Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea mays L.)
Abstract
1. Introduction
2. Materials and Methods
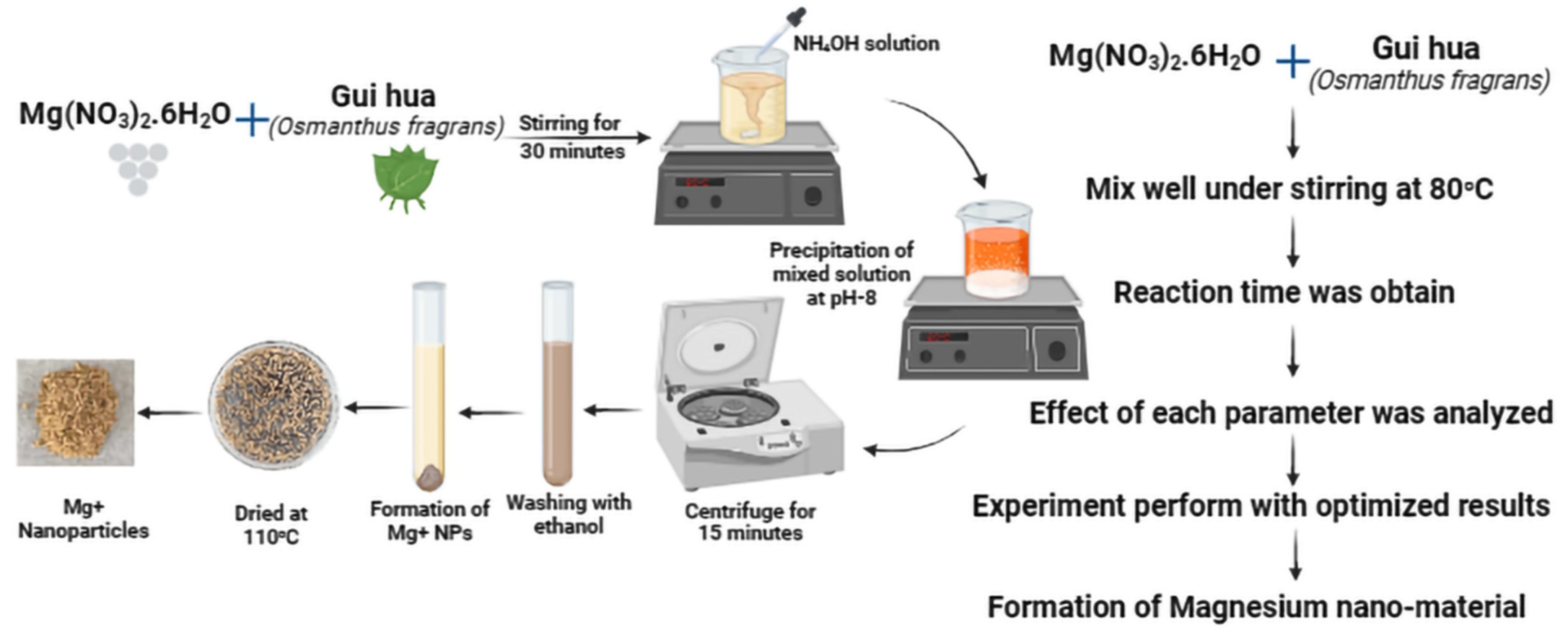
2.1. Leaves Extract and MgO NP Synthesis
2.2. Composition of Magnesium Nano-Material
2.3. Seed Germination Exposure
Seed Germination Percentage
2.4. Seedlings Growth in a Greenhouse
2.5. Plant Growth in the Field
3. Results
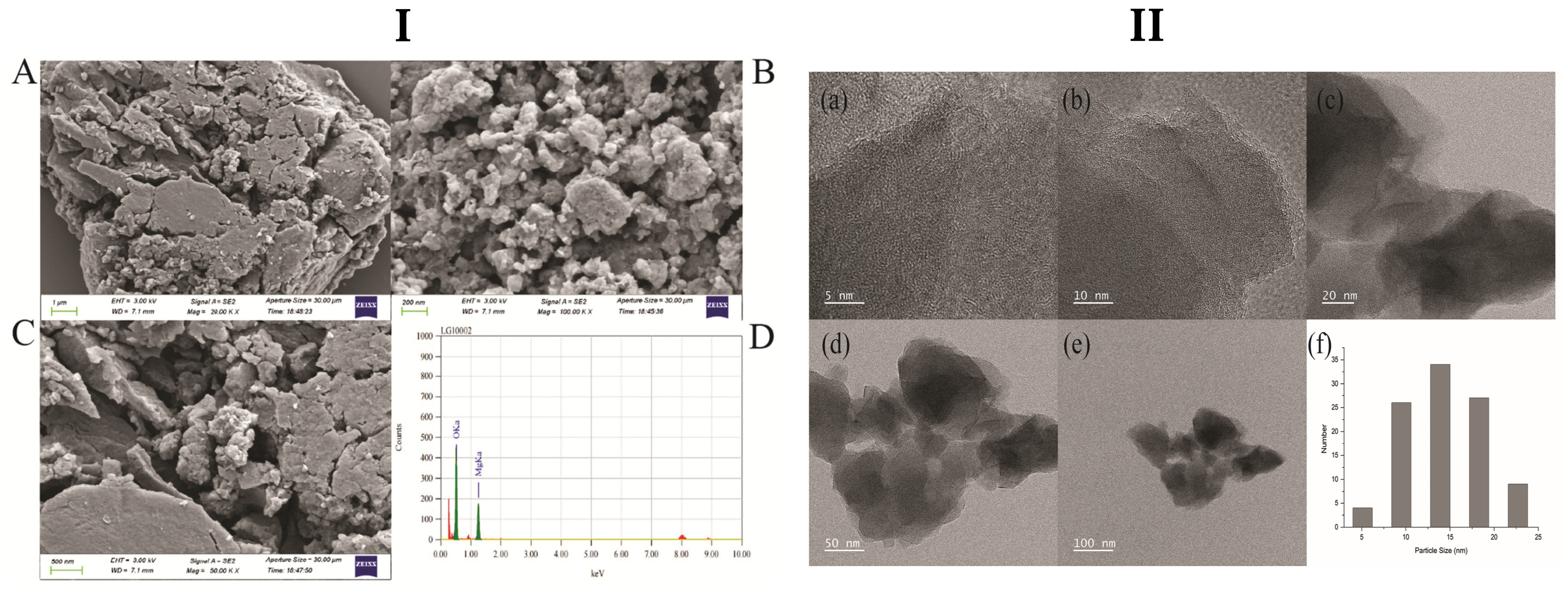
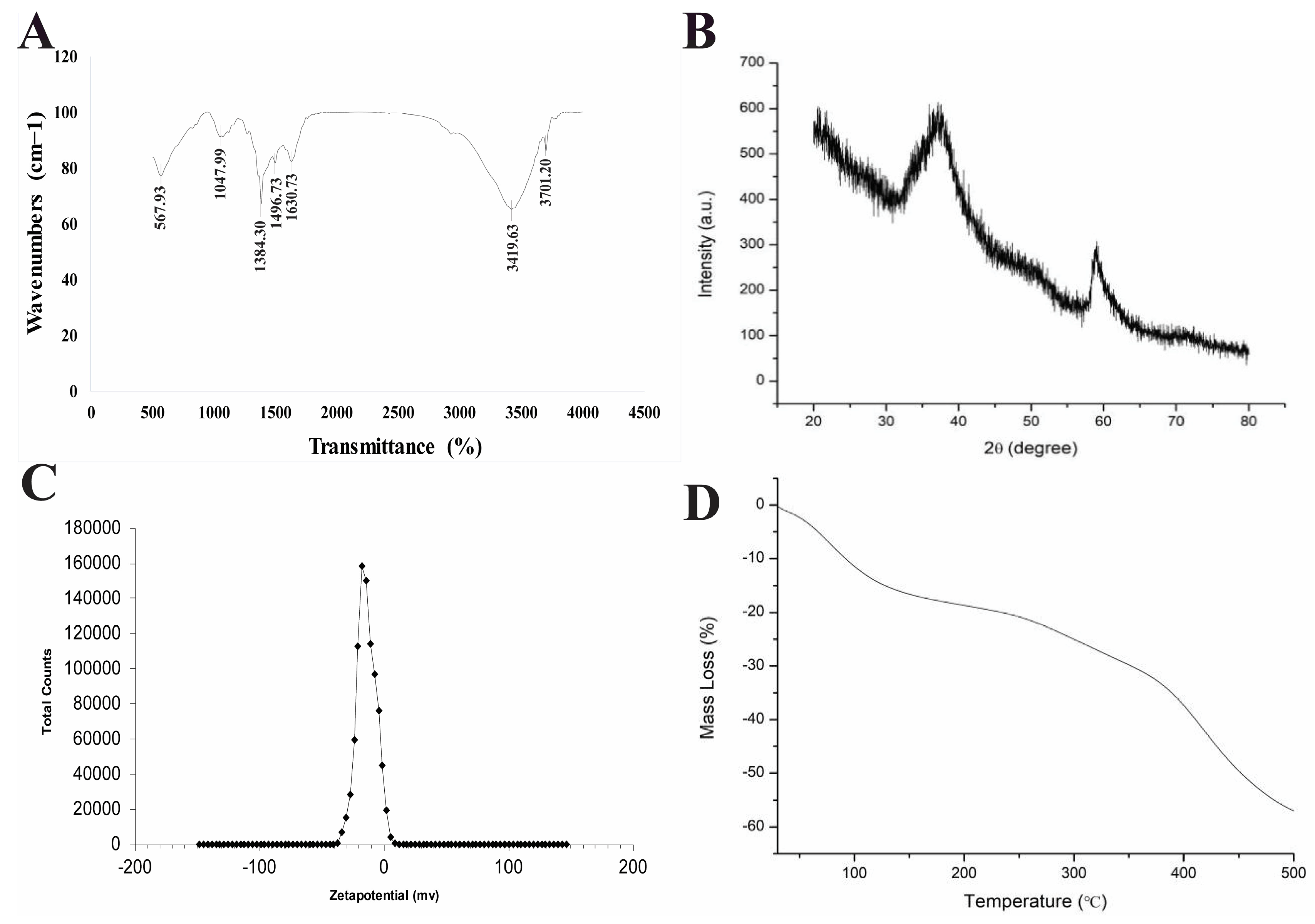
3.1. Characterization of Synthesized MgO NPs
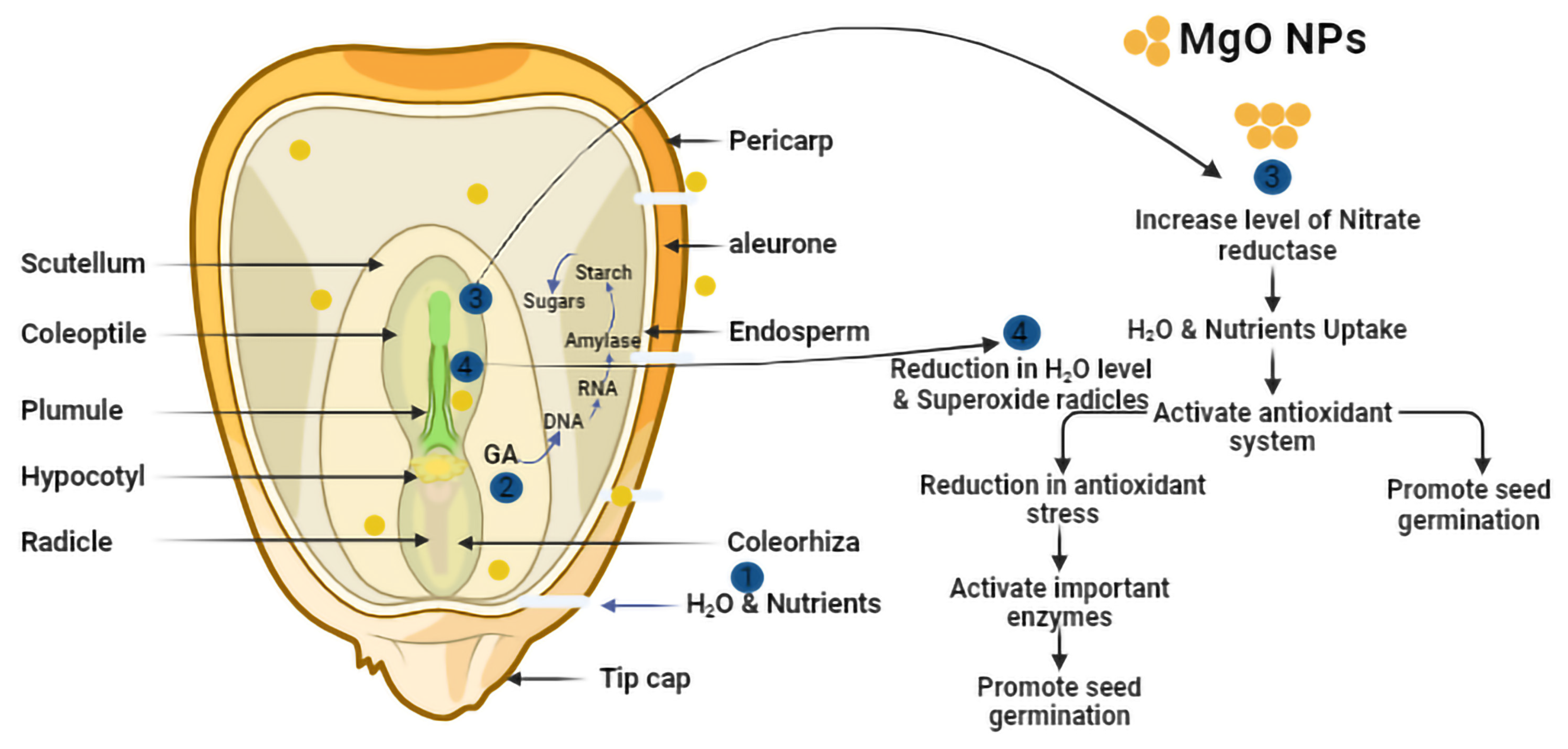
3.2. Effect of MgO NPs on Z. mays Seed Germination
3.3. Efficacy of MgO NPs on Seedling Growth in Greenhouse
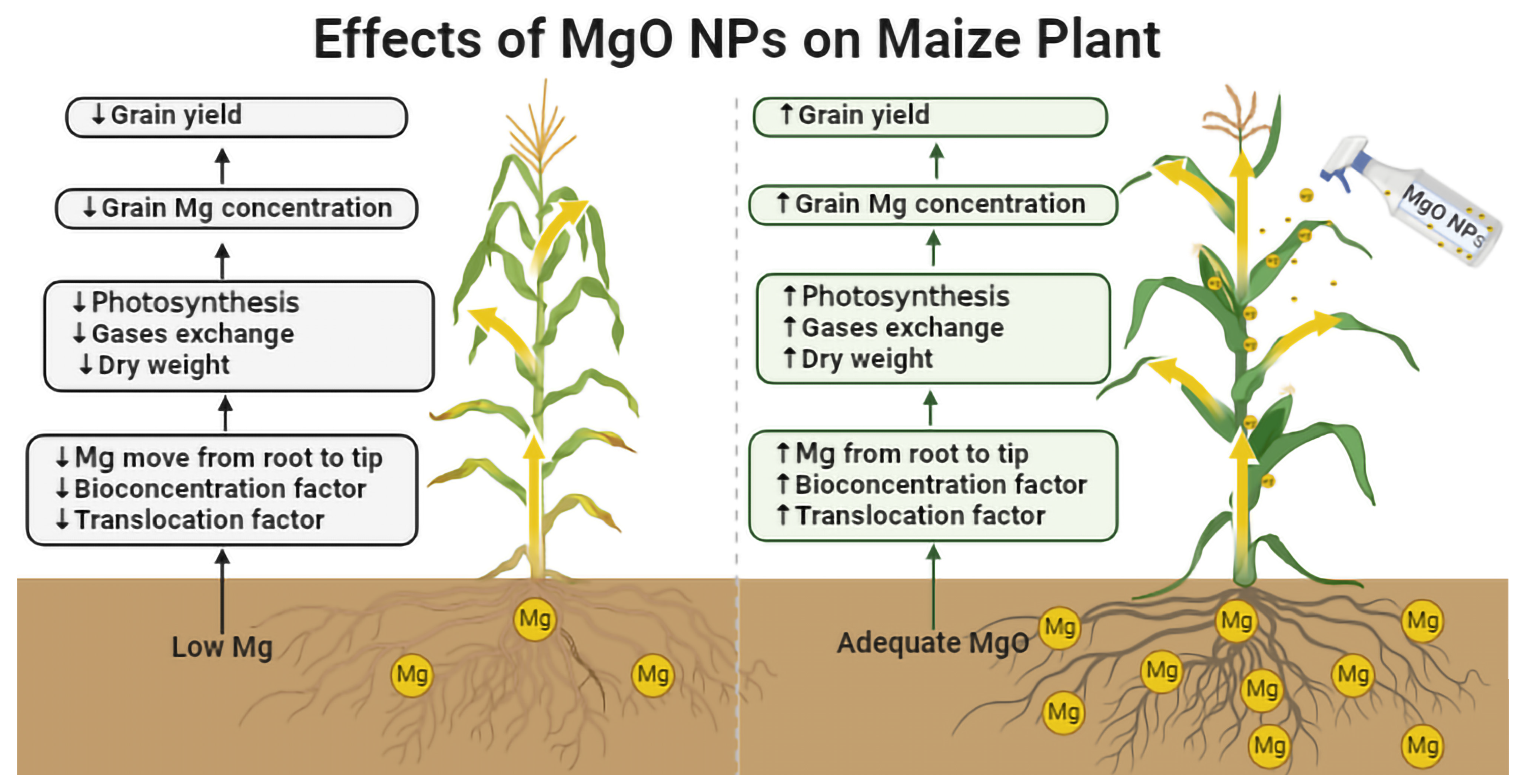
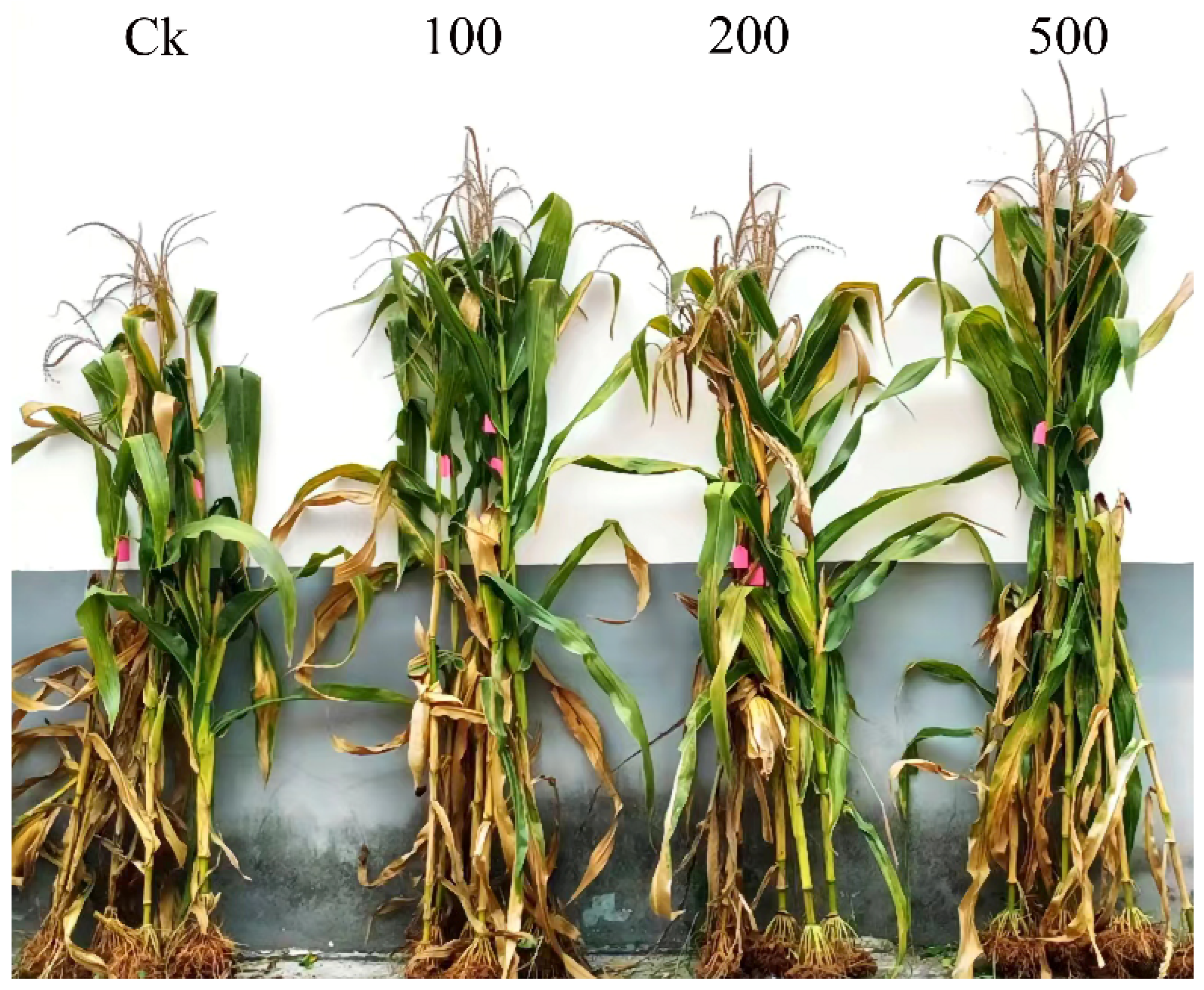
3.4. Effect of MgO NPs on Maize Plants’ Growth
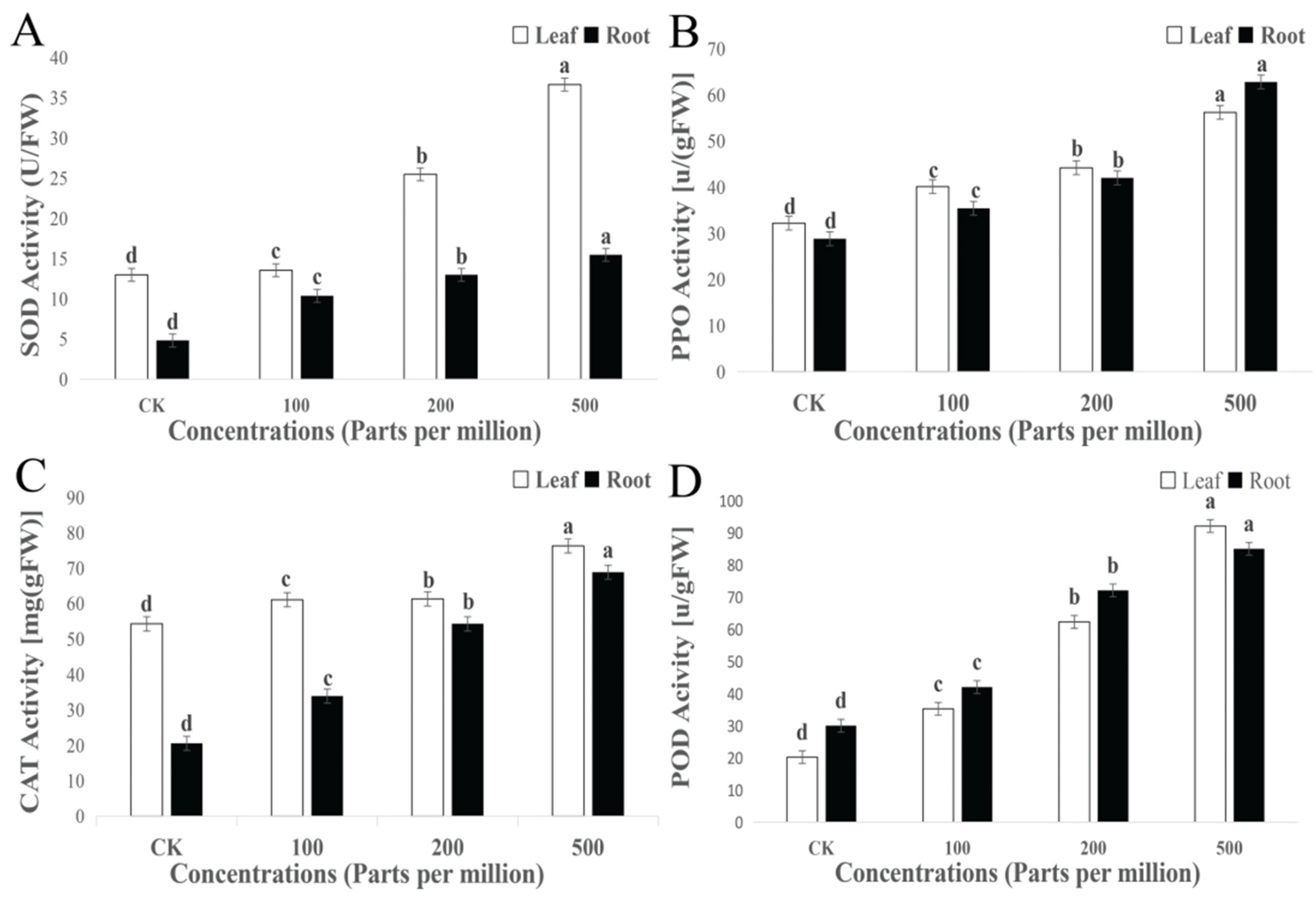
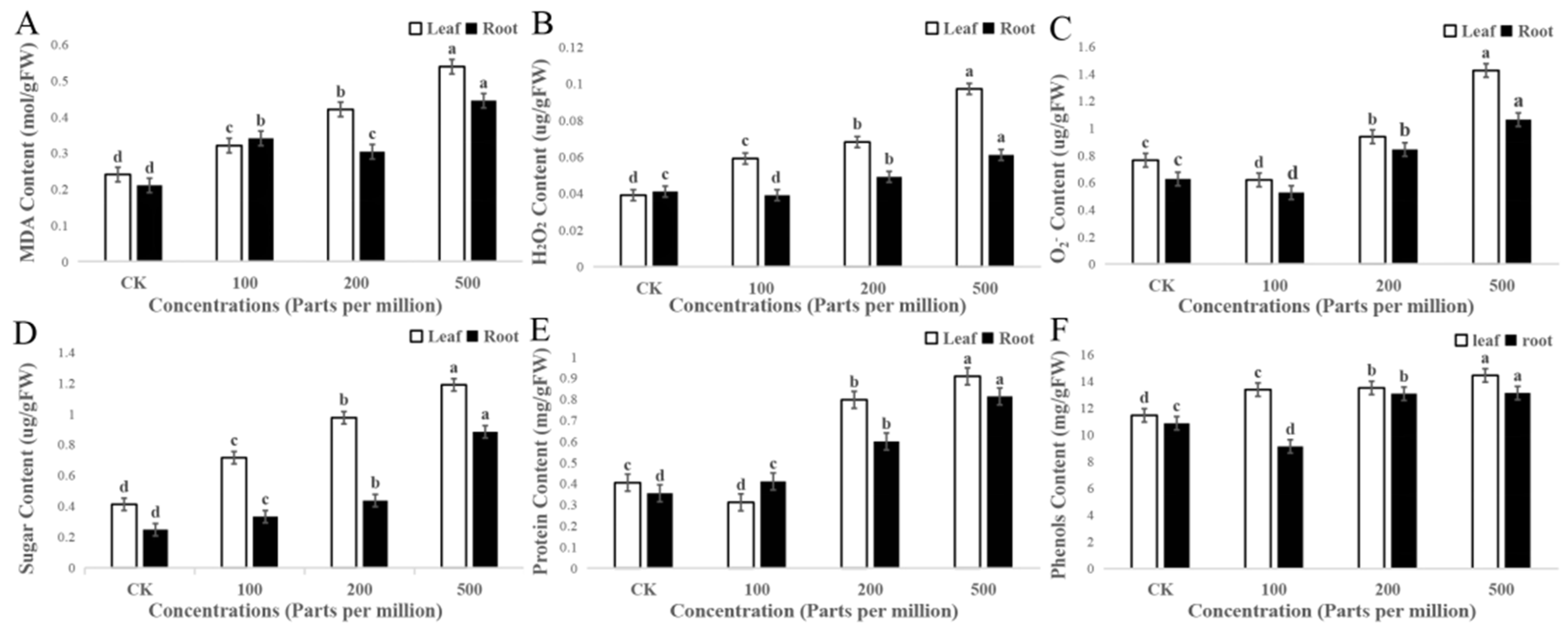
3.5. Biochemical Characterization of Maize Treated with MgO NPs
3.5.1. Antioxidant Enzymes
3.5.2. Membrane Lipid Peroxidation
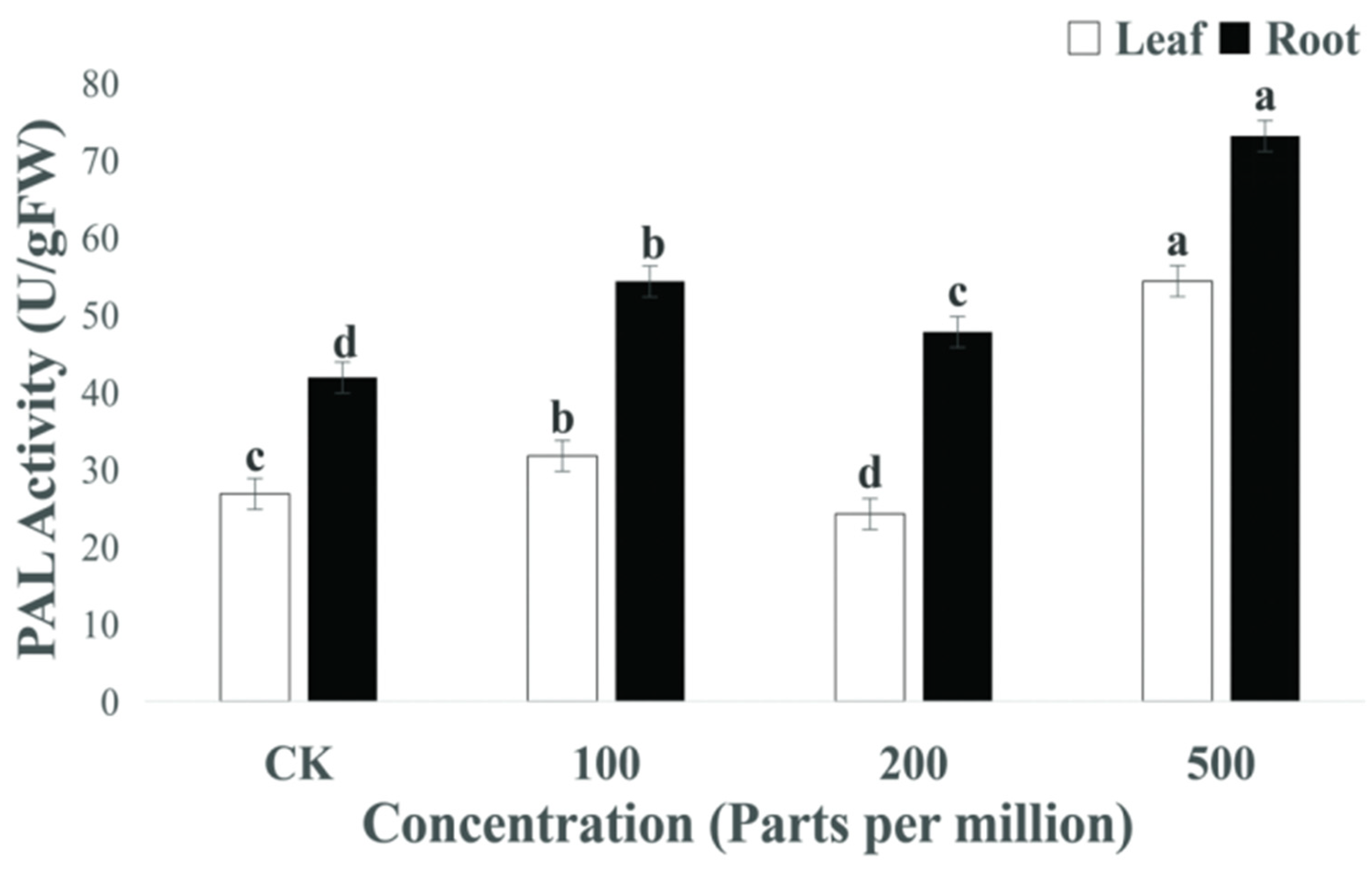
3.5.3. Plant Defense Enzymes
3.5.4. Phenolics
3.5.5. Acetylcholinesterase (AchE) Activity
4. Discussion
4.1. Physical and Chemical Properties of MgO NPs
4.2. Morphological Changes in Germination
4.3. Exposed to MgO NPs in Greenhouse
4.4. Exposure to MgO NPs in Field
4.5. Interpretation to MgO NPs in Biochemical Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ditta, A. How helpful is nanotechnology in agriculture? Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 033002. [Google Scholar] [CrossRef]
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural Nanotechnologies: What are the current possibilities? Nano Today 2015, 10, 124–127. [Google Scholar] [CrossRef]
- Kaul, R.K.; Kumar, P.; Burman, U.; Joshi, P.; Agrawal, A.; Raliya, R.; Tarafdar, J.C. Magnesium and iron nanoparticles production using microorganisms and various salts. Mater. Sci. 2012, 30, 254–258. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Appu, M.; Ramalingam, P.; Sathiyanarayanan, A.; Huang, J. An overview of plant defense-related enzymes responses to biotic stresses. Plant Gene 2021, 27, 100302. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Yahya, R.; Bakri, M.M.; Al Abboud, M.A.; Yahya, R.; Qanash, H.; Bazaid, A.S.; Salem, S.S. Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass-Convers. Biorefinery 2022, 13, 417–430. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.; Salem, S.S.; Alharbi, A.A.; Abdelghany, T. Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms. Arab. J. Chem. 2022, 15, 103927. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Correction to: Eco-Friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2800–2801. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Di, D.-R.; He, Z.-Z.; Sun, Z.-Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1233–1241. [Google Scholar] [CrossRef]
- Patel, M.K.; Zafaryab; Alam Rizvi, M.M.; Agrawal, V.V.; Ansari, Z.A.; Malhotra, B.D.; Ansari, S.G. Antibacterial and Cytotoxic Effect of Magnesium Oxide Nanoparticles on Bacterial and Human Cells. J. Nanoeng. Nanomanufacturing 2013, 3, 162–166. [Google Scholar] [CrossRef]
- Cai, L.; Liu, M.; Liu, Z.; Yang, H.; Sun, X.; Chen, J.; Xiang, S.; Ding, W. MgONPs Can Boost Plant Growth: Evidence from Increased Seedling Growth, Morpho-Physiological Activities, and Mg Uptake in Tobacco (Nicotiana tabacum L.). Molecules 2018, 23, 3375. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Singh, S.K.; Gautam, R.; Choudhary, K.; Maurino, V.G.; Saharan, V. MgO Nanoparticles Biosynthesis and Its Effect on Chlorophyll Contents in the Leaves of Clusterbean (Cyamopsis tetragonoloba L.). Adv. Sci. Eng. Med. 2014, 6, 538–545. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Sekhon, B. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Mohammad, F. Role of Nanoparticles in Plants, in Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–35. [Google Scholar]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Chen, J.; Sun, L.; Cheng, Y.; Lu, Z.; Shao, K.; Li, T.; Hu, C.; Han, H. Graphene Oxide-Silver Nanocomposite: Novel Agricultural Antifungal Agent against Fusarium graminearum for Crop Disease Prevention. ACS Appl. Mater. Interfaces 2016, 8, 24057–24070. [Google Scholar] [CrossRef]
- Horst, A.M.; Vukanti, R.; Priester, J.H.; Holden, P.A. An Assessment of Fluorescence- and Absorbance-Based Assays to Study Metal-Oxide Nanoparticle ROS Production and Effects on Bacterial Membranes. Small 2013, 9, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.R. Review on Problems and its Remedy in Plant Tissue Culture. Asian J. Biol. Sci. 2018, 11, 165–172. [Google Scholar] [CrossRef]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent Against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Paralikar, P.; Ingle, A.P.; Rai, M. Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger. Arab. J. Chem. 2020, 13, 3172–3182. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop. J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Martínez-Mera, I.; Espinosa-Pesqueira, M.; Pérez-Hernández, R.; Arenas-Alatorre, J. Synthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperature. Mater. Lett. 2007, 61, 4447–4451. [Google Scholar] [CrossRef]
- Camtakan, Z.; Erenturk, S.; Yusan, S. Magnesium oxide nanoparticles: Preparation, characterization, and uranium sorption properties. Environ. Prog. Sustain. Energy 2012, 31, 536–543. [Google Scholar] [CrossRef]
- Sharma, G.; Soni, R.; Jasuja, N.D. Phytoassisted synthesis of magnesium oxide nanoparticles with Swertia chirayaita. J. Taibah Univ. Sci. 2017, 11, 471–477. [Google Scholar] [CrossRef]
- Sushma, N.J.; Prathyusha, D.; Swathi, G.; Madhavi, T.; Raju, B.D.P.; Mallikarjuna, K.; Kim, H.-S. Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea—characterization and in vitro antioxidant studies. Appl. Nanosci. 2016, 6, 437–444. [Google Scholar] [CrossRef]
- Mast, J.; Verleysen, E.; Hodoroaba, V.D.; Kaegi, R. Chapter 2.1.2-Characterization of nanomaterials by transmission electron microscopy: Measurement procedures. In Characterization of Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–48. [Google Scholar]
- Pilarska, A.; Wysokowski, M.; Markiewicz, E.; Jesionowski, T. Synthesis of magnesium hydroxide and its calcinates by a precipitation method with the use of magnesium sulfate and poly(ethylene glycols). Powder Technol. 2013, 235, 148–157. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Thakar, M.A.; Jha, S.S.; Phasinam, K.; Manne, R.; Qureshi, Y.; Babu, V.H. X ray diffraction (XRD) analysis and evaluation of antioxidant activity of copper oxide nanoparticles synthesized from leaf extract of Cissus vitiginea. Mater. Today Proc. 2022, 51, 319–324. [Google Scholar] [CrossRef]
- Hirayama, T.; Mochida, K. Plant Hormonomics: A Key Tool for Deep Physiological Phenotyping to Improve Crop Productivity. Plant Cell Physiol. 2023, 63, 1826–1839. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Khalofah, A.; Migdadi, H.; El-Harty, E. Antioxidant Enzymatic Activities and Growth Response of Quinoa (Chenopodium quinoa Willd) to Exogenous Selenium Application. Plants 2021, 10, 719. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Enhancement of disease resistance, growth potential, and biochemical markers in maize plants by inoculation with plant growth-promoting bacteria under biotic stress. J. Plant Pathol. 2023, 105, 729–748. [Google Scholar] [CrossRef]
- Kumar, C.; Chand, P.; Choudhary, C.S.; Maurya, S.; Kumar, A.; Priya, S. Activity of different defense related antioxidative biochemical compound upon exogenous application of different elicitors on maize against Helminthosporium maydis Y. Nisik & C. Miyake. Cereal Res. Commun. 2023, 1–11. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Inafuku, M.; Nahar, K.; Fujita, M.; Oku, H. Nitric Oxide Regulates Plant Growth, Physiology, Antioxidant Defense, and Ion Homeostasis to Confer Salt Tolerance in the Mangrove Species, Kandelia obovata. Antioxidants 2021, 10, 611. [Google Scholar] [CrossRef]
- Mpanza, T.D.E.; Mani, S. Effects of Vachellia mearnsii Tannin Extract as an Additive on Fermentation Quality, Aerobic Stability, and Microbial Modulation of Maize Silage. Microorganisms 2023, 11, 2767. [Google Scholar] [CrossRef]
- Yucharoen, R.; Srisuksomwong, P.; Julsrigival, J.; Mungmai, L.; Kaewkod, T.; Tragoolpua, Y. Antioxidant, Anti-Tyrosinase, and Anti-Skin Pathogenic Bacterial Activities and Phytochemical Compositions of Corn Silk Extracts, and Stability of Corn Silk Facial Cream Product. Antibiotics 2023, 12, 1443. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, Y.; Xu, C.; Wang, X.; Wang, P.; Huang, S. Abscisic acid collaborates with lignin and flavonoid to improve pre-silking drought tolerance by tuning stem elongation and ear development in maize (Zea mays L.). Plant J. 2023, 114, 437–454. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Lucy, A.; Sarayut, P.; Patcharin, K. Entomopathogenic fungi based microbial insecticides and their physiological and biochemical effects on Spodoptera frugiperda (J.E. Smith). Front. Cell. Infect. Microbiol. 2023, 13, 1254475. [Google Scholar] [CrossRef]
- Mihali, C.; Frumuzachi, O.; Nicolescu, A.; Babotă, M.; Păltinean, R.; Tanase, C.; Mocan, A. Valorization of Corn Silk as an Agricultural By-Product through the Optimization of Ultrasound-Assisted Extraction. Appl. Sci. 2024, 14, 1516. [Google Scholar] [CrossRef]
- Henrist, C.; Mathieu, J.-P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef]
- Susi, H. [17] The Strength of Hydrogen Bonding: Infrared Spectroscopy. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1972; pp. 381–391. [Google Scholar]
- Sheng, X.; Jiang, X.; Zhao, H.; Wan, D.; Liu, Y.; Ngwenya, C.A.; Du, L. FTIR study of hydrogen bonding interaction between fluorinated alcohol and unsaturated esters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 198, 239–247. [Google Scholar] [CrossRef]
- Guerin, A.C.; Riley, K.; Rupnik, K.; Kuroda, D.G. Determining the Energetics of the Hydrogen Bond through FTIR: A Hands-On Physical Chemistry Lab Experiment. J. Chem. Educ. 2016, 93, 1124–1129. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis and characterization of MgO nanoparticles from plant extracts via induced molecular nucleation. New J. Chem. 2017, 41, 2800–2814. [Google Scholar] [CrossRef]
- Fuliaş, A.; Vlase, G.; Vlase, T.; Soica, C.; Heghes, A.; Craina, M.; Ledeti, I. Comparative kinetic analysis on thermal degradation of some cephalosporins using TG and DSC data. Chem. Cent. J. 2013, 7, 70. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Negrão, C.A.B.; Camelo, S.R.P.; Ribeiro-Costa, R.M.; Barbosa, W.L.R.; da Costa, C.E.F.; Silva Júnior, J.O.C. Investigation of thermal behavior of Heliotropium indicum L. lyophilized extract by TG and DSC. J. Therm. Anal. Calorim. 2013, 111, 1959–1964. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Kumari, S.B.; Rao, V.K.; Prabhu, Y.T. Enhancement of Growth In Maize By Biogenic- Synthesized Mgo Nanoparticles. Int. J. Pure Appl. Zool. 2016, 4, 262–270. [Google Scholar]
- Segatto, C.; Souza, C.A.; Lajús, C.R.; Fiori, M.A.; Silva, L.L.; Riella, H.G.; Coelho, C.M.M. Pretreatment of maize seeds with different magnesium nanoparticles improves the germinating performance and storability. Aust. J. Crop Sci. 2020, 14, 1473–1478. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Rajendran, V.; Kannan, N. Influence of ZrO2, SiO2, Al2O3 and TiO2 nanoparticles on maize seed germination under different growth conditions. IET Nanobiotechnol. 2016, 10, 171–177. [Google Scholar] [CrossRef]
- Almutairi, Z.; Alharbi, A. Effect of Silver Nanoparticles on Seed Germination of Crop Plants. World Academy of Science, Engineering and Technology. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015, 4, 280–285. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Wen, J.; Wu, G.; Tao, M. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–171. [Google Scholar]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- Biazus, J.P.M.; Santana, J.C.C.; Souza, R.R.; Tambourgi, E.B. Maximizing of production stages of malt from Zea mays. Braz. J. Food Technol. Spec. 2005, 138–145. Available online: https://bjft.ital.sp.gov.br/especiais/ed_especial/24.pdf (accessed on 22 February 2024).
- Biazus, J.P.M.; de Souza, R.R.; Márquez, J.E.; Franco, T.T.; Santana, J.C.C.; Tambourgi, E.B. Production and characterization of amylases from Zea mays malt. Braz. Arch. Biol. Technol. 2009, 52, 991–1000. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Julkapli, N.M.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef] [PubMed]
- Muleya, M.; Li, D.; Chiutsi-Phiri, G.; Botoman, L.; Brameld, J.M.; Salter, A.M. In vitro determination of the protein quality of maize varieties cultivated in Malawi using the INFOGEST digestion method. Heliyon 2023, 9, e19797. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, S.; Du, S.; Zhang, J.; Song, Z.; Jiang, J. Analysis of the main antioxidant enzymes in the roots of Tamarix ramosissima under NaCl stress by applying exogenous potassium (K+). Front. Plant Sci. 2023, 14, 1114266. [Google Scholar] [CrossRef]
- Zeng, C.-Q.; Liu, W.-X.; Hao, J.-Y.; Fan, D.-N.; Chen, L.-M.; Xu, H.-N.; Li, K.-Z. Measuring the expression and activity of the CAT enzyme to determine Al resistance in soybean. Plant Physiol. Biochem. 2019, 144, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Kitamura, R.S.A.; Marques, R.Z.; Barbato, M.L.; Zámocký, M. The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna minor to Antibiotics: Implications for Phytoremediation. Antioxidants 2022, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.-H.; Liu, J.-H.; Zhang, N.; Yang, J.-H.; Sa, R.-L.; Wu, L. Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat. J. Integr. Agric. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.; Shabala, L.; Ahmed, H.A.I.; Deng, S.; Zhang, H.; Song, P.; et al. Antioxidant Enzymatic Activity and Osmotic Adjustment as Components of the Drought Tolerance Mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef]
- Melicher, P.; Dvořák, P.; Šamaj, J.; Takáč, T. Protein-protein interactions in plant antioxidant defense. Front. Plant Sci. 2022, 13, 1035573. [Google Scholar] [CrossRef]
- Luqman, M.; Shahbaz, M.; Maqsood, M.F.; Farhat, F.; Zulfiqar, U.; Siddiqui, M.H.; Masood, A.; Aqeel, M.; Haider, F.U. Effect of strigolactone on growth, photosynthetic efficiency, antioxidant activity, and osmolytes accumulation in different maize (Zea mays L.) hybrids grown under drought stress. Plant Signal. Behav. 2023, 18, 2262795. [Google Scholar] [CrossRef]
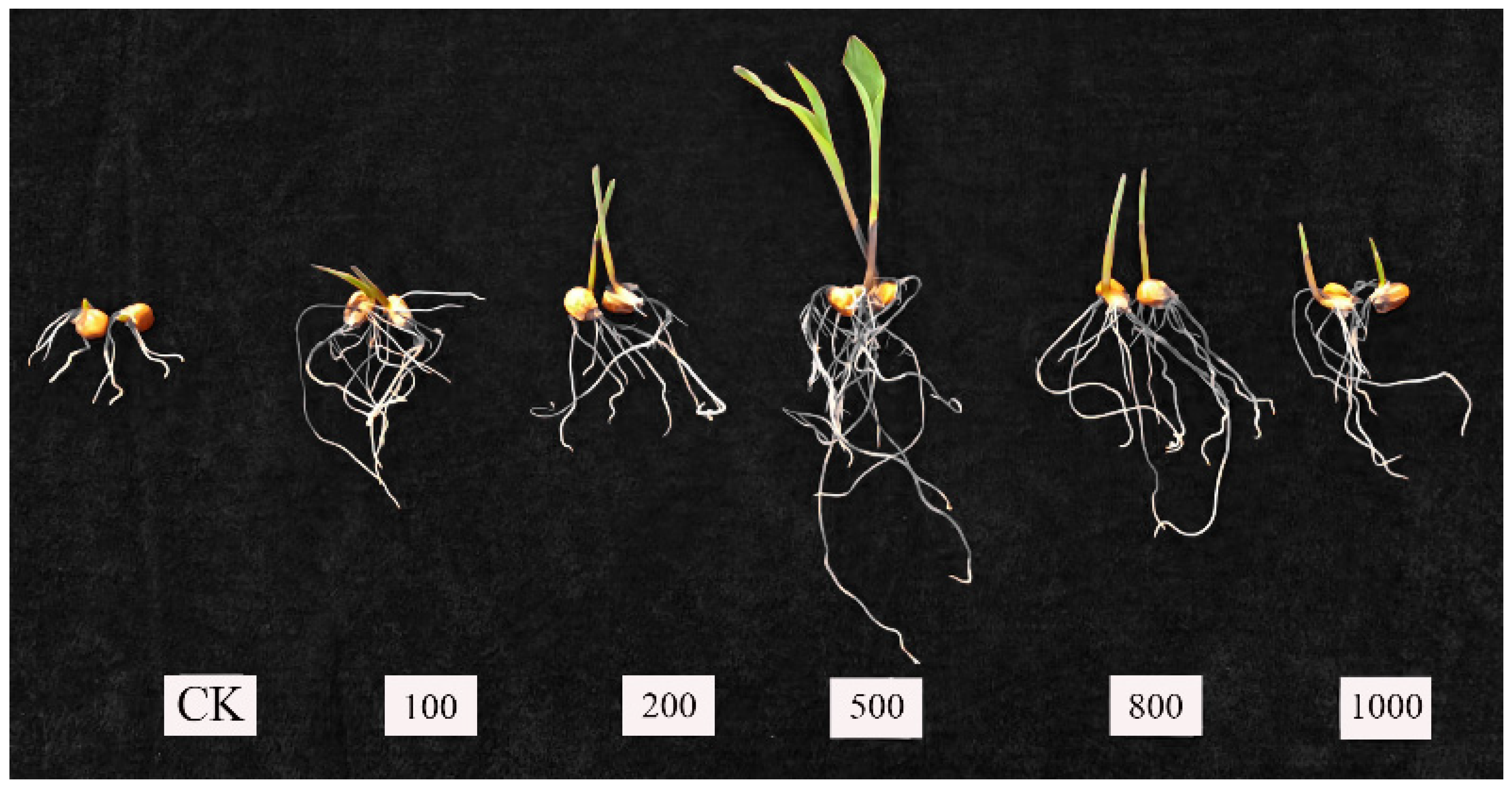


| Treatments | R1 (cm) | R2 (cm) | R3 (cm) | GS (Numbers) | GP (%) |
|---|---|---|---|---|---|
| CK | 11 | 14 | 16 | 41 | 27.33 |
| 100 ppm | 18 | 22 | 16 | 56 | 37.33 |
| 200 ppm | 25 | 27 | 21 | 73 | 48.67 |
| 500 ppm | 34 | 45 | 46 | 125 | 83.33 |
| 800 ppm | 16 | 22 | 12 | 50 | 33.33 |
| 1000 ppm | 14 | 12 | 18 | 44 | 29.33 |
| Treatments | Germination Rate (%) | Germination Potential (%) | Germination Index (%) |
|---|---|---|---|
| CK | 40.67 | 20.33 | 8.74 |
| 100 ppm | 48.67 | 24.33 | 10.43 |
| 200 ppm | 58.12 | 29.37 | 12.43 |
| 500 ppm | 75.33 | 37.67 | 16.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Z.; Hassan, M.A.; Huang, W.; Yu, H.; Xu, M.; Chang, X.; Fang, X.; Liu, L. Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea mays L.). Agronomy 2024, 14, 617. https://doi.org/10.3390/agronomy14030617
Abbas Z, Hassan MA, Huang W, Yu H, Xu M, Chang X, Fang X, Liu L. Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea mays L.). Agronomy. 2024; 14(3):617. https://doi.org/10.3390/agronomy14030617
Chicago/Turabian StyleAbbas, Zain, Muhammad Ahmad Hassan, Weidong Huang, Haibing Yu, Mengqin Xu, Xiaoyu Chang, Xisheng Fang, and Liqin Liu. 2024. "Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea mays L.)" Agronomy 14, no. 3: 617. https://doi.org/10.3390/agronomy14030617
APA StyleAbbas, Z., Hassan, M. A., Huang, W., Yu, H., Xu, M., Chang, X., Fang, X., & Liu, L. (2024). Influence of Magnesium Oxide (MgO) Nanoparticles on Maize (Zea mays L.). Agronomy, 14(3), 617. https://doi.org/10.3390/agronomy14030617







