Abstract
Calcium (Ca) plays a vital role as a macronutrient in the growth and development of plants. In order of decreasing solubility, Ca can be found in vegetal tissues as soluble Ca (Fraction I), bound Ca (mainly pectates, Fraction II), inorganic insoluble Ca (mainly phosphates and carbonates, Fraction III) and organic insoluble Ca or oxalate (Fraction IV). To explore the impact of Ca fertilizer application on plant growth and its allocation among different fractions, young citrus trees were fed over a complete vegetative cycle with a 44Ca labeled fertilizer (T1-Ca), while control plants (T2) received no Ca fertilizer. The results showed that plants receiving Ca exhibited significantly greater biomass. 44Ca derived from the fertilizer was localized mainly in sink organs (new flush leaves–twigs and fibrous roots). The primary fraction responsible for total Ca partitioning was Fraction II, followed by Fraction III or IV. Citrus plants, commonly found in calcareous soils, demonstrated improved growth with calcium treatments, indicating a positive link between calcium supplementation and enhanced development. The calcium supplied through the fertilizer (44Ca) was predominantly concentrated in sink organs (mainly in Ca-pectate fraction), including new flush leaves and twigs above ground, as well as fibrous roots below ground.
1. Introduction
Calcium (Ca) is an essential macronutrient in plants, which is involved in several biochemical and physiological processes needed for growth and development as both a structural component and an intracellular second messenger in a variety of processes [1,2,3]. The versatility of this macronutrient is derived from Ca being able to interact with membranes, proteins and organic acids. The ability of Ca to form different coordination bonds, from six to nine, results in high affinity for carboxylate oxygen, rapid binding kinetics and complex geometries [4,5].
Ca uptake from soil and its translocation to different plant organs, including fruit, are controlled by distinct factors along the soil–root–fruit pathway. Ca is taken up by plants in the form of Ca2+ ions through channels in the membrane of fibrous roots on both the symplastic (for root nutrition and cell signaling) and apoplastic (for transfer to shoots) pathways [6]. As Ca is a practically immobile element in phloem, it does not move easily from old to young tissues. Therefore, the majority of Ca uptake in plants is largely passive. Accordingly, the transpiration and movement of water through plants are crucial for Ca uptake [2,7].
Once inside plants, Ca plays the above-mentioned intracellular messenger role. As such, plant cells reprogram their cellular setup by triggering a network of signaling events, which start with stress perception at the cell membrane level and end with a cellular response [8,9]. Many extracellular and environmental cues (light, biotic or abiotic stress factors) elicit changes in cellular Ca levels, termed calcium signatures [10]. Initially, cytosolic-free Ca, [Ca2+]cyt, is low as a toxic cellular compound because Ca triggers the aggregation of proteins and nucleic acids, as well as the precipitation of phosphates (present in ATP), and can affect lipid membrane integrity [5]. After stress perception, however, signaling is triggered by this stimulus to generate various Ca2+-mobilizing signals, and this activates the ON mechanisms, which feed Ca2+ into the cytoplasm [11]. In this regard, part of Ca can be found in the free Ca2+ form (Fraction I) in plants (Figure 1). Despite the Ca flux in the cytosol being provided by external medium and subcellular compartments, part of Ca interacts with the middle lamella in the cell wall to synthesize Ca-pectate, a pectin cross-linking polymer (Fraction II). Ca2+ plays a key role in the cross-linking acidic pectin residues in the cell wall by acting as the bridge of antiparallel homogalacturonan chains with negatively charged carboxyl groups to form structures called “eggboxes” [12]. This helps facilitate extrusion among pectin polymers to form the cell wall network, enhance mechanic strength and limit access for cell wall hydrolases [2]. Ca is also linked with the carboxylic and phosphorylate groups, which are present as, respectively, Ca-carbonate and Ca-phosphate (Fraction III), mainly in the cell membrane [13].
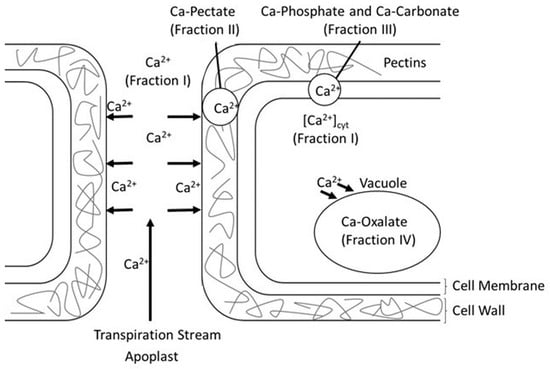
Figure 1.
Scheme of the main calcium stores and cellular compartments in a plant cell.
Then, [Ca2+]cyt functions as a messenger to stimulate numerous Ca2+-sensitive processes. The OFF mechanisms (pumps and exchangers) remove Ca2+ from the cytoplasm to restore the resting state [11]. Ca can be sequestered by the endoplasmic reticulum, mitochondria, plastids and vacuole, or it can be pumped out of the protoplast to keep [Ca2+]cyt low [14,15]. Part of this Ca precipitates in Ca-oxalate crystals (Fraction IV). Although these crystals can be found in cell walls [16,17], they are often formed in the vacuoles of remarkably specialized cells called crystal idioblasts [18,19]. It has been hypothesized that the function of these crystals is Ca regulation and homeostasis, defense against herbivores and Ca reserve [15,20,21]. In citrus crops, it has been reported that large Ca-oxalate-containing palisade cells are produced in the upper epidermis of mature leaves [22].
Ca deficiency in cell walls results in pectin solubilization, with the acceleration of senescence and the development of some physiological disorders, such as albedo breakdown in sweet oranges [23,24].
Despite Ca being naturally present under Mediterranean conditions, and soil being potentially able to cover the nutritional needs of citrus, it is necessary to treat plants with Ca to prevent physio-pathologies because much of the Ca present in soil comes in forms, which are unavailable to or cannot be assimilated by plants [25,26], and this may not always synchronize with the plants’ demand for calcium. Moreover, Ca applications can significantly increase the cell-wall Ca content in the peel. They can also inhibit the degradation of pectin, cellulose and hemicellulose, reduce arabinose and galactose, and increase water-soluble pectin in the peel cell wall [27]. Some studies have analyzed the effect of Ca treatments on fruit quality and on the incidence of fruit physio-pathologies [28,29]. Nevertheless, no works have focused on Ca fertilization influence on Ca uptake from fertilizers and its accumulation in different Ca fractions in plant organs—a dynamic, which could be linked to the incidence of those fruit pathologies. This information would allow us to not only establish the differences in plant parts but also the Ca requirements in different organs, depending on the structuring function as a messenger, or those, which have to accumulate more Ca content in storage compartments.
Stable isotope techniques with nitrogen (N), Ca and iron (Fe) (15N, 44Ca, 57Fe) have been widely used to understand nutrient dynamics and cycling, also known as non-traditional isotopes [30]. With these techniques, it is possible to analyze the fate of some nutrients, which derive from mineral and organic fertilizer applications or are fixed in the atmosphere. With N, 15N-tracing techniques are the methods of choice for quantifying N transformation dynamics, including the processes of gaseous N emissions, nitrate lixiviation and plant N uptake rates [31,32,33]. However, very few references are found, which trace the movement of fertilizer Ca in plant–water–soil. We are able to ascertain this based on the use of labeled fertilizers with 44Ca. In citrus fruit, the uptake and distribution of Ca deriving from fertilizers in “Clemenules” and “Fukumoto” mandarin trees have been studied using the isotope labeling technique with radioactive isotope 45Ca [34,35]. This approach opens new possibilities for gathering insights into calcium nutrition in crops, offering a valuable alternative to radioactive isotopes. Therefore, in the present work, the quantification of Ca uptake from fertilizers labeled with stable isotopes (44Ca) was carried out in young citrus plants grown in soil throughout a vegetative cycle. In addition, the partitioning of Ca uptake was studied by means of Ca fraction quantification in different plant organs and both labeled and unlabeled fractions. The “non-application” of Ca and its effect on plant growth and partitioning in plants was also analyzed.
2. Materials and Methods
2.1. Experimental Conditions and Plant Material
The study was carried out at the experimental station of the Valencian Institute of Agricultural Research (IVIA) in Moncada (Valencia), Spain (39°33′ N; 24°24′ W). In March, fifteen 2-year-old “Salustiana” sweet orange plants (Citrus sinensis L. Osbeck) grown in a greenhouse were transplanted into 4 kg pots containing loam soil to be left in a greenhouse for a complete vegetative cycle. Soil typical of a Mediterranean citrus cultivation area—characterized by 45.6% sand, 37.3% silt, 17.1% clay, pH 8.5 (slightly alkaline), total carbonates 23.1 (calcareous), 5.0% active limestone (low chlorophyll), low organic matter content (0.69%), slightly low Olsen phosphorus (P) (19.2%), low potassium (K; 0.30 meq 100 g soil−1), normal magnesium (Mg) and Ca (1.7 and 8.5 meq 100 g soil−1, respectively) and electrical conductivity of 290 µS cm−1—was used as substrate. In agricultural soil, the extractable Ca values ranging between 5 and 10 meq/100 g of soil are considered optimal. Soil determinations were made following the Official Methods of the Spanish Ministry of Agriculture, Food and Fisheries [36], with minor modifications (using reactive Panreac Co., Ltd., Barcelona, Spain): granulometry (25 mm, 5 mm and 2 mm), pH (1:25 water extract), electrical conductivity ((EC), 1:5 water extract), oxidizable organic carbon (OOC), oxidization with K2Cr2O7, organic N (Kjeldahl method), ammonium and nitrate N (2 N KCl extract), macronutrients (HCl digestion), micronutrients and heavy metals (aqua regia digestion). The pH was measured with a pH meter (Basic 20, Crison, Barcelona, Spain), EC with a conductometer (Sensor+ EC7, Hach, Barcelona, Spain), WSOC content using a Total Organic Carbon Analyzer (TOC-VCSN, Shimadzu, Kyoto, Japan), organic and mineral N using a 8200 Kjeltec (Foss, Tecator AB, Hoeganaes, Sweden), and the total concentrations of phosphorous (P), potassium (K), Ca and magnesium (Mg) were measured with simultaneous inductively coupled plasma atomic emission spectrometry (iCAP-AES 6000, Thermo Scientific, Cambridge, UK).
Regarding the initial state, at the beginning of April, three plants were removed to ascertain their biomass and nutrient content. Old leaves and branches, the trunk, coarse and fibrous roots were separated. The biomass of all these fractions was freshly weighed, and a sample of each one was washed with deionized water and frozen with liquid nitrogen; humidity was removed by lyophilization, and dry weight (DW) was noted.
2.2. Ca Uptake Assay
During the complete vegetative cycle, two treatments were performed to study the effect of applying Ca with the isotope dilution technique. Citrus trees were fertilized from March to October with the doses of nutrients indicated in Table 1 depending on plant size and soil fertility. Two treatments were carried out: one with (T1-Ca) and one without calcium (T2) applications. In the T1-Ca treatment, six plants were fertilized using fertilizers labeled with stable 44Ca isotopes during a vegetative period (March to October) to evaluate annual Ca uptake by young citrus plants. N was provided by the sources indicated at the bottom of Table 1: Ca nitrate, ammonium nitrate and potassium nitrate. Two sources were used—the 44Ca form in Ca(NO3)2 (Cambridge Isotope Laboratories, Inc., Andover, MA, USA) and YaraLiva™ Calcinit (Yara Ca nitrate)—to provide 156 mg 44Ca per tree with 10.35% 44Ca in excess. P was supplied as phosphoric acid, K as potassium nitrate and Mg as magnesium sulphate. Fe, Zinc (Zn) and manganese (Mn) were supplied in multiple chelate forms (4.5% Fe, 0.5% Zn and 1.0% Mn).

Table 1.
Annual dose and monthly distribution of fertilizers in standard nutrient solution.
For T2, in the absence of Ca, the fertilizers indicated in Table 1 were applied (potassium nitrate and ammonium nitrate) to provide the N doses. The remaining macros (K, P and Mg) and micros (Fe, Zn and Mn) were supplied with the same fertilizers described in T1.
2.3. Collection and Extraction of Plants
Throughout the growth cycle, petals, ovaries and fruit at different growth stages, along with leaves from the previous flush (old leaves), were naturally abscised from the plants. These organs—petals, ovaries, developing fruit and old leaves—were collected weekly through a mesh system to quantify the amounts of 44Ca uptake by the litter organs (abscised organs) in both treatments during the greatest physiological drop period (from beginning of May to end of July). During lethargy (January of the subsequent growing season), plants were extracted from soil, and young organs (ripe fruit, leaves and branches of the different sprouts (spring, summer and autumn), old organs (leaves, old branches and trunk) and the root system (coarse and fibrous roots) were separated. Then, they were weighed, and a sample was taken from each one of these fractions. Samples were washed with deionized water, lyophilized to constant DW, and DW was recorded. Plant samples were ground with a water-refrigerated mill, sieved through a 0.3 mm mesh sieve and stored at −20 °C for further analyses.
2.4. Calcium Analysis
The Ca concentration was measured with simultaneous inductively coupled plasma atomic emission spectrometry (ICAP-AES 6000, Thermo Scientific, Cambridge, UK) after nitric-perchloric digestion [37]. The dried plant material (0.5 g) was predigested overnight with 10 mL HNO3 on a digestion block at 120 °C. Samples were cooled down to room temperature, and 2.0 mL of 70% ultratrace-metal grade HClO4 was added and redigested at 220 °C until white fumes appeared. The digested product was diluted to 25 mL with ultrapure water, and Ca concentrations were subsequently measured [38].
2.5. Separation and Analysis of Ca Fractions
In order to determine the distribution of the different Ca fractions in plants, the biomass (DW) of each fraction, the total Ca concentration of these organs and the Ca concentration in each separate fraction were measured.
In the plant samples, different Ca fractions were separated according to the Ca fractionation analytical procedure described by Ohta et al. [39], with slight modifications to update and adapt it to our format (Figure 2). Four fractions were determined as opposed to five, given that the fraction extracted with water was negligible and was incorporated into the first fraction extracted. Therefore, the determination of the different Ca forms consisted of a sequential extraction process, which separated four Ca fractions in order of increasing insolubility: soluble Ca (Fraction I); linked Ca, mainly forming pectates (Fraction II); inorganic insoluble Ca, primarily forming phosphates and carbonates (Fraction III); insoluble organic Ca in the form of oxalate (Fraction IV) [40]. To treat the samples, a lyophilizer (TELSTAR, LyoAlfa 6, Terrassa, Spain), an Eppendorf 5810R centrifuge (Eppendorf Iberica, Madrid, Spain), a homogenizer (vortex) and an orbital shaker (Orbital Shape, Shaker, Thermo) were employed. The solvents used, LC-MS quality water and absolute ethanol were purchased from Sharlab S. L (Spain). The reagents (sodium chloride, acetic acid and hydrochloric acid) were supplied by Panreac Química (Barcelona, Spain).
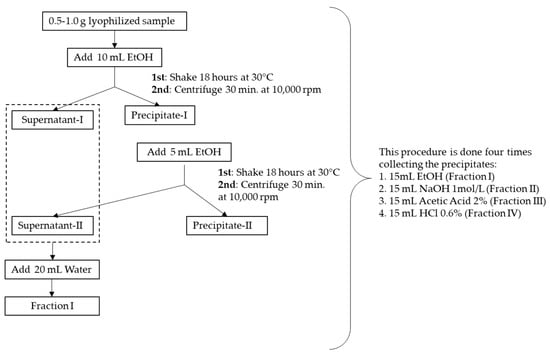
Figure 2.
Calcium fraction determination process.
To obtain Fraction I, 0.5–1.0 g of lyophilized and pulverized sample of each organ was weighed. Then, 10 mL of absolute ethanol was added and homogenized for 5 min before being stirred in an orbital shaker for 18 h at 30 °C. Subsequently, the samples were centrifuged at 10,000 rpm for 30 min, which resulted in supernatant-I and precipitate-I. The extraction was repeated with 5 mL of absolute ethanol (added to precipitate-I) and stirred in an orbital shaker for 2 h at 30 °C under the same conditions by subsequently combining the two supernatants, which were diluted with water to 20 mL. This produced Fraction I, water-soluble Ca, associated with water-soluble components, such as organic acids, chlorides and nitrates.
To determine Fraction II, the resulting precipitate-I was washed, and 10 mL of 1 mol·L−1 NaCl aqueous solution was added. This was homogenized for 5 min and stirred in an orbital shaker for 18 h at 30 °C. Subsequently, the samples were centrifuged at 10,000 rpm for 30 min, which produced supernatant-II and precipitate-II. The extraction was repeated with 5 mL of 1 mol·L−1 NaCl aqueous solution (added to precipitate-II) by combining the two supernatants, which were diluted with water to 20 mL. This produced Fraction II, in which Ca pectates were subsequently determined.
To quantify Fraction III, the resulting precipitate-II was washed, and 10 mL of 2% aqueous acetic acid solution was added, homogenized for 5 min and stirred in an orbital shaker for 18 h at 30 °C. Then, the samples were centrifuged at 10,000 rpm for 30 min to produce supernatant-III and precipitate-III. The extraction was repeated with 5 mL of 2% acetic acid aqueous solution (added to precipitate-II) by combining the two supernatants, which were diluted with water to 20 mL. This produced Fraction III, in which the phosphates and carbonates of Ca were subsequently determined.
Finally, to analyze Fraction IV, the resulting precipitate-III was washed, and 10 mL of 0.6% aqueous solution of hydrochloric acid was added, homogenized for 5 min and stirred in an orbital shaker for 18 h at 30 °C. Subsequently, the samples were centrifuged at 10,000 rpm for 30 min to produce supernatant-IV and precipitate-IV. The extraction was repeated with 5 mL of 0.6% aqueous hydrochloric acid solution (added to precipitate-III) by bringing together the two supernatants, which were diluted with water to 20 mL, thus producing Fraction IV, in which Ca oxalates were finally determined.
2.6. Calcium-Labeled Analysis
The total 44Ca concentration in each fraction was determined using a mass spectrometer with a multiple collector and inductive coupling plasma source (MC-ICP MS, Thermo Finnigan Neptune, California, USA). The natural abundance of this isotope (2.086 atoms % of 44Ca) was subtracted from the 44Ca results from different plant parts. The results presented in the tables and text refer to the excess or enrichment of this isotope.
Based on the DW (mg) and the total Ca concentration ([Ca] data and the %, w/w) for each plant compartment, the Ca content was calculated as
Ca (mg) = [Ca] × DW/100
The 44Ca content or Ca uptake from the fertilizer (Cauff) per plant compartment was determined as follows:
where atom 44Ca excess was calculated by subtracting the natural 44Ca abundance from the atom % 44Ca in each sample. Natural 44Ca abundance was taken as 2.086% [41].
44Cauff plant compartment (mg) = DW × [Ca] × atom 44Ca excess/10
Ca use efficiency or Ca uptake from the applied Ca [CaUE (%)] was calculated as
CaUE (%) organ = 44Cauff (mg) × 100/44Ca (mg) solution (156 mg of 44Ca).
2.7. Statistical Analysis
Data were subjected to an analysis of variance (ANOVA) to test for significant differences between treatments (with and without Ca) and to compare the means using Fisher’s LSD test at the 95% confidence level. Both tests were performed using version 5.1 of the Statgraphics Plus statistical program (Statgraphics Technologies, The plains, Virginia, USA). Before carrying out any statistical analysis, the normality of all data was studied using the Kolmogorov–Smirnov test. Otherwise, data analyses were carried out with the variables measured on their natural scales.
3. Results and Discussion
Knowledge of nutrient partitioning in trees is important for better fertilizer management practices in citrus production. In the case of Ca nutrient, the presence of Ca in the soil and water is not typically small because it is present in water irrigation, or it can also be found in the form of Ca carbonate in lime soils. However, the availability for plants could be low because Ca in soil comes in the form of a solid compound, it is poorly soluble. When it comes in ionic forms contained in the cation exchange complex, Ca dominates this complex because it is a divalent cation, which limits its mobility in soil [27].
As previously mentioned, Ca is taken up by plants in the form of Ca2+ ions. Therefore, the entry of Ca2+ into the cell is carried out exclusively through channels in the membrane, and its movement is produced by the xylem flow from roots to organs, such as leaves and fruit, via the transpiration stream [2]. Therefore, Ca distribution may depend on the amount of Ca2+ absorbed by the roots and may vary during fruit development [6].
3.1. Effect of Ca Fertilization on Ca Content Distribution in Citrus Trees
In addition to K, Ca, P and N are the dominant nutrients in citrus tree biomass, although their proportions may vary among different cultivars and with tree age and horticultural practices in the orchards [42]. In the present study, the plants extracted at the beginning of the assay for investigation of the initial nutrient content showed a mean total biomass (old leaves, branches, trunk, coarse and fibrous roots) of almost 50 g DW (Table 2). The trunk and coarse roots displayed the highest biomass values, with 32% and 23% of the total biomass, respectively, and fibrous roots had the lowest value, with 9%.

Table 2.
Biomass and Ca concentration of the main organs of the “Salustiana” orange trees harvested at the beginning of the trial Z.
The Ca content in trees at the beginning was 637 mg Ca, with an average concentration in the total plant of 1.39%. Old leaves exhibited the highest Ca concentration and the highest Ca content despite having a lower biomass than the trunk or coarse roots. These values will serve as a loading state for the uptake of nutrients from the complete cycle.
Table 3 shows the dry biomass (g DW) distribution of the main organs of the “Salustiana” orange trees harvested in January, 10 months after fertilization with and without Ca began. The plants fertilized with Ca (T1-Ca) grew into significantly larger trees (total plant 128 g DW) and were nearly 10% higher than the Ca-unfertilized plants (T2). It was noteworthy, in both treatments, that the DW of the newly developed leaves surpassed the old leaves’ weight. However, in T1 plants, the increase was 7-fold, while in T2 plants, it was 4.5-fold. The effect of Ca contribution on the biomass of plants has been reported previously after observing a positive impact on growth, root development, leaf mineral concentration and fruit quality [3,43]. The biomass values obtained in this study also agree with these results.

Table 3.
Distribution of dry biomass (g DW), Ca concentration (% DW) and Ca content (mg) of the main organs of the “Salustiana” orange trees harvested in January at dormancy and fertilized with Ca (T1-Ca) or without Ca (T2) Z.
In tissues, the effect of Ca application induced significant differences in the dry biomass (g DW) both in the coarse and fibrous roots systems and also in young tissues despite the optimum calcium concentration of the soil. The greater development of coarse and fibrous roots’ organs in T1-Ca compared to T2 can be attributed to the importance of Ca for healthy root development. When Ca is deficient, root growth is severely inhibited [44]. In severe Ca scarcity cases, root tip growth may even cease, which results in a poor root system [45]. Tadayon [46] also observed that soil supplemental application of Ca-nitrate and Mg-nitrate significantly increased the fibrous root density of “Valencia” orange trees. In the present study, the same effect was noted in aerial young tissues, including mature fruit, young leaves and twigs. The “Salustiana” trees fertilized with Ca (T1-Ca) obtained a larger biomass in these organs compared to trees without Ca. This could possibly be due to the greater root development observed, which would contribute to higher aerial growth.
For the Ca concentration, the lowest values in both treatments were obtained for mature fruit. Given that Ca absorption is closely related to transpiration, the highest Ca contents were found in the organs of plants with high transpiration rates (young organs, mainly leaves), while the concentration was lower in organs with a low transpiration rate, such as fruit [45]. In young above-ground organs, a higher Ca concentration was also previously observed in young leaves relative to mature fruit in “W. Murcott” mandarin [47].
Significant differences were observed among both treatments, but only in abscised organs. In these organs, the higher Ca concentration in T1-Ca resulted in significant variations in Ca content compared to T2 trees, with no differences in biomass among treatments. According to Bonomelli et al. [35], during fruit set, more Ca transport to the fruit pulp occurs (up to 39% of applied 45Ca). However, as the fruit grows, more Ca is retained in the calyx zone, which decreases the rise in Ca in fruit (between 17 and 19% of applied 45Ca).
In the total plant and in both root system fractions, despite there being no significant differences in the average total Ca concentration between the trees treated with or without Ca (Table 3), Ca application using fertilizers had a significant effect on Ca content. Ca-fertilized plants (T1-Ca) exhibited a significantly higher content in the coarse and fibrous roots and in the total plant but with no higher Ca concentration compared to T2. This was primarily due to their higher DW values. Thus, while Ca content in the total plant increased in both treatments (T1-Ca and T2) compared to the initially described values, it increased by 3.8-fold in T1-Ca and by 3.4-fold in T2.
3.2. Labeled Ca Distribution
Table 4 presents the labeled Ca uptake results for T1-Ca plants. The average excess 44Ca concentration in the entire plant was 0.466%, where 11.12 mg of labeled Ca (44Ca) was recovered from 156 mg of applied 44Ca. Similar to the total Ca, labeled Ca distribution was not homogeneous across all plant organs.

Table 4.
Distribution of labeled Ca concentration (44Ca % in excess) and content (44Ca mg) between the main organs and store organs of the “Salustiana” orange trees harvested in January at dormancy Z.
Unlike the enrichment observed in Ca concentration, the greatest 44Ca enrichments (% in excess) were found in the later developed flush of the aerial part, with 1.013% and 1.253% in the autumn flush leaves and twigs, respectively. However, the 44Ca content depended on the dry biomass. As can be observed in the results, the significantly higher dry biomass value of the summer leaves in these trees was reflected in the higher 44Ca content from the fertilizer compared to the autumn leaves, which had the highest 44Ca concentration but a lower dry biomass value. This higher accumulation pattern was repeated both in the aerial and root parts of plants, with significantly and consistently higher 44Ca content values in young organs (above ground and roots system) than their corresponding older organs. However, the total Ca present in young leaves and twigs, mainly from reserves (% DW), was significantly lower the younger the organs were. Ca is considered, as mentioned previously, to be an element with poor mobility within the plant, and its ability to translocate from older tissues to younger organs is limited. This translocation primarily occurs throughout the transpiration process and results in an age-dependent increase in Ca levels in leaves. Despite relying on the transpiration stream, Ca tends to be preferentially routed to meristematic zones and younger tissue [48].
Regarding Ca use efficiency (CaUE), similar to 44Ca content, young leaves exhibited the highest CaUE along with fibrous roots (Table 4). For leaves, however, summer leaves showed the highest CaUE. This can be attributed to the convergence of two conditions. First, these leaves sprouted later than spring leaves, allowing the plants to accumulate a higher 44Ca content as the labeled Ca supply continued. Second, this delayed sprouting also provided the plants with an extended period of assimilation from the beginning of fertilizer application. Additionally, summer leaves exhibit a larger biomass compared to autumn leaves, which emerge later and contribute less to the overall sprouting biomass in citrus trees.
The lowest labeled concentration, content and recovery from fertilizer were found in abscised and mature fruit. These results are consistent with those of Bonomelli et al. [34], who observed the smallest amount of 45Ca from fertilizer applied to soil in “Clemenules” mandarin fruit. This can possibly be attributed to the low transpiration rate in fruit because citrus peel has a lower stomatal density compared to leaves and a higher proportion of cuticle per unit area.
In this study, the young tissues from above-ground organs exhibited the highest percentages of Ca amount absorbed from fertilizer by plants (CaUE 47%) (Figure 3). In root systems, 19% of the Ca applied with the fertilizer was found in fibrous roots despite having a lower biomass (only 10% of the total) than coarse roots. This behavior demonstrates that the Ca derived from the fertilizer (44Ca) was localized mainly in sink organs.
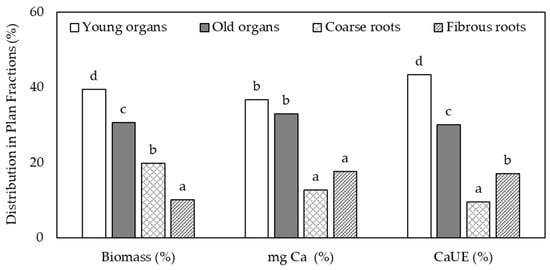
Figure 3.
Distribution (%) of dry biomass, total Ca content (mg Ca) and Ca uptake from the fertilizer among the different fractions of the plant. Lowercase letters on top of the bars show significant differences (p < 0.05) among organs in each different parameter.
3.3. Effect of Ca Fertilization on Ca Partitioning in Different Forms in Citrus Trees
Ca exists in plants in a number of forms, including Ca(NO3)2, Ca pectate, Ca phosphate, Ca oxalate, Ca carbonate (CaCO3) and undissolved Ca compounds, such as Ca silicate [49]. Given the importance of the relation between free Ca and bound Ca, a sequential extraction process was employed to isolate four Ca fractions separated in order of increasing insolubility: soluble Ca, bound Ca (mainly in the form of pectates and carbonates), inorganic insoluble Ca (predominantly phosphates), and finally, organic insoluble Ca or oxalate. This fraction is particularly pertinent due to the considerable presence of calcium in plants as precipitated calcium oxalate.
To determine the effect of Ca fertilization on Ca partitioning, the Ca content in the fractions in “Salustiana” orange trees was evaluated at the beginning of the trial (Table 5), prior to Ca treatment and also during vegetative lethargy, after applying the fertilization with Ca (Table 6). During lethargy, total 44Ca partitioning was also analyzed (Table 7).

Table 5.
Total Ca partitioning (percentage) in different forms of “Salustiana” orange trees harvested at the beginning of the trial Z.

Table 6.
Total calcium partitioning (percentage) in different forms of the main organs of “Salustiana” orange trees harvested in January at dormancy (uptake trial with and without Ca) Z.

Table 7.
Total labeled Ca partitioning (% 44Ca) in different forms of the main organs of “Salustiana” orange trees harvested in January at dormancy (uptake trial with Ca) Z.
Throughout both periods analyzed and the total and labeled Ca partitioning, regardless of Ca application, Fraction II was the largest fraction in most “Salustiana” orange tree organs, while Fraction I was the smallest. These findings agree with previously reported data [50,51].
The application of Ca fertilization affected partitioning in different organs. Fraction IV (insoluble Ca) was larger in the spring flush twigs and in the old branches and trunks of plants without Ca (T2) (Table 6). Such detriment was reflected as a slight increase in the other fractions, with significant differences only observed in Fraction I for the trunk. In Ca-treated plants, this indicates that there was a higher Ca content in more soluble forms in laminar tissue, which was then translocated to sink organs, such as new sprouting leaves and fruit.
The results also showed that annually developed organs (abscised organs, mature fruits, spring, summer and autumn flush leaves, and fibrous roots) from the trees fertilized with Ca exhibited a significantly lower percentage of Fraction II compared to the trees without Ca. This decrease was reflected only as a significant increase in Fraction IV for the mature fruits fertilized with Ca. Accordingly, the Ca supply in T1-Ca plants covered the Ca needs in Fraction II, which allowed the extra Ca supply to be utilized for other functions in leaves. As a result, these findings indicate that newly developed organs, such as leaves, prioritize the formation of cell walls. Fertilization with Ca enables a larger amount of soluble Ca to be passively transported to areas of higher transpiration, such as leaves. The increase in [Ca2+]apo adjacent to the guard cells regulates stomatal aperture [52]. At higher concentrations, extracellular Ca2+ is involved in regulating stomatal aperture by directly influencing water channels to slow down aperture change [53]. Moreover, the increase in Ca-pectate content due to Ca fertilization would contribute to greater fruit firmness, since in other crops, a relationship has been observed between the calcium concentration in this fraction and fruit firmness [54].
The highest percentage of Fraction III in T1-Ca trees was observed in all leaves, which developed during the growth cycle, with significant differences in summer and spring leaves. This indicates that a greater soluble Ca flux in the leaves allows for a larger amount of Ca to be deposited in cell membranes in the form of phosphates or carbonates or as part of Ca-binding proteins (Fraction III). Among these proteins, calreticulin plays a role in intracellular Ca2+ homeostasis by modulating endoplasmic reticulum Ca2+ storage and transport [55]. It has also been reported that changes in intracellular Ca2+ levels may activate Ca2+-dependent protein kinases (CDPK), calmodulin (CaM) and calcineurin B-like (CBL) proteins, which are involved in regulation of the water channels and subsequent stomatal movement [56]. These results suggest that T1-Ca trees may have improved vegetative development by exhibiting a wide distribution of Ca fractions in their leaves, which potentially leads to an enhanced photosynthesis rate. Indeed, as illustrated in Table 3, T1-Ca plants exhibited significantly higher DW values (128.18 g) compared to those without Ca supplementation (T2) (117.83 g).
Regarding the fibrous root organ (Table 6), T1-Ca plants—compared with T2 plants without Ca—had the lowest Ca proportion in Fraction II and the highest Ca proportion in Fraction IV (the latter exhibiting no significant differences). However, when considering the Ca content (Table 3), the percentages of Fraction II corresponded to 125.5 mg and 124 mg of Ca-pectate in the trees with and without Ca, respectively. Therefore, it is likely that Ca-pectate saturation in the fibrous roots of cv. “Salustiana” fell within the range of approximately 125 mg of Ca-pectate. Once this concentration is reached, fibrous roots store Ca in other forms. This phenomenon was observed in T1-Ca plants, which exhibited a higher percentage of Fraction IV. The formation of Ca-oxalate (Fraction IV) and the presence of oxalic acid are possibly influenced by ionic balance because crystal formation dynamically fluctuates according to free Ca availability [20,57]. These results are consistent with the findings of Schadel and Walter [58] and of Gouveia et al. [59], who reported that sweet potatoes can increase Ca-oxalate levels as a mechanism to isolate high Ca accumulation.
Fraction II mainly represents the Ca present in the cell wall, which participates in cross-linking negative charges, especially in the carboxylic residues of pectins [60]. The Ca-pectate structure is abundant in the middle lamella and in cell corners, where it can retain or even attract proteins with an affinity for it [61]. Aghdam et al. [62] reported that this fraction accounts for 60–75% of the total tissue Ca2+content, which serves as the largest calcium pool in plant tissue. In the present study, however, only old leaves at the beginning of the trial (Table 5) and old leaves (spring and summer flush leaves) without Ca treatment at the end of the study period (Table 6) accounted for over 60% of the total Ca content. This suggests that as the leaves mature in citrus trees, the primary function of Ca is structural, while this element is required for more functions in other organs.
Among the evaluated organs, independent of Ca treatment, Fraction II increased as it moved further away from the coarse roots and eventually reached the old leaves. This increase was primarily attributed to a decrease in Fraction IV. It has been suggested that Ca-oxalate crystals (Fraction IV) play a functional role in excess Ca excretion because these crystals are found in the organs or tissues, which will be discarded, thus facilitating the elimination of excess Ca [15]. Therefore, the results showed that the older the organ is, the longer it will have had to accumulate Ca in Fraction IV. The lower percentage of Ca-pectate in roots than in the upper parts of the plant, which turn into old leaves, has also been observed by Schmitt et al. [63] in beech trees (Fagus sylvatica L.). These authors suggested that if the Ca circulating in the xylem sap will undergo ion exchange processes and will preferentially bind to the pectins in the middle lamella of the wood cell wall, then the suction for the xylem sap circulation in old wood would be greater than that for the xylem sap circulation in younger branches. Thus, lower sap circulation could be the result of a larger number of binding sites, which could result in higher Ca-pectate values (Fraction II) at the end of the pathway.
Moreover, Fraction II was smaller in young organs (leaves and twigs) than in old leaves, which favored an increase in Fraction IV (Table 6). The highest percentage of the oxalate fraction in young leaves can be explained in agreement with Giannopoulos et al. [64]. These authors established that the formation of inclusions of Ca oxalate crystals or idioblasts containing Ca carbonate takes place at very early development stages, with a maximum density of idioblasts observed in very young leaves. This is in agreement with our results, since Fraction II increased as the leaves matured. This can be explained by increased cell-wall Ca requirements in developing cells and by the transformation of the small leaf primordium into a mature leaf, which is controlled by at least six distinct processes: cytoplasmic growth, cell division, endoreduplication, transition between division and expansion, cell expansion, cell differentiation into stomata, vascular tissue and trichomes [65].
Similar to the content observed in total Ca (Table 6), regarding 44Ca content, Fraction II (Ca-pectates) was the main significant fraction observed in most organs (Table 7), followed by Fraction III (Ca-phosphate/Ca-carbonates). Fraction II exhibited higher levels in the woody tree parts (i.e., twigs, branches, trunk, coarse roots). Notably, the closer the region was to the zone of labeled Ca entry, the more significant the increase in 44Ca percentage in Fraction II it exhibited. For instance, in fibrous roots, Fraction II constituted 29.87% of the total Ca content (Table 6), while Ca derived from the fertilizer represented 40.53% of the total 44Ca content (Table 7). The higher 44Ca percentage in these organs supports limited mobility, since Ca is transported passively through transpiration processes from roots to shoots.
Additionally, in fibrous roots, Fraction III exhibited the highest percentage of 44Ca compared to the other organs analyzed. Ca2+ absorption occurs primarily at the apical and lateral root sites. When the Casparian band is absent or disrupted, Ca2+ must be taken up through the apoplastic pathway [2]. However, when the impermeable Casparian band is present, Ca2+ movement to the xylem must occur via the symplastic pathway [6]. In both cases, Ca2+ entry in the xylem parenchyma cells is mediated by the membrane transporters associated with Ca2+, including Ca channels, cation/H+ antiporters and ATPases [66], which represent an important part of Fraction III. The presence of Ca2+-ATPases in the plasma membrane of root cells has been demonstrated both biochemically and electrophysiologically [67]. It has also been reported that Ca promotes increased ATPase activity [68], which would explain why Ca-phosphate (Fraction III) was predominant in fibrous roots, since Ca2+-ATPases are rich in P.
The lowest 44Ca percentage in Fraction IV was found in old branches, with 9.71%. This is because these tissues do not need significant precipitation of the Ca incorporated during the season in the form of calcium oxalate crystals—either because the Ca concentration in the sap is high and the cation-exchange complex of the xylem wall is saturated, which leads to substantial Ca movement to the upper part of the plant, or because the vacuoles already contain a considerable amount of Ca oxalate to maintain cell homeostasis [69]. However, in fibrous roots, the 44Ca percentage in Fraction IV (23.28%) was similar to that observed in the previous total Ca content (22.36%). This suggests that the Ca oxalate crystals accumulate in roots, especially in the cells associated with xylem or near this tissue and are prone to their Ca reserves being mobilized and transported over short distances via the apoplast until they reach tracheary cells, as reported by Paiva [15] previously.
In leaves, twigs and fruit, the 44Ca percentage in the different fractions was similar to that determined in the total Ca of T1-Ca plants, with the majority being present in Fraction II, followed by Fractions III and IV together.
In summary, 44Ca distribution in the different fractions showed variations compared to the total Ca distribution in existing tree tissues. However, the 44Ca fractions’ percentages followed the same distribution pattern in the newly formed tissues during the current season.
4. Conclusions
Citrus plants, typically growing in calcareous soils, exhibited enhanced growth when subjected to calcium applications, suggesting a positive correlation between calcium supplementation and increased development. Therefore, the total Ca content rose in the entire plant and in some organs during both trials due to the larger biomass obtained with Ca supply. Ca derived from the fertilizer (44Ca) was localized mainly in sink organs both above (new flush leaves and twigs) and below (fibrous roots) ground plant.
For total Ca partitioning in different forms, the most abundant fraction corresponded to Ca pectates (Fraction II), followed by Fractions III or IV. The lowest values were found in Fraction I in the most soluble fraction form. However, 44Ca partitioning in the different fractions showed variations compared to the total Ca distribution in existing tree tissues. The percentages of these fractions followed the same distribution pattern in the newly formed tissues during the current season.
Moreover, it was observed that Fraction I, which corresponds to soluble calcium, was lower in the labeled calcium with respect to total calcium. This indicates that the Ca supplied during the year is arranged within the plant in a less insoluble form (phosphates, pectates and oxalates). In addition, it also shows that there is an active Ca metabolism in the plant, since the Ca already present is remobilized.
This research sheds light on the essential role of calcium in plant growth and highlights the significance of proper Ca supplementation for enhancing plant biomass. By understanding the mechanisms of Ca partitioning, fertilizer application methods could be optimized and contribute to more efficient agricultural practices.
Author Contributions
Conceptualization, A.Q.; methodology, B.M-A., A.B., J.M. (Jorge Millos), F.L. and A.Q.; software, A.Q. and J.M. (Julia Morales); validation, A.Q. and J.M. (Julia Morales); formal analysis, J.M. (Julia Morales), B.M-A., A.B, J.M. (Jorge Millos) and A.Q.; investigation, B.M.-A., F.L. and A.Q.; resources, F.L. and A.Q.; data curation, J.M. (Julia Morales) and A.Q.; writing—original draft preparation, J.M. (Julia Morales) and A.Q.; writing—review and editing, J.M. (Julia Morales) and A.Q.; funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported via a private research contract (IVIA-7213) with YARA Iberian, Madrid (Spain).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hepler, P.K.; Wayne, R.O. Calcium and plant development. Annu. Rev. Plant Physiol. 1985, 36, 397–439. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, S.S. Calcium signalling system in plants. Russ. J. Plant Physiol. 2005, 52, 249–270. [Google Scholar] [CrossRef]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. The pathways of calcium movement to the xylem. J. Exp. Bot. 2001, 52, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef]
- Ravi, B.; Sanyal, S.K.; Pandey, G.K. Calcium decoders and their targets: The holy alliance that regulate cellular responses in stress signaling. Adv. Prot. Chem. Struct. Biol. 2023, 134, 371–439. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Ca signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Lionetti, V.; Francocci, F.; Ferrari, S.; Volpi, C.; Bellincampi, D.; Galletti, R.; Ovidio, R.; Lorenzo, G.; Cervone, F. Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc. Natl. Acad. Sci. USA 2010, 107, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N. Role of plant nutrients in plant growth and physiology. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer Nature Ltd.: Singapore, 2018; pp. 51–93. [Google Scholar] [CrossRef]
- Daverkausen-Fischer, L.; Pröls, F. Regulation of calcium homeostasis and flux between the endoplasmic reticulum and the cytosol. J. Biol. Chem. 2022, 298, 102061. [Google Scholar] [CrossRef] [PubMed]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2009, 223, 1707–1711. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, S.V.; McConnell, D.B.; Gower, L.B.; Kane, M.E.; Lucansky, T. Periplasmic cuticular calcium oxalate crystal deposition in Dracaena sanderiana. New Phytol. 2001, 149, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hudgins, J.W.; Krekling, T.; Franceschi, V.R. Distribution of calcium oxalate crystals in the secondary phloem of conifers: A constitutive defense mechanism? New Phytol. 2003, 159, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Pandith, S.A.; Shah, M.A.; Reshi, Z.A. Calcium Oxalate Crystals, the Plant ‘Gemstones’: Insights into Their Synthesis and Physiological Implications in Plants. Plant Cell Physiol. 2023, 64, 1124–1138. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.M.; Goss, L.J.; Franceschi, V.R. Calcium channels are involved in calcium oxalate crystal formation in specialized cells of Pistia stratiotes L. Ann. Bot. 2004, 93, 741–753. [Google Scholar] [CrossRef][Green Version]
- Nakata, P.A. Advances in our understanding of calcium oxalate crystal formation and function in plants. Plant Sci. 2003, 164, 901–909. [Google Scholar] [CrossRef]
- Nakata, P.A. Plant calcium oxalate crystal formation, function, and its impact on human health. Front. Biol. 2012, 7, 254–266. [Google Scholar] [CrossRef]
- Schneider, A. The probable function of calcium oxalate crystals in plants. Bot. Gaz. 1901, 32, 142–144. [Google Scholar] [CrossRef][Green Version]
- Storey, R.; Treeby, M.T. Nutrient uptake into navel oranges during fruit development. J. Hort. Sci. Biotechnol. 2002, 77, 91–99. [Google Scholar] [CrossRef]
- Treeby, M.T.; Storey, R. Calcium-spray treatments for ameliorating albedo breakdown in navel oranges. Aust. J. Exp. Agric. 2002, 42, 495–502. [Google Scholar] [CrossRef]
- Quiñones, A.; Martínez-Alcántara, B.; Primo-Millo, E.; Legaz, F. Fertigation: Concept and application in citrus. In Advances in Citrus Nutrition; Springer: Dordrecht, The Netherlands, 2012; pp. 281–301. [Google Scholar] [CrossRef]
- Zekri, M.; Obreza, T.A. Calcium (Ca) and Sulfur (S) for Citrus Trees; The Soil and Water Science Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2013. [Google Scholar]
- Juan, L.I.; Jiezhong, C.H.E.N. Citrus fruit-cracking: Causes and occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Pham, T.T.M.; Singh, Z.; Behboudian, M.H. Different surfactants improve calcium uptake into leaf and fruit of ‘Washington navel’ sweet orange and reduce albedo breakdown. J. Plant Nutr. 2012, 35, 889–904. [Google Scholar] [CrossRef]
- Meena, M.K.; Jain, M.C.; Singh, J.; Sharma, M.; Shing, B.; Maurya, I.B. Effect of pre-harvest spray of calcium nitrate, boric acid and zinc sulphate on yield and quality of Nagpur mandarin (Citrus reticulata Blanco). Int. J. Hortic. Sci. 2016, 22, 23–28. [Google Scholar] [CrossRef]
- Wiggenhauser, M.; Moore, R.E.; Wang, P.; Bienert, G.P.; Laursen, K.H.; Blotevogel, S. Stable isotope fractionation of metals and metalloids in plants: A review. Front. Plant Sci. 2022, 13, 840941. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Martínez-Cuenca, M.R.; Fernández, C.; Legaz, F.; Quiñones, A. Production of 15N-Labelled liquid organic fertilisers based on manure and crop residue for use in fertigation studies. PLoS ONE 2016, 11, e0150851. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Martínez-Cuenca, M.R.; Bermejo, A.; Legaz, F.; Quinones, A. Liquid organic fertilizers for sustainable agriculture: Nutrient uptake of organic versus mineral fertilizers in citrus trees. PLoS ONE 2016, 11, e0161619. [Google Scholar] [CrossRef]
- Craswell, E.T.; Chalk, P.M.; Kaudal, B.B. Role of 15N in tracing biologically driven nitrogen dynamics in soils amended with biochar: A review. Soil Biol. Biochem. 2021, 162, 108416. [Google Scholar] [CrossRef]
- Bonomelli, C.; Fernández, V.; Martiz, J.; Videla, X.; Arias, M.I.; Rojas-Silva, X.; Nario, A. Absorption and distribution of root, fruit, and foliar-applied 45Ca in ‘Clemenules’ mandarin trees. J. Sci. Food Agric. 2020, 100, 4643–4650. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, C.; Fernández, V.; Capurro, F.; Palma, C.; Videla, X.; Rojas-Silva, X.; Nario, A.; Mártiz, J. Absorption and Distribution of Calcium (45Ca) Applied to the Surface of Orange (Citrus sinensis) Fruits at Different Developmental Stages. Agronomy 2022, 12, 150. [Google Scholar] [CrossRef]
- MAPA. Métodos Oficiales de Análisis de Suelos y Aguas para el Riego. In Plantas, Productos Orgánicos Fertilizantes, Suelos, Aguas, Productos Fitosanitarios, Fertilizantes Inorgánicos; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994; Volume 3, p. 662. [Google Scholar]
- Isaac, R.A.; Johnson, W.C., Jr. Elemental determination by inductively coupled plasma atomic emission spectrometry. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 165–170. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. In Handbook of Reference Method for Plant Analysis; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 37–49. [Google Scholar]
- Ohta, Y.; Yamamoto, K.; Deguchi, M. Chemical fractionation of calcium in the fresh leaf blade and influences of deficiency or over supply of calcium and age of leaf on the content of each calcium fraction. J. Sci. Soil Manure 1970, 41, 19–26. [Google Scholar]
- Minamide, T.; Goto, M.; Iwata, T. Forms of calcium compounds and their changes after harvest in fruits and vegetables. J. Jpn. Soc. Hort. Sci. 1986, 54, 507–513. [Google Scholar] [CrossRef][Green Version]
- Stürup, S.; Bendahl, L.; Gammelgaard, B. Optimisation of dynamic reaction cell (DRC)-ICP-MS for the determination of 42Ca/43Ca and 44Ca/43Ca isotope ratios in human urine. J. Anal. At. Spectrom. 2006, 21, 297–304. [Google Scholar] [CrossRef]
- Mattos, D., Jr.; Quaggio, J.A.; Cantarella, H.; Alva, A.K. Nutrient content of biomass components of Hamlin sweet orange trees. Sci. Agric. 2003, 60, 155–160. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Zhang, D.; Shu, B.; Xiao, J.; Xia, R. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
- Eticha, D.; Kwast, A.; De Souza, C.T.R.; Horowitz, N.; Stützel, H. Calcium nutrition of orange and its impact on growth, nutrient uptake and leaf cell wall. Citrus Res. Technol. 2017, 38, 62–70. [Google Scholar] [CrossRef]
- Tadayon, M.S. The role of nutritional management in improving the symptoms of citrus decline. J. Plant Nutr. 2020, 43, 1555–1570. [Google Scholar] [CrossRef]
- Fan, Z.; Xiong, H.; Luo, Y.; Wang, Y.; Zhao, H.; Li, W.; He, X.; Wang, J.; Shi, X.; Zhang, Y. Fruit yields depend on biomass and nutrient accumulations in new shoots of citrus trees. Agronomy 2020, 10, 1988. [Google Scholar] [CrossRef]
- Storey, R.; Leigh, R.A. Processes modulating calcium distribution in citrus leaves. An investigation using X-ray microanalysis with strontium as a tracer. Plant Physiol. 2004, 136, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, C.; Arias, M.I.; Villalobos, L. Adaptation and validation of a methodology for the measurement of calcium fractions in fruits. Commun. Soil Sci. Plant Anal. 2018, 49, 735–744. [Google Scholar] [CrossRef]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Xia, R.; Xiao, Z.; Wang, P.; Song, W. Effect of pre-harvest application of calcium and boron on dietary fibre, hydrolases, and ultrastructure in ‘Cara Cara’ navel orange (Citrus sinensis L. Osbeck) fruit. Sci. Hort. 2009, 121, 272–277. [Google Scholar] [CrossRef]
- Wilkinson, S.; Clephan, A.L.; Davies, W.J. Rapid low temperature-induced stomatal closure occurs in cold-tolerant Commelina communis leaves but not in cold-sensitive tobacco leaves, via a mechanism that involves apoplastic calcium but not abscisic acid. Plant Physiol. 2001, 126, 1566–1578. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, X.Y.; Tang, Q.L.; Wang, G.X. Extracellular calcium is involved in stomatal movement through the regulation of water channels in broad bean. J. Plant Growth Regul. 2006, 50, 79–83. [Google Scholar] [CrossRef]
- Vilhena, N.Q.; Quinones, A.; Rodríguez, I.; Gil, R.; Fernández-Serrano, P.; Salvador, A. Leaf and fruit nutrient concentration in Rojo Brillante persimmon grown under conventional and organic management, and its correlation with fruit quality parameters. Agronomy 2022, 12, 237. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, X.Y.; Wang, G.X. Cytosolic calcium oscillation signalling in guard cell. Plant Sci. 2004, 166, 549–556. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Schadel, W.E.; Walter, W.M., Jr. Calcium oxalate crystals in the roots of sweet potato. J. Am. Soc. Hortic. Sci. 1980, 105, 851–854. [Google Scholar] [CrossRef]
- Gouveia, C.S.; Ganança, J.F.; Lebot, V.; Pinheiro de Carvalho, M.A. Changes in oxalate composition and other nutritive traits in root tubers and shoots of sweet potato (Ipomoea batatas L. [Lam.]) under water stress. J. Sci. Food Agric. 2020, 100, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.K.; Winship, L.J. Calcium at the cell wall-cytoplast interface. J. Integr. Plant Biol. 2010, 52, 147–160. [Google Scholar] [CrossRef]
- Jarvis, M.C.; Briggs, S.P.H.; Knox, J.P. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Hassanpouraghdam, M.B.; Paliyath, G.; Farmani, B. The language of calcium in postharvest life of fruits, vegetables and flowers. Sci. Hortic. 2012, 144, 102–115. [Google Scholar] [CrossRef]
- Schmitt, A.D.; Borrelli, N.; Ertlen, D.; Gangloff, S.; Chabaux, F.; Osterrieth, M. Stable calcium isotope speciation and calcium oxalate production within beech tree (Fagus sylvatica L.) organs. Biogeochemistry 2018, 137, 197–217. [Google Scholar] [CrossRef]
- Giannopoulos, A.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G.; Fasseas, C.; Karabourniotis, G. Changes in the properties of calcium-carbon inclusions during leaf development and their possible relationship with leaf functional maturation in three inclusion-bearing species. Protoplasma 2019, 256, 349–358. [Google Scholar] [CrossRef]
- Kalve, S.; De Vos, D.; Beemster, G.T. Leaf development: A cellular perspective. Front. Plant Sci. 2014, 5, 362. [Google Scholar] [CrossRef]
- Boudsocq, M.; Lauriere, C. Osmotic signaling in plants. Multiple pathways mediated by emerging kinase families. Plant Physiol. 2005, 138, 1185–1194. [Google Scholar] [CrossRef]
- Costa, A.; Resentini, F.; Buratti, S.; Bonza, M.C. Plant Ca2+-ATPases: From biochemistry to signalling. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2023, 1870, 119508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, H.; Xu, F.; Yan, F.; Xu, W. H+-ATPases in plant growth and stress responses. Ann. Rev. Plant Biol. 2022, 73, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, C.; Luo, Y.; Shi, H.; Li, Q.; PinChu, C.; Li, X.; Jang, J.; Fan, W. Oxalate in Plants: Metabolism, Function, Regulation, and Application. J. Agric. Food Chem. 2022, 70, 16037–16049. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).