Soybean GmVIT1 Gene Confers Plant Tolerance to Excess Fe/Mn Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Iron Deficiency Tolerance Detection
2.3. Cloning of GmVIT1 and GmVIT1 Promoter
2.4. Subcellular Localization of GmVIT1
2.5. Gene Expression Analysis
2.6. Generation and Character Analysis of GmVIT1 Transgenic Arabidopsis
2.7. Statistical Analysis
3. Results
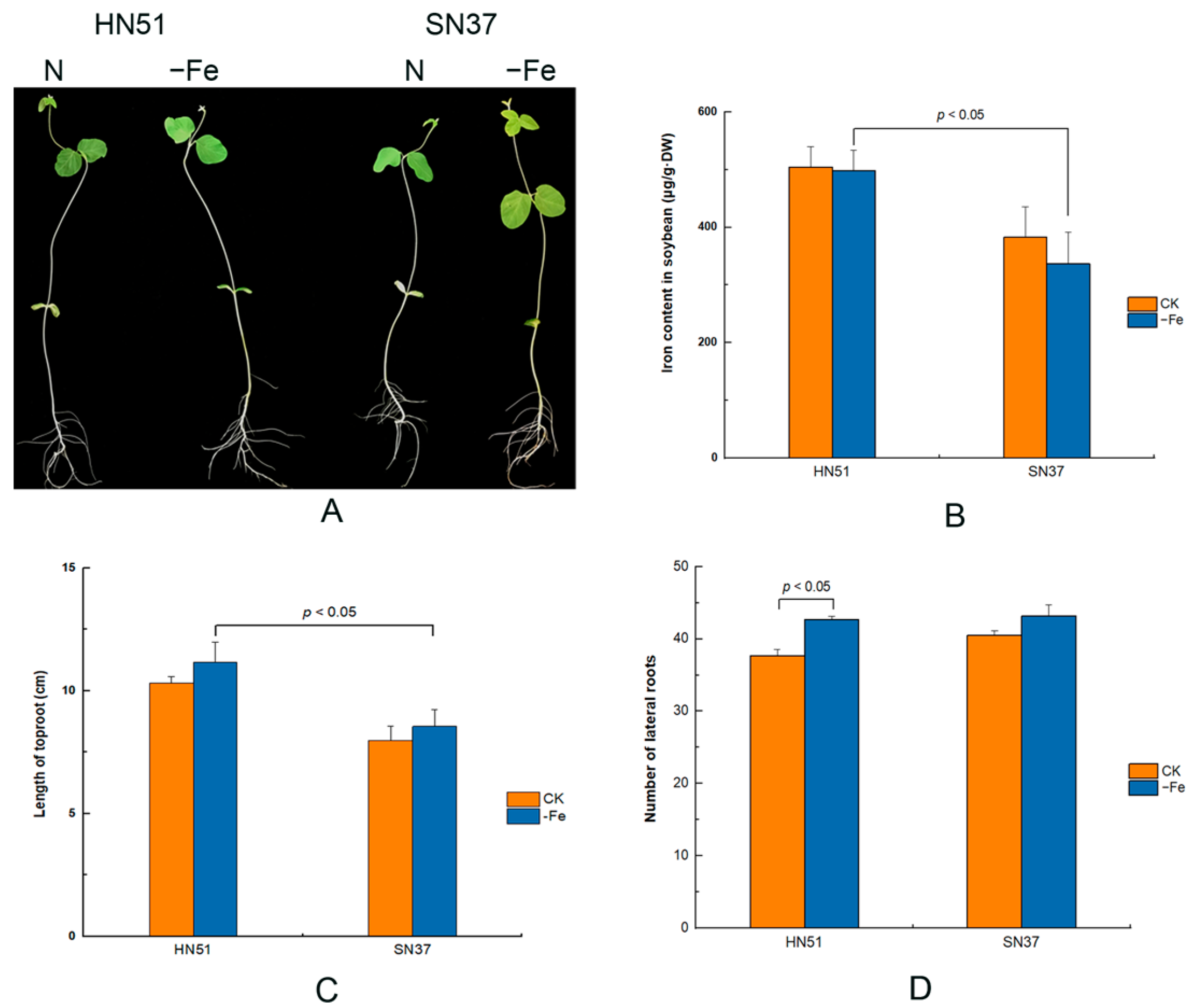
3.1. The Tolerance of HN51 and SN37 to Fe Deficiency
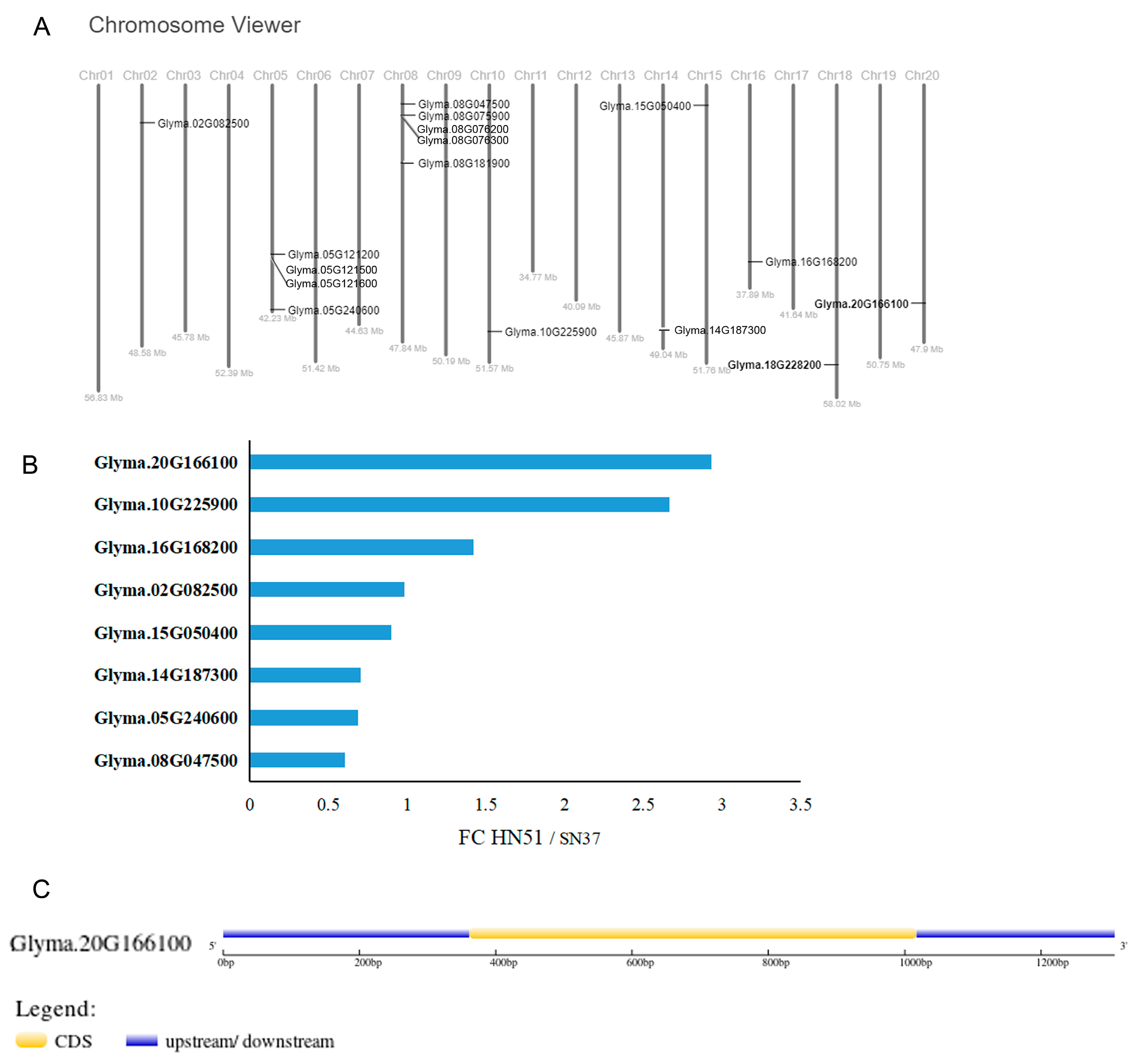
3.2. The Expression of GmVITs in Transcriptomic Analysis
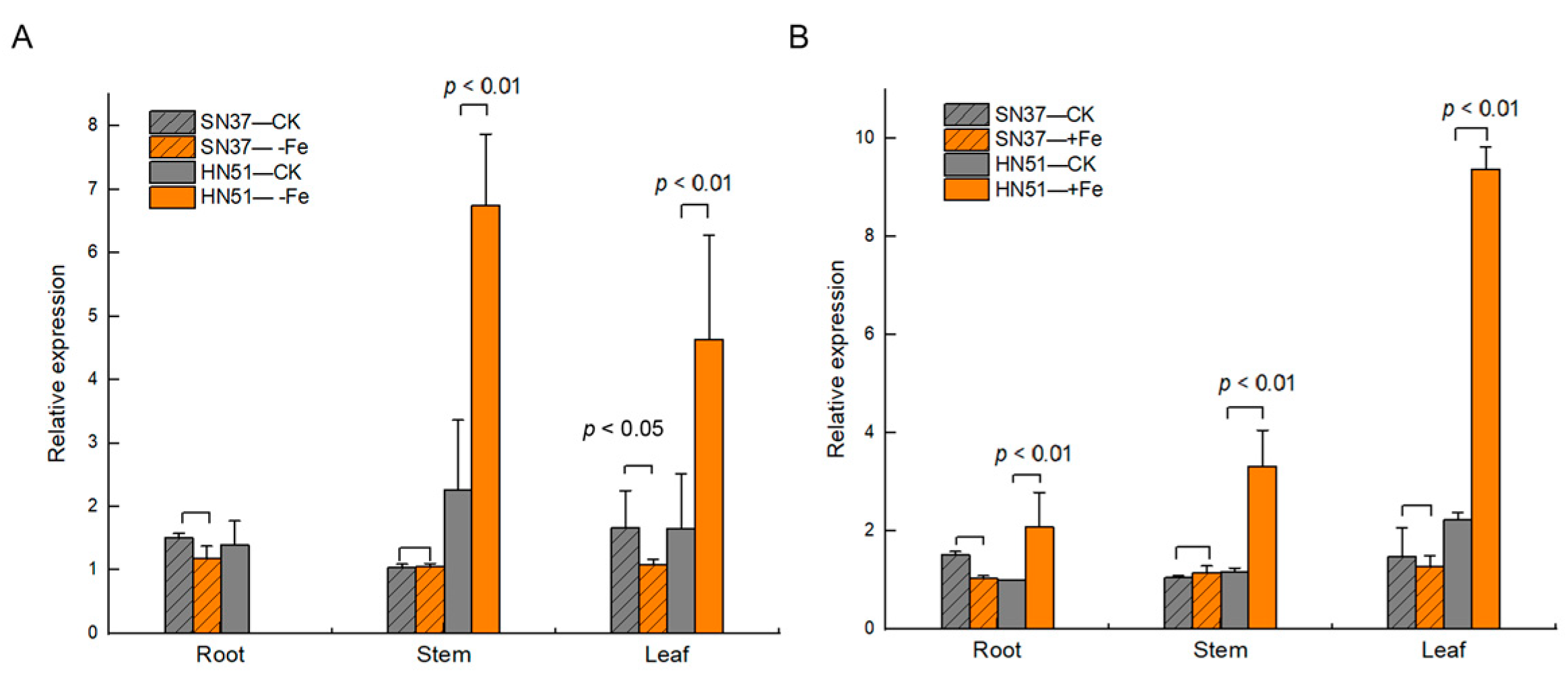
3.3. GmVIT1 Response to Iron Deficiency and Excess Iron in HN51 and SN37
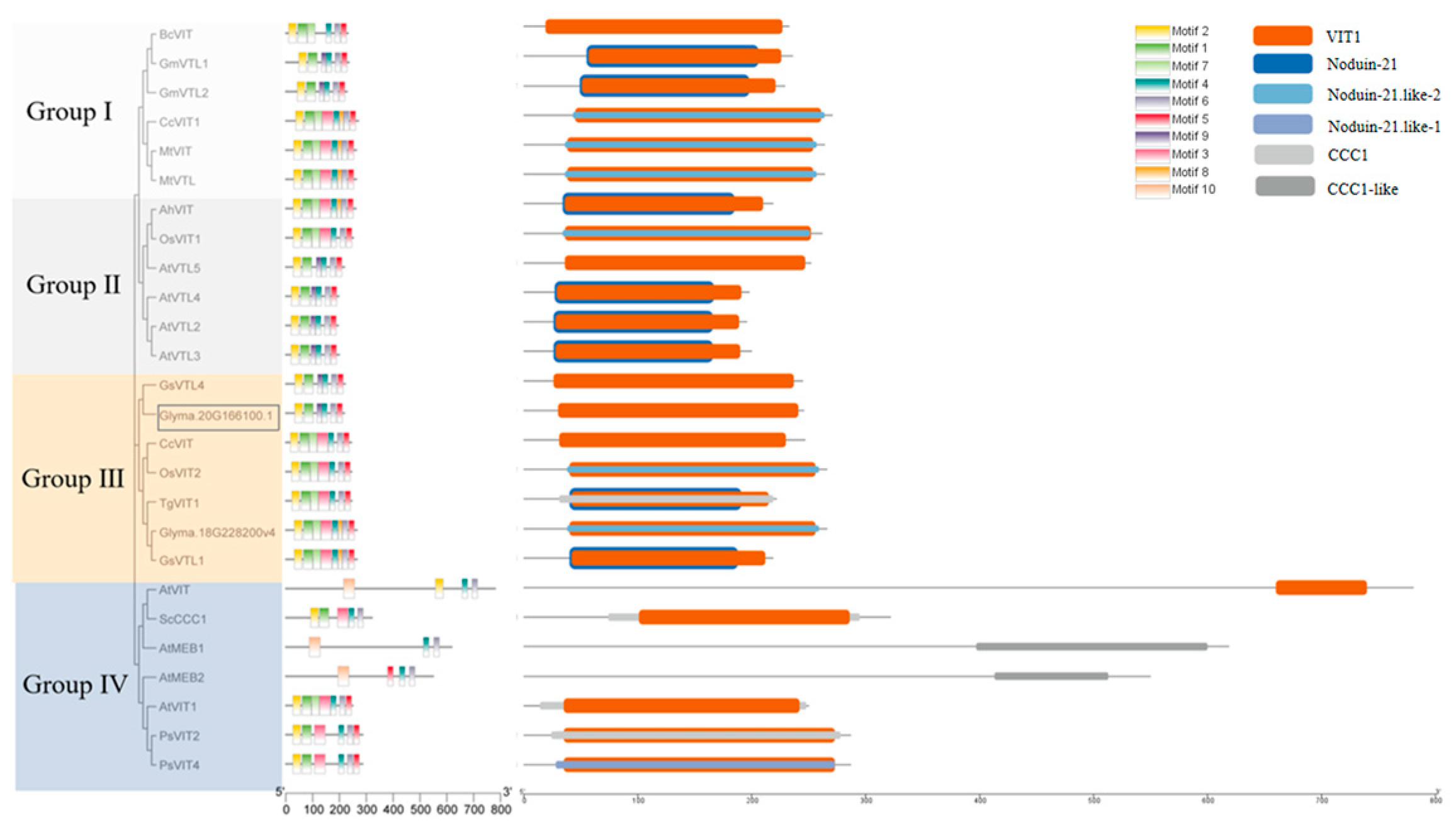
3.4. Cloning and Bioinformatics Analysis of GmVIT1
3.5. Subcellular Localization of GmVIT1 Protein
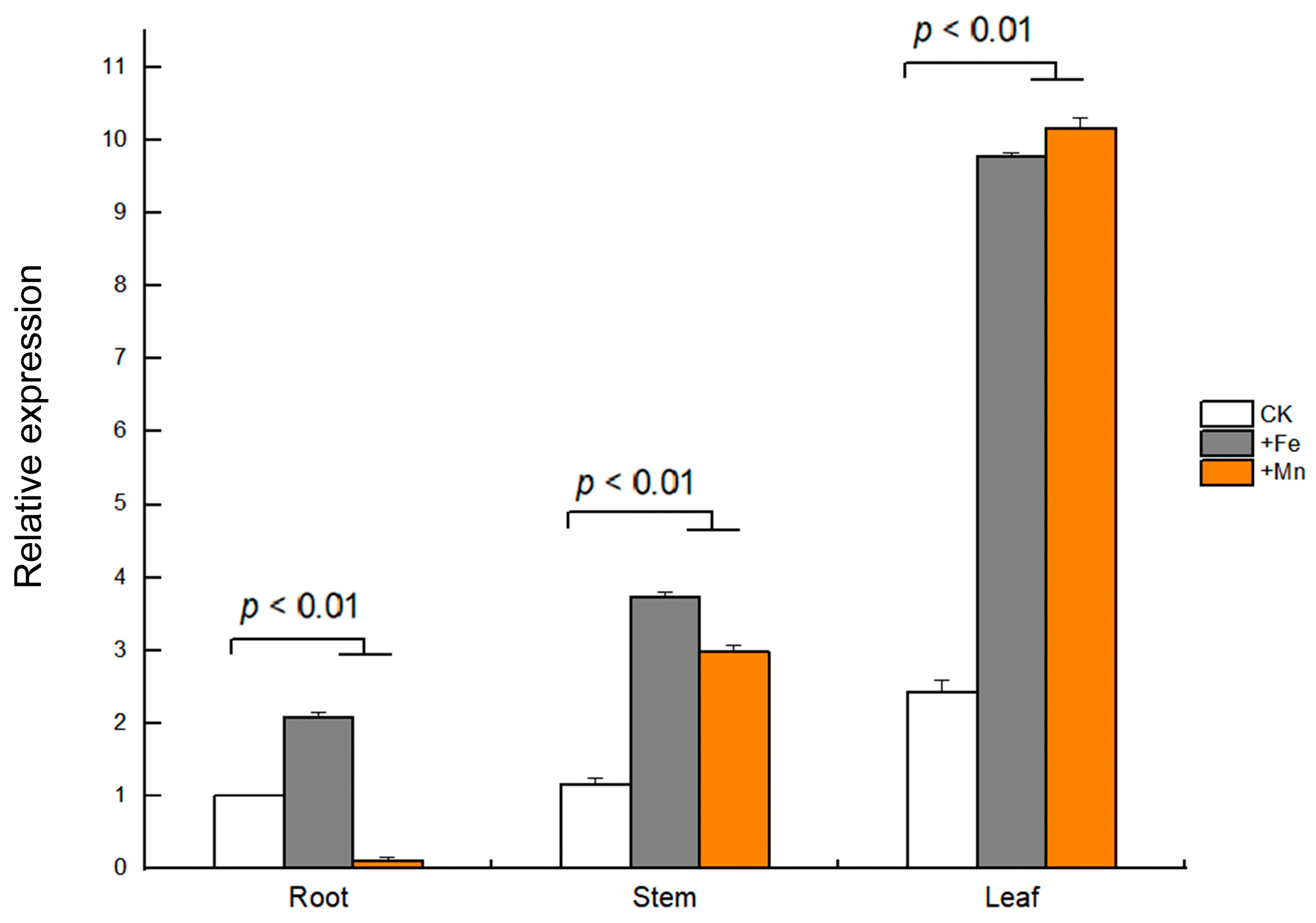
3.6. Expression Patterns of GmVIT1 Gene in Soybean
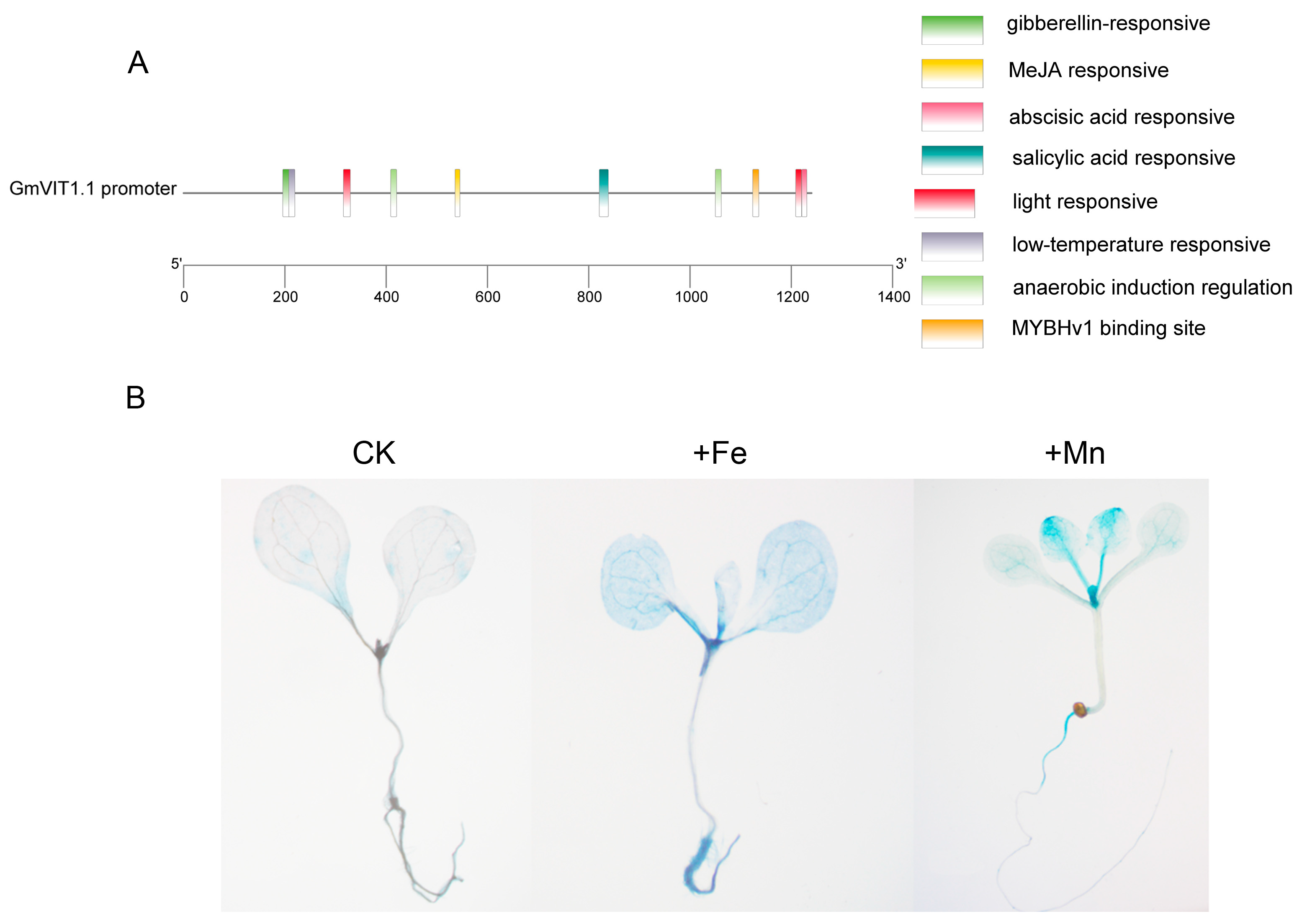
3.7. GmVIT1 Promoter Activity in Response to +Fe or +Mn Stress in Arabidopsis
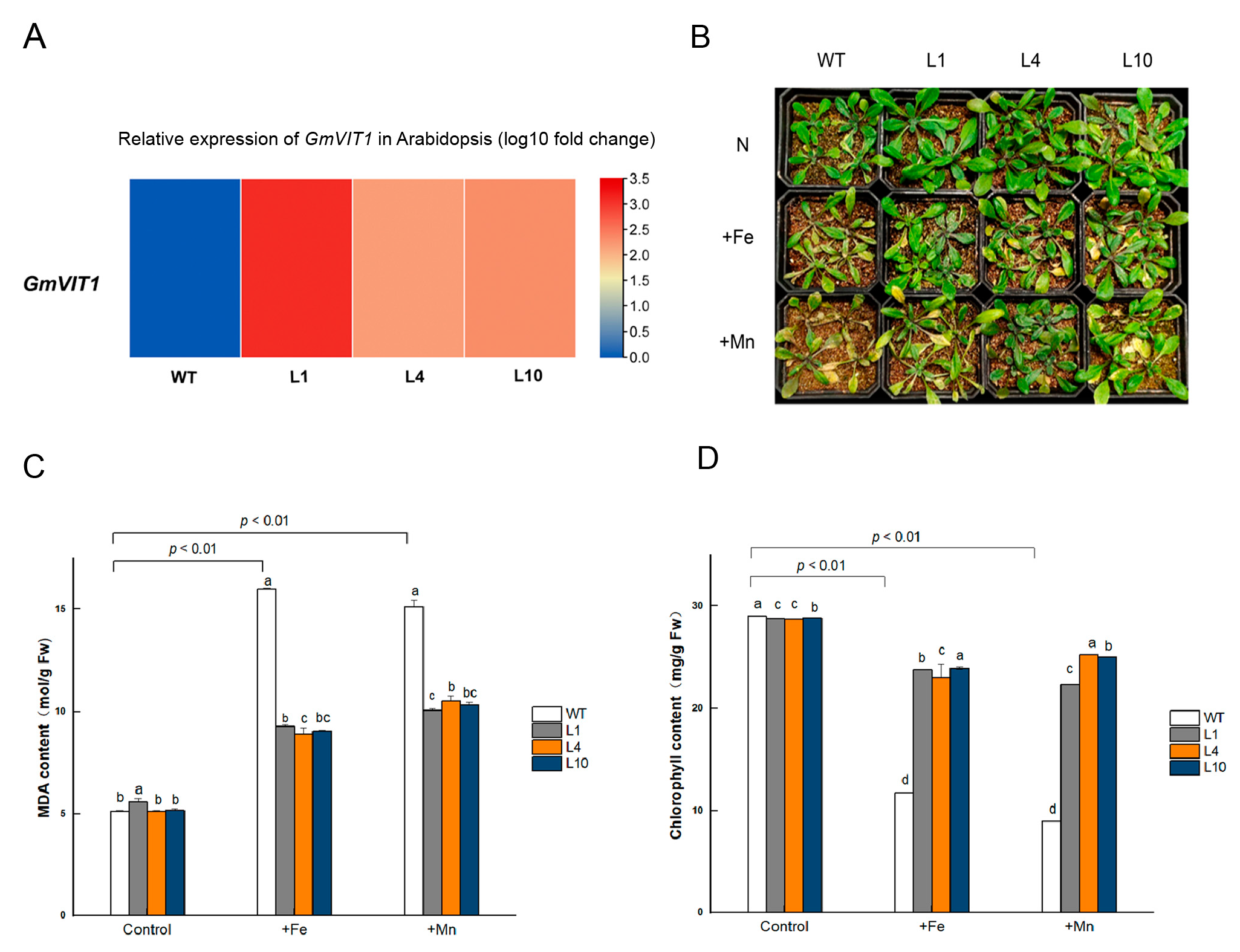
3.8. GmVIT1 Transgenic Arabidopsis Exhibit Enhancement of Tolerance to +Fe or +Mn Stress
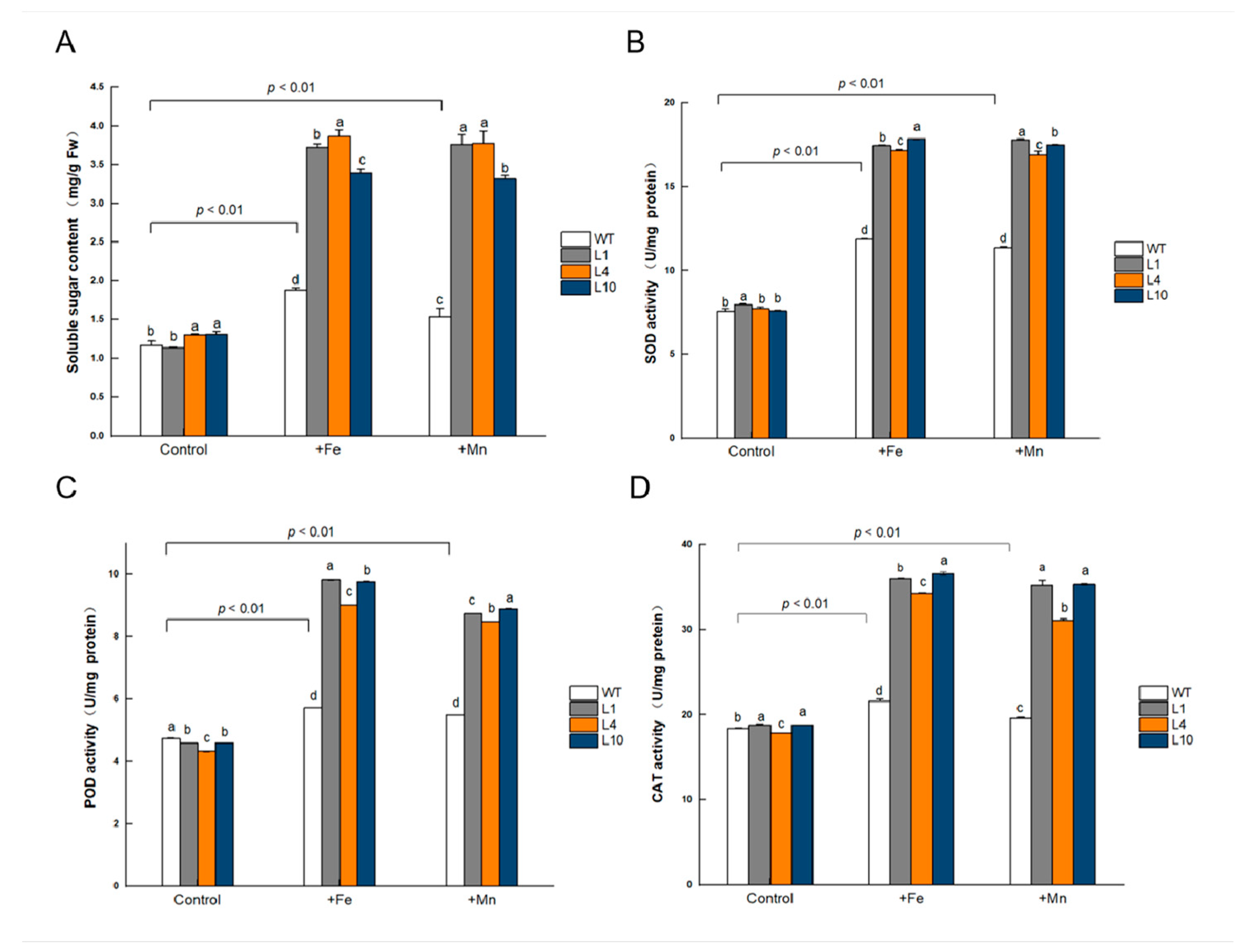
3.9. GmVIT1 Transgenic Arabidopsis Showed Higher Soluble Sugar Content and Antioxidant Enzyme Activity under +Fe or +Mn Stress
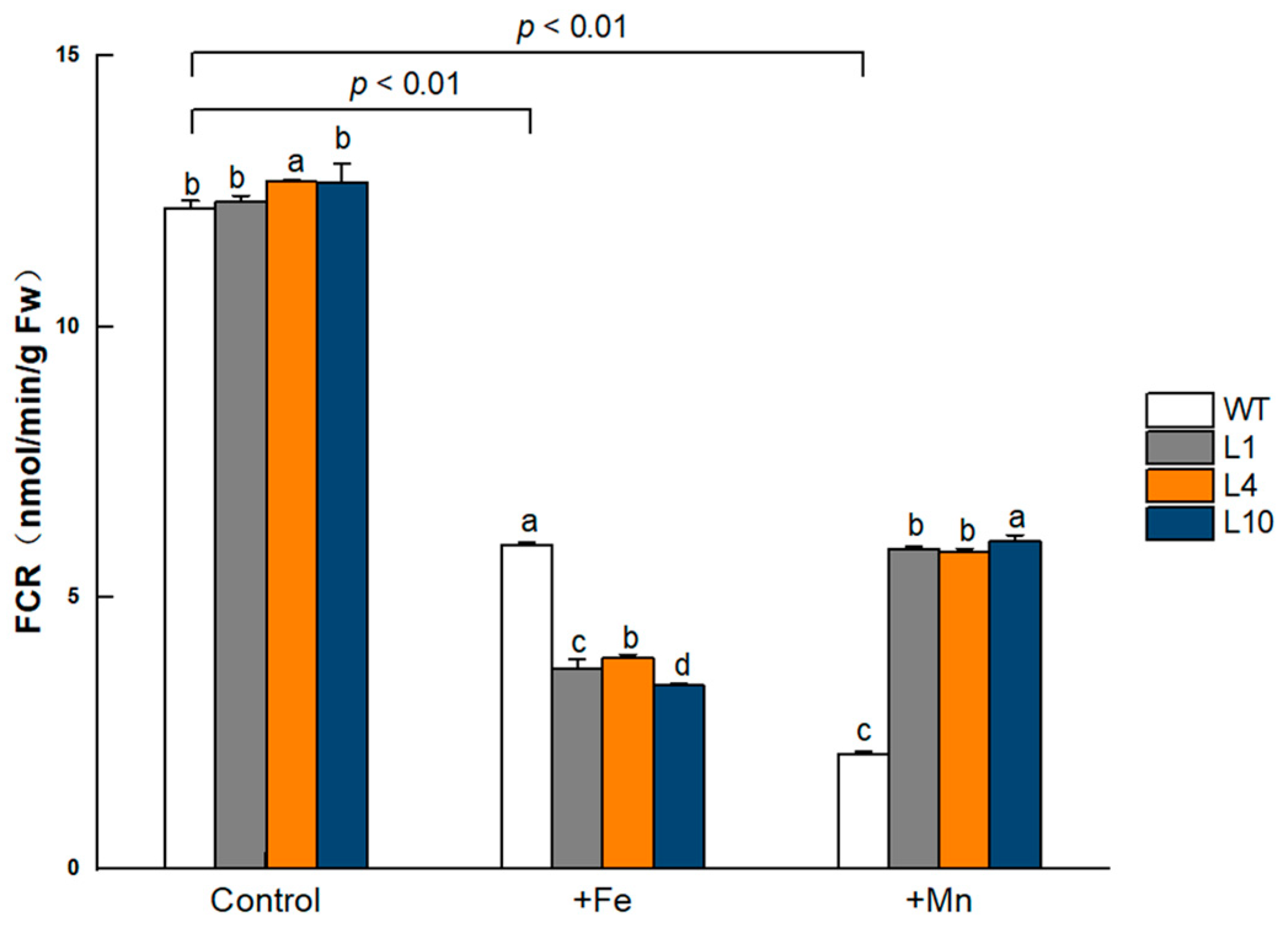
3.10. FCR Activity Changed in GmVIT1 Transgenic Plants under +Fe or +Mn Stress
4. Discussion
4.1. GmVIT1 Is a Unique Gene in Soybean VIT Family
4.2. GmVIT1 Was Highly Expressed in Leaves and Induced by Fe/Mn Stress
4.3. Overexpression of GmVIT1 Enhances the Tolerance to +Fe/+Mn Stress in Arabidopsis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef] [PubMed]
- Nsch, H.R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant. Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Ramirez, L.; Simontacchi, M.; Murgia, I.; Zabaleta, E.; Lamattina, L. Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: A well equipped team to preserve plant iron homeostasis. Plant Sci. 2001, 181, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Briat, J.F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Neelapu, N.; Surekha, C. Iron, Zinc, and Copper Application in Overcoming Environmental Stress. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress, 1st ed.; Aryadeep, R., Durgesh, K.T., Eds.; Wiley: Noida, India, 2020; Volume 29, pp. 582–596. [Google Scholar]
- Pereira, E.G.; Oliva, M.A.; Rosado-Souza, L.; Mendes, G.C.; Colares, D.S.; Stopato, C.H.; Almeida, A.M. Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci. 2013, 202, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Asch, F. Iron toxicity in rice-Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- Khabaz, S.H.; Rengel, Z.; Wilson, R.; Setter, T.L. Variation for tolerance to high concentration of ferrous iron (Fe2+) in Australian hexaploid wheat. Euphytica 2010, 172, 275–283. [Google Scholar] [CrossRef]
- Cho, U.H.; Park, J.O. Mercury-induced oxidative stress in tomato seedlings. Plant Sci. 2000, 156, 1–9. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Palma, J.M.; Rodriguez-Ruiz, M. Plant peroxisomes: A nitro-oxidative cocktail. Redox Biol. 2017, 11, 535–542. [Google Scholar] [CrossRef]
- Li, P.; Song, A.L.; Li, Z.J.; Fan, F.L.; Liang, Y.C. Silicon ameliorates manganese toxicity by regulating manganese transport and antioxidant reactions in rice (Oryza sativa L.). Plant Soil 2012, 354, 407–419. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Díaz, M.M.; Alberdi, M.R.; Alvarez-Cortez, D.A.; Rengel, Z.; Mora, M.D.L.L. Photosynthetic impairment caused by manganese toxicity and associated antioxidative responses in perennial ryegrass. Crop Pasture Sci. 2013, 64, 696–707. [Google Scholar] [CrossRef]
- Santos, E.F.; Santini, K.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 56, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300–323. [Google Scholar] [CrossRef] [PubMed]
- Lovyagina, E.R.; Semin, B.K. Competitive interaction of Mn(II) and Fe(II) cations with the high-affinity Mn-binding site of the photosystem II: Evolutionary aspect. Orig. Life Evol. Biosph. 2022, 52, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Mishra, A.K.; Biswal, U.C. Manganese induced peroxidation of thylakoid lipids and changes in chlorophyll-α flfluorescence during aging of cell free chloroplasts in light. Phytochemistry 1987, 26, 3217–3219. [Google Scholar] [CrossRef]
- Hauck, M.; Paul, A.; Gross, S.; Raubuch, M. Manganese toxicity in epiphytic lichens: Chlorophyll degradation and interaction with iron and phosphorus. Environ. Exp. Bot. 2003, 49, 181–191. [Google Scholar] [CrossRef]
- Peng, J.S.; Gong, J.M. Vacuolar sequestration capacity and long-distance metal transport in plants. Front. Plant Sci. 2014, 5, 19–32. [Google Scholar] [CrossRef]
- Agorio, A.; Giraudat, J.; Bianchi, M.W.; Marion, J.; Espagne, C.; Castaings, L.; Lelièvre, F.; Curie, C.; Thomine, S.; Merlot, S. Phosphatidylinositol 3-phosphate-binding protein AtPH1 controls the localization of the metal transporter NRAMP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 3354–3363. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelievre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Kraemer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef]
- Chang, J.-D.; Huang, S.; Yamaji, N.; Zhang, W.; Ma, J.F.; Zhao, F. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ. 2020, 43, 2476–2491. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, S.; Giehl, R.F.H.; Meier, B.; Takahashi, M.; Terada, Y.; Ignatiev, K.; Andresen, E.; Kupper, H.; Peiter, E.; Wiren, N.V. Metal Tolerance Protein 8 Mediates Manganese Homeostasis and Iron Reallocation during Seed Development and Germination. Plant Physiol. 2017, 174, 1633–1647. [Google Scholar] [CrossRef]
- Eckhardt, U.; Marques, A.; Buckhout, T.J. Two iron-regulated cation transporters from tomato complement metal uptake-defificient yeast mutants. Plant Mol. Biol. 2001, 45, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Park, J.; Mendoza-Cozatl, D.J.; Suter-Grotemeyer, M.; Shim, D.; Hortensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D.; et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, O.S.; Ward, D.M.; Kaplan, J. Ccc1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 2021, 31, 29515–29519. [Google Scholar] [CrossRef] [PubMed]
- Sorribes-Dauden, R.; Peris, D.; Martínez-Pastor, M.T.; Puig, S. Structure and function of the vacuolar Ccc1/VIT1 family of iron transporters and its regulation in fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Kurt, F.; Filiz, E. Subcellular iron transport genes in Arabidopsis thaliana: Insights into iron homeostasis. J. BioSci. Biotechnol. 2020, 9, 1–10. [Google Scholar]
- Bashir, K.; Takahashi, R.; Akhtar, S.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. The knockdown of OsVIT2 and MIT affects iron localization in rice seed. Rice 2013, 6, 31–38. [Google Scholar] [CrossRef]
- Zhu, W.; Zuo, R.; Zhou, R.; Huang, J.; Tang, M.; Cheng, X.; Liu, Y.; Tong, C.; Xiang, Y.; Dong, C.; et al. Vacuolar Iron Transporter BnMEB2 Is Involved in Enhancing Iron Tolerance of Brassica napus. Front. Plant Sci. 2016, 13, 1353–1366. [Google Scholar] [CrossRef]
- Connorton, J.M.; Jones, E.R.; Rodríguez-Ramiro, I.; Fairweather-Tait, S.; Uauy, C.; Balk, J. Wheat Vacuolar Iron Transporter TaVIT2 Transports Fe and Mn and Is Effective for Biofortification. Plant Physiol. 2017, 174, 2434–2444. [Google Scholar] [CrossRef]
- Borg, S.; Brinchpedersen, H.; Tauris, B.; Holm, P.B. Iron Transport, Deposition and Bioavailability in the Wheat and Barley Grain. Plant Soil 2009, 325, 15–24. [Google Scholar] [CrossRef]
- Slavic, K.; Krishna, S.; Lahree, A.; Bouyer, G.; Hanson, K.K.; Vera, I.; Pittman, J.K.; Staines, H.M.; Mota, M.M. A vacuolar iron-transporter homologue acts as a detoxifier in Plasmodium. Nat. Commun. 2016, 7, 10403–10413. [Google Scholar] [CrossRef] [PubMed]
- Cao, J. Molecular Evolution of the Vacuolar Iron Transporter (VIT) Family Genes in 14 Plant Species. Genes 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.T.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Jeet, R.; Singh, S.P.; Tiwari, S.; Pathak, P. Wheat TaVIT2D restores phenotype and mediates iron homeostasis during growth of Arabidopsis thaliana in iron-deficient conditions. Plant Physiol. Rep. 2019, 1, 24–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.H.; Yi, H.Y.; Gong, J.-M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef]
- Ram, H.; Sardar, S.; Gandass, N. Vacuolar Iron Transporter (Like) proteins: Regulators of cellular iron accumulation in plants. Physiol. Plant. 2021, 171, 823–832. [Google Scholar] [CrossRef]
- Labarbuta, P.; Duckett, K.; Botting, C.H.; Chahrour, O.; Malone, J.; Dalton, J.P.; Law, C.J. Recombinant vacuolar iron transporter family homologue PfVIT from human malaria-causing Plasmodium falciparum is a Fe2+/H+ exchanger. Sci. Rep. 2017, 7, 42850. [Google Scholar] [CrossRef]
- Liu, S.; Liao, L.L.; Nie, M.M.; Peng, W.T.; Zhang, M.S.; Lei, J.N.; Zhong, Y.J.; Liao, H.; Chen, Z.C. A VIT-like transporter facilitates iron transport into nodule symbiosomes for nitrogen fixation in soybean. New Phytol. 2020, 226, 1413–1428. [Google Scholar] [CrossRef]
- Li, L.; Ward, D.M. Iron toxicity in yeast: Transcriptional regulation of the vacuolar iron importer CCC1. Curr. Genet. 2018, 64, 413–416. [Google Scholar] [CrossRef]
- Eroglu, S.; Karaca, N.; Vogel-Mikus, K.; Kavčič, A.; Filiz, E.; Tanyolac, B. The Conservation of VIT1-Dependent Iron Distribution in Seeds. Front. Plant Sci. 2019, 10, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Gollhofer, J.; Timofeev, R.; Lan, P.; Schmidt, W.; Buckhout, T.J. Vacuolar-Iron-Transporter1-Like proteins mediate iron homeostasis in Arabidopsis. PLoS ONE 2014, 9, e110468. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yamaji, N.; Ma, J.F. Role of a vacuolar iron transporter OsVIT2 in the distribution of iron to rice grains. New Phytol. 2021, 230, 1049–1062. [Google Scholar] [CrossRef]
- Momonoi, K.; Yoshida, K.; Mano, S.; Takahashi, H.; Nakamori, C.; Shoji, K.; Nitta, A.; Nishimura, M. A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J. 2009, 59, 437–447. [Google Scholar] [CrossRef]
- Yoshida, K.; Negishi, T. The identification of a vacuolar iron transporter involved in the blue coloration of cornflower petals. Phytochemistry 2013, 94, 60–67. [Google Scholar] [CrossRef]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solis, E.; Grusak, M.A.; Taylor, N.; Anderson, P. Overexpression of Arabidopsis VIT1 increases accumulation of iron in cassava roots and stems. Plant Sci. 2015, 240, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Andrew, C.S.; Hegarty, M.P. Comparative responses to manganese excess of eight tropical and four temperate pasture legume species. Aust. J. Agric. Res. 1969, 20, 687–696. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physiol. 2015, 167, 176–188. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, W.; Sun, L.; Tian, J.; Liao, H. Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. J. Proteom. 2016, 143, 151–160. [Google Scholar] [CrossRef]
- Brear, E.M.; Bedon, F.; Gavrin, A.; Kryvoruchko, I.S.; Torres-Jerez, I.; Udvardi, M.K.; Day, D.A.; Smith, P.M.C. GmVTL1a is an iron transporter on the symbiosome membrane of soybean with an important role in nitrogen fixation. New Phytol. 2020, 228, 667–681. [Google Scholar] [CrossRef]
- Rajinder, S.; Dhindsa, P.L.; Plumb, D.; David, M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar]
- Jun, S.E.; Okushima, Y.; Nam, J.; Umeda, M.; Kim, G.T. Kip-Related Protein 3 Is Required for Control of Endoreduplication in the Shoot Apical Meristem and Leaves of Arabidopsis. Mol. Cells 2013, 35, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Manara, A. Plant Responses to Heavy Metal Toxicity. In Plants and Heavy Metals, 1st ed.; Antonella, F., Ed.; Springer: Dordrecht, The Netherlands, 2012; Volume 3, pp. 27–53. [Google Scholar]
- Aung, M.S.; Masuda, H. How Does Rice Defend Against Excess Iron?: Physiological and Molecular Mechanisms. Front. Plant Sci. 2020, 11, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Y.; Xie, B.; Zhu, S.; Lu, X.; Liang, C.; Tian, J. Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiol. Biochem. 2020, 155, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Buckhout, T.J.; Yang, T.J.; Schmidt, W. Early iron-deficiency-induced transcriptional changes in Arabidopsis roots as revealed by microarray analyses. BMC Genom. 2009, 10, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Lapinskas, P.J.; Lin, S.J.; Culotta, V.C. The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol. Microbiol. 1996, 21, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, G.; Kumar, A.; Meena, V.; Ram, H.; Kaur, J.; Pandey, A.K. Gene Expression Pattern of Vacuolar-Iron Transporter-Like (VTL) Genes in Hexaploid Wheat during Metal Stress. Plants 2020, 9, 229. [Google Scholar] [CrossRef]
- Kisku, G.C.; Kumar, V.; Sahu, P. An Over View of Metal Toxicity in Agricultural Soil and Plants. In Metallic Contamination and Its Toxicity, 1st ed.; Gautam, A., Pathak, C., Eds.; Daya Publishing House: New Delhi, India, 2020; Volume 12, pp. 2451–2456. [Google Scholar]
- Becana, M.; Moran, J.F.; Iturbe-Ormaetxe, I. Iron-dependent oxygen free radical generation in plants subjected to environmental stress: Toxicity and antioxidant protection. Plant Soil 1998, 201, 137–147. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef]
- Harrington, S.A.; Connorton, J.M.; Nyangoma, N.I.M.; McNelly, R.; Morgan, Y.M.L.; Aslam, M.F.; Sharp, P.A.; Johnson, A.A.T.; Uauy, C.; Balk, J. A two-gene strategy increases iron and zinc concentrations in wheat flour, improving mineral bioaccessibility. Plant Physiol. 2023, 191, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Tissot, N.; Robe, K.; Gao, F.; Grant-Grant, S.; Boucherez, J.; Bellegarde, F.; Maghiaoui, A.; Marcelin, R.; Izquierdo, E.; Benhamed, M.; et al. Transcriptional integration of the responses to iron availability in Arabidopsis by the bHLH factor ILR3. New Phytol. 2019, 223, 1433–1446. [Google Scholar] [CrossRef]

| Motif | Width | Sites | E-Value | Sequences |
|---|---|---|---|---|
| 1 | 35 | 23 | 1.6 × 10−333 | DSKLVLLAGFAGLVAGAISMGIGEFVSASSZRDVE |
| 2 | 29 | 23 | 2.3 × 10−250 | YSWRGZWVRAAVLGANDGLVSTFSLMMGI |
| 3 | 41 | 11 | 2.0 × 10−162 | MEYNDATPVVNIFRKYPDILVDQRMVADKGLLPADQEVKPW |
| 4 | 21 | 26 | 9.5 × 10−136 | ALASALSFSLGGLVPLLSAIF |
| 5 | 21 | 22 | 2.9 × 10−122 | SAVRTLLGGAJAMAIAFGLTK |
| 6 | 21 | 26 | 3.6 × 10−106 | IVSLLALVLFGVAKARJGGAP |
| 7 | 29 | 11 | 6.3 × 10−102 | RERRVTZWDVINVPDTEQAEJVEIYQQLG |
| 8 | 15 | 6 | 2.9 × 10−33 | IPFTDNDSVKFLGAC |
| 9 | 18 | 8 | 9.3 × 10−26 | REDEDEIEKEKLPNPLQA |
| 10 | 41 | 3 | 1.7 × 10−14 | DDDVENLJDNQENYDLYCPSCGSCITKNVILKKRKRPKHVN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhang, X.-M.; Gao, J.-L.; Wang, L.; Si, L.; Shu, Y.-J.; Guo, C.-H.; Lai, Y.-C.; Bi, Y.-D.; Guo, D.-L. Soybean GmVIT1 Gene Confers Plant Tolerance to Excess Fe/Mn Stress. Agronomy 2023, 13, 384. https://doi.org/10.3390/agronomy13020384
Li T, Zhang X-M, Gao J-L, Wang L, Si L, Shu Y-J, Guo C-H, Lai Y-C, Bi Y-D, Guo D-L. Soybean GmVIT1 Gene Confers Plant Tolerance to Excess Fe/Mn Stress. Agronomy. 2023; 13(2):384. https://doi.org/10.3390/agronomy13020384
Chicago/Turabian StyleLi, Tong, Xue-Meng Zhang, Jia-Lu Gao, Ling Wang, Liang Si, Yong-Jun Shu, Chang-Hong Guo, Yong-Cai Lai, Ying-Dong Bi, and Dong-Lin Guo. 2023. "Soybean GmVIT1 Gene Confers Plant Tolerance to Excess Fe/Mn Stress" Agronomy 13, no. 2: 384. https://doi.org/10.3390/agronomy13020384
APA StyleLi, T., Zhang, X.-M., Gao, J.-L., Wang, L., Si, L., Shu, Y.-J., Guo, C.-H., Lai, Y.-C., Bi, Y.-D., & Guo, D.-L. (2023). Soybean GmVIT1 Gene Confers Plant Tolerance to Excess Fe/Mn Stress. Agronomy, 13(2), 384. https://doi.org/10.3390/agronomy13020384







