The Supply of Macro- and Microelements to Cotton Plants at Different Distances from a Fertilizer Production Factory
Abstract
1. Introduction
- To investigate the composition of technogenic dust from the pipes of the factory;
- To determine the ash contents in washed and in unwashed cotton leaves;
- To determine the chemical compositions of the above- and below-ground organs of cotton at different distances from the pollutant source, and to determine the protein content in the leaves of the plants;
- To develop empirical models describing the relationships among the concentrations of P, K, Mg, Ca, S, Mn, and F in the roots and leaves of cotton plants;
- To identify zones of technogenic stress.
2. Materials and Methods
2.1. Site Description
2.2. Sampling
2.3. Analyses
2.4. Data Analyses
3. Results
3.1. Compositions of Cotton Leaves and Roots
3.2. Contents of Fluorine in Cotton Leaves and in Roots
3.3. Microelements
3.4. Alkali and Alkaline Earth Metals
3.5. Cotton Yield as Affected by the Distance from the Pollutant
4. Discussion
4.1. Contents of Phosphorus in Cotton Leaves and in Roots
4.2. Contents of Fluorine in Cotton Leaves and in Roots
4.3. Microelements
4.3.1. Manganese
4.3.2. Sulfur
4.4. Alkali and Alkaline Earth Metals
4.5. Cotton Yield Affected by the Distance from the Pollutant
4.6. Impact Zones of the Factory as an Emission Source of Pollutants
4.6.1. Zone of Strong Technogenic Impact (0–3 km from the Factory)
4.6.2. Zone of Moderate Technogenic Impact (5–15 km from the Factory)
4.6.3. Zone of Low Technogenic Impact (15–20 km from the Factory)
5. Conclusions
- In the impact zone of the factory, the chemical and biochemical compositions of cotton plants change;
- The fluorine concentrations made it possible to precisely delimit the radius of the most intense atmospheric dispersion of emissions from the pipes of the fertilizer factory. Two impact zones were identified in the study area: <5 km and >5 km from the pollution source. These zones correspond to a strong and a moderate technogenic impact;
- The physiological mechanisms of cotton make it possible to control the negative impacts of technogenic emissions by regulating the metabolic processes in its tissues. It is possible to grow cotton at a distance > 5 km from a nitrogen–phosphate fertilizer factory;
- Considering the results obtained, it is recommended that the analysis of the chemical compositions of plants located in an impact zone be carried out only after removing dust particles from their surface;
- The recommendation is to continuously monitor the concentrations of microelements in agricultural crops in the area most vulnerable to technogenic pollution;
- The results of this study can be applied to other cotton-growing areas with similar climatic and soil conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anand, P.; Mina, U.; Khare, M.; Kumar, P.; Kota, S.H. Air pollution and plant health response-current status and future directions. Atmos. Pollut. Res. 2022, 13, 101508. [Google Scholar] [CrossRef]
- Fowler, D.; Brimblecombe, P.; Burrows, J.; Heal, M.R.; Grennfelt, P.; Stevenson, D.S.; Jowett, A.; Nemitz, E.; Coyle, M.; Liu, X.; et al. A chronology of global air quality. Philos. Trans. R. Soc. A 2020, 378, 2019031420190314. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Ustyuzhanina, P. Decomposition of air pollution emissions from Swedish manufacturing. Environ. Econ. Policy Stud. 2022, 24, 195–223. [Google Scholar] [CrossRef]
- Al-Hasnawi, S.S.; Hussain, H.M.; Al-Ansari, N.; Knutsson, S. The Effect of the Industrial Activities on Air Pollution at Baiji and Its Surrounding Areas, Iraq. Engineering 2016, 8, 34–44. [Google Scholar] [CrossRef][Green Version]
- Assanov, D.; Zapasnyi, V.; Kerimray, A. Air Quality and Industrial Emissions in the Cities of Kazakhstan. Atmosphere 2021, 12, 314. [Google Scholar] [CrossRef]
- Belozertseva, I.A.; Milić, M.; Tošić, S.; Saljnikov, E. Environmental Pollution in the Vicinity of an Aluminium Smelter in Siberia. In Advances in Understanding Soil Degradation; Saljnikov, E., Mueller, L., Lavrishchev, A., Eulenstein, F., Eds.; Innovations in Landscape Research; Springer: Cham, Switzerland, 2022; pp. 379–402. [Google Scholar] [CrossRef]
- Idrees, M.; Nergis, Y.; Irfan, M. Industrial Emission Monitoring and Assessment of Air Quality in Karachi Coastal City, Pakistan. Atmosphere 2023, 14, 1515. [Google Scholar] [CrossRef]
- Lukyanets, A.; Gura, D.; Savinova, O.; Kondratenko, L.; Lushkov, R. Industrial emissions effect into atmospheric air quality: Mathematical modeling. Rev. Environ. Health 2023, 38, 385–393. [Google Scholar] [CrossRef]
- Saljnikov, E.; Mrvic, V.; Cakmak, D.; Jaramaz, D.; Perovic, V.; Antic-Mladenovic, S.; Pavlovic, P. Pollution indices and sources apportionment of heavy metal pollution of agricultural soils near the thermal power plant. Environ. Geochem. Health 2019, 41, 2265–2279. [Google Scholar] [CrossRef]
- Westley, M.L.; Hicks, B.B. Some factors that affect the deposition rates of sulfur dioxide and similar gases on vegetation. J. Air Pollut. Control Assoc. 1977, 27, 1110–1116. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Zhai, J.; Cong, L.; Wang, Y.; Ma, W.; Zhang, Z.; Li, C. Comparison of dry and wet deposition of particulate matter in near surface waters during summer. PLoS ONE 2018, 13, e0199241. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Bessonova, V.B.; Lyzhenko, I.I. Accumulation of Heavy Metals by Ecosystem Components in Industrial Areas. Heavy Metals in the Environment and Nature Conservation; Moscow State University Publisher: Moscow, Russia, 1988; Part 2; 240p. [Google Scholar]
- Bui, H.-T.; Jeong, N.-R.; Park, B.-J. Seasonal Variations of Particulate Matter Capture and the Air Pollution Tolerance Index of Five Roadside Plant Species. Atmosphere 2023, 14, 138. [Google Scholar] [CrossRef]
- Chatterjee, N.; Sahu, G.; Bag, A.G.; Plal, B.; Hazra, G.C. Role of Fluoride on Soil. Plant and Human Health: A Review on Its Sources. Toxicity and Mitigation Strategies. Int. J. Environ. Clim. Chang. 2020, 10, 77–90. [Google Scholar] [CrossRef]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Syed Ali, M.N.V.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mat. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Cronin, S.J.; Manoharan, V.; Hedley, M.J.; Loganathan, P. Fluoride: A review of its fate: Bioavailability and risks of fluorosis in grazed-pasture systems in New Zealand. N. Z. J. Agric. Res. 2000, 43, 295–321. [Google Scholar] [CrossRef]
- Darral, N.M. The effect of air pollutants on physiological processes in plants. Plant Cell Environ. 1989, 12, 1–30. [Google Scholar] [CrossRef]
- Ilkun, G.N. Air Pollution and Plants; Nauka, Dumka Publishing: Kyiv, Ukraine, 1978; 247p. [Google Scholar]
- Makete, N.; Rizzu, M.; Seddaiu, G.; Gohole, L.; Otinga, A. Fluoride toxicity in cropping systems: Mitigation, adaptation strategies and related mechanisms. A review. Sci. Total Environ. 2022, 883, 155129. [Google Scholar] [CrossRef]
- Minkina, T.; Mandzhieva, S.; Chapligin, V.; Motuzova, G.; Sushkova, S.; Fedorov, Y.; Kolesnikov, S.; Bauer, T. Accumulation and distribution of heavy metals in plants within the technogenesis zone. Environ. Eng. Manag. J. 2014, 13, 1307–1315. [Google Scholar] [CrossRef]
- Stevens, C.J.; Bell, J.N.B.; Brimblecombe, P.; Clark, C.M.; Dise, N.B.; Fowler, D.; Lovett, G.M.; Wolseley, P.A. The impact of air pollution on terrestrial managed and natural vegetation. Philos. Trans. R. Soc. A 2020, 378, 2019031720190317. [Google Scholar] [CrossRef]
- Tressaud, A. Fluorine, a Key Element for the 21St Century. In Progress in Fluorine Science; Tressaud, A.B.T.-F., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 5, pp. 77–150. [Google Scholar] [CrossRef]
- Chaudhry, M.R. Cotton Production and Processing, Industrial Applications of Natural Fibres; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 219–234. [Google Scholar]
- Nix, A.; Paull, C.; Colgrave, M. Flavonoid Profile of the Cotton Plant, Gossypium hirsutum: A Review. Plants 2017, 6, 43. [Google Scholar] [CrossRef]
- Rogers, G.M.; Poore, M.H.; Paschal, J.C. Feeding cotton products to cattle. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.R.; Guitchounts, A. Cotton Facts. Technical Paper No.25, Common Fund for Commodities, International Cotton Advisory Committee. 2003. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.icac.org/Content/LearningCorner/Cotton%20Facts/Cotton%20Facts.pdf (accessed on 28 May 2023).
- Ibragimov, B.O. Factors affecting growth and development of cotton. Univers. Tech. Sci. 2020, 8, 77. Available online: https://7universum.com/ru/tech/archive/item/10604 (accessed on 10 November 2023).
- International Cotton Advisory Committee (ICAC). Cotton World Statistics; International Cotton Advisory Committee (ICAC): Washington, DC, USA, 2017. [Google Scholar]
- Brewer, R.F.; Ferry, G. Effects of air pollution on cotton in the San Joaquin Valley. Calif. Agric. 1974, 28, 6–7. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://calag.ucanr.edu/download_pdf.cfm?article=ca.v028n06p6 (accessed on 29 May 2023).
- Uzbekistan in Numbers 2006. State Statistical Committee of the Republic of Uzbekistan, Tashkent. 2007. Available online: https://stat.uz/ru/ (accessed on 29 May 2023).
- FAO. Recent Trends and Prospects in the World Cotton Market and Policy Developments; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Li, L.; Wu, H.; Gao, Y.; Zhang, S. Predicting Ecologically Suitable Areas of Cotton Cultivation Using the MaxEnt Model in Xinjiang, China. Ecologies 2023, 4, 654–670. [Google Scholar] [CrossRef]
- Saini, D.K.; Impa, S.M.; McCallister, D.; Patil, G.B.; Abidi, N.; Ritchie, G.; Jaconis, S.Y.; Jagadish, K.S.V. High day and night temperatures impact on cotton yield and quality—Current status and future research direction. J. Cotton Res. 2023, 6, 16. [Google Scholar] [CrossRef]
- Radhakrishnan, S. Sustainable cotton production. In Sustainable Finres and Textiles; Senthuikannan, M.S., Ed.; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar] [CrossRef]
- Wright, D.L.; Esquivel, I.; George, S.; Small, I. Cotton Growth and Development; IFAS Extension, University of Florida: Gainesville, FL, USA, 2022; SS-agr-238; Available online: https://edis.ifas.ufl.edu/publication/ag235 (accessed on 29 May 2023).
- WRB. World reference base for soil resources 2014. In World Soil Resources Reports; No. 106; FAO: Rome, Italy, 2014; 189p. [Google Scholar]
- Novitsky, M.V.; Lavrishchev, A.V.; Nazarova, A.V.; Shabanov, M.V.; Rodicheva, T.V.; Melnikov, S.P.; Bayeva, N.N.; Kolodka, V.P. Laboratory and Practical Classes in Soil Science; St. Petersburg Nauka Avenue Publishing: St. Petersburg, Russia, 2021; 332p. [Google Scholar]
- Bure, V.M. Methodology of Statistical Analysis of Experimental Data; Agrophysical Research Institute, St. Petersburg State University: St. Petersburg, Russia, 2007; 141p. [Google Scholar]
- Nawaz, M.; Dawood, M.; Javaid, K.; Imran, M.; Zhao, F.; Saima, S.; Shah, S.T.H. Evaluating the environmental impacts of fluoride on the growth and physiology of cotton (Gossypium hirsutum). Pak. J. Agric. Res. 2021, 34, 425–430. [Google Scholar] [CrossRef]
- Mondal, P.; George, S. Removal of fluoride from drinking water using novel adsorbent magnesia-hydroxyapatite. Water Air Soil Pollut. 2015, 226, 241. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Jernstedt, J. Morphology and Anatomy of the Cotton Plant Chapter 2.1. In Cotton: Origin, Hystory, Technology, and Production; Smith, W.C., Cothren, J.T., Eds.; Smith, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of manganese nanoparticle exposure on nutrient acquisition in wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. [Google Scholar] [CrossRef]
- Narayan, O.P.; Kumar, P.; Yadav, B.; Dua, M.; Johri, A.K. Sulfur nutrition and its role in plant growth and development. Plant Signal. Behav. 2022, 2030082. [Google Scholar] [CrossRef]
- Domingos, M.; Klumpp, A.; Rinaldi, M.C.S.; Modesto, I.F.; Klumpp, G.; Delitti, W.B.C. Combined effects of air and soil pollution by fluoride emissions on Tibouchina pulchra Cogn., at Cubatão, SE Brazil, and their relations with aluminium. Plant Soil 2003, 249, 297–308. [Google Scholar] [CrossRef]
- Kropff, M.J. Long-term effect of SO2 on plants, SO2 metabolism and regulation of intracellular pH. Plant Soil 1991, 131, 235–245. [Google Scholar] [CrossRef]
- Shao, J.; Dong, H.; Jin, Y.; Li, P.; Sun, M.; Feng, W.; Zheng, C. Effects of soil potassium levels on dry matter and nutrient accumulation and distribution in cotton. J. Cotton Res. 2023, 6, 10. [Google Scholar] [CrossRef]
- Dong, H.; Tang, W.; Li, Z.; Zhang, D. On potassium deficiency in cottone disorder, cause and tissue diagnosis. Agric. Conspect. Sci. 2004, 69, 77–85. [Google Scholar]
- Encyclopedia of cotton growing. Tashkent 1985, 1, 561.
- Bednarz, C.W.; Oosterhuis, D.M. Physiological changes associated with potassium deficiency in cotton. J. Plant Nutr. 1999, 22, 303–313. [Google Scholar] [CrossRef]
- Hu, W.; Dai, Z.; Yang, J.; Snider, J.L.; Wang, S.; Meng, Y.; Wang, Y.; Chen, B.; Zhao, W.; Zhou, Z. Cultivar sensitivity of cotton seed yield to potassium availability is associated with differences in carbohydrate metabolism in the developing embryo. Field Crop Res. 2017, 214, 301–309. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Prasad, S.M. Sulfur and Calcium Simultaneously Regulate Photosynthetic Performance and Nitrogen Metabolism Status in As-Challenged Brassica juncea L. Seedlings. Front. Plant Sci. 2018, 9, 772. [Google Scholar] [CrossRef]
- Zakari, S.; Jiang, X.; Zhu, X.; Liu, W.; Gloriose, M.; Allakonon, B.; Singh, A.K.; Chen, C.; Zou, X.; Irénikatché Akponikpè, P.B.; et al. Influence of sulfur amendments on heavy metals phytoextraction from agricultural contaminated soils: A meta-analysis. Environ. Pollut. 2021, 288, 117820. [Google Scholar] [CrossRef] [PubMed]
| Distance from the Factory, km | Latitude | Longitude |
|---|---|---|
| 0.6 | 39°40′24.1″ | 66°48′32.7″ |
| 3.0 | 39°41′31.6″ | 66°47′43.7″ |
| 5.0 | 39°42′20.3″ | 66°46′48.5″ |
| 7.5 | 39°43′30.5″ | 66°45′55.9″ |
| 10.0 | 39°44′28.0″ | 66°44′34.4″ |
| 12.5 | 39°45′38.0″ | 66°43′44.0″ |
| 15.0 | 39°46′40.7″ | 66°42′35.3″ |
| 20.0 | 39°48′55.9″ | 66°40′34.2″ |
| Distance from the Factory, km | Ash Content of Unwashed Leaves | Ash Content of Washed Leaves | Difference | |
|---|---|---|---|---|
| g | % | |||
| 0.6 | 21.1 a | 19.1 a | 2.0 | 9.5 |
| 3 | 21.4 a | 17.1 a | 4.3 | 20.1 |
| 5 | 23.5 a | 21.2 ab | 2.3 | 9.8 |
| 10 | 22.6 a | 21.0 ab | 1.6 | 7.1 |
| 15 | 21.7 a | 20.6 a | 1.1 | 5.1 |
| 20 | 20.9 a | 19.2 a | 1.7 | 8.1 |
| Distance from the Factory, km | Mg | P | S | K | Ca | Protein |
|---|---|---|---|---|---|---|
| 0.6 | 1.08 0.66 a | 0.57 0.12 a | 1.37 1.30 a | 1.37 1.10 a | ND 5.65 a | 10.6 a |
| 3 | 0.67 a | 0.11 a | 1.29 a | 1.40 a | 6.05 a | 10.3 a |
| 7.5 | 0.61 a | 0.08 b | 1.04 b | 1.51 ba | 5.21 ab | 12.0 a |
| 10 | 0.59 0.60 a | 0.37 0.11 a | 0.94 1.16 b | 1.83 1.63 b | ND 5.02 b | 12.9 ab |
| 12.5 | 0.53 b | 0.16 a | 0.91 b | 1.56 ba | 5.01 b | 12.9 ab |
| 15 | 0.46 0.59 ab | 0.40 0.12 a | 1.00 1.20 ab | 1.53 1.68 b | ND 5.07 b | 14.1 b |
| 20 | 0.49 b | 0.09 b | 0.84 b | 1.68 b | 4.52 b | 14.2 b |
| Control | 0.48 b | 0.08 b | 0.83 b | 1.61 b | 4.69 b |
| Distance from the Factory, km | Mg | P | S | K | Ca |
|---|---|---|---|---|---|
| 0.6 | 0.46 a | 0.18 a | 0.09 a | 1.64 a | 0.77 a |
| 3 | 0.40 a | 0.16 a | 0.11 a | 1.58 a | 1.15 b |
| 7.5 | 0.31 b | 0.17 a | 0.11 a | 1.41 b | 0.58 a |
| 10 | 0.30 b | 0.19 a | 0.10 a | 1.45 b | 0.68 a |
| 12.5 | 0.33 b | 0.18 a | 0.11 a | 1.42 b | 0.69 a |
| 15 | 0.37 ab | 0.16 a | 0.10 a | 1.45 b | 0.71 a |
| 20 | 0.28 b | 0.11 b | 0.06 ba | 1.39 b | 0.51 a |
| Control | 0.27 b | 0.13 b | 0.10 a | 1.30 b | 0.60 a |
| Model | p-Value | R2 | v |
|---|---|---|---|
| 0.14 | 0.38 | −0.0024 | |
| 0.9 | 0.0014 | −0.00015 | |
| 0.31 | 0.2 | 0.46 | |
| 0.008 | 0.945 | − | |
| 0.02 | 0.9999 | − | |
| 0.23 | 0.27 | −0.0014 | |
| 0.04 | 0.58 | −0.02 | |
| 0.36 | 0.16 | 0.04 | |
| 0.053 | 0.558 | −0.007 | |
| 0.0038 | 0.837 | −0.0088 | |
| 0.05 | 0.559 | 0.73 | |
| 0.12 | 0.41 | −0.019 | |
| 0.0056 | 0.81 | −0.66 | |
| 0.007 | 0.79 | 0.369 | |
| 0.017 | 0.71 | −0.012 | |
| 0.01 | 0.76 | 0.027 | |
| 0.008 | 0.781 | −0.41 | |
| 0.0006 | 0.92 | 0.22 |
| Distance from the Factory, km | Roots | Leaves | |
|---|---|---|---|
| Without Washing | With Washing | ||
| 0.6 | 26.5 a | 70.0 a | 17.5 a |
| 3 | 7.0 b | 300.0 b | 72.0 b |
| 5 | 3.5 c | 50.0 a | 12.0 a |
| 7.5 | 2.4 c | 40.0 a | 12.0 a |
| 10 | 2.25 c | 23.0 ac | 7.0 ac |
| 12.5 | 2.65 c | 20.0 c | 5.2 c |
| 15 | 5.6 bc | 21.0 c | 4.8 c |
| 20 | 3.6 c | 1805 d | 4.2 c |
| 25 | 4.2 bc | 12.2 c | 4.1 c |
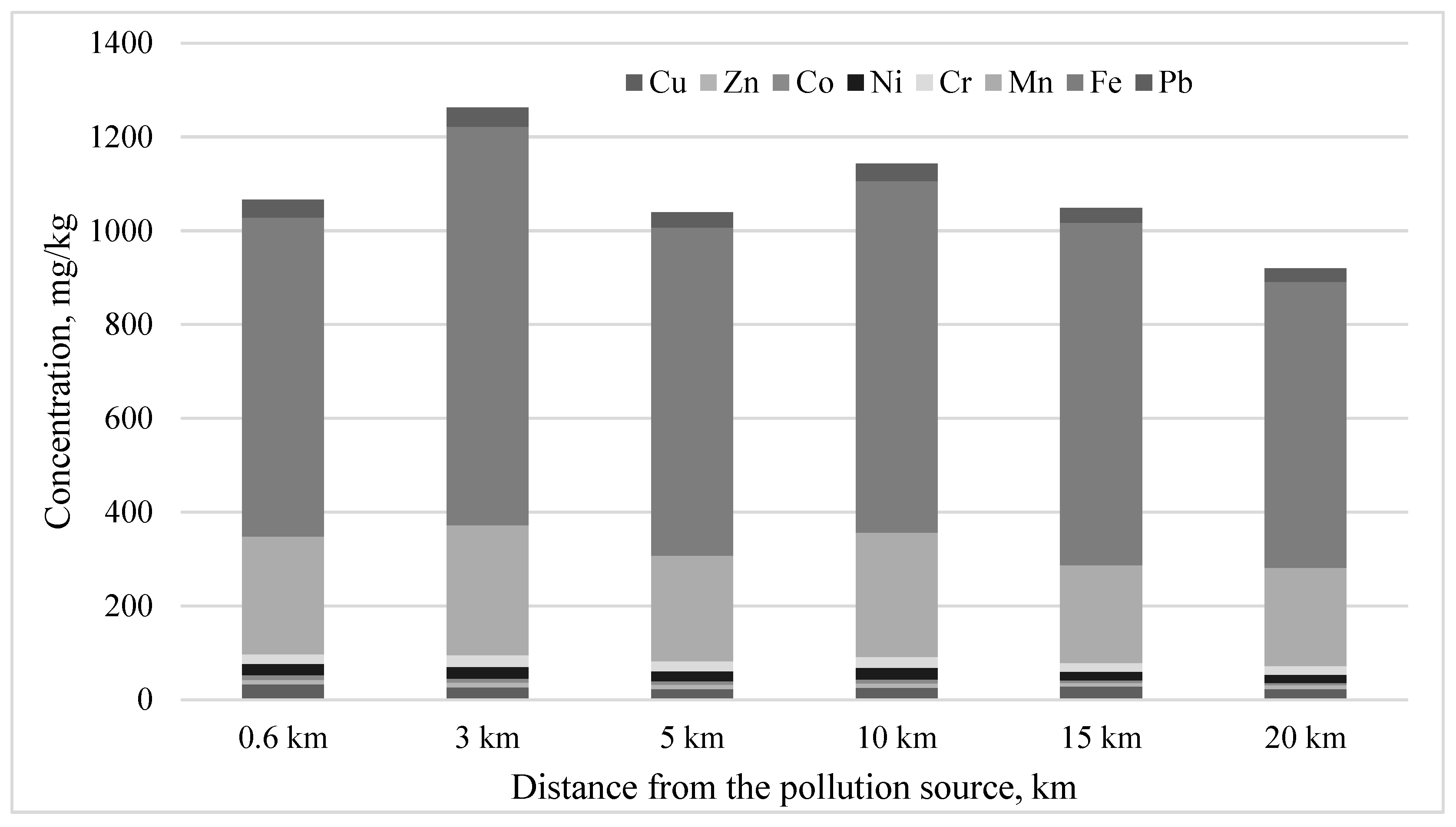
| Element | Distance from the Factory, km | |||||
|---|---|---|---|---|---|---|
| 0.6 | 3 | 5 | 10 | 15 | 20 | |
| Zn | 22.0 a | 16.2 a | 16.2 a | 15.0 a | 21.8 a | 21.8 a |
| Co | 2.0 a | 2.0 a | 2.0 a | 1.8 a | 1.9 a | 1.8 a |
| Pb | 10.2 a | 10.5 a | 10.0 a | 10.5 a | 11.0 a | 10.2 a |
| Ni | 3.7 a | 3.6 a | 3.5 a | 3.6 a | 3.7 a | 3.3 a |
| Cr | 1.3 a | 1.2 a | 1.5 a | 1.6 a | 1.4 a | 1.2 a |
| Mn | 101.0 a | 56.0 b | 50.0 b | 61.0 b | 63.5 b | 57.5 b |
| Fe | 135.0 b | 140.0 b | 165.0 a | 150.0 ab | 140.0 b | 100.0 c |
| Cu | 8.4 a | 5.4 b | 5.2 b | 5.8 b | 7.4 ab | 7.4 ab |
| Distance from the Factory, km | Average Plant Height, cm | Productivity, t/ha |
|---|---|---|
| 0.6 | 63.7 | 2.0 |
| 3 | 64.8 | 2.1 |
| 5 | 70.1 | 2.8 |
| 10 | 79.3 | 2.6 |
| 15 | 89.1 | 2.6 |
| 20 | 75.5 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litvinovich, A.; Lavrishchev, A.; Bure, V.M.; Turebayeva, S.; Kenzhegulova, S.; Dutbayev, A.; Slyamova, N.; Zhapparova, A.; Saljnikov, E. The Supply of Macro- and Microelements to Cotton Plants at Different Distances from a Fertilizer Production Factory. Agronomy 2023, 13, 3063. https://doi.org/10.3390/agronomy13123063
Litvinovich A, Lavrishchev A, Bure VM, Turebayeva S, Kenzhegulova S, Dutbayev A, Slyamova N, Zhapparova A, Saljnikov E. The Supply of Macro- and Microelements to Cotton Plants at Different Distances from a Fertilizer Production Factory. Agronomy. 2023; 13(12):3063. https://doi.org/10.3390/agronomy13123063
Chicago/Turabian StyleLitvinovich, Andrey, Anton Lavrishchev, Vladimir M. Bure, Sagadat Turebayeva, Sayagul Kenzhegulova, Ayan Dutbayev, Nazira Slyamova, Aigul Zhapparova, and Elmira Saljnikov. 2023. "The Supply of Macro- and Microelements to Cotton Plants at Different Distances from a Fertilizer Production Factory" Agronomy 13, no. 12: 3063. https://doi.org/10.3390/agronomy13123063
APA StyleLitvinovich, A., Lavrishchev, A., Bure, V. M., Turebayeva, S., Kenzhegulova, S., Dutbayev, A., Slyamova, N., Zhapparova, A., & Saljnikov, E. (2023). The Supply of Macro- and Microelements to Cotton Plants at Different Distances from a Fertilizer Production Factory. Agronomy, 13(12), 3063. https://doi.org/10.3390/agronomy13123063






