Low-Temperature Fermented Straw Compost Regulates Rice Growth and Yield by Affecting Soil Physicochemical Properties and the Expression of Important Signaling Pathway Genes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials in This Study
2.2. Measurement of Rice Physiological Indices
2.3. DAB (3,3′-Diaminobenzidine) and NBT (Nitrotetrazolium Blue Chloride) Staining of Rice Leaves
2.4. Measurement of Grain Shape and Hundred-Grain Weight of Songjing 2 Rice
2.5. Determination of Soil Physiochemical Properties
2.6. Determination of Soil Microbial Diversity
2.7. Rice Transcriptome Sequencing and Data Analysis
2.8. qRT-PCR Analysis
2.9. Statistical Analysis
3. Results
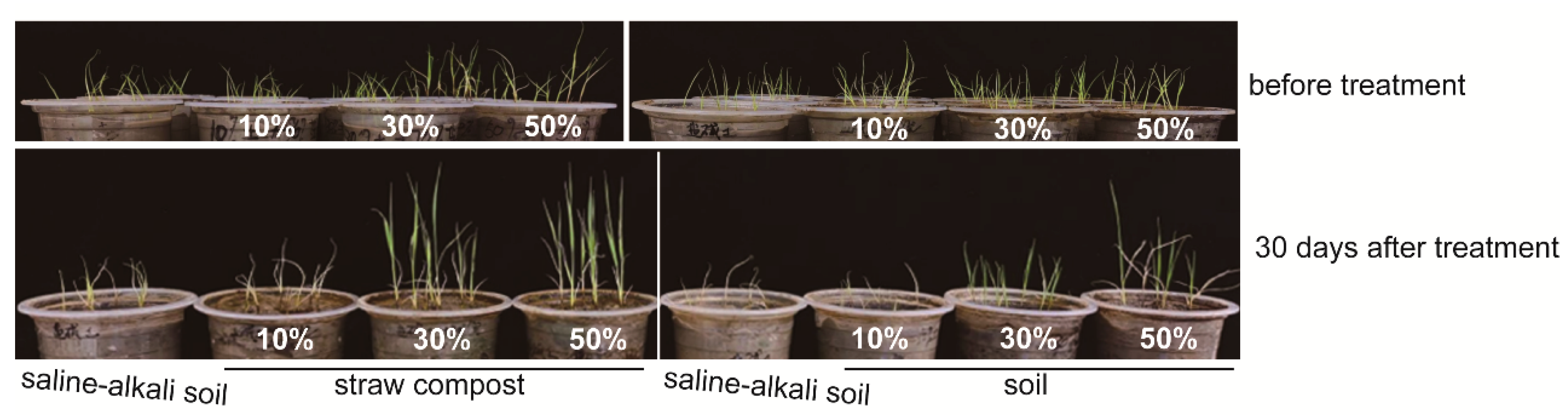
3.1. Straw Compost Affected the Rice Biomass and Yield
3.2. Effects of Straw Compost on Various Rice Physiological Indices
3.3. The Application of Straw Compost Improved the Soil Physicochemical Properties
3.4. The Addition of Straw Compost Increased the Soil Microbial Abundance
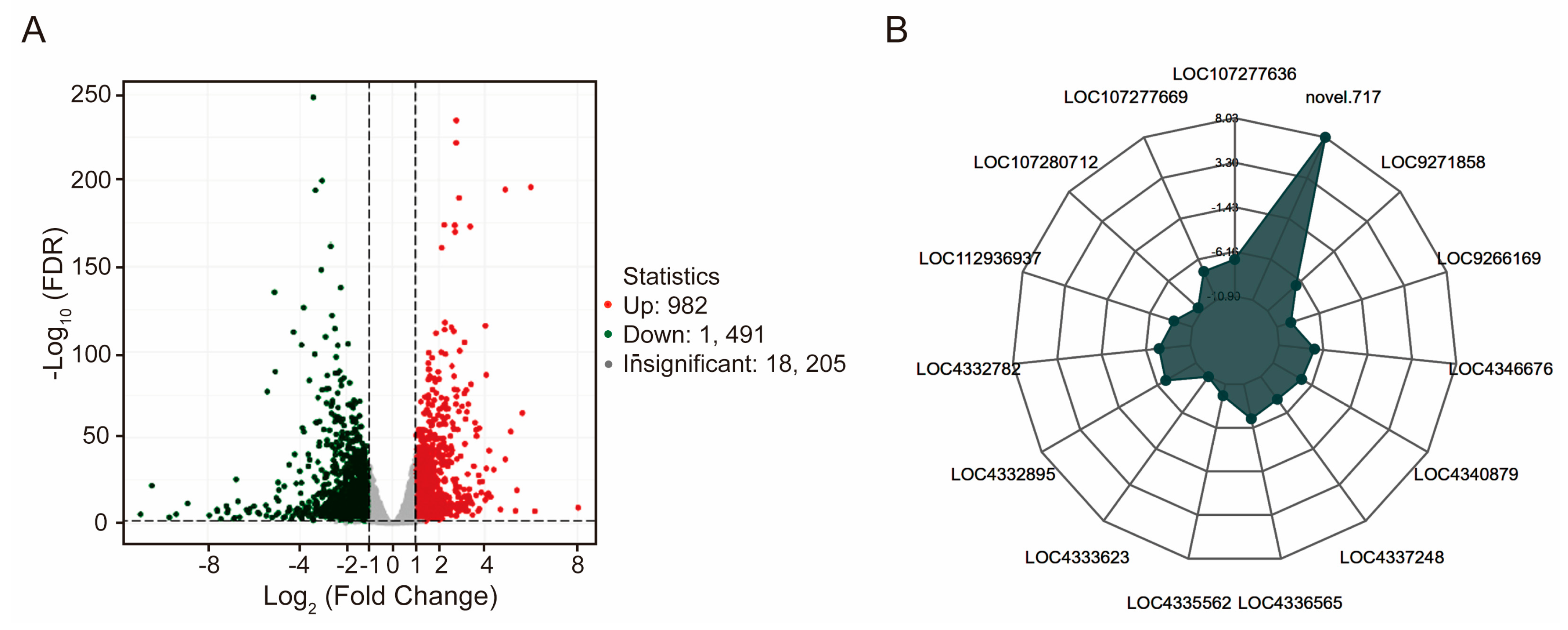
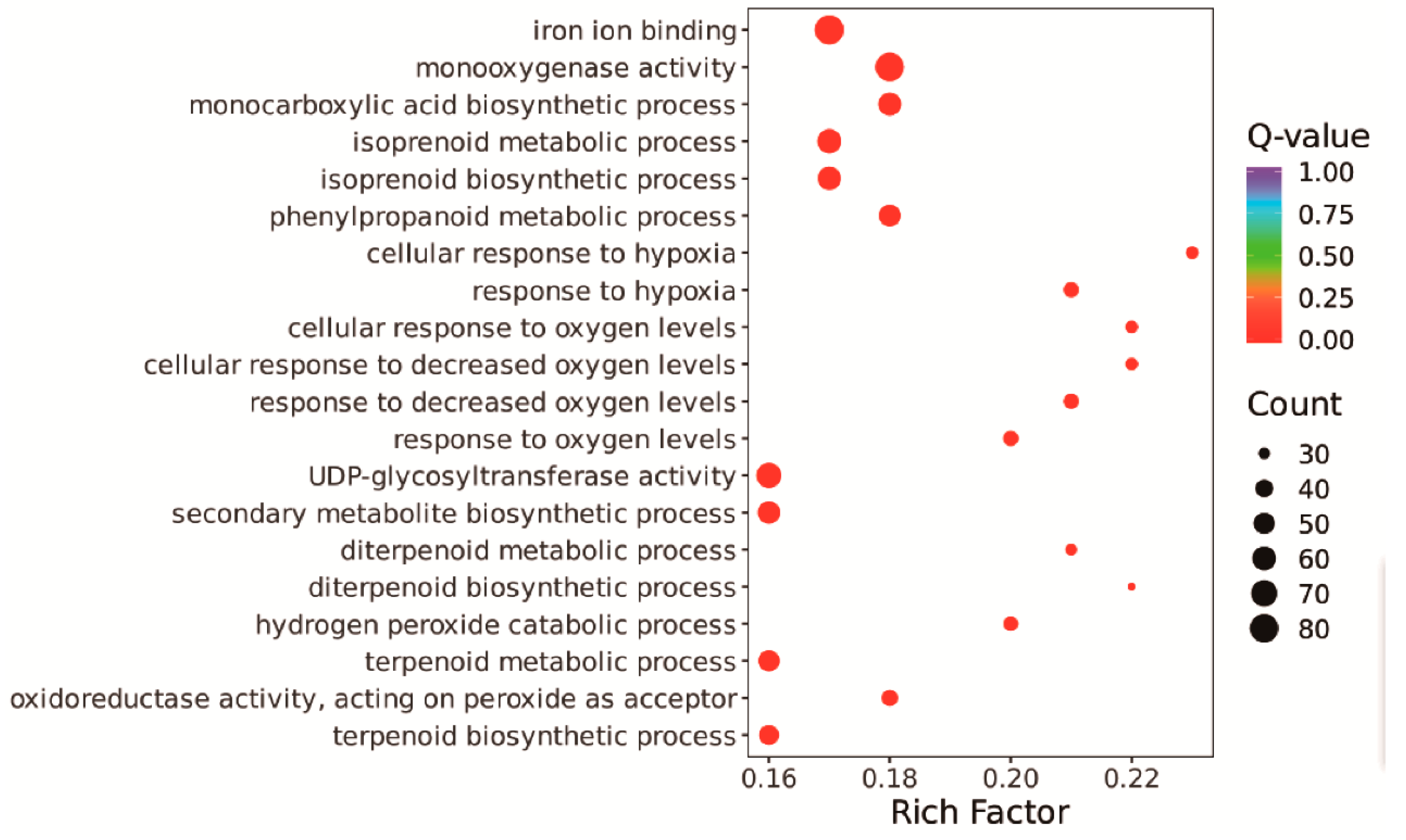
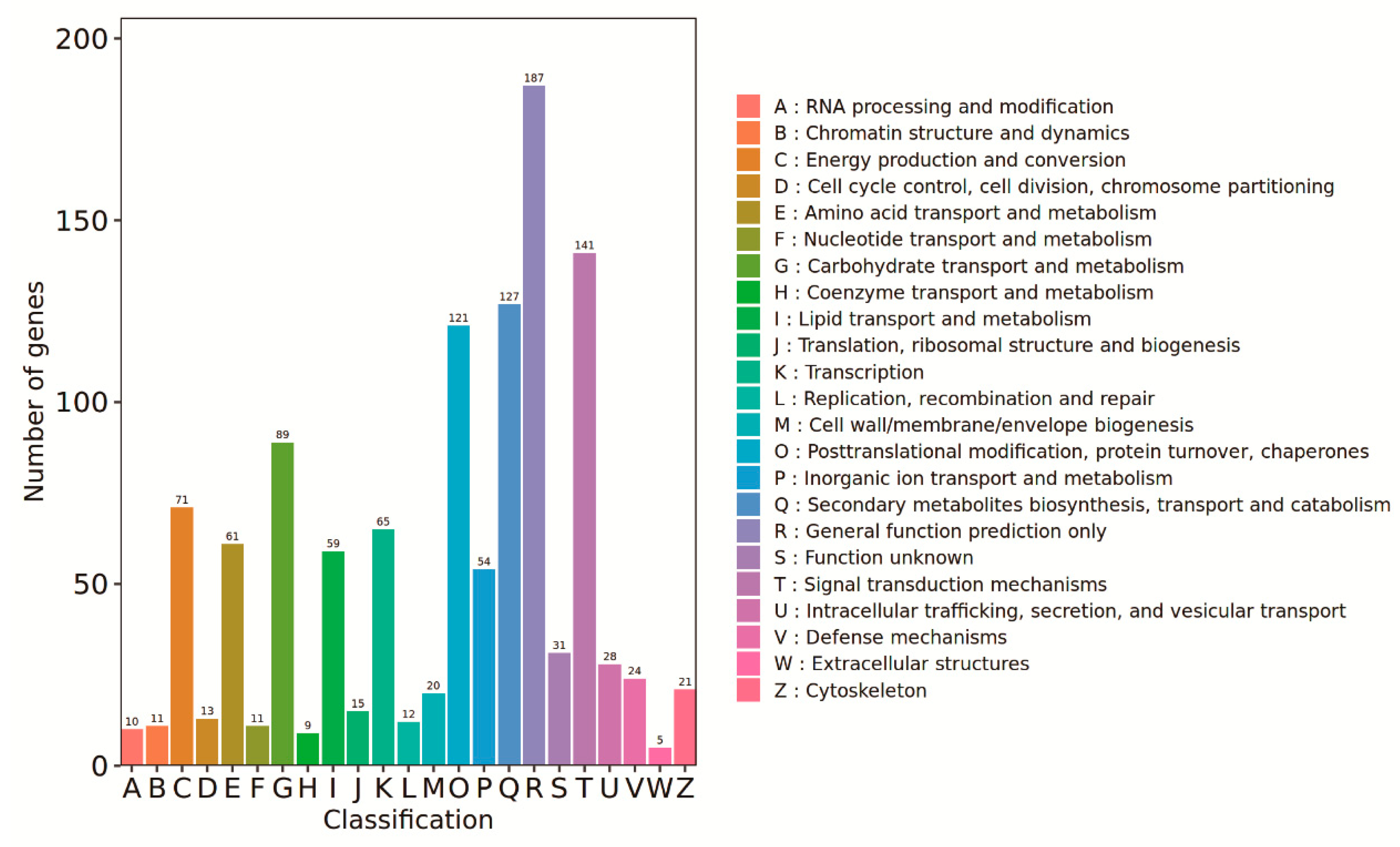
3.5. Changes in the Rice Leaf Transcriptome in Response to the Straw Compost Treatment
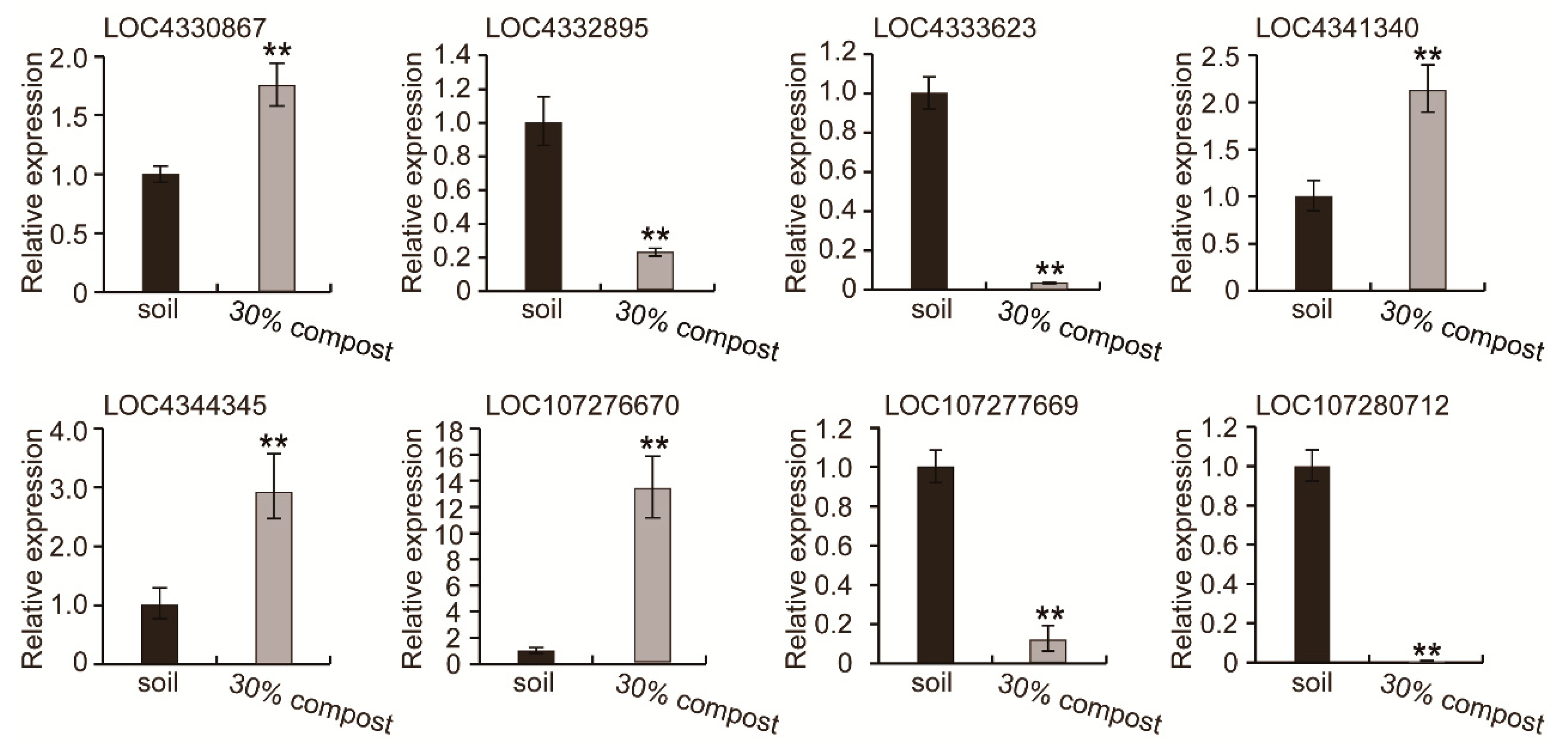
3.6. qRT-PCR Verification of the RNA-Seq Results
4. Discussion
4.1. Utility of the Low-Temperature Fermented Straw Compost for Promoting Rice Growth and Increasing Yield
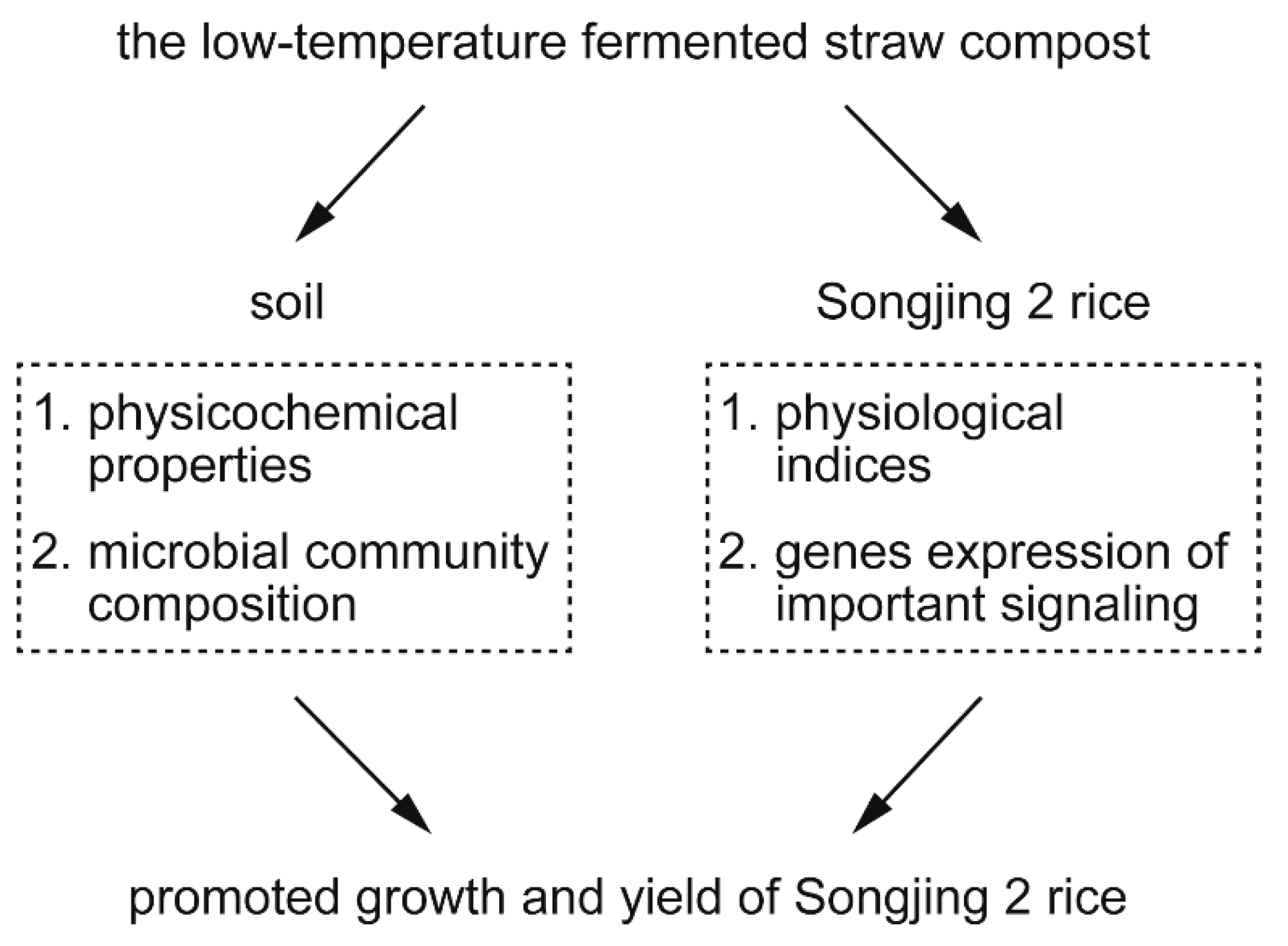
4.2. The Low-Temperature Fermented Straw Compost Promoted Rice Growth and Increased the Grain Yield by Altering Soil Physicochemical Properties and the Expression of Important Signaling Pathway Genes in Rice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.L.; Van Der Heijden, M.G.A.; Zhang, F.S.; Bender, S.F. Soil biodiversity and crop diversification are vital components of healthy soils and agricultural sustainability. Front. Agric. Sci. Eng. 2020, 7, 236–242. [Google Scholar] [CrossRef]
- Rong, G.H. Experimental Study on Effects of Erosion and Tillage Measures on Soil Quality. Ph.D Thesis, Northwest A&F University, Xianyang, China, 2022. [Google Scholar]
- Jacopo De Rosa, J.; Pontolillo, D.M.; Di Maio, C.; Vassallo, R. Chemical Clay Soil Improvement: From Laboratory to Field Test. Procedia Eng. 2016, 158, 284–289. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.X.; Xiang, H.Y. Research progress on effects and mechanisms of straw returning on soil fertility. Jiangsu Agric. Sci. 2018, 46, 22–28. [Google Scholar]
- Wang, C.Q.; Xie, D.T.; Li, B.; Zhou, Y.; Li, H.X.; Li, T.X.; Zhang, X.Z. Effect of Different Kinds and Dosage of Organic Manure on the Yield and Quality of Celery. Chin. Agric. Sci. Bull. 2005, 21, 192–195. [Google Scholar]
- Cao, G.L.; Zhang, X.Y.; Wang, D.; Zheng, F.C. Inventory of Emissions of Pollutants from Open Burning Crop Residue. J. Agro-Environ. Sci. 2005, 4, 800–804. [Google Scholar]
- Hrynkiewicz, K.; Baum, C.; Leinweber, P. Density, metabolic activity, and identity of cultivable rhizosphere bacteria on Salix viminalis in disturbed arable and landfill soils. Plant Nutr. Soil Sci. 2010, 173, 747–756. [Google Scholar] [CrossRef]
- Liu, J.P.; Ju, M.T.; Liu, Y.H.; Wang, P.; Wu, W.T.; Tong, S.M. The Analysis of China’s Utilization Technology of Agricultural Straw and Development of Biomass Industry. Ecol. Econ. 2011, 5, 136–141. [Google Scholar]
- Li, N.; Shao, T.Y.; Zhou, Y.J.; Cao, Y.C.; Hu, H.Y.; Sun, Q.K.; Long, X.H.; Yue, Y.; Gao, X.M.; Rengel, Z. Effects of planting Melia azedarach L. on soil properties and microbial community in saline-alkali soil. Land Degrad. Dev. 2021, 32, 2951–2961. [Google Scholar] [CrossRef]
- Shi, Y. The Comprehensive Utilization of Crop Straw and in Binzhou Demonstration Application. Master’s Thesis, Shandong University, Jinan, China, 2012. [Google Scholar]
- Yin, J.; Liu, Y.; Yu, F.; Cai, J.; Liu, T. Screening and identification of a lignin degrading bacterium and its application in composting. China Soil Fertil. 2019, 281, 179–185. [Google Scholar]
- Zhang, P.F.; Li, S.Y.; Yu, K.F.; Jiang, X.F. Screening of lignin-degrading bacteria and study on degradation of garden waste. J. Anhui Agric. Univ. 2018, 45, 676–681. [Google Scholar]
- Wan, Y.; Liu, J.; Deng, F.; Xie, Z.J.; Chen, Y.C.; Li, J.B.; Li, D. Screening of lignin-degrading fungi and bioaugmentation on the directional humification of garden waste composting. Ind. Crops Prod. 2023, 203, 117208. [Google Scholar] [CrossRef]
- Peng, X.G.; Yang, L.E.; Zhang, L. Study Progress on Biological Degrading of Lignin in Straws. Xiandai Nongye Keji 2010, 1, 18–20. [Google Scholar]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, G.; Heitman, J.L. The effects of compost incorporation on soil physical properties in urban soils–A concise review. J. Environ. Manag. 2020, 261, 110209. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Li, L.X.; Wang, Z.G.; Sun, J.; Zhang, H.L. Impacts of Corn Straw Compost on Rice Growth and Soil Microflora under Saline-Alkali Stress. Agronomy 2023, 13, 1525. [Google Scholar] [CrossRef]
- Sarangi, S.; Swain, H.; Adak, T.; Bhattacharyya, P.; Mukherjee, A.K. Trichoderma-mediated rice straw compost promotes plant growth and imparts stress tolerance. Environ. Sci. Pollut. Res. 2021, 28, 44014–44027. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Naveed, M.; Azeem, M.; Yaseen, M.; Ullah, R.; Alamri, S.; Ain, F.; Qurrat, U. Siddiqui Manzer H Efficiency of wheat straw biochar in combination with compost and biogas slurry for enhancing nutritional status and productivity of soil and plant. Plants 2020, 9, 1516. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Zhang, X.; Liu, J.Q.; Hu, Q.L.; Zhang, J.F. Study of compound microbial agents in the improvement of soda saline-alkali soil. J. Jilin Agric. Univ. 2023, 9, 1–12. [Google Scholar]
- Li, F.L.; Xu, Y.Y.; Xu, Y.Q.; Feng, Y.Z.; Yang, X.M.; Yuan, Q.; Wang, L.J. Bacterial Strains of Low Temperature Fermentation of Corn Stover and Their Fermentation and Cultivation Methods and Applications. Chinese Patent CN108865927B, 6 April 2021. [Google Scholar]
- Wang, H. Screening and Genetic Analysis of Saline-Alkali Tolerant Rice Varieties. Ph.D Thesis, Northeast Forestry University, Harbin, China, 2020. [Google Scholar]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Guo, L.F.; Zhang, X.C.; Zhao, J.W.; Zhang, A.Q.; Pang, Q.Y. Enhancement of sulfur metabolism and antioxidant machinery confers Bacillus sp. Jrh14-10-induced alkaline stress tolerance in plant. Plant Physiol. Biochem. 2023, 203, 108063. [Google Scholar] [CrossRef]
- Ding, S.J.; Zhang, X.F.; Yang, W.L.; Xin, X.L.; Zhu, A.N.; Huang, S.M. Soil Nutrients and Aggregate Composition of Four Soils with Contrasting Textures in a Long-Term Experiment. Eurasian Soil Sci. 2021, 54, 1746–1755. [Google Scholar] [CrossRef]
- Yang, L.; Tan, L.L.; Zhang, F.H.; Gale, W.J.; Cheng, Z.B.; Sang, W. Duration of continuous cropping with straw return affects the composition and structure of soil bacterial communities in cotton fields. Can. J. Microbiol. 2018, 64, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.F.; Wang, Z.Y.; Leng, X.Y.; Liu, J.M.; Cao, J.M. Improvement of soil phosphatase activity measurement method. Exp. Technol. Manag. 2016, 33, 48–49+54. [Google Scholar]
- Luo, M.X.; Hu, Z.D.; Liu, X.L.; Li, Y.F.; Hu, J.; Ou, D.H.; Wu, D.H. Characteristics of soil microbial biomass carbon, nitrogen and enzyme activities in Picea asperata plantations with different ages in subalpine of western Sichuan, China. Acta Ecol. Sin. 2021, 41, 5632–5642. [Google Scholar]
- Yu, J.L.; Shi, H.X. Changes of Microbes Population in the Different Degraded Alpine Meadows on the Qinghai-Tibetan Plateau. Acta Agric. Boreali-Occident. Sin. 2011, 20, 77–81. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 4, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2- and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Xie, Q.J.; Liu, B.C.; Dong, W.F.; Li, J.H.; Wang, D.N.; Liu, Z.Y.; Gao, C.Q. Comparative transcriptomic and metabolomic analyses provide insights into the responses to NaCl and Cd stress in Tamarix hispida. Sci. Total Environ. 2023, 884, 163889. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Zhang, L.; Guo, X.P.; Zhang, S.X.; Cao, X.C. The Effects of Straw Incorporation on Physicochemical Properties of Soil: A Review. J. Irrig. Drain. 2022, 41, 1–11. [Google Scholar]
- Wang, Z. Effects of Compound Microbial Agents on the Growth and Development of Rice. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2017. [Google Scholar]
- Li, J.; Li, M.; Gao, X.X.; Fang, F. Corn straw mulching affects Parthenium hysterophorus and rhizosphere organisms. Crop Prot. 2018, 113, 90–96. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Xu, Y.Q.; Li, F.L.; Liu, D.; Hao, L.B.; Wang, M.J.; Wang, X.; Dong, S.J.; Hu, B.Z. Effects of Composting Mulch and Organic Fertilizer Fermented by EM on Cucumber Quality. Crops 2015, 3, 104–110. [Google Scholar]
- Chen, J.J.; Zhang, T. Effects of banana stalk and leaf straw fertilizer on the growth and quality of mustard. Shanghai Veg. 2022, 3, 42–44. [Google Scholar]
- Liang, X.; Huang, C.Z.; Deng, Z.W.; Liang, A.; Yu, T. Study on the application effect of organic material decomposition agent in returning banana stems and leaves to field. Agric. Technol. 2017, 37, 59–61. [Google Scholar]
- Ding, Z.L.; Han, L.N.; Zeng, H.C.; Zheng, W.; He, Y.D.; Zang, X.P. Effect of Banana Stalk Organic Fertilizer on Chinese Cabbage Growth. Chin. Agric. Sci. Bull. 2016, 32, 73–78. [Google Scholar]
- Mu, C.L.; Wu, X.S.; Li, S.N.; Ma, M.C.; Li, J.; Shen, B.L.; Zhu, B.C. Screening and identification of a cold-adapted cellulase-producing strains and characterization of cellulose. Acta Microbiol. Sin. 2013, 40, 1193–1201. [Google Scholar]
- Shao, N.N.; Xuan, H.L.; Luo, F.; Dai, X.Z. Isolation, identification and characterization of a cold-adapted cellulaseprodu Cing psychrotrophic Streptomyces sp. Sci. Technol. Food Ind. 2015, 36, 159–163. [Google Scholar]
- Feng, X.X.; Li, F.L.; Xu, Y.Q. Screening of cellulase producing strains from rotten wood in Xinjiang cold area and analysis of their characteristics of enzyme production at low temperature. Acta Agric. Zhejiangensis 2021, 33, 1468–1476. [Google Scholar]
- Yi, Y.K.; Zhou, Z.B.; Chen, G.H. Biomass-based dynamic model for rice root system. J. Agro-Environ. Sci. 2017, 36, 428–436. [Google Scholar]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biocharamendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van der Voort, M.; Schneider, J.H.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Wang, L.; Pang, X.; Li, N.; Qi, K.; Yin, C. Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Sun, H.Y.; Li, X.K.; Ren, T.; Cong, R.H.; Lu, J.W. Effects of Fertilizer in Shallow Soils on Growth and Distribution of Rice Roots at Seedling Stage. Sci. Agric. Sin. 2014, 47, 2476–2484. [Google Scholar]
- Cogger, C.G. Potential compost benefits for restoration of soils disturbed by urban development. Compos. Sci. Util. 2005, 13, 243–251. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Xuan, T.; Mekawy, A.; Wang, H.; Khanh, T. Relationship of Salinity Tolerance to Na+ Exclusion, Proline Accumulation, and Antioxidant Enzyme Activity in Rice Seedlings. Agriculture 2020, 8, 166. [Google Scholar] [CrossRef]
- Kibria, M.G.; Hossain, M.; Murata, Y.; Hoque, M.A. Antioxidant Defense Mechanisms of Salinity Tolerance in Rice Genotypes. Rice Sci. 2017, 24, 155–162. [Google Scholar] [CrossRef]
- Chi, K.W. Effects of Maize Straw Composting on Maize Growth and Development and Transcriptome Analysis under Saline-Alkali Stress. Master’s Thesis, Northeast Forestry University, Harbin, China, 2021. [Google Scholar]
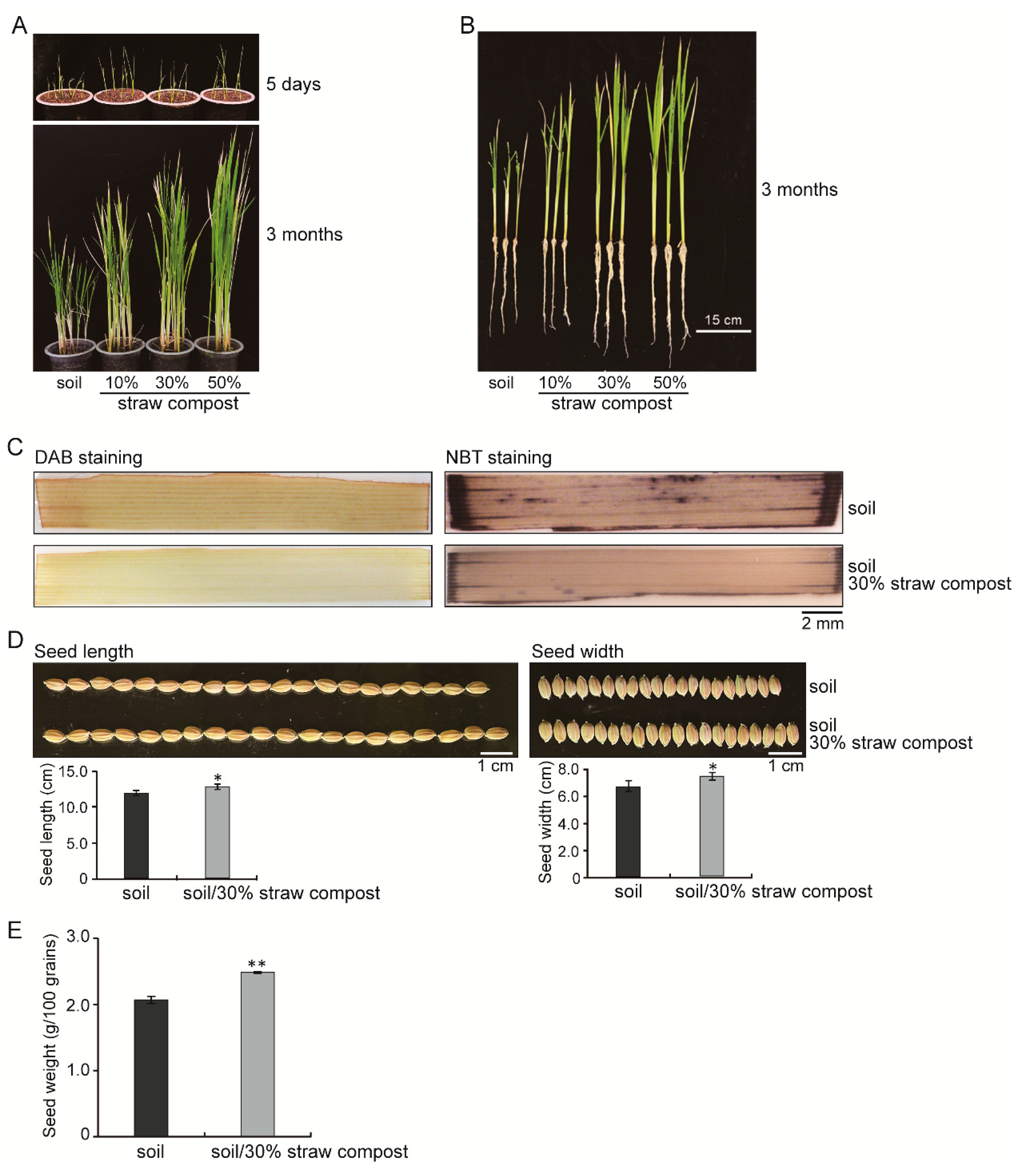

| Sample | Soluble Protein Content (µg/mL) | POD Activity (U/mg) | CAT Activity (U/mg) | APX Activity (U/mg) | SOD Activity (U/mg) | H2O2 Content (µmol/mL) |
|---|---|---|---|---|---|---|
| Soil | 44.074 ± 2.631 a | 23.314 ± 1.066 b | 0.054 ± 0.004 b | 0.005 ± 0 b | 1.209 ± 0.007 b | 0.213 ± 0 a |
| Soil/30%-straw compost | 47.909 ± 1.047 a | 49.239 ± 0.482 a | 0.113 ± 0.014 a | 0.006 ± 0 a | 1.444 ± 0.058 a | 0.075 ± 0.005 b |
| Sample | Chlorophyll a (µg/mL) | Chlorophyll b (µg/mL) | Carotenoid (µg/mL) |
|---|---|---|---|
| Soil | 8.81 ± 0.24 b | 3.54 ± 0.30 a | 2.96 ± 0.06 b |
| Soil/30%-straw compost | 11.04 ± 0.05 a | 3.98 ± 0.09 a | 3.81 ± 0.22 a |
| Sample | Soil pH | Organic Matter (g/kg) | Total Nitrogen (N) % | Total Phosphorus (P) (g/kg) |
|---|---|---|---|---|
| Soil | 4.87 ± 0.12 b | 206 ± 6.08 b | 0.73 ± 0.04 a | 0.63 ± 0.02 b |
| Soil/30%-straw compost | 6.35 ± 0.02 a | 252 ± 4.58 a | 0.87 ± 0.10 a | 1.11 ± 0.03 a |
| Sample | Sucrase Gmg/(g·24 h) | Urease mg/(g·24 h) | Cellulase µg/(mL·min) | Phosphatase mg/(g·24 h) |
|---|---|---|---|---|
| Soil | 8.48 ± 0.11 b | 1.15 ± 0.03 b | 0.46 ± 0.03 b | 25.59 ± 0.12 b |
| Soil/30%-straw compost | 15.17 ± 0.09 a | 1.45 ± 0.05 a | 2.28 ± 0.03 a | 33.00 ± 1.73 a |
| Sample | Bacteria (CFU/g) | Fungus (CFU/g) | Actinomycetes (CFU/g) |
|---|---|---|---|
| Soil | 1.53 × 107 ± 3.46 × 105 b | 7.07 × 104 ± 9.53 × 102 a | 1.19 × 105 ± 9.64 × 103 b |
| Soil/30%-straw compost | 2.30 × 107 ± 2.65 ×105 a | 7.08 × 104 ± 4.58 × 102 a | 4.27 × 105 ± 1.35 × 104 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, Z.; Zhao, Z.; Xu, K.; Chen, H.; Feng, Y.; Wang, W.; Zhang, N.; Liu, D.; He, X.; et al. Low-Temperature Fermented Straw Compost Regulates Rice Growth and Yield by Affecting Soil Physicochemical Properties and the Expression of Important Signaling Pathway Genes. Agronomy 2023, 13, 3066. https://doi.org/10.3390/agronomy13123066
Liu T, Liu Z, Zhao Z, Xu K, Chen H, Feng Y, Wang W, Zhang N, Liu D, He X, et al. Low-Temperature Fermented Straw Compost Regulates Rice Growth and Yield by Affecting Soil Physicochemical Properties and the Expression of Important Signaling Pathway Genes. Agronomy. 2023; 13(12):3066. https://doi.org/10.3390/agronomy13123066
Chicago/Turabian StyleLiu, Tongtong, Ziguang Liu, Ziyi Zhao, Kai Xu, Heshu Chen, Yanzhong Feng, Wentao Wang, Nan Zhang, Di Liu, Xinmiao He, and et al. 2023. "Low-Temperature Fermented Straw Compost Regulates Rice Growth and Yield by Affecting Soil Physicochemical Properties and the Expression of Important Signaling Pathway Genes" Agronomy 13, no. 12: 3066. https://doi.org/10.3390/agronomy13123066
APA StyleLiu, T., Liu, Z., Zhao, Z., Xu, K., Chen, H., Feng, Y., Wang, W., Zhang, N., Liu, D., He, X., & Wu, J. (2023). Low-Temperature Fermented Straw Compost Regulates Rice Growth and Yield by Affecting Soil Physicochemical Properties and the Expression of Important Signaling Pathway Genes. Agronomy, 13(12), 3066. https://doi.org/10.3390/agronomy13123066





