C.-F.; Sheng, R.; Zhang, L.-M.; Geisen, S. Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol. Biochem. 2020, 148, 107863. [Google Scholar] [CrossRef]
- Angus, J.; Gupta, V.; Pitson, G.; Good, A. Effects of banded ammonia and urea fertiliser on soil properties and the growth and yield of wheat. Crop Pasture Sci. 2014, 65, 337–352. [Google Scholar] [CrossRef]
- Asiloglu, R.; Shiroishi, K.; Suzuki, K.; Turgay, O.C.; Murase, J.; Harada, N. Protist-enhanced survival of a plant growth promoting rhizobacteria, Azospirillum sp. B510, and the growth of rice (Oryza sativa L.) plants. Appl. Soil Ecol. 2020, 154, 103599. [Google Scholar] [CrossRef]
- Asiloglu, R.; Sevilir, B.; Samuel, S.O.; Aycan, M.; Akca, M.O.; Suzuki, K.; Murase, J.; Turgay, O.C.; Harada, N. Effect of protists on rhizobacterial community composition and rice plant growth in a biochar amended soil. Biol. Fertil. Soils 2021, 57, 293–304. [Google Scholar] [CrossRef]
- Murase, J.; Frenzel, P. Selective grazing of methanotrophs by protozoa in a rice field soil. FEMS Microbiol. Ecol. 2008, 65, 408–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, J.; Frenzel, P. A methane-driven microbial food web in a wetland rice soil. Environ. Microbiol. 2007, 9, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Murase, J.; Noll, M.; Frenzel, P. Impact of protists on the activity and structure of the bacterial community in a rice field soil. Appl. Environ. Microbiol. 2006, 72, 5436–5444. [Google Scholar] [CrossRef] [Green Version]
- Herdler, S.; Kreuzer, K.; Scheu, S.; Bonkowski, M. Interactions between arbuscular mycorrhizal fungi (Glomus intraradices, Glomeromycota) and amoebae (Acanthamoeba castellanii, Protozoa) in the rhizosphere of rice (Oryza sativa). Soil Biol. Biochem. 2008, 40, 660–668. [Google Scholar] [CrossRef]
- Santos, S.S.; Schoeler, A.; Nielsen, T.K.; Hansen, L.H.; Schloter, M.; Winding, A. Land use as a driver for protist community structure in soils under agricultural use across Europe. Sci. Total Environ. 2020, 717, 137228. [Google Scholar] [CrossRef]
- Asiloglu, R.; Samuel, S.O.; Sevilir, B.; Akca, M.O.; Acar Bozkurt, P.; Suzuki, K.; Murase, J.; Turgay, O.C.; Harada, N. Biochar affects taxonomic and functional community composition of protists. Biol. Fertil. Soils 2021, 57, 15–29. [Google Scholar] [CrossRef]
- Murase, J.; Hida, A.; Ogawa, K.; Nonoyama, T.; Yoshikawa, N.; Imai, K. Impact of long-term fertilizer treatment on the microeukaryotic community structure of a rice field soil. Soil Biol. Biochem. 2015, 80, 237–243. [Google Scholar] [CrossRef]
- Asiloglu, R.; Honjo, H.; Saka, N.; Asakawa, S.; Murase, J. Community structure of microeukaryotes in a rice rhizosphere revealed by DNA-based PCR-DGGE. Soil Sci. Plant Nutr. 2015, 61, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Asiloglu, R.; Murase, J. Active community structure of microeukaryotes in a rice (Oryza sativa L.) rhizosphere revealed by RNA-based PCR-DGGE. Soil Sci. Plant Nutr. 2016, 62, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Asiloglu, R.; Shiroishi, K.; Suzuki, K.; Turgay, O.C.; Harada, N. Soil properties have more significant effects on the community composition of protists than the rhizosphere effect of rice plants in alkaline paddy field soils. Soil Biol. Biochem. 2021, 161, 108397. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, H.; Ma, M.; Bu, Y.; Shao, W.; Huang, W.; Ji, Q.; Yao, Y. An apple fruit fermentation (AFF) treatment improves the composition of the rhizosphere microbial community and growth of strawberry (Fragaria × ananassa Duch ‘Benihoppe’) seedlings. PLoS ONE 2016, 11, e0164776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Fekete, I.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérész, B. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, W.; Zhang, R.; Hang, X.; Wang, D.; Li, R.; Shen, Q. Continuous application of different organic additives can suppress tomato disease by inducing the healthy rhizospheric microbiota through alterations to the bulk soil microflora. Plant Soil 2018, 423, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Escuadra, G.M.E.; Amemiya, Y. Suppression of Fusarium wilt of spinach with compost amendments. J. Gen. Plant Pathol. 2008, 74, 267–274. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, D.; Ruan, Y.; Xue, C.; Zhang, J.; Li, R.; Shen, Q. Deep 16S rRNA pyrosequencing reveals a bacterial community associated with banana Fusarium wilt disease suppression induced by bio-organic fertilizer application. PLoS ONE 2014, 9, e98420. [Google Scholar] [CrossRef]
- Mazzola, M. Manipulation of rhizosphere bacterial communities to induce suppressive soils. J. Nematol. 2007, 39, 213. [Google Scholar]
- Gómez Expósito, R.; De Bruijn, I.; Postma, J.; Raaijmakers, J.M. Current insights into the role of rhizosphere bacteria in disease suppressive soils. Front. Microbiol. 2017, 8, 2529. [Google Scholar] [CrossRef]
- Weller, D.M.; Raaijmakers, J.M.; Gardener, B.B.M.; Thomashow, L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef] [Green Version]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease suppressive soils: New insights from the soil microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.-Y.; Han, S.; Hong, H.-J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.-K.; Crüsemann, M.; Bok Lee, Y.; Kim, J.F. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Zhang, N.; Shen, Z.; Zhu, C.; Li, R.; Salles, J.F.; Shen, Q. Rhizosphere bacteria assembly derived from fumigation and organic amendment triggers the direct and indirect suppression of tomato bacterial wilt disease. Appl. Soil Ecol. 2020, 147, 103364. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- Neeno-Eckwall, E.C.; Kinkel, L.L.; Schottel, J.L. Competition and antibiosis in the biological control of potato scab. Can. J. Microbiol. 2001, 47, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lemanceau, P.; Alabouvette, C.; Couteaudier, Y. Recherches sur la résistance des sols aux maladies. XIV. Modification du niveau de réceptivité d’un sol résistant et d’un sol sensible aux fusarioses vasculaires en réponse à des apports de fer ou de glucose. Agronomie 1988, 8, 155–162. [Google Scholar] [CrossRef]
- Couteaudier, Y.; Alabouvette, C. Quantitative comparison of Fusarium oxysporum competitiveness in relation to carbon utilization. FEMS Microbiol. Lett. 1990, 74, 261–267. [Google Scholar] [CrossRef]
- Klein, E.; Ofek, M.; Katan, J.; Minz, D.; Gamliel, A. Soil suppressiveness to Fusarium disease: Shifts in root microbiome associated with reduction of pathogen root colonization. Phytopathology 2013, 103, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Burges, A.; Epelde, L.; Garbisu, C. Impact of repeated single-metal and multi-metal pollution events on soil quality. Chemosphere 2015, 120, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qiao, M.; Wang, F.-H.; Zhu, Y.-G. Use of commercial organic fertilizer increases the abundance of antibiotic resistance genes and antibiotics in soil. Environ. Sci. Pollut. Res. 2017, 24, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Shuaib, M.; Azam, N.; Bahadur, S.; Romman, M.; Yu, Q.; Xuexiu, C. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microb. Pathog. 2021, 150, 104713. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wang, H.; Cai, L.; Yu, Y. Prevalence of antibiotic resistance genes and bacterial pathogens in long-term manured greenhouse soils as revealed by metagenomic survey. Environ. Sci. Technol. 2015, 49, 1095–1104. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T. Pathogenic bacteria in sewage treatment plants as revealed by 454 pyrosequencing. Environ. Sci. Technol. 2011, 45, 7173–7179. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Standards for the Use and Disposal of Sewage Sludge: Final Rules, Federal Register, Part II; Environmental Protection Agency: Washington, DC, USA, 1993; pp. 9247–9415.
- Shrivas, V.L.; Singh, U.; Weisskopf, L.; Hariprasad, P.; Sharma, S. Effect of Organic Farming on Structural and Functional Diversity of Soil Microbiome: Benefits and Risks. In Plant Biotic Interactions; Springer: Berlin/Heidelberg, Germany, 2019; p. 129. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O. Below-ground-above-ground plant-microbial interactions: Focusing on soybean, rhizobacteria and mycorrhizal fungi. Open Microbiol. J. 2018, 12, 261. [Google Scholar] [CrossRef]
- Yan, Y.; Kuramae, E.E.; de Hollander, M.; Klinkhamer, P.G.; van Veen, J.A. Functional traits dominate the diversity-related selection of bacterial communities in the rhizosphere. ISME J. 2017, 11, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic amendments, beneficial microbes, and soil microbiota: Toward a unified framework for disease suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Ashworth, A.; DeBruyn, J.; Allen, F.; Radosevich, M.; Owens, P. Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Biol. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Riegel, C.; Noe, J. Chicken litter soil amendment effects on soilborne microbes and Meloidogyne incognita on cotton. Plant Dis. 2000, 84, 1275–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Li, G.; Cotner, J.B.; Wei, L.; Wang, Y.; Pan, T.; Ding, K. Long-term organic fertilization changes soil active bacterial composition and multifunctionality: RNA-based bacterial community and qPCR-based SmartChip analysis. J. Soils Sed. 2021, 21, 799–809. [Google Scholar] [CrossRef]
- Suleiman, A.K.A.; Harkes, P.; Van Den Elsen, S.; Holterman, M.; Korthals, G.W.; Helder, J.; Kuramae, E.E. Organic amendment strengthens interkingdom associations in the soil and rhizosphere of barley (Hordeum vulgare). Sci. Total Environ. 2019, 695, 133885. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Das, S.; Jeong, S.T.; Das, S.; Kim, P.J. Composted cattle manure increases microbial activity and soil fertility more than composted swine manure in a submerged rice paddy. Front. Microbiol. 2017, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, J.; Liang, H.; Huang, J.; Chen, Z.; Nie, Y.; Wang, C.; Wang, Y. Manipulation of the rhizosphere microbial community through application of a new bio-organic fertilizer improves watermelon quality and health. PLoS ONE 2018, 13, e0192967. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. Effects of inorganic and organic treatments on the microbial community of maize rhizosphere by a shotgun metagenomics approach. Ann. Microbiol. 2020, 70, 49. [Google Scholar] [CrossRef]
- Harkes, P.; Suleiman, A.K.; van den Elsen, S.J.; de Haan, J.J.; Holterman, M.; Kuramae, E.E.; Helder, J. Conventional and organic soil management as divergent drivers of resident and active fractions of major soil food web constituents. Sci. Rep. 2019, 9, 13521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, K.; Wu, L.; Deng, Y.; He, Z.; Van Nostrand, J.; Robertson, P.G.; Schmidt, T.M.; Zhou, J. Functional gene differences in soil microbial communities from conventional, low-input, and organic farmlands. Appl. Environ. Microbiol. 2013, 79, 1284–1292. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.L.; Sheaffer, C.C.; Wyse, D.L.; Staley, C.; Gould, T.J.; Sadowsky, M.J. Associations between soil bacterial community structure and nutrient cycling functions in long-term organic farm soils following cover crop and organic fertilizer amendment. Sci. Total Environ. 2016, 566, 949–959. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Lennon, J.T. The generation and maintenance of diversity in microbial communities. Am. J. Bot. 2011, 98, 439–448. [Google Scholar] [CrossRef]
- Hoorman, J. The Role of Soil Fungus (Report No. SAG-14-11); Ohio State University: Ohio, CO, USA, 2011. [Google Scholar]
- Yaseen, T.; Burni, T.; Hussain, F. Effect of arbuscular mycorrhizal inoculation on nutrient uptake, growth and productivity of cowpea (Vigna unguiculata) varieties. Afr. J. Biotechnol. 2011, 10, 8593–8598. [Google Scholar]
- Chaverri, P.; Gazis, R.O. Perisporiopsis lateritia, a new species on decaying leaves of Hevea spp. from the Amazon basin in Peru. Mycotaxon 2010, 113, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.N. Using trichoderma species for biological control of plant pathogens in Viet Nam. J. ISSAAS (Int. Soc. Southeast Asian Agric. Sci.) 2010, 16, 17–21. [Google Scholar]
- Chen, Y.-L.; Zhang, X.; Ye, J.-S.; Han, H.-Y.; Wan, S.-Q.; Chen, B.-D. Six-year fertilization modifies the biodiversity of arbuscular mycorrhizal fungi in a temperate steppe in Inner Mongolia. Soil Biol. Biochem. 2014, 69, 371–381. [Google Scholar] [CrossRef]
- Marinho, F.; Oehl, F.; da Silva, I.R.; Coyne, D.; da Nobrega Veras, J.S.; Maia, L.C. High diversity of arbuscular mycorrhizal fungi in natural and anthropized sites of a Brazilian tropical dry forest (Caatinga). Fungal Ecol. 2019, 40, 82–91. [Google Scholar] [CrossRef]
- Polme, S.; Öpik, M.; Moora, M.; Zobel, M.; Kohout, P.; Oja, J.; Koljalg, U.; Tedersoo, L. Arbuscular mycorrhizal fungi associating with roots of Alnus and Rubus in Europe and the Middle East. Fungal Ecol. 2016, 24, 27–34. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, R.; Wang, J.; Liu, J.; Li, M.; Lin, X.; Hu, J. Long-term moderate carbon input benefited arbuscular mycorrhizal fungal community diversity and vitality in a sandy loam soil. Ecol. Indic. 2022, 136, 108679. [Google Scholar] [CrossRef]
- Posada, R.H.; Sánchez de Prager, M.; Heredia-Abarca, G.; Sieverding, E. Effects of soil physical and chemical parameters, and farm management practices on arbuscular mycorrhizal fungi communities and diversities in coffee plantations in Colombia and Mexico. Agrofor. Syst. 2018, 92, 555–574. [Google Scholar] [CrossRef]
- Frew, A. Arbuscular mycorrhizal fungal diversity increases growth and phosphorus uptake in C3 and C4 crop plants. Soil Biol. Biochem. 2019, 135, 248–250. [Google Scholar] [CrossRef]
- Liu, W.; Ma, K.; Wang, X.; Wang, Z.; Negrete-Yankelevich, S. Effects of no-tillage and biologically-based organic fertilizer on soil arbuscular mycorrhizal fungal communities in winter wheat field. Appl. Soil Ecol. 2022, 178, 104564. [Google Scholar] [CrossRef]
- Qin, H.; Lu, K.; Strong, P.; Xu, Q.; Wu, Q.; Xu, Z.; Xu, J.; Wang, H. Long-term fertilizer application effects on the soil, root arbuscular mycorrhizal fungi and community composition in rotation agriculture. Appl. Soil Ecol. 2015, 89, 35–43. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Graaff, M.-A.; Hornslein, N.; Throop, H.L.; Kardol, P.; van Diepen, L.T. Effects of agricultural intensification on soil biodiversity and implications for ecosystem functioning: A meta-analysis. Adv. Agron. 2019, 155, 1–44. [Google Scholar]
- Liu, Y.; Johnson, N.C.; Mao, L.; Shi, G.; Jiang, S.; Ma, X.; Du, G.; An, L.; Feng, H. Phylogenetic structure of arbuscular mycorrhizal community shifts in response to increasing soil fertility. Soil Biol. Biochem. 2015, 89, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Van der Gast, C.J.; Gosling, P.; Tiwari, B.; Bending, G.D. Spatial scaling of arbuscular mycorrhizal fungal diversity is affected by farming practice. Environ. Microbiol. 2011, 13, 241–249. [Google Scholar] [CrossRef]
- Manoharan, L.; Rosenstock, N.P.; Williams, A.; Hedlund, K. Agricultural management practices influence AMF diversity and community composition with cascading effects on plant productivity. Appl. Soil Ecol. 2017, 115, 53–59. [Google Scholar] [CrossRef]
- Gottshall, C.B.; Cooper, M.; Emery, S.M. Activity, diversity and function of arbuscular mycorrhizae vary with changes in agricultural management intensity. Agric. Ecosyst. Environ. 2017, 241, 142–149. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef] [Green Version]
- Hodge, A. Arbuscular mycorrhizal fungi influence decomposition of, but not plant nutrient capture from, glycine patches in soil. New Phytol. 2001, 151, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wang, T.; Liu, Y.; Jia, S.; Gao, Y.; Liu, S. Effects of Different Fertilizer Treatments on Rhizosphere Soil Microbiome Composition and Functions. Land 2020, 9, 329. [Google Scholar] [CrossRef]
- Hruby, C.E.; Soupir, M.L.; Moorman, T.B.; Pederson, C.; Kanwar, R. Salmonella and fecal indicator bacteria survival in soils amended with poultry manure. Water Air Soil Pollut. 2018, 229, 32. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; He, Z.; Powell, C.A.; Stoffella, P.J. Survival of Escherichia coli in soil with modified microbial community composition. Soil Biol. Biochem. 2011, 43, 1591–1599. [Google Scholar] [CrossRef]
- Semenov, M.V.; Krasnov, G.S.; Semenov, V.M.; Ksenofontova, N.; Zinyakova, N.B.; van Bruggen, A.H. Does fresh farmyard manure introduce surviving microbes into soil or activate soil-borne microbiota? J. Environ. Manag. 2021, 294, 113018. [Google Scholar] [CrossRef]
- Semenov, A.M.; Kuprianov, A.A.; Van Bruggen, A.H. Transfer of enteric pathogens to successive habitats as part of microbial cycles. Microb. Ecol. 2010, 60, 239–249. [Google Scholar] [CrossRef]
- Franz, E.; Semenov, A.V.; Termorshuizen, A.J.; De Vos, O.; Bokhorst, J.G.; Van Bruggen, A.H. Manure-amended soil characteristics affecting the survival of E. coli O157: H7 in 36 Dutch soils. Environ. Microbiol. 2008, 10, 313–327. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, L.; Fang, L.; Su, Y.; Zhu, W. Responses in colonic microbial community and gene expression of pigs to a long-term high resistant starch diet. Front. Microbiol. 2015, 6, 877. [Google Scholar] [CrossRef] [Green Version]
- Rieke, E.L.; Soupir, M.L.; Moorman, T.B.; Yang, F.; Howe, A.C. Temporal dynamics of bacterial communities in soil and leachate water after swine manure application. Front. Microbiol. 2018, 9, 3197. [Google Scholar] [CrossRef]
- Pandey, P.; Chiu, C.; Miao, M.; Wang, Y.; Settles, M.; Del Rio, N.S.; Castillo, A.; Souza, A.; Pereira, R.; Jeannotte, R. 16S rRNA analysis of diversity of manure microbial community in dairy farm environment. PLoS ONE 2018, 13, e0190126. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.R.; Morris, J.G., Jr. One Health-Cycling of diverse microbial communities as a connecting force for soil, plant, animal, human and ecosystem health. Sci. Total Environ. 2019, 664, 927–937. [Google Scholar] [CrossRef]
- Suleiman, A.K.; Gonzatto, R.; Aita, C.; Lupatini, M.; Jacques, R.J.; Kuramae, E.E.; Antoniolli, Z.I.; Roesch, L.F. Temporal variability of soil microbial communities after application of dicyandiamide-treated swine slurry and mineral fertilizers. Soil Biol. Biochem. 2016, 97, 71–82. [Google Scholar] [CrossRef]
- Lourenço, K.S.; Suleiman, A.K.; Pijl, A.; Van Veen, J.; Cantarella, H.; Kuramae, E. Resilience of the resident soil microbiome to organic and inorganic amendment disturbances and to temporary bacterial invasion. Microbiome 2018, 6, 142. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Mengual, C.; Schoebitz, M.; Azcón, R.; Roldán, A. Microbial inoculants and organic amendment improves plant establishment and soil rehabilitation under semiarid conditions. J. Environ. Manag. 2014, 134, 1–7. [Google Scholar] [CrossRef]
- Caravaca, F.; Figueroa, D.; Barea, J.; Azcon-Aguilar, C.; Roldan, A. Effect of mycorrhizal inoculation on nutrient acquisition, gas exchange, and nitrate reductase activity of two Mediterranean-autochthonous shrub species under drought stress. J. Plant Nutr. 2004, 27, 57–74. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Glick, B.R., Ed.; Springer: Cham, Switzerland, 2020; p. 383. [Google Scholar]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Chandra, S.; Askari, K.; Kumari, M. Optimization of indole acetic acid production by isolated bacteria from Stevia rebaudiana rhizosphere and its effects on plant growth. J. Genet. Eng. Biotechnol. 2018, 16, 581–586. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, M.; Upadhyay, R. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2018, 16, 155–162. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Harikrishnan, H.; Shanmugaiah, V.; Balasubramanian, N. Optimization for production of Indole acetic acid (IAA) by plant growth promoting Streptomyces sp VSMGT1014 isolated from rice rhizosphere. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 158–171. [Google Scholar]
- Reetha, S.; Bhuvaneswari, G.; Thamizhiniyan, P.; Mycin, T.R. Isolation of indole acetic acid (IAA) producing rhizobacteria of Pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allim cepa L.). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 568–574. [Google Scholar]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen-fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef]
- Shokri, D.; Emtiazi, G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr. Microbiol. 2010, 61, 217–225. [Google Scholar] [CrossRef]
- Deng, S.; Wipf, H.M.-L.; Pierroz, G.; Raab, T.K.; Khanna, R.; Coleman-Derr, D. A plant growth-promoting microbial soil amendment dynamically alters the strawberry root bacterial microbiome. Sci. Rep. 2019, 9, 17677. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, C.; Gao, W.; Xue, C.; Guo, Z.; Jiang, L.; Li, F.; Liu, Y. Impact of biochar amendment on the abundance and structure of diazotrophic community in an alkaline soil. Sci. Total Environ. 2019, 688, 944–951. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Sugiyama, A. The soybean rhizosphere: Metabolites, microbes, and beyond—A review. J. Adv. Res. 2019, 19, 67–73. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Yang, F.; Meng, J.; Chen, W.; Li, X. Influence of biochar application on potassium-solubilizing Bacillus mucilaginosus as potential biofertilizer. Prep. Biochem. Biotechnol. 2017, 47, 32–37. [Google Scholar] [CrossRef]
- Walpola, B.; Hettiarachchi, R. Organic manure amended with phosphate solubilizing bacteria on soil phosphorous availability. J. Agric. Sci. 2018, 15, 142–153. [Google Scholar] [CrossRef]
- Cherni, M.; Ferjani, R.; Mapelli, F.; Boudabous, A.; Borin, S.; Ouzari, H.-I. Soil parameters drive the diversity of Citrus sinensis rhizosphere microbiota which exhibits a potential in plant drought stress alleviation. Appl. Soil Ecol. 2019, 135, 182–193. [Google Scholar] [CrossRef]
- Gomez-Ramirez, L.F.; Uribe-Velez, D. Phosphorus solubilizing and mineralizing Bacillus spp. contribute to rice growth promotion using soil amended with rice straw. Curr. Microbiol. 2021, 78, 932–943. [Google Scholar] [CrossRef]
- Watson, T.T.; Nelson, L.M.; Neilsen, D.; Neilsen, G.H.; Forge, T.A. Soil amendments influence Pratylenchus penetrans populations, beneficial rhizosphere microorganisms, and growth of newly planted sweet cherry. Appl. Soil Ecol. 2017, 117, 212–220. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Złoch, M.; Thiem, D.; Gadzała-Kopciuch, R.; Hrynkiewicz, K. Synthesis of siderophores by plant-associated metallotolerant bacteria under exposure to Cd2+. Chemosphere 2016, 156, 312–325. [Google Scholar] [CrossRef]
- Pawar, S.; Chaudhari, A.; Prabha, R.; Shukla, R.; Singh, D.P. Microbial pyrrolnitrin: Natural metabolite with immense practical utility. Biomolecules 2019, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, P.B.; Beneduzi, A.; de Souza, R.; Schoenfeld, R.; Vargas, L.K.; Passaglia, L.M. The effects of different fertilization conditions on bacterial plant growth promoting traits: Guidelines for directed bacterial prospection and testing. Plant Soil 2013, 368, 267–280. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ververidis, P.; John, P. Complete recovery in vitro of ethylene-forming enzyme activity. Phytochemistry 1991, 30, 725–727. [Google Scholar] [CrossRef]
- Spence, C.; Bais, H. Role of plant growth regulators as chemical signals in plant–microbe interactions: A double edged sword. Curr. Opin. Plant Biol. 2015, 27, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Trivellini, A.; Fatma, M.; Masood, A.; Francini, A.; Iqbal, N.; Ferrante, A.; Khan, N.A. Role of ethylene in responses of plants to nitrogen availability. Front. Plant Sci. 2015, 6, 927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Wang, J.; Feng, X.; Lin, L.; Zhao, Y.; Jiang, W. Effects of 1-MCP and exogenous ethylene on fruit ripening and antioxidants in stored mango. Plant Growth Regul. 2009, 57, 185–192. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. New Perspect. Approaches Plant Growth-Promot. Rhizobact. Res. 2007, 329–339. [Google Scholar] [CrossRef]
- Shah, S.; Li, J.; Moffatt, B.A.; Glick, B.R. Isolation and characterization of ACC deaminase genes from two different plant growth-promoting rhizobacteria. Can. J. Microbiol. 1998, 44, 833–843. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Danish, S.; Zafar-ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Khalid, M.; Jones, D.; Ahmad, R.; Zahir, Z. Relative efficacy of Pseudomonas spp., containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of organic fertilizer. Pak. J. Bot. 2008, 40, 1243–1251. [Google Scholar]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Nandi, M.; Selin, C.; Brassinga, A.K.C.; Belmonte, M.F.; Fernando, W.D.; Loewen, P.C.; De Kievit, T.R. Pyrrolnitrin and hydrogen cyanide production by Pseudomonas chlororaphis strain PA23 exhibits nematicidal and repellent activity against Caenorhabditis elegans. PLoS ONE 2015, 10, e0123184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugtenberg, B.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Y.; Liang, H.; Huang, J.; Chen, Z.; Nie, Y. The rhizosphere microbial community response to a bio-organic fertilizer: Finding the mechanisms behind the suppression of watermelon Fusarium wilt disease. Acta Physiol. Plant. 2018, 40, 17. [Google Scholar] [CrossRef]
- Lutz, S.; Thuerig, B.; Oberhaensli, T.; Mayerhofer, J.; Fuchs, J.G.; Widmer, F.; Freimoser, F.M.; Ahrens, C.H. Harnessing the microbiomes of suppressive composts for plant protection: From metagenomes to beneficial microorganisms and reliable diagnostics. Front. Microbiol. 2020, 11, 1810. [Google Scholar] [CrossRef]
- Yu, D.; Sinkkonen, A.; Hui, N.; Kurola, J.M.; Kukkonen, S.; Parikka, P.; Vestberg, M.; Romantschuk, M. Molecular profile of microbiota of Finnish commercial compost suppressive against Pythium disease on cucumber plants. Appl. Soil Ecol. 2015, 92, 47–53. [Google Scholar] [CrossRef]
- Oberhaensli, T.; Hofer, V.; Tamm, L.; Fuchs, J.; Koller, M.; Herforth-Rahmé, J.; Maurhofer, M.; Thuerig, B. Aeromonas media in compost amendments contributes to suppression of Pythium ultimum in cress. In Proceedings of the III International Symposium on Organic Greenhouse Horticulture 1164, Izmir, Turkey, 11–14 April 2016; pp. 353–360. [Google Scholar]
- Tsolakidou, M.-D.; Stringlis, I.A.; Fanega-Sleziak, N.; Papageorgiou, S.; Tsalakou, A.; Pantelides, I.S. Rhizosphere-enriched microbes as a pool to design synthetic communities for reproducible beneficial outputs. FEMS Microbiol. Ecol. 2019, 95, fiz138. [Google Scholar] [CrossRef]
- Du, N.; Shi, L.; Yuan, Y.; Sun, J.; Shu, S.; Guo, S. Isolation of a potential biocontrol agent Paenibacillus polymyxa NSY50 from vinegar waste compost and its induction of host defense responses against Fusarium wilt of cucumber. Microbiol. Res. 2017, 202, 1–10. [Google Scholar] [CrossRef]
- Chin, C.F.S.; Furuya, Y.; Zainudin, M.H.M.; Ramli, N.; Hassan, M.A.; Tashiro, Y.; Sakai, K. Novel multifunctional plant growth–promoting bacteria in co-compost of palm oil industry waste. J. Biosci. Bioeng. 2017, 124, 506–513. [Google Scholar] [CrossRef]
- Cuesta, G.; García-de-la-Fuente, R.; Abad, M.; Fornes, F. Isolation and identification of actinomycetes from a compost-amended soil with potential as biocontrol agents. J. Environ. Manag. 2012, 95, S280–S284. [Google Scholar] [CrossRef] [PubMed]
- Scotti, R.; Mitchell, A.L.; Pane, C.; Finn, R.D.; Zaccardelli, M. Microbiota characterization of agricultural green waste-based suppressive composts using omics and classic approaches. Agriculture 2020, 10, 61. [Google Scholar] [CrossRef]
- Achari, G.A.; Ramesh, R. Characterization of quorum quenching enzymes from endophytic and rhizosphere colonizing bacteria. Biocatal. Agric. Biotechnol. 2018, 13, 20–24. [Google Scholar] [CrossRef]
- Huang, P.-M.; Wang, M.-K.; Chiu, C.-Y. Soil mineral–organic matter–microbe interactions: Impacts on biogeochemical processes and biodiversity in soils. Pedobiologia 2005, 49, 609–635. [Google Scholar] [CrossRef]
- Różyło, K.; Bohacz, J. Microbial and enzyme analysis of soil after the agricultural utilization of biogas digestate and mineral mining waste. Int. J. Environ. Sci. Technol. 2020, 17, 1051–1062. [Google Scholar] [CrossRef] [Green Version]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [CrossRef]
- Giacometti, C.; Cavani, L.; Baldoni, G.; Ciavatta, C.; Marzadori, C.; Kandeler, E. Microplate-scale fluorometric soil enzyme assays as tools to assess soil quality in a long-term agricultural field experiment. Appl. Soil Ecol. 2014, 75, 80–85. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Song, G.C.; Ryu, C.-M. Two volatile organic compounds trigger plant self-defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Tang, J.; Karuppiah, V.; Li, Y.; Xu, N.; Chen, J. Co-culture of Trichoderma atroviride SG3403 and Bacillus subtilis 22 improves the production of antifungal secondary metabolites. Biol. Control 2020, 140, 104122. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Marczak, M.; Mazur, A.; Koper, P.; Żebracki, K.; Skorupska, A. Synthesis of rhizobial exopolysaccharides and their importance for symbiosis with legume plants. Genes 2017, 8, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janczarek, M.; Rachwał, K.; Cieśla, J.; Ginalska, G.; Bieganowski, A. Production of exopolysaccharide by Rhizobium leguminosarum bv. trifolii and its role in bacterial attachment and surface properties. Plant Soil 2015, 388, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Qiao, C.; Penton, C.R.; Xiong, W.; Liu, C.; Wang, R.; Liu, Z.; Xu, X.; Li, R.; Shen, Q. Reshaping the rhizosphere microbiome by bio-organic amendment to enhance crop yield in a maize-cabbage rotation system. Appl. Soil Ecol. 2019, 142, 136–146. [Google Scholar] [CrossRef]
- Beardmore, R.E.; Gudelj, I.; Lipson, D.A.; Hurst, L.D. Metabolic trade-offs and the maintenance of the fittest and the flattest. Nature 2011, 472, 342–346. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, S.; Liu, X.; Jiang, Q.; Ding, W. Soil acidification amendments change the rhizosphere bacterial community of tobacco in a bacterial wilt affected field. Appl. Microbiol. Biotechnol. 2018, 102, 9781–9791. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yu, Z.; Sun, Y. Effects of oyster shell soil amendmenton fruit auality and soil chemical properties in greenhouse tomato acidic soils. Agric. Sci. Technol. 2016, 17, 2096. [Google Scholar]
- Bakker, P.A.; Berendsen, R.L.; Doornbos, R.F.; Wintermans, P.C.; Pieterse, C.M. The rhizosphere revisited: Root microbiomics. Front. Plant Sci. 2013, 4, 165. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [Green Version]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Poudel, R.; Jumpponen, A.; Schlatter, D.C.; Paulitz, T.; Gardener, B.M.; Kinkel, L.L.; Garrett, K. Microbiome networks: A systems framework for identifying candidate microbial assemblages for disease management. Phytopathology 2016, 106, 1083–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Piqueres, A.; Edel-Hermann, V.; Alabouvette, C.; Steinberg, C. Response of soil microbial communities to compost amendments. Soil Biol. Biochem. 2006, 38, 460–470. [Google Scholar] [CrossRef]
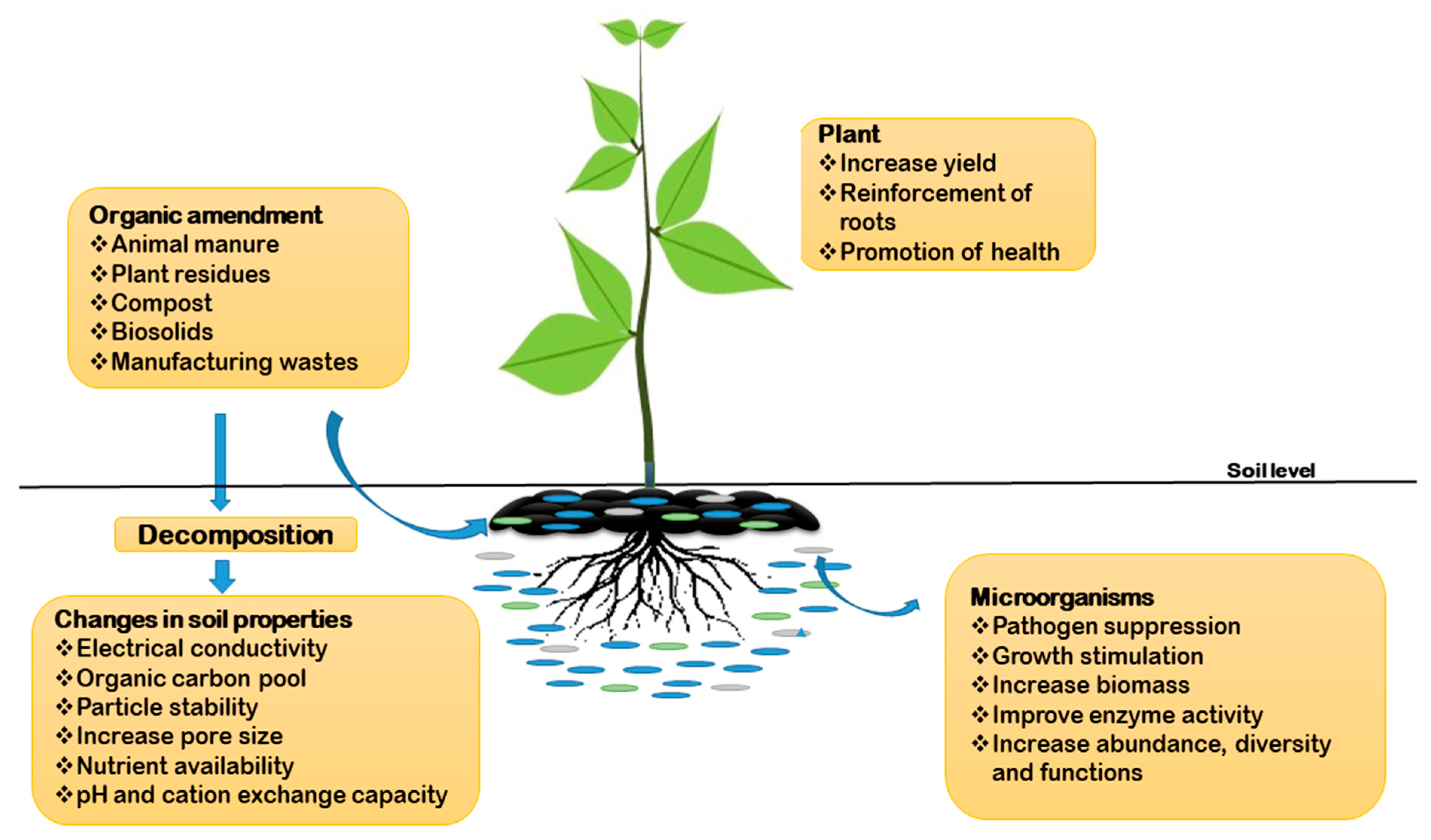
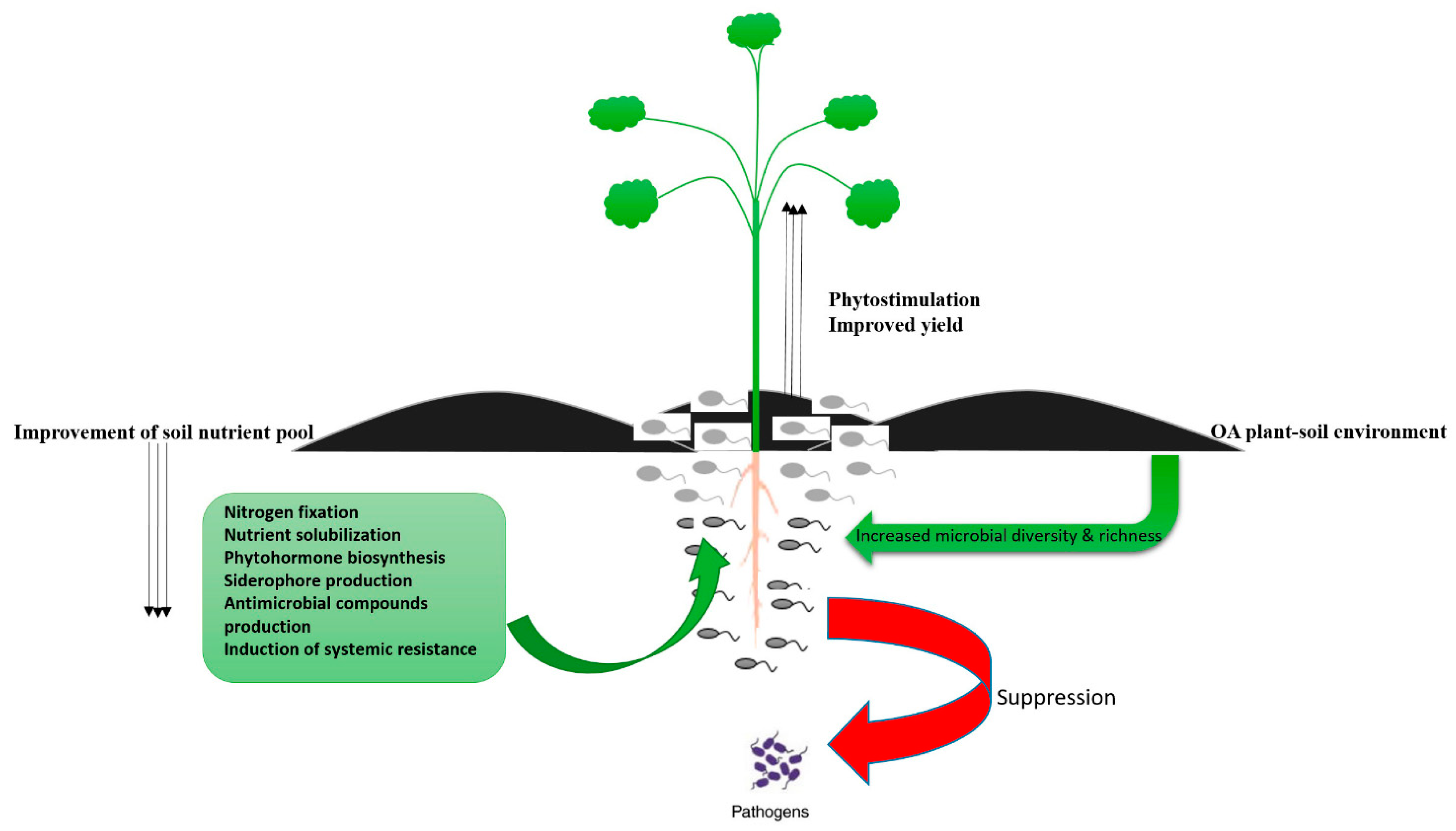
| Organic Amendment | Plant | Impact on Microbial Structure | Reference |
|---|---|---|---|
| Pelletized spent mushroom substrate and biochar | Hordeum vulgare | The dominant phyla in the rhizosphere were Proteobacteria, Acidobacteria, Actinobacteria, Gemmatimonadetes, Chloroflexi, and Bacteroidetes. An increase in abundance of Acidobacteria, whereas a decrease in the abundances of Actinobacteria, Chloroflexi, and Bacteroidetes were observed. | Obermeier, Minarsch, Durai Raj, Rineau and Schröder [28] |
| Mixtures of soybean oil cake, cotton cake, and wheat straw | Triticum aestivum and Zea mays |
Proteobacteria, Firmicutes, Acidobacteria, Actinobacteria, and Cyanobacteria were the most dominant. There was a six-fold increase in the abundance of Firmicutes. | Su, et al. [118] |
| Cow manure | Hordeum vulgare | There was an increase in the abundance of the phyla Zygomycota and Glomeromycota, whereas the phyla Ascomycota (order Chaetothyriales), Deinococcus-Thermus, and Actinobacteria decreased significantly. | Suleiman, et al. [119] |
| Corn straw and pig manure | Zea mays | The relative abundance of most Gram-negative bacteria and saprotrophic fungi increased. Ktedonobacteria, Acidobacteria, Solibacteres, and Alphaproteobacteria increased with organic amendments. The fungal communities were predominantly composed of Hypocreales, Sordariales, and Eurotiales. Organic amendments significantly increased Sordariales but decreased Hypocreales. | Wang, et al. [120] |
| Composted cattle manure and swine manure | rice paddy | A significant increase in Alphaproteobacteria, Betaproteobacteria, Firmicutes, and Bacteroidetes and decrease in Actinobacteria and Acidobacteria in composted cattle manure were observed, whereas a significant increase in Gammaproteobacteria, Bacteroidetes, and Gemmatimonadetes and decrease in Acidobacteria were observed in composted swine manure. | Das, et al. [121] |
| Cow and chicken manure compost | Watermelon |
Proteobacteria, Firmicutes, Planctomycetes, Actinobacteria, Bacteroidetes, Gemmatimonadetes, Acidobacteria, Chloroflexi, Verrucomicrobia, and Nitrospirae were the dominant phyla. There was a decrease in the abundance of Proteobacteria and Verrucomicrobia, whereas Firmicutes, Planctomycetes, Actinobacteria, and Bacteroidetes increased. | Zhao, et al. [122] |
| Compost | Zea mays | There was an increase in the abundance of Glomeromycota, Ascomycota, and Basidiomycota. | Enebe and Babalola [123] |
| Farmyard manure and cow slurry | Hordeum vulgare | Glomeromycetes, Cantharellales, Saccharomycetales, Trichosporonales, Agaricales, and Onygenales were indicators of OA, whereas Paraglomerales, Eurotiales, Neocallimastigales, and Chaetothyriales were observed in the control. | Harkes, et al. [124] |
| Bacteria | Mechanisms | Effects | References |
|---|---|---|---|
| Acintobacter spp. | Possible production of antibiotics | Suppression of Pythium spp. causing damping-off of seedlings in cucumber | Yu et al. [205] |
| Aeromonas media | Possible production of antibiotics | Control of Pythium ultimum that causes damping-off disease in cress | Oberhaensli et al. [206] |
| Bacillus amyloliquefaciens JDF35 | Nitrogen fixation, phosphate solubilization, and enzyme synthesis | Control of Fusarium oxysporum that causes Fusarium wilt in watermelon | Zhao, Wang, Liang, Huang, Chen, and Nie [203] |
| Bacillus amyloliquefaciens, B. licheniformis, B. subtilis | Enzyme synthesis, production of secondary metabolites, indole acetic acid, and 1-aminoclopropane-1-carboxylate (ACC) deaminase activity | Control of Fusarium oxysporum that causes Fusarium wilt in cucumber and Verticillium dahliae that cause Verticillium wilt in tomato | Tsolakidou et al. [207,208] |
| Burkholderia spp. | Possible production of secondary metabolites | Control of Rosellinia necatrix that cause white root rot in avocado | [204] |
| Chryseobacterium spp. | Synthesis of secondary metabolites, ACC deaminase activity, and indole acetic acid | Control of Vericillium dahlia, and Fusarium oxysporum that cause Verticillium-Fusarium wilt in tomato | Tsolakidou, Stringlis, Fanega-Sleziak, Papageorgiou, Tsalakou, and Pantelides [207] |
| Enterobacter spp. | Possible production of antibiotics | Control of Pythium spp., Fusarium oxysporum, and Verticillium daliae wilt in plants | Chin et al. [209] |
| Lechevlieria spp. | Production of antibiotics | Control of Phytophtoria cinnamomi, Sclerotinia sclerotiorum, Agrobacterium tumefaciens, Pythium debaryanum, Thanatephorus cucumeri that cause stem rot in tomato | Cuesta et al. [210] |
| Ochrobacterium spp. | Production of secondary metabolites, IAA, and ACC deaminase activity | Verticillium dahlia and Fusarium oxysporum control of wilt in tomato | Tsolakidou, Stringlis, Fanega-Sleziak, Papageorgiou, Tsalakou, and Pantelides [207] |
| Paenibacillus polymyxa | Enzyme synthesis, and ACC deaminase activity | Control of Fusarium wilt caused by Fusarium oxysporum in cucumber | Du, Shi, Yuan, Sun, Shu, and Guo [208] |
| Pseudomonas spp. | - | Control of Rhizoctonia solani, Sclerotinia minor and Rosellinia necatrix white root rot in cress and avocado | Scotti et al. [211] |
| Stenotrophomonas maltophilia | Secondary metabolites production, IAA, and ACC deaminase activities | Control of Verticillium dahlia and Fusarium oxysporum that cause wilting in tomato | Tsolakidou, Stringlis, Fanega-Sleziak, Papageorgiou, Tsalakou, and Pantelides [207] |
| Streptomyces lusitarus, S. aureoverticillatus, S. griseoruber, S. albogriseolus, S. variegatus | ACC deaminase activity, and antibiotic production | Suppresses activity of Phytophtoria cinnamomi, Sclerotinia sclerotiorum, Agrobacterium tumefaciens, Pythium debaryanum, and Thanatephorus cucumeris in plants | [204], Cuesta, García-de-la-Fuente, Abad, and Fornes [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayangbenro, A.S.; Chukwuneme, C.F.; Ayilara, M.S.; Kutu, F.R.; Khantsi, M.; Adeleke, B.S.; Glick, B.R.; Babalola, O.O. Harnessing the Rhizosphere Soil Microbiome of Organically Amended Soil for Plant Productivity. Agronomy 2022, 12, 3179. https://doi.org/10.3390/agronomy12123179
Ayangbenro AS, Chukwuneme CF, Ayilara MS, Kutu FR, Khantsi M, Adeleke BS, Glick BR, Babalola OO. Harnessing the Rhizosphere Soil Microbiome of Organically Amended Soil for Plant Productivity. Agronomy. 2022; 12(12):3179. https://doi.org/10.3390/agronomy12123179
Chicago/Turabian StyleAyangbenro, Ayansina Segun, Chinenyenwa Fortune Chukwuneme, Modupe Stella Ayilara, Funso Raphael Kutu, Motlagomang Khantsi, Bartholomew Saanu Adeleke, Bernard R. Glick, and Olubukola Oluranti Babalola. 2022. "Harnessing the Rhizosphere Soil Microbiome of Organically Amended Soil for Plant Productivity" Agronomy 12, no. 12: 3179. https://doi.org/10.3390/agronomy12123179
APA StyleAyangbenro, A. S., Chukwuneme, C. F., Ayilara, M. S., Kutu, F. R., Khantsi, M., Adeleke, B. S., Glick, B. R., & Babalola, O. O. (2022). Harnessing the Rhizosphere Soil Microbiome of Organically Amended Soil for Plant Productivity. Agronomy, 12(12), 3179. https://doi.org/10.3390/agronomy12123179









