Genome-Wide Characterization of HSP90 Gene Family in Chinese Pumpkin (Cucurbita moschata Duch.) and Their Expression Patterns in Response to Heat and Cold Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification of the HSP90 Genes in Cucurbita moschata
2.2. Physical and Chemical Properties and Position on Chromosomes
2.3. Construction of Phylogenetic Tree of HSP90 Proteins
2.4. Structure Analysis of HSP90 Gene in Pumpkin
2.5. Analysis of Gene Duplication and Synteny of HSP90 Genes
2.6. Prediction of Cis-Regulatory Elements (CREs) for Pumpkin HSP90 Gene Promoters
2.7. The Network of Protein-Protein Interaction
2.8. Stress Treatments
2.9. Expression Profile Analysis of CmoHSP90 Genes by Real-Time Quantitative PCR (RT-qPCR)
3. Results
3.1. Genome-Wide Identification and Chromosomal Location of HSP90 Family Genes in Pumpkin
3.2. Phylogenetic Relationship of the CmoHSP90 Proteins in Pumpkin
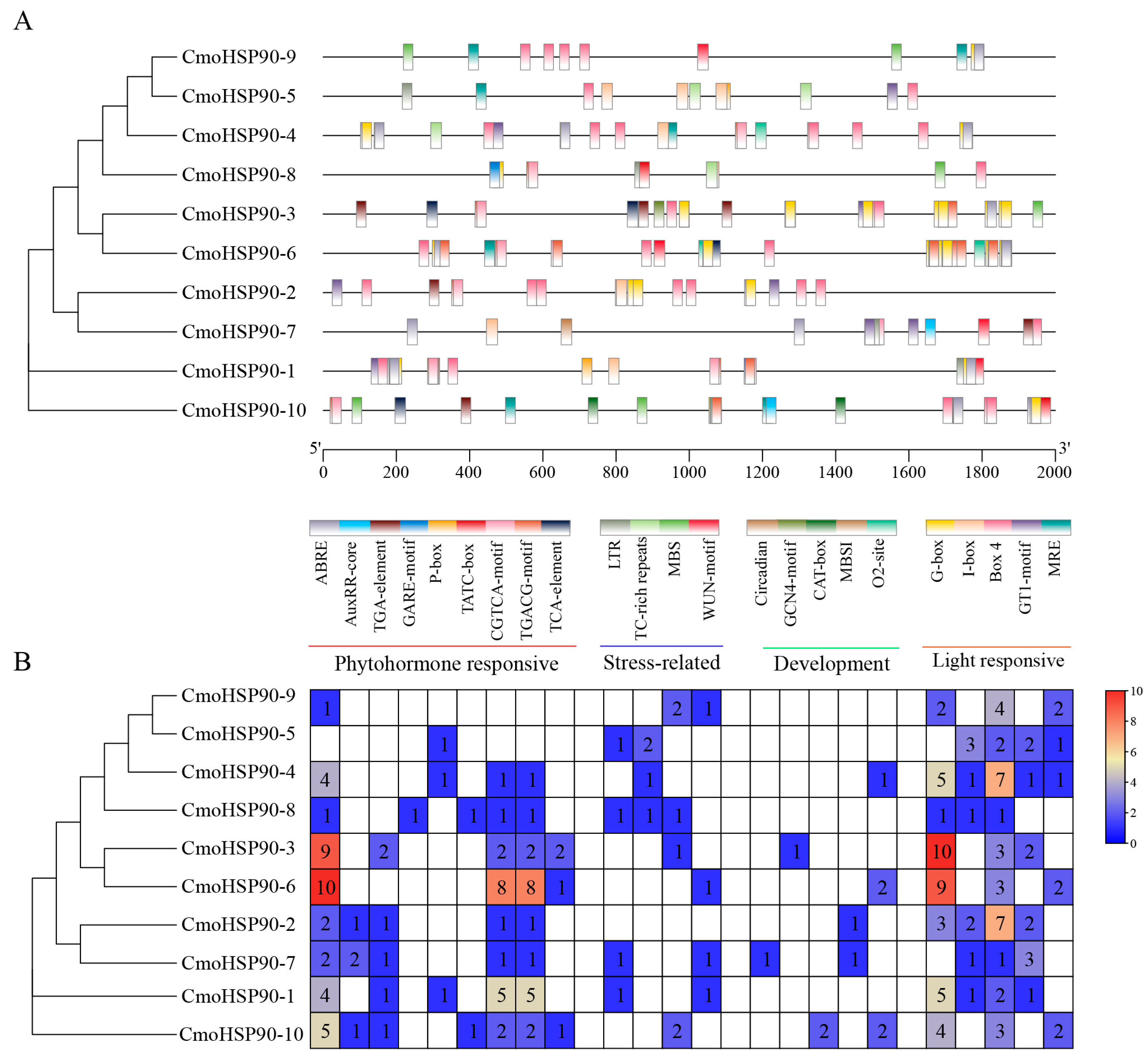
3.3. Conserved Gene Structures and Motifs of the CmoHSP90 Genes
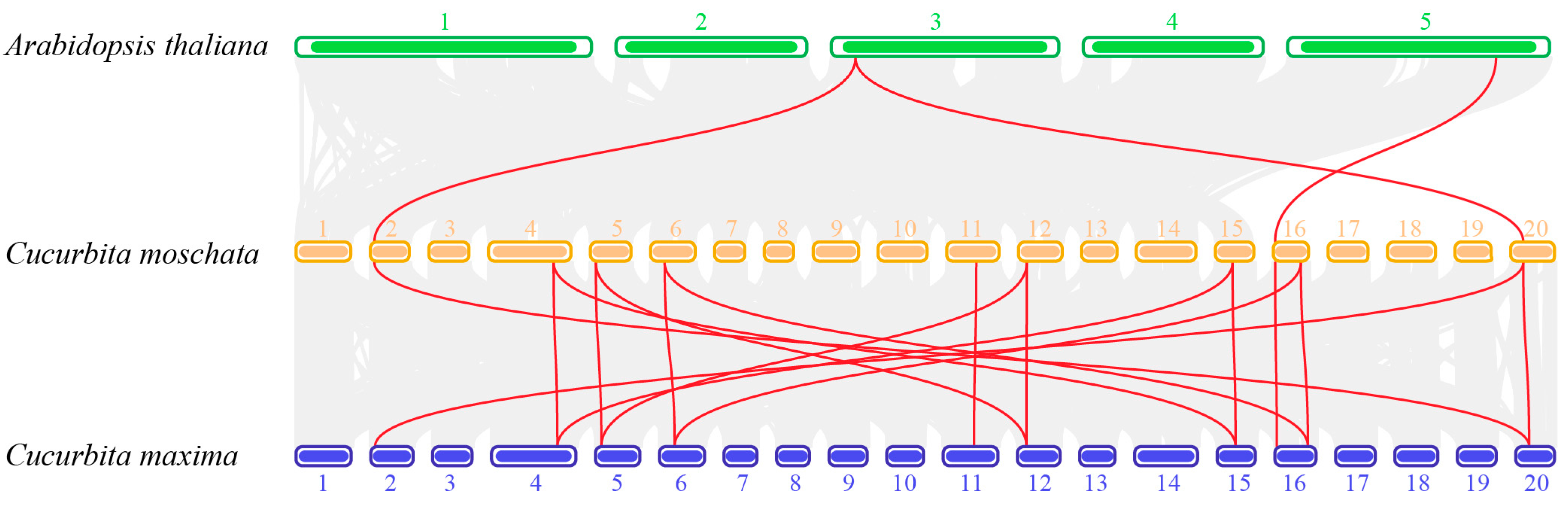
3.4. Chromosome Location and Synteny Analysis of CmoHSP90 Genes
3.5. Analysis of CmoHSP90 Promoters
3.6. Expression Profiling of CmoHSP90 Genes under Heat and Cold Stresses
3.7. Protein-Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerr, R.A. Global warming is changing the world. Science 2007, 316, 188–190. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Driedonks, N.; Xu, J.; Peters, J.L.; Park, S.; Rieu, I. Multi-level interactions between heat shock factors, heat shock proteins, and the redox system regulate acclimation to heat. Front. Plant Sci. 2015, 6, 999. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heatshock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Swindell, W.R.; Huebner, M.; Weber, A.P. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genom. 2007, 8, 125. [Google Scholar] [CrossRef]
- Johnson, J.L.; Brown, C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperon. 2009, 14, 83–94. [Google Scholar] [CrossRef]
- Sangster, T.A.; Queitsch, C. The HSP90 chaperone complex, an emerging force in plant development and phenotypic plasticity. Curr. Opin. Plant Biol. 2005, 8, 86–92. [Google Scholar] [CrossRef]
- Reddy, R.K.; Chaudhary, S.; Patil, P.; Krishna, P. The 90 kDa heat shock protein (hsp90) is expressed throughout Brassica napus seed development and germination. Plant Sci. 1998, 131, 131–137. [Google Scholar] [CrossRef]
- Wang, G.F.; Wei, X.; Fan, R.; Zhou, H.; Wang, X.; Yu, C.; Dong, L.; Dong, Z.; Wang, X.; Kang, Z.; et al. Molecular analysis of common wheat genes encoding three types of cytosolic heat shock protein 90 (Hsp90): Functional involvement of cytosolic Hsp90s in the control of wheat seedling growth and disease resistance. New Phytol. 2011, 191, 418–431. [Google Scholar] [CrossRef]
- Chen, J.; Gao, T.; Wan, S.; Zhang, Y.; Yang, J.; Yu, Y.; Wang, W. Genome-wide identification, classification and expression analysis of the HSP gene superfamily in tea plant (Camellia sinensis). Int. J. Mol. Sci. 2018, 19, 2633. [Google Scholar] [CrossRef]
- Hu, W.H.; Hu, G.C.; Han, B. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci. 2009, 176, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Kozeko, L.Y. The role of HSP90 chaperonesin stability and plasticity of ontogenesis of plants under normal and stressful conditions (Arabidopsis thaliana). Cytol. Genet. 2019, 53, 143–161. [Google Scholar] [CrossRef]
- Chaudhary, R.; Baranwal, V.K.; Kumar, R.; Sircar, D.; Chauhan, H. Genome-wide identification and expression analysis of Hsp70, Hsp90, and Hsp100 heat shock protein genes in barley under stress conditions and reproductive development. Funct. Integr. Genomic. 2019, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Li, W.H.; Wei, Z.W.; Qiao, Z.H.; Wu, Z.; Cheng, L.; Wang, Y. Proteomics analysis of alfalfa response to heat stress. PLoS ONE 2013, 8, e82725. [Google Scholar] [CrossRef]
- Prasad, B.D.; Goel, S.; Krishna, P. In silico identification of carboxylate clamp type tetratricopeptide repeat proteins in Arabidopsis and rice as putative co-chaperones of Hsp90/Hsp70. PLoS ONE 2010, 5, e12761. [Google Scholar] [CrossRef]
- Song, Z.; Pan, F.; Yang, C.; Jia, H.; Jiang, H.; He, F.; Li, N.; Lu, X.; Zhang, H. Genome-wide identification and expression analysis of HSP90 gene family in Nicotiana tabacum. BMC Genet. 2019, 20, 35. [Google Scholar] [CrossRef]
- Xu, J.; Xue, C.; Xue, D.; Zhao, J.; Gai, J.; Guo, N.; Xing, H. Overexpression of GmHsp90s, a heat shock protein 90 (Hsp90) gene family cloningfrom soybean, decrease damage of abiotic stressesin Arabidopsis thaliana. PLoS ONE 2013, 8, e69810. [Google Scholar] [CrossRef]
- Huang, Y.; Xuan, H.; Yang, C.; Guo, N.; Wang, H.; Zhao, J.; Xing, H. GmHsp90A2 is involved in soybean heat stress as a positive regulator. Plant Sci. 2019, 285, 26–33. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.H.; Seo, K.I.; Ryu, B.; Sung, Y.; Chung, T.; Deng, X.W.; Lee, J.H. Characterization of a Novel DWD protein that participates in heat stress response in Arabidopsis. Mol. Cells 2014, 37, 833–840. [Google Scholar] [CrossRef]
- Agarwal, G.; Garg, V.; Kudapa, H.; Doddamani, D.; Pazhamala, L.T.; Khan, A.W.; Thudi, M.; Lee, S.H.; Varshney, R.K. Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol. J. 2016, 14, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, W.; Hu, W.; Yan, Y.; Shi, H. The chaperone MeHSP90 recruits MeWRKY20 and MeCatalase 1 to regulate drought stress resistance in cassava. New Phytol. 2020, 226, 476–491. [Google Scholar] [CrossRef]
- Song, H.; Zhao, R.; Fan, P.; Wang, X.; Chen, X.; Li, Y.X. Overexpression of AtHsp90.2, AtHsp90.5 and AtHsp90.7 in Arabidopsis thaliana enhances plant sensitivity to salt and drought stresses. Planta 2009, 229, 955–964. [Google Scholar] [CrossRef]
- Xu, X.; Song, H.; Zhou, Z.; Shi, N.; Ying, Q.; Wang, H. Functional characterization of AtHsp90.3 in Saccharomyces cerevisiae and Arabidopsis thaliana under heat stress. Biotechnol. Lett. 2010, 32, 979–987. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, E.J.; Kim, Y.N.; Choi, C.; Lee, B.H. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr. Res. Pract. 2012, 6, 21–27. [Google Scholar] [CrossRef]
- Balkaya, A.; Ozbakir, M.; Karaagac, O. Evaluation of variation and fruit characterization of pumpkin (Cucurbita moschata Duch.) populations collected from Black Sea region. Tarim. Bilimleri. Dergisi. 2010, 16, 17–25. [Google Scholar] [CrossRef]
- Zhi, H.Y.; Yue, Q.; Chen, M.; Ma, H. Heat resistance comparison of four species and interspecific hybrids of Cucurbita. J. Shanxi Agric. Sci. 2020, 48, 327–329. (in Chinese). [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Gu, B.; Li, L.; Huang, Y.; Zhang, X. Evaluation of heat tolerance in recombination inbred lines derived from inter-specific hybrids of Cucurbita moschata Duch. × Cucurbita maximam Duch. J. Plant Genet. Res. 2020, 21, 1577–1585. (in Chinese). [Google Scholar] [CrossRef]
- Cao, H.; Wang, L.; Nawaz, M.A.; Niu, M.; Sun, J.; Xie, J.; Kong, Q.; Huang, Y.; Cheng, F.; Bie, Z. Ectopic expression of pumpkin NAC transcription factor CmNAC1 improves multiple abiotic stress tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 2052. [Google Scholar] [CrossRef]
- Hu, Y.P.; Zhang, T.T.; Liu, Y.; Li, Y.X.; Wang, M.; Zhu, B.; Liao, D.; Yun, T.; Huang, W.; Zhang, W.; et al. Pumpkin (Cucurbita moschata) HSP20 gene family identification and expression under heat stress. Front. Genet. 2021, 12, 753953. [Google Scholar] [CrossRef]
- Zhang, K.; He, S.; Sui, Y.; Gao, Q.; Jia, S.; Lu, X.; Jia, L. Genome-wide characterization of HSP90 gene family in cucumber and their potential roles in response to abiotic and biotic stresses. Front Genet. 2021, 12, 584886. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, J.; Tan, F.; Liang, C.; Zhang, X.; Ou, L.; Niran, J.; Wang, F.; Jiao, C.; Zou, X.; Chen, W. Genomewide identification and analysis of HSP90 gene family in pepper. Acta Horti. Sinica 2020, 47, 665–674. (in Chinese). [Google Scholar]
- Liu, Y.; Wan, H.; Yang, Y.; Wei, Y.; Li, Z.; Ye, Q.; Wang, R.; Ruan, M.; Yao, Z.; Zhou, G. Genome-wide identification and analysis of heat shock protein 90 in tomato. Hereditas 2014, 36, 1043–1052. (in Chinese). [Google Scholar] [CrossRef] [PubMed]
- Krishna, P.; Gloor, G. The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperon. 2001, 6, 238–246. [Google Scholar] [CrossRef]
- Babenko, V.N.; Rogozin, I.B.; Mekhedov, S.L.; Koonin, E.V. Prevalence of intron gain over intron loss in the evolution of paralogous gene families. Nucleic Acids Res. 2004, 32, 3724–3733. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.W.; Penny, D. On the incidence of intron loss and gain in paralogous gene families. Mol. Biol. Evol. 2007, 24, 1579–1581. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Maere, S.; De Bodt, S.; Raes, J.; Casneuf, T.; Van Montagu, M.; Kuiper, M.; Van de Peer, Y. Modeling gene and genome duplications in eukaryotes. Proc. Natl. Acad. Sci. USA 2005, 102, 5454–5459. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, S.; Zhang, G.; Jiao, C.; Guo, S.; Ren, Y.; Zhang, J.; Zhang, H.; Gong, G.; Jia, Z.; et al. Karyotype stability and unbiased fractionation in the paleo-allotetraploid Cucurbita genomes. Mol. Plant. 2017, 10, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.J. Molecular Cytogenetic Studies on Siraitai grosvenoorii and 15 Genera of Cucurbitaceous Plant. Ph.D. Thesis, Guangxi University, Nanning, China, 2018; pp. 74–77. (in Chinese). [Google Scholar]
- Richter, K.; Buchner, J. Hsp90: Chaperoning signal transduction. J. Cell. Physiol. 2001, 188, 281–290. [Google Scholar] [CrossRef]
- Young, J.C.; Moarefi, I.; Hartl, F.U. Hsp90: A specialized but essential protein-folding tool. J. Cell Biol. 2001, 154, 267–273. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 516. [Google Scholar] [CrossRef]
- Appiah, C.; Yang, Z.F.; He, J.; Wang, Y.; Zhou, J.; Xu, W.Z.; Nie, G.; Zhu, Y. Genome-wide identification of Hsp90 Gene family in perennial ryegrass and expression analysis under various abiotic stresses. Plants 2021, 10, 2509. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Liu, Y.; Wu, Y.; Xie, Q. The sHSP22 heat shock protein requires the ABI1 protein phosphatase to modulate polar auxin transport and downstream responses. Plant Physiol. 2018, 176, 2406–2425. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamdi, J.; Bouktila, D. Genome-wide analysis of HSP90 gene family in the Mediterranean olive (Olea europaea subsp. europaea) provides insight into structural patterns, evolution and functional diversity. Physiol. Mol. Biol. Plants 2020, 26, 2301–2318. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Betsuyaku, S.; Peart, J.; Takahashi, A.; Noel, L.; Sadanandom, A.; Casais, C.; Parker, J.; Shirasu, K. Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J. 2006, 25, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.J.; Muskett, P.; Kahn, K.; Feys, B.J.; Jones, J.D.G.; Parker, J.E. Regulatory role of SGT1 in early R gene-mediated plan defenses. Science 2002, 295, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Boter, M.; Amigues, B.; Peart, J.; Breuer, C.; Kadota, Y.; Casais, C.; Moore, G.; Kleanthous, C.; Ochsenbein, F.; Shirasu, K.; et al. Structural and functional analysis of SGT1 reveals that its interaction with HSP90 is required for the accumulation of Rx, an R protein involved in plant immunity. Plant Cell 2007, 19, 3791–3804. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Yamaguchi, K.; Nishiuchi, T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007, 58, 3373–3383. [Google Scholar] [CrossRef]
- Nishizawa-Yokoi, A.; Tainaka, H.; Yoshida, E.; Tamoi, M.; Yabuta, Y.; Shigeoka, S. The 26S proteasome function and Hsp90 activity involved in the regulation of HsfA2 expression in response to oxidative stress. Plant Cell Physiol. 2010, 51, 486–496. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef]






| Gene Name | Gene ID | Locus | Length (aa) | MW (kDa) | pI | No. of Transmembrane | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| CmoHSP90-1 | CmoCh02G002280 | Chr02: 1063366..1072967 | 567 | 64.32 | 6.24 | - | mitochondrial |
| CmoHSP90-2 | CmoCh04G022830 | Chr04: 17001131..17008245 | 793 | 89.91 | 4.89 | - | mitochondrial |
| CmoHSP90-3 | CmoCh05G003160 | Chr05: 1403562..1408218 | 816 | 93.26 | 4.92 | - | endoplasmic reticulum |
| CmoHSP90-4 | CmoCh06G007160 | Chr06: 3650095..3653148 | 700 | 80.02 | 4.97 | - | cytoplasmic, nuclear |
| CmoHSP90-5 | CmoCh11G012410 | Chr11: 7760957..7763733 | 701 | 80.22 | 4.99 | - | cytoplasmic |
| CmoHSP90-6 | CmoCh12G003530 | Chr12: 2180257..2185125 | 813 | 93.16 | 4.95 | - | endoplasmic reticulum |
| CmoHSP90-7 | CmoCh15G008820 | Chr15: 4464899..4470946 | 832 | 94.64 | 4.95 | - | mitochondrial |
| CmoHSP90-8 | CmoCh16G001050 | Chr16: 480853..483672 | 704 | 80.87 | 4.95 | - | cytoplasmic, nuclear |
| CmoHSP90-9 | CmoCh16G010310 | Chr16: 7195032..7198329 | 700 | 80.26 | 4.97 | - | cytoplasmic |
| CmoHSP90-10 | CmoCh20G006750 | Chr20: 3297642..3309582 | 865 | 97.36 | 8.22 | 2 | endoplasmic reticulum |
| Gene Name_1 | Gene Name_2 | Ka | Ks | Ka/Ks | Data (Mya) a |
|---|---|---|---|---|---|
| CmoHSP90-1 | CmoHSP90-10 | 0.449832238 | 0.967877472 | 0.464761554 | 269 |
| CmoHSP90-2 | CmoHSP90-7 | 0.034303315 | 0.328100391 | 0.104551277 | 91 |
| CmoHSP90-3 | CmoHSP90-6 | 0.03377163 | 0.395340434 | 0.085424175 | 110 |
| CmoHSP90-4 | CmoHSP90-9 | 0.014596781 | 0.548892504 | 0.026593151 | 152 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhang, T.; Wang, P.; Li, Y.; Wang, M.; Zhu, B.; Liao, D.; Yun, T.; Huang, W.; Chen, Y.; et al. Genome-Wide Characterization of HSP90 Gene Family in Chinese Pumpkin (Cucurbita moschata Duch.) and Their Expression Patterns in Response to Heat and Cold Stresses. Agronomy 2023, 13, 430. https://doi.org/10.3390/agronomy13020430
Hu Y, Zhang T, Wang P, Li Y, Wang M, Zhu B, Liao D, Yun T, Huang W, Chen Y, et al. Genome-Wide Characterization of HSP90 Gene Family in Chinese Pumpkin (Cucurbita moschata Duch.) and Their Expression Patterns in Response to Heat and Cold Stresses. Agronomy. 2023; 13(2):430. https://doi.org/10.3390/agronomy13020430
Chicago/Turabian StyleHu, Yanping, Tingting Zhang, Peng Wang, Yuxin Li, Min Wang, Baibi Zhu, Daolong Liao, Tianhai Yun, Wenfeng Huang, Yisong Chen, and et al. 2023. "Genome-Wide Characterization of HSP90 Gene Family in Chinese Pumpkin (Cucurbita moschata Duch.) and Their Expression Patterns in Response to Heat and Cold Stresses" Agronomy 13, no. 2: 430. https://doi.org/10.3390/agronomy13020430
APA StyleHu, Y., Zhang, T., Wang, P., Li, Y., Wang, M., Zhu, B., Liao, D., Yun, T., Huang, W., Chen, Y., Zhang, W., & Zhou, Y. (2023). Genome-Wide Characterization of HSP90 Gene Family in Chinese Pumpkin (Cucurbita moschata Duch.) and Their Expression Patterns in Response to Heat and Cold Stresses. Agronomy, 13(2), 430. https://doi.org/10.3390/agronomy13020430







