Effects of Nitrogen Fertilizer on Nitrospira- and Nitrobacter-like Nitrite-Oxidizing Bacterial Microbial Communities under Mulched Fertigation System in Semi-Arid Area of Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection
2.3. Assessment of Soil Chemical Properties and Soil Nitrite Oxidoreductase Activity
2.4. Determination of Soil Nitrite Oxidation Potential (PNO)
2.5. Soil DNA Extraction and Quantification of Nitrospira- and Nitrobacter-like NOB Abundance
2.6. High-Throughput Sequencing and Data Analysis Method for nxrB Gene of Nitrospira-like NOB
2.7. Statistical Method and Data Analysis
3. Results
3.1. Soil Chemical Properties
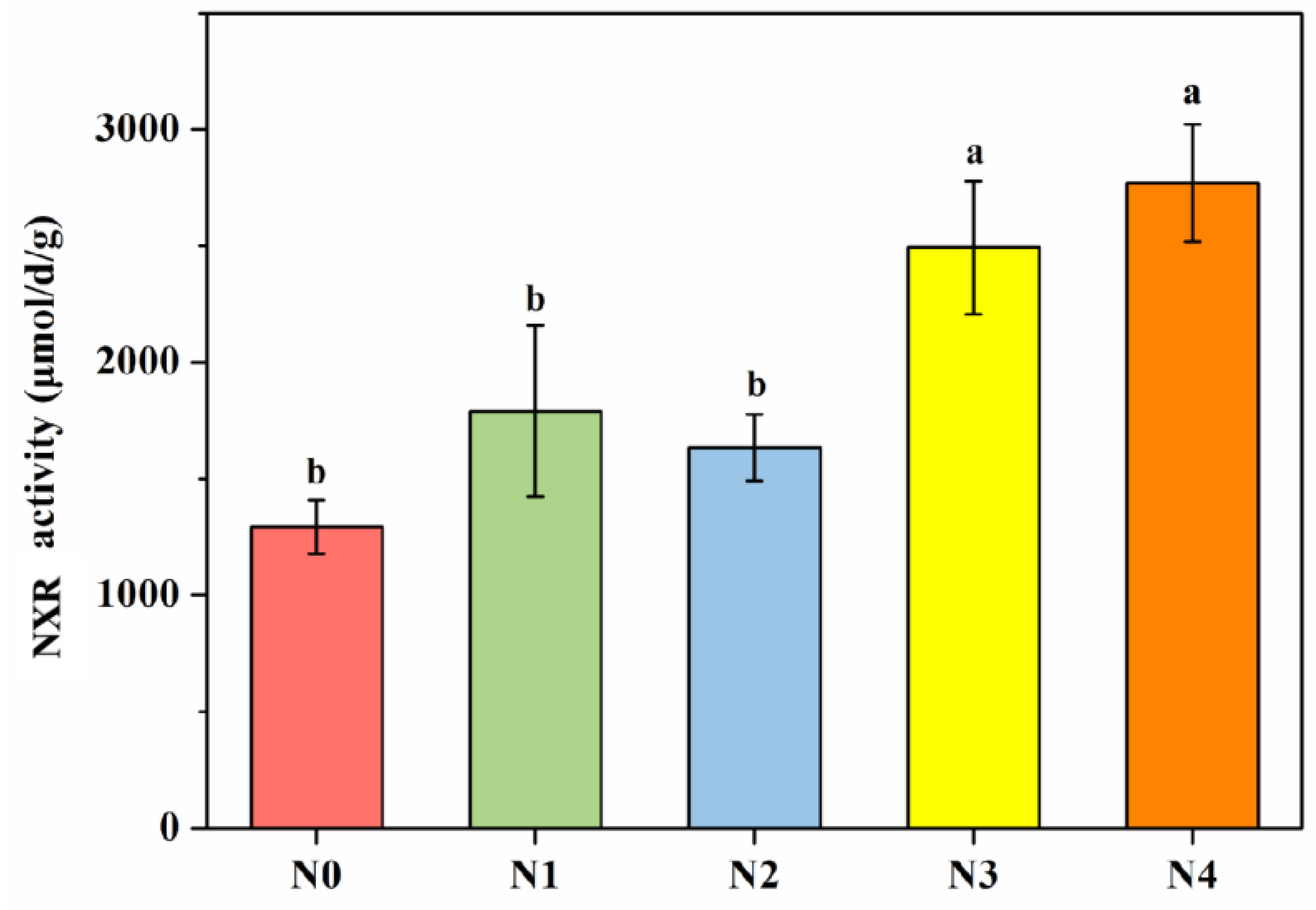
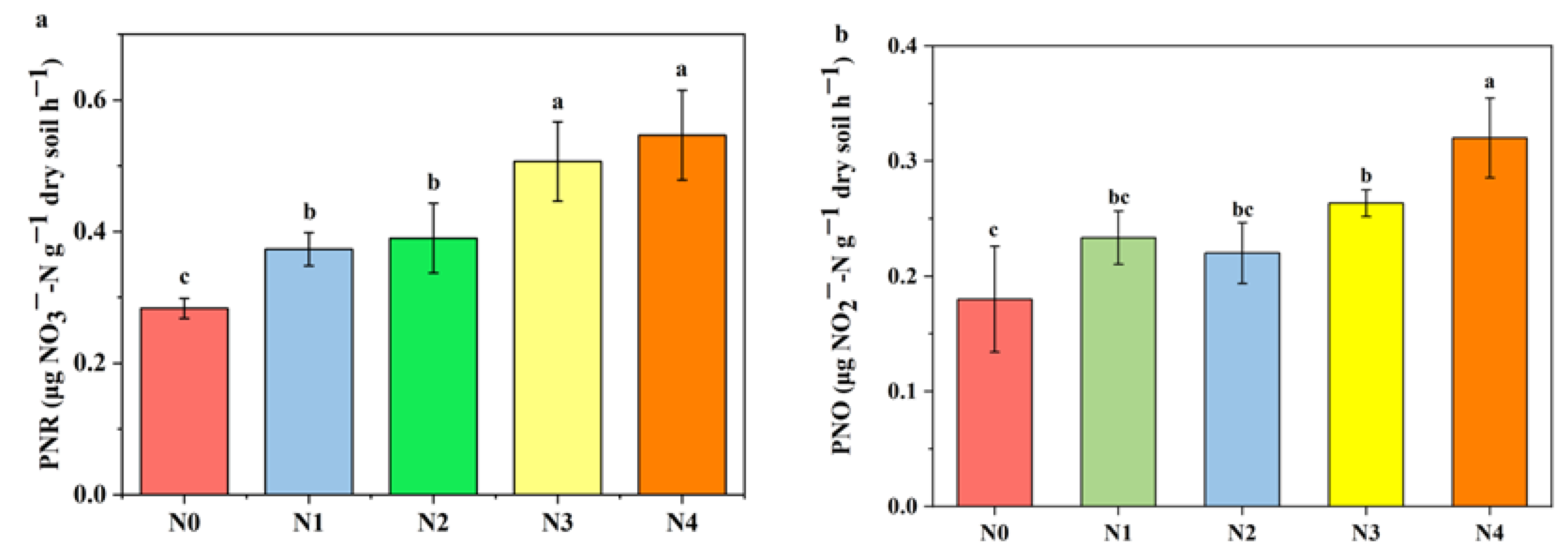
3.2. Soil NXR Activity and PNO
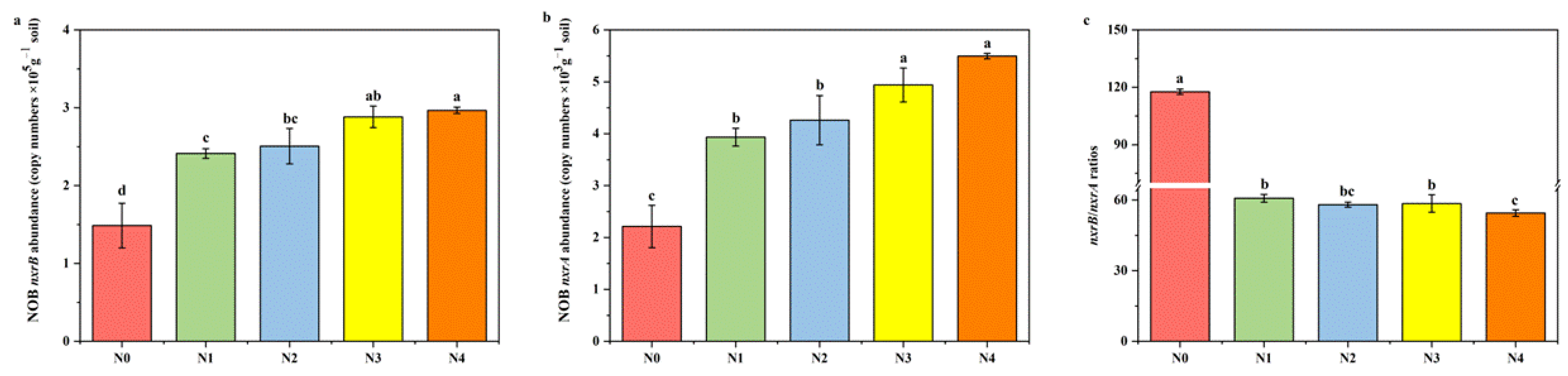
3.3. Abundance of Soil Nitrospira- and Nitrobacter-like NOB
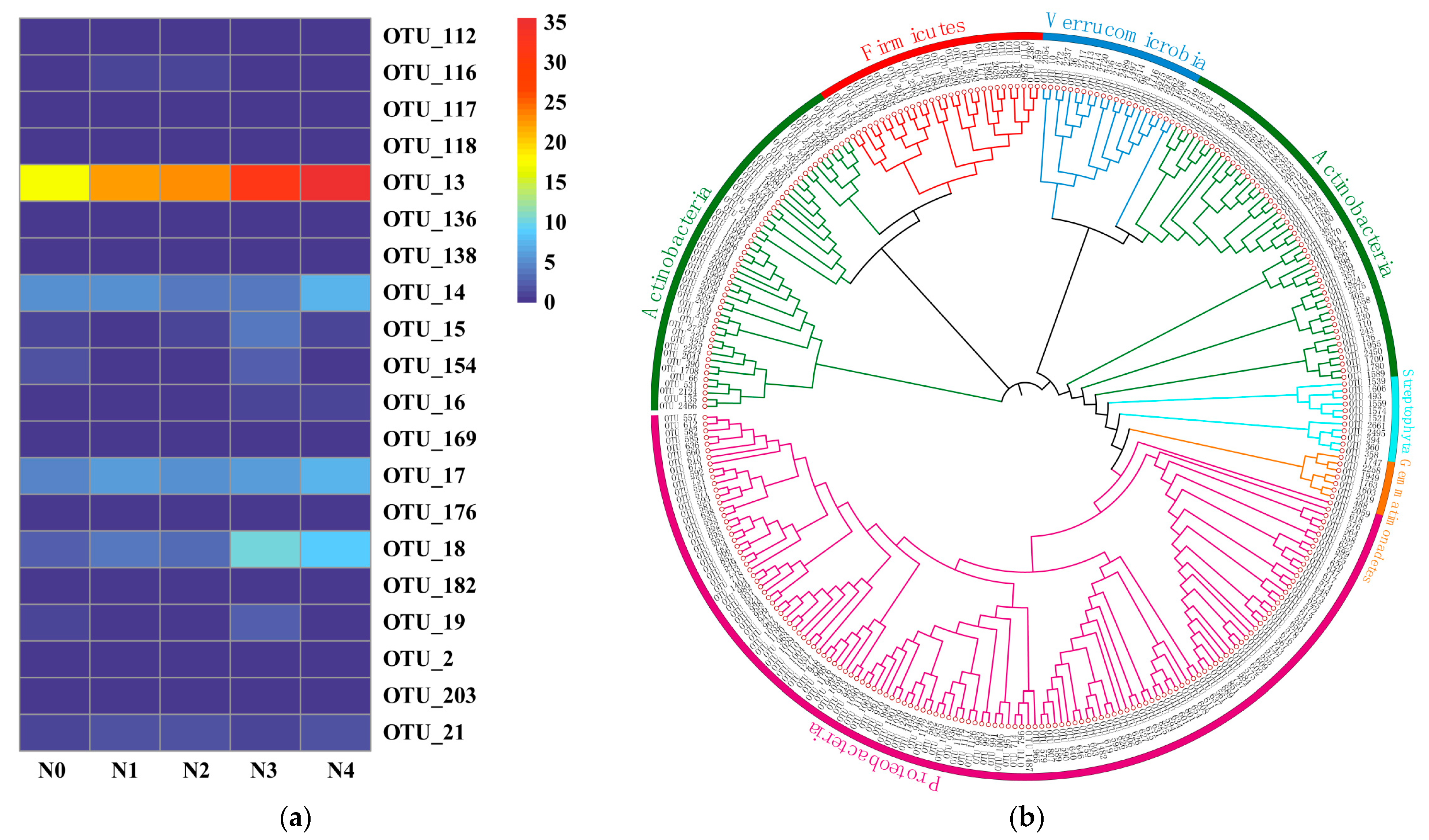
3.4. Compositions of Soil Nitrospira-like NOB Taxa
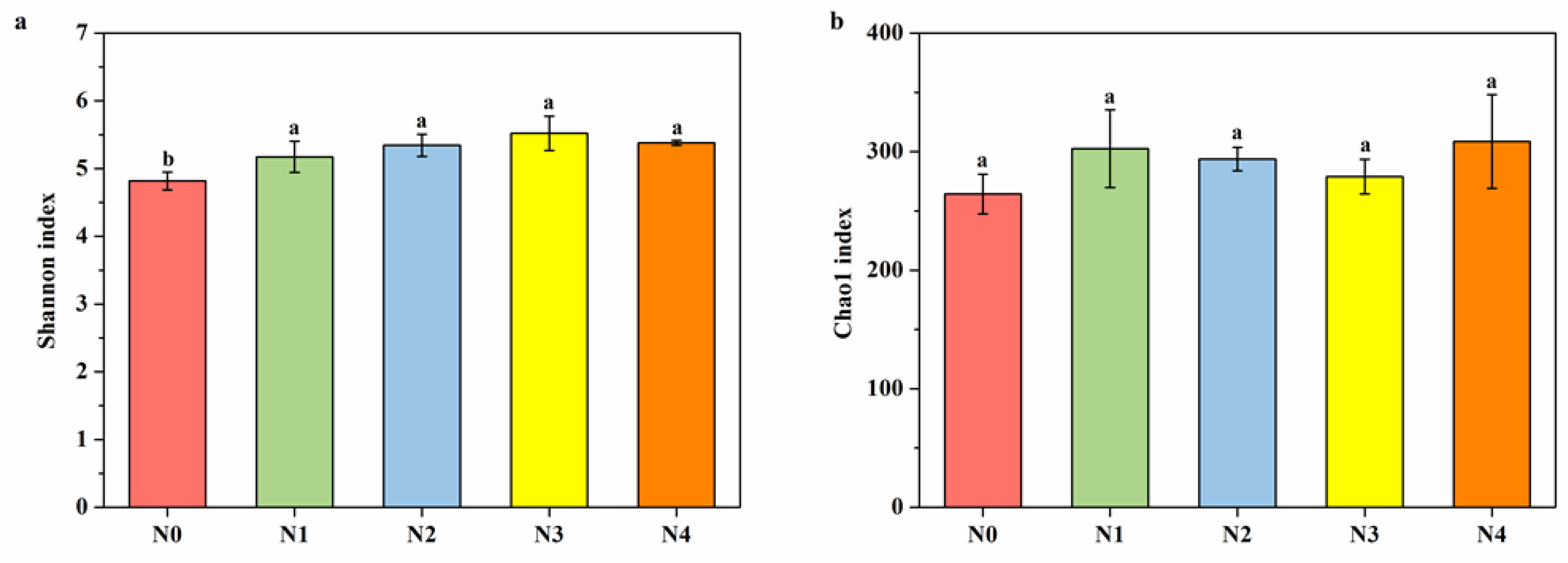
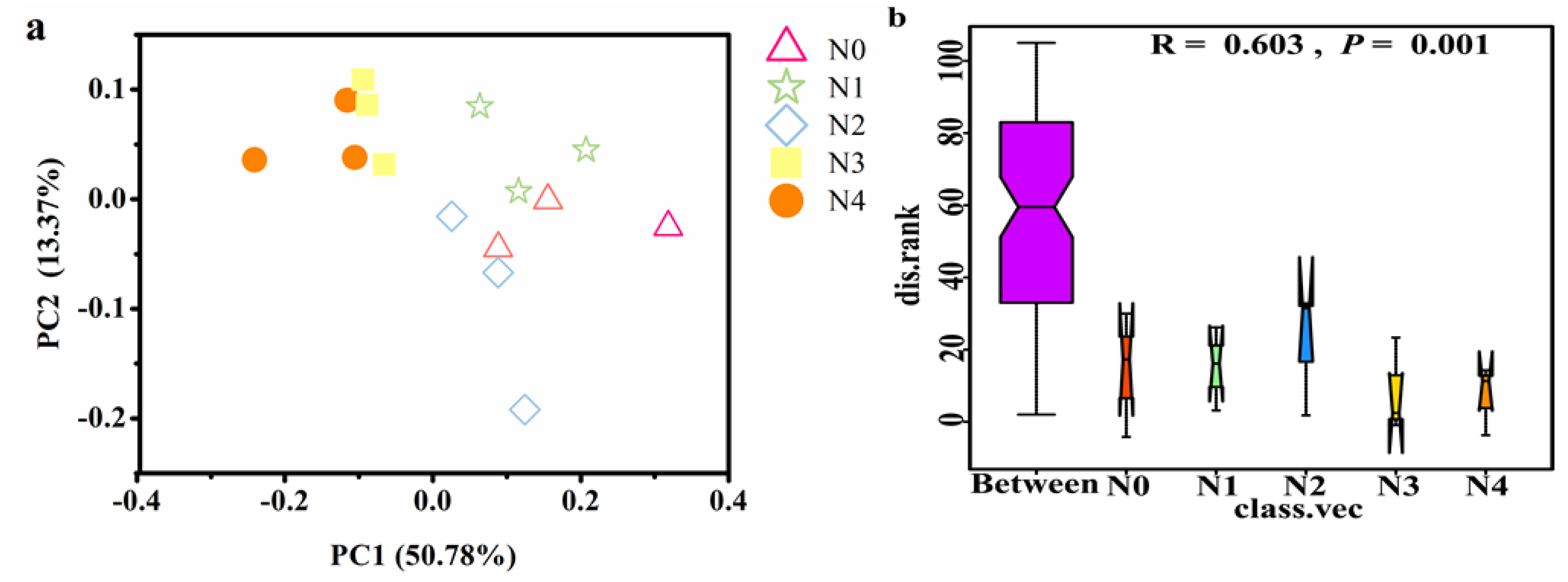
3.5. Diversities of Soil Nitrospira-like NOB
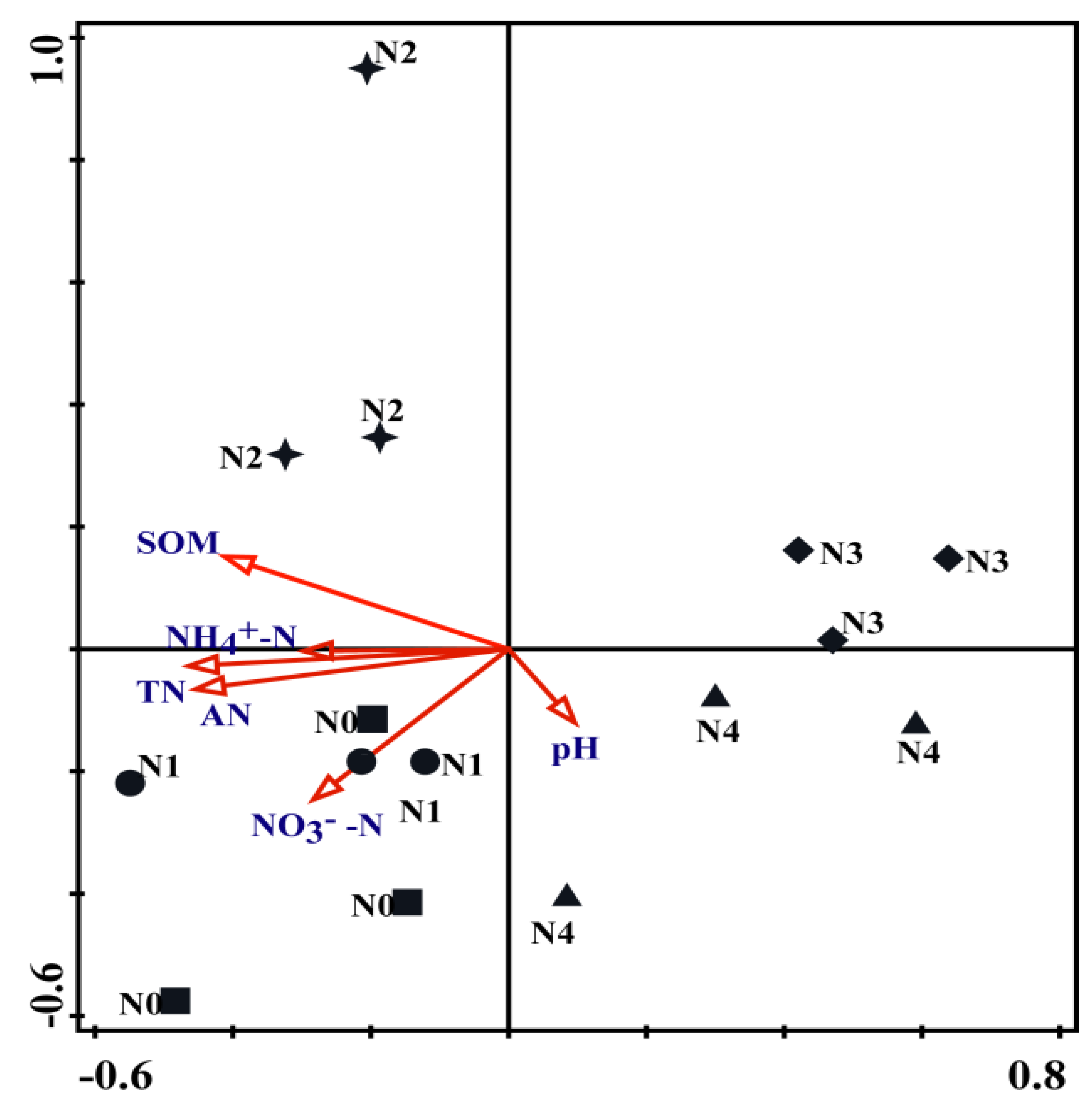
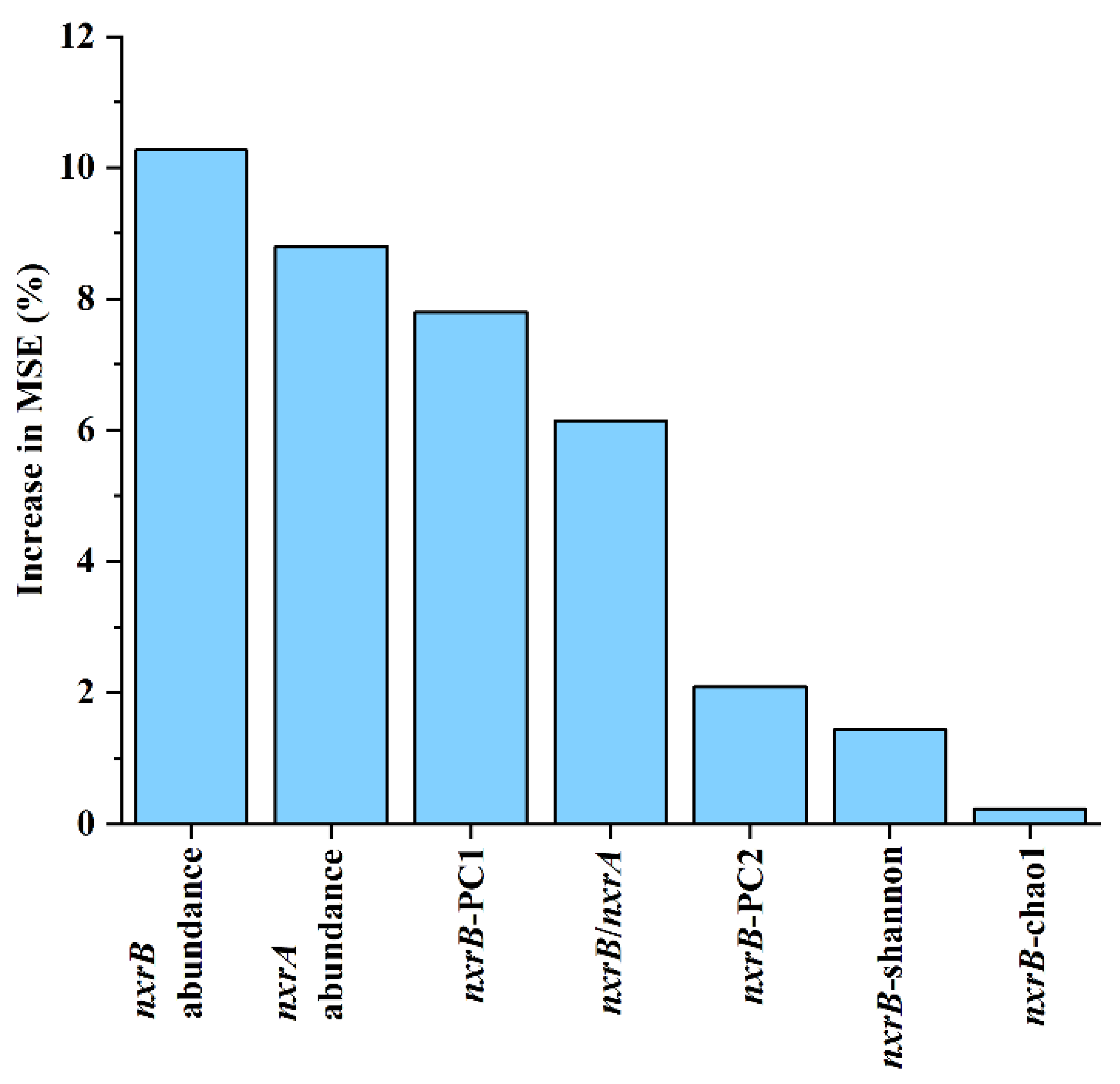
3.6. Drivering Factors of Changes in Soil Nitrospira-like NOB Community
4. Discussion
4.1. Variations in N Application Rates’ Impact on the Chemical Properties of Soil and Enzyme Activity
4.2. Effects of Different N Application Rates on Soil Nitrospira- and Nitrobacter-like NOB Abundance
4.3. Relationship between Environmental Factors and Nitrospira-like NOB Community Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vesco, L.G.; Santos, D.F.C.C.; Bruno, S.E.S.D. Interactive effects of increased plant density, cultivars and N rates in environments with different cotton yield recovery potential. Ind. Crops Prod. 2022, 176, 176. [Google Scholar] [CrossRef]
- Pasley, H.R.; Cairns, J.E.; Camberato, J.J.; Vyn, T.J. Nitrogen fertilizer rate increases plant uptake and soil availability of essential nutrients in continuous maize production in Kenya and Zimbabwe. Nutr. Cycl. Agroecosyst. 2019, 115, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Pentangelo, A.; Parisi, M. Optimizing N fertilization to improve yield, technological and nutritional quality of tomato grown in high fertility soil conditions. Plants 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Babita, A.; Roshni, G.F.A.C. Nitrous oxide emission in altered nitrogen cycle and implications for climate change. Environ. Pollut. 2022, 314, 120272. [Google Scholar] [CrossRef]
- Cao, M.; Zhu, Y.; Lv, G. Spatial distribution of global cultivated land and its variation between 2000 and 2010, from both agro-eecological and geopolitical perspectives. Sustainability 2019, 11, 1242. [Google Scholar] [CrossRef]
- Yan, D.H.; Wu, S.H.; Tang, Y.S. Arable land and water footprints for food consumption in China: From the perspective of urban and rural dietary change. Sci. Total Environ. 2022, 838, 155749. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Shao, C.; Shi, R. Development trend and driving factors of aagricultural chemical fertilizer efficiency in China. Sustainability 2020, 12, 4607. [Google Scholar] [CrossRef]
- Wei, W.; Yang, M.; Liu, Y.; Huang, H.; Ye, C.; Zheng, J.; Guo, C.; Hao, M.; He, X.; Zhu, S. Fertilizer N application rate impacts plant-soil feedback in a sanqi production system. Sci. Total Environ. 2018, 633, 796–807. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef]
- Shitu, A.; Zhu, S.; Qi, W. Performance of novel sponge biocarrier in MBBR treating recirculating aquaculture systems wastewater: Microbial community and kinetic study. J. Environ. Manag. 2020, 275, 111264. [Google Scholar] [CrossRef]
- Bohsale, R.R.; Almomani, F. Optimizing nutrient removal of moving bed biofilm reactor process using response surface methodology. Bioresour. Technol. 2020, 305, 123059. [Google Scholar] [CrossRef]
- Mehrani, M.J.; Lu, X.; Kowal, P. Incorporation of the complete ammonia oxidation (comammox) process for modeling nitrification in suspended growth wastewater treatment systems. J. Environ. Manag. 2021, 297, 113223. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.Y.; Zhang, L.; Yu, D.S.; Zhang, J.H.; Zhang, W.K.; Ma, G.C.; Zhao, X.C.; Peng, Y.Z. Application of intermittent aeration in nitrogen removal process: Development, advantages and mechanisms. Chem. Eng. 2021, 10, 1016. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Myrold, D.D.; Bottomley, P.J. Temperature affects the kinetics of nitrite oxidation and nitrification coupling in four agricultural soils. Soil Biol. Biochem. 2019, 136, 107523. [Google Scholar] [CrossRef]
- Gelfand, I.; Yakir, D. Influence of nitrite accumulation in association with seasonal patterns and mineralization of soil nitrogen in a semi-arid pine forest. Soil Biol. Biochem. 2008, 40, 415–424. [Google Scholar] [CrossRef]
- Noriega-Hevia, G.; Mateo, o.; Maciá, A.; Lardín, C.; Pastor, L.; Serralta, J.; Bouzas, A. Experimental sulphide inhibition calibration method in nitrification processes: A case-study. J. Environ. Manag. 2020, 274, 111191. [Google Scholar] [CrossRef]
- Wu, J.; Kong, Z.; Luo, Z.B.; Qin, Y.; Rong, C.; Wang, T.J.; Hanaoka, T.; Sakemi, S.; Ito, M.; Kobayashi, S.; et al. A successful start-up of an anaerobic membrane bioreactor (AnMBR) coupled mainstream partial nitritation-anammox (PN/A) system: A pilot-scale study on insitu NOB elimination, AnAOB growth kinetics, and mainstream treatment performance. Water Res. 2021, 207, 117783. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Lu, Q. Ammonia oxidizer abundance in paddy soil profile with different fertilizer regimes. Appl. Soil Ecol. 2014, 84, 38–44. [Google Scholar] [CrossRef]
- Spieck, E.; Bock, E. The lithoautotrophic nitrite oxidizing bacteria. Bergey’s Man. Syst. Bacteriol. 2005, 2, 149–153. [Google Scholar] [CrossRef]
- Poly, F.; Wertz, S.; Brothier, E.; Degrange, V. First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol. Ecol. 2008, 63, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lucker, S. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wan, W.; Zheng, L. Community assembly mechanisms and co-occurrence patterns of nitrite-oxidizing bacteria communities in saline soils. Sci. Total Environ. 2021, 772, 145472. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wen, X.; Zhang, B. Diversity and assembly patterns of activated sludge microbial communities: A review. Biotechnol. Adv. 2018, 36, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Leigh, A.K.; Grayston, S.J. Effects of long-term fertilization of forest soils on potential nitrification and on the abundance and community structure of ammonia oxidizers and nitrite oxidizers. FEMS Microbiol. Ecol. 2012, 79, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Angel, R.; Lu, Y.; Conrad, R. Niche differentiation of ammonia oxidizers and nitrite oxidizers in rice paddy soil. Environ. Microbiol. 2013, 15, 2275–2292. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Luo, X.; Liao, H.; Nie, H.; Chen, W.; Huang, Q. Nitrospira are more sensitive than Nitrobacter to land management in acid, fertilized soils of a rapeseed-rice rotation field trial. Sci. Total Environ. 2017, 599–600, 135–144. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Takamoto, A.; Takahashi, T.; Togami, K. Estimation models from soil pH with a solid-to-liquid ratio of 1,2.5 to pH measured by other methods using soils in Japan. Soil Sci. Plant Nutr. 2023, 69, 190–198. [Google Scholar] [CrossRef]
- Michatowski, T.; Tadeusz, A.G. The Titration in the Kjeldahl Method of Nitrogen Determination: “Base or Acid as Titrant?”. J. Chem. Educ. 2013, 90, 191–197. [Google Scholar] [CrossRef]
- Lu, R. Soil and Agro-Chemical Analytical Methods; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Jaynes, D.B.; Colvin, T.S.; Karlen, D.L. Nitrate loss in subsurface drainage as affected by nitrogen fertilizer rate. Environ. Qual. 2001, 30, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Porteous, L.A.; Armstrong, J.L.; Seidler, R.J.; Watrud, L.S. An effective method to extractDNA from environmental—Samples for polymerase chain-reaction amplification andDNA fingerprint analysis. Curr. Microbiol. 1994, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Degrange, V.; Prosser, J.I. Decline of soil microbial diversity does not influence the resistance and resilience of key soil microbial functional groups following a model disturbance. Environ. Microbiol. 2007, 9, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Attard, E.; Poly, F.; Commeaux, C.; Laurent, F.; Terada, A.; Smets, B.F. Shifts between Nitrospira- and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ. Microbiol. 2010, 12, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.W.; Wang, M.; Li, Q.; Qin, Y.B.; Sun, B.; Tan, P.; Liu, H.; Li, C.L.; Zhang, J.J. Comammox dominate soil nitrification under different N fertilization regimes in semi-arid areas of Northeast China. Appl. Soil Ecol. 2024, 193, 105119. [Google Scholar]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Reay Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Mary, B.; Recous, S.; Darwis, D.; Robin, D. Interactions between decomposition of plant residues and nitrogen cycling in soil. Plant Soil 1996, 181, 71–82. [Google Scholar] [CrossRef]
- Cong, R.; Xu, M.; Wang, X.; Zhang, W.; Yang, X.; Wang, H.B. An analysis of soil carbon dynamics in long-term soil fertility trials in China. Nutr. Cycl. Agroecosystems 2012, 93, 201–213. [Google Scholar] [CrossRef]
- Ghimire, R.; Lamichhane, S.; Acharya, B.S. Tillage, crop residue, and nutrient management effects on soil organic carbon in rice-based cropping systems: A review. J. Integr. Agric. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, t.; Gong, Y. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat–maize cropping system in northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Manna, M.C.; Swarup, A.; Wanjari, R.H.; Mishra, B.; Shahi, D.K. Long-term fertilization, manure and liming effects on soil organic matter and crop yields. Soil Tillage Res. 2007, 94, 397–409. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-term impact of fertilizers on soil organic carbon pools and sequestration rates in maize–wheat–cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Tian, J.; Lou, Y.; Gao, Y.; Fang, H.; Liu, S. Response of soil organic matter fractions and composition of microbial community to long-term organic and mineral fertilization. Biol. Fertil. Soils 2017, 53, 523–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y. Soil acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108–116. [Google Scholar] [CrossRef]
- Bolan, N.S.; Hedley, M.J. Role of carbon, nitrogen, and sulfur cycles in soil acidification. In Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; pp. 43–70. [Google Scholar] [CrossRef]
- Dal Molin, S.J.; Ernani, P.R.; Gerber, J.M. Soil acidification and nitrogen release following application of nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2020, 51, 2551–2558. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Liu, S. Effect of long-term mineral fertilizer application on soil enzyme activities and bacterial community composition. Plant Soil Environ. 2018, 64, 571–577. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, J.; Cui, Y. Metagenomics insights into the selective inhibition of NOB and comammox by phenacetin: Transcriptional activity, nitrogen metabolism and mechanistic understanding. Sci. Total Environ. 2022, 803, 150068. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Schlatter, D.C.; Kroese, D.R. Impacts of lime application on soil bacterial microbiome in dryland wheat soil in the Pacific Northwest. Appl. Soil Ecol. 2021, 168, 104113. [Google Scholar] [CrossRef]
- Zhou, X.; Fornara, D.; Wasson, E.A.; Wang, D.; Ren, G.; Christie, P.; Jia, Z. Effects of 44 years of chronic nitrogen fertilization on the soil nitrifying community of permanent grassland. Soil Biol. Biochem. 2015, 91, 76–83. [Google Scholar] [CrossRef]
- Le, T.T.H.; Fettig, J.; Meon, G. Kinetics and simulation of nitrification at various pH values of a polluted river in the tropics. Ecohydrol. Hydrobiol. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Daebeler, A.; Kitzinger, K.; Koch, H. Exploring the upper pH limits of nitrite oxidation: Diversity, ecophysiology, and adaptive traits of haloalkalitolerant Nitrospira. ISME J. 2020, 14, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- De Boer, W.; Kowalchuk, G.A. Nitrification in acid soils: Micro-organisms and mechanisms. Soil Biol. Biochem. 2001, 33, 853–866. [Google Scholar] [CrossRef]
- Zhong, W.; Bian, B.; Gao, N. Nitrogen fertilization induced changes in ammonia oxidation are attributable mostly to bacteria rather than archaea in greenhouse-based high N input vegetable soil. Soil Biol. Biochem. 2016, 93, 150–159. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, M.; Zhao, Z.; Zhou, N.; He, S.; Liu, S.; Wang, J.; Wang, X. Transcriptional activity and diversity of comammox bacteria as a previously overlooked ammonia oxidizing prokaryote in full-scale wastewater treatment plants. Sci. Total Environ. 2019, 656, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Ren, X.; Shen, C.; Wang, F.; Wu, D. Influences of nitrogen forms on abundances and community structures of ammonia and nitrite oxidizers in a slightly alkaline upland soil. Arch. Agron. Soil Sci. 2021, 67, 152–165. [Google Scholar] [CrossRef]
- Han, S.; Li, X.; Luo, X.; Wen, S.; Chen, W.; Huang, Q. Nitrite-oxidizing bacteria community composition and diversity are influenced by fertilizer regimes, but are independent of the soil aggregate in acidic subtropical red soil. Front. Microbiol. 2018, 9, 885. [Google Scholar] [CrossRef] [PubMed]
- Freitag, T.E.; Chang, L.; Clegg, C.D.; Prosser, J.I. Influence of inorganic nitrogen management regime on the diversity of nitrite-oxidizing bacteria in agricultural grassland soils. Appl. Environ. Microbiol. 2005, 71, 8323–8334. [Google Scholar] [CrossRef]

| pH | SOM (g kg−1) | TN (g kg−1) | AN (mg kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | |
|---|---|---|---|---|---|---|
| N0 | 6.89 ± 0.09 a | 17.6 ± 1.85 c | 1.11 ± 0.01 d | 105.64 ± 0.92 d | 0.33 ± 0.07 b | 2.74 ± 0.22 d |
| N1 | 6.79 ± 0.26 ab | 21.23 ± 2.15 b | 1.42 ± 0.02 c | 127.32 ± 4.48 c | 0.59 ± 0.09 a | 3.54 ± 0.99 d |
| N2 | 6.64 ± 0.11 a | 23.38 ± 0.33 ab | 1.53 ± 0.06 b | 150.4 ± 7.88 b | 0.7 ± 0.15 a | 5.97 ± 0.43 c |
| N3 | 6.62 ± 0.31 ab | 23.82 ± 0.55 a | 1.58 ± 0.02 b | 153.83 ± 1.18 b | 0.74 ± 0.03 a | 7.96 ± 1.47 b |
| N4 | 6.45 ± 0.29 b | 23.42 ± 1.25 ab | 1.71 ± 0.05 a | 163.3 ± 11.47 a | 0.77 ± 0.13 a | 10.03 ± 1.58 a |
| Explains % | Contribution % | Pseudo-F | p | |
|---|---|---|---|---|
| AN | 15.6 | 31.6 | 2.4 | 0.014 |
| NH4+-N | 9.3 | 18.7 | 1.5 | 0.21 |
| NO3−-N | 7.8 | 15.8 | 1.2 | 0.28 |
| pH | 6.3 | 12.7 | 1 | 0.37 |
| TN | 5.6 | 11.4 | 0.8 | 0.404 |
| SOM | 4.9 | 9.8 | 0.7 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Wang, M.; Feng, X.; Li, Q.; Qin, Y.; Sun, B.; Li, C.; Zhang, J.; Liu, H. Effects of Nitrogen Fertilizer on Nitrospira- and Nitrobacter-like Nitrite-Oxidizing Bacterial Microbial Communities under Mulched Fertigation System in Semi-Arid Area of Northeast China. Agronomy 2023, 13, 2909. https://doi.org/10.3390/agronomy13122909
Yuan Y, Wang M, Feng X, Li Q, Qin Y, Sun B, Li C, Zhang J, Liu H. Effects of Nitrogen Fertilizer on Nitrospira- and Nitrobacter-like Nitrite-Oxidizing Bacterial Microbial Communities under Mulched Fertigation System in Semi-Arid Area of Northeast China. Agronomy. 2023; 13(12):2909. https://doi.org/10.3390/agronomy13122909
Chicago/Turabian StyleYuan, Yuhan, Meng Wang, Xuewan Feng, Qian Li, Yubo Qin, Bo Sun, Cuilan Li, Jinjing Zhang, and Hang Liu. 2023. "Effects of Nitrogen Fertilizer on Nitrospira- and Nitrobacter-like Nitrite-Oxidizing Bacterial Microbial Communities under Mulched Fertigation System in Semi-Arid Area of Northeast China" Agronomy 13, no. 12: 2909. https://doi.org/10.3390/agronomy13122909
APA StyleYuan, Y., Wang, M., Feng, X., Li, Q., Qin, Y., Sun, B., Li, C., Zhang, J., & Liu, H. (2023). Effects of Nitrogen Fertilizer on Nitrospira- and Nitrobacter-like Nitrite-Oxidizing Bacterial Microbial Communities under Mulched Fertigation System in Semi-Arid Area of Northeast China. Agronomy, 13(12), 2909. https://doi.org/10.3390/agronomy13122909





