Increased Accumulation of Recombinant Proteins in Soybean Seeds via the Combination Strategy of Polypeptide Fusion and Suppression of Endogenous Storage Proteins
Abstract
1. Introduction
2. Materials and Methods
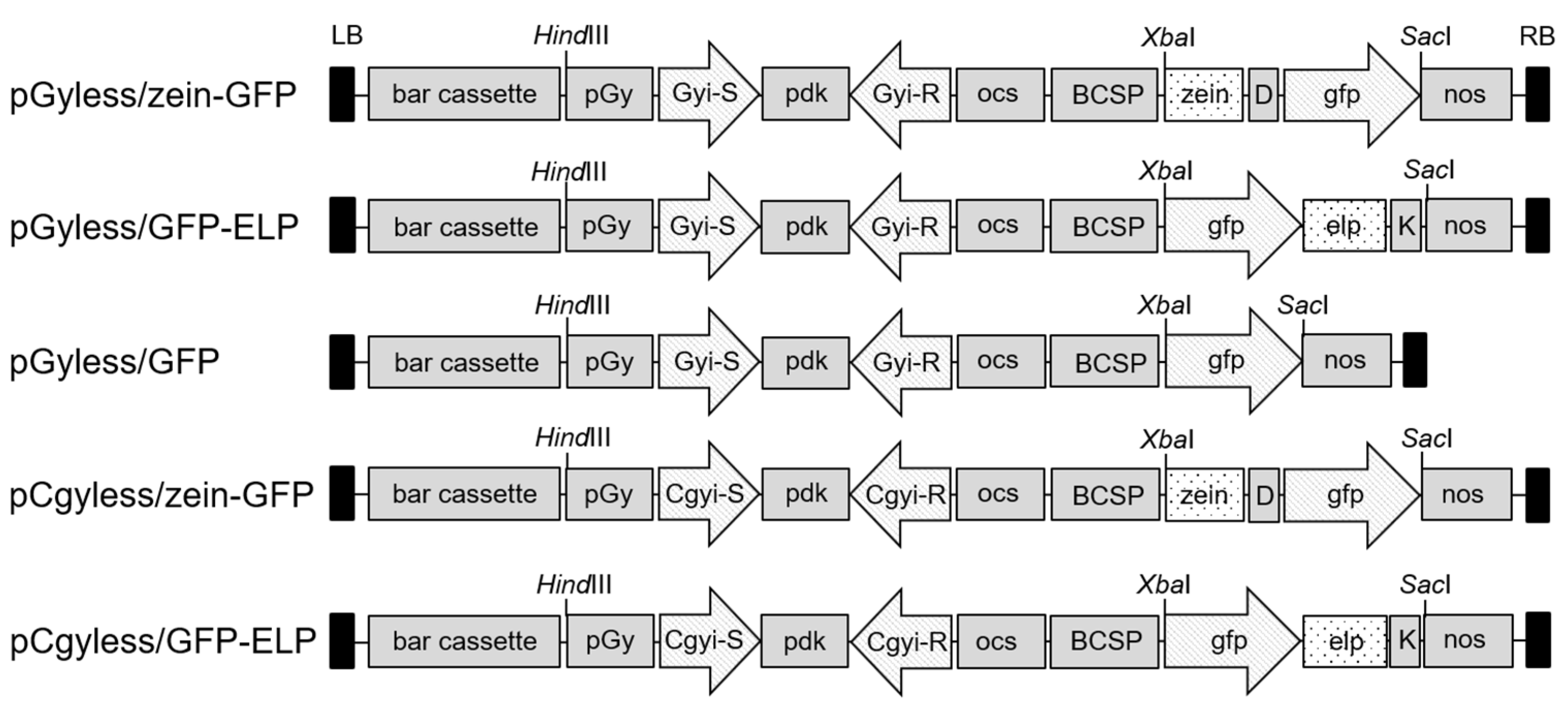
2.1. Vector Constructs for Soybean Genetic Transformation
2.2. Generation of Transgenic Soybean Plants
2.3. Transcription Analysis of Transgenic Soybean Seeds
2.4. Protein Expression and Quantification Analysis
2.5. Analysis of Total Protein and Oil Content in Soybean Seeds
2.6. Statistical Analysis
3. Results
3.1. Generation and Molecular Analysis of Transgenic Soybeans
3.2. Suppression of Glycinin and Conglycinin Expression in the Transgenic Soybean Seeds
3.3. Influence of RNAi-Mediated Suppression of Glycinin or Conglycinin Synthesis on the Accumulation of Recombinant Polypeptides
3.4. Increased Accumulation of Recombinant Proteins Did Not Affect Total Protein and Oil Contents in Soybean Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, J.K.C.; Christou, P.; Chikwamba, R.; Haydon, H.; Paul, M.; Ferrer, M.P.; Ramalingam, S.; Rech, E.; Rybicki, E.; Wigdorowitz, A. Realising the value of plant molecular pharming to benefit the poor in developing countries and emerging economies. Plant Biotechnol. J. 2013, 11, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Stoger, E.; Fischer, R.; Moloney, M.; Ma, J.K.-C. Plant Molecular Pharming for the Treatment of Chronic and Infectious Diseases. Annu. Rev. Plant Biol. 2014, 65, 743–768. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Habibi, P.; Haq, A.N.U.; Saeed, M.; Gulghutay Amjad, N.; Khan, I. Seed-Based System for Cost-Effective Production of Vaccine Against Chronic Respiratory Disease in Chickens. Mol. Biotechnol. 2023, 65, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Vianna, G.R.; Cunha, N.B.; Murad, A.M.; Rech, E.L. Soybeans as bioreactors for biopharmaceuticals and industrial proteins. Genet. Mol. Res. 2011, 10, 1733–1752. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.C.; Garg, R.; Bost, K.L.; Piller, K.J. Soybean Seeds: A Practical Host for the Production of Functional Subunit Vaccines. Biomed. Res. Int. 2014, 2014, 340804. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.N.; Maldaner, F.R.; Mendes, É.A.; Sousa, A.R.; D’Allastta, R.C.; Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L. Evaluation of lettuce chloroplast and soybean cotyledon as platforms for production of functional bone morphogenetic protein 2. Transgenic Res. 2019, 28, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Vianna, G.R.; Cunha, N.B.; Rech, E.L. Soybean seed protein storage vacuoles for expression of recombinant molecules. Curr. Opin. Plant Biol. 2023, 71, 102331. [Google Scholar] [CrossRef]
- Khan, M.S.; Joyia, F.A.; Mustafa, G. Seeds as Economical Production Platform for Recombinant Proteins. Protein Pept. Lett. 2020, 27, 89–104. [Google Scholar] [CrossRef]
- Müntz, K. Deposition of storage proteins. Plant Mol. Biol. 1998, 38, 77–99. [Google Scholar] [CrossRef]
- Cunha, N.B.; Murad, A.M.; Ramos, G.L.; Maranhão, A.Q.; Brígido, M.M.; Araújo, A.C.G.; Lacorte, C.; Aragão, F.J.L.; Covas, D.T.; Fontes, A.M. Accumulation of functional recombinant human coagulation factor IX in transgenic soybean seeds. Transgenic Res. 2011, 20, 841–855. [Google Scholar] [CrossRef]
- Morandini, F.; Avesani, L.; Bortesi, L.; Van, D.B.; De Wilde, K.; Arcalis, E.; Bazzoni, F.; Santi, L.; Brozzetti, A.; Falorni, A.; et al. Non-food/feed seeds as biofactories for the high-yield production of recombinant pharmaceuticals. Plant Biotechnol. J. 2011, 9, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.H.; Huang, L.Y.; Wang, Y.D.; Sun, H.C.; Xiang, Z.H. High-level Expression of Basic Fibroblast Growth Factor in Transgenic Soybean Seeds and Characterization of its Biological Activity. Biotechnol. Lett. 2006, 28, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Moravec, T.; Schmidt, M.A.; Herman, E.M.; Woodford-Thomas, T. Production of Escherichia coli heat labile toxin (LT) B subunit in soybean seed and analysis of its immunogenicity as an oral vaccine. Vaccine 2007, 25, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nishizawa, K.; Yokoo, M.; Zhao, H.; Onishi, K.; Teraishi, M.; Utsumi, S.; Ishimoto, M.; Yoshikawa, M. Anti-hypertensive activity of genetically modified soybean seeds accumulating novokinin. Peptides 2008, 29, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Cunha, N.B.; Murad, A.M.; Cipriano, T.M.; Araújo, A.C.; Aragão, F.J.; Leite, A.; Vianna, G.R.; McPhee, T.R.; Souza, G.H.; Waters, M.J.; et al. Expression of functional recombinant human growth hormone in transgenic soybean seeds. Transgenic Res. 2011, 20, 811–826. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Murad, A.M.; Vianna, G.R.; Ramessar, K.; Saucedo, C.J.; Wilson, J.; Buckheit, K.W.; da Cunha, N.B.; Araújo, A.C.; Lacorte, C.C.; et al. Engineering soya bean seeds as a scalable platform to produce cyanovirin-N, a non-ARV microbicide against HIV. Plant Biotechnol. J. 2015, 13, 884–892. [Google Scholar] [CrossRef]
- Sirko, A.; Vaněk, T.; Góra-Sochacka, A.; Redkiewicz, P. Recombinant Cytokines from Plants. Int. J. Mol. Sci. 2011, 12, 3536–3552. [Google Scholar] [CrossRef]
- Kusnadi, A.R.; Nikolov, Z.L.; Howard, J.A. Production of recombinant proteins in transgenic plants: Practical considerations. Biotechnol. Bioeng. 1997, 56, 473–484. [Google Scholar] [CrossRef]
- Yang, J.; Xun, H.; Niu, L.; He, H.; Cheng, Y.; Zhong, X.; Zhao, Q.; Xing, G.; Liu, J.; Yang, X. Elastin-like polypeptide and γ-zein fusions significantly increase recombinant protein accumulation in soybean seeds. Transgenic Res. 2021, 30, 675–686. [Google Scholar] [CrossRef]
- He, Y.; Ning, T.; Xie, T.; Qiu, Q.; Zhang, L.; Sun, Y.; Jiang, D.; Fu, K.; Yin, F.; Zhang, W.; et al. Large-scale production of functional human serum albumin from transgenic rice seeds. Proc. Natl. Acad. Sci. USA 2011, 108, 19078–19083. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.F.; Pettit, S.C.; Zaro, J.L.; Huang, N.; Shen, W.C. Characterization of transferrin receptor-mediated endocytosis and cellular iron delivery of recombinant human serum transferrin from rice (Oryza sativa L. ). BMC Biotechnol. 2012, 12, 92. [Google Scholar]
- Scheller, J.; Leps, M.; Conrad, U. Forcing single-chain variable fragment production in tobacco seeds by fusion to elastin-like polypeptides. Plant Biotechnol. J. 2006, 4, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Llompart, B.; Lasserre-Ramassamy, S.; Llop-Tous, I.; Ludevid, M.D. Eukaryotic protein production in designed storage organelles. BMC Biol. 2009, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, A.; Peters, J.; Arcalis, E.; Rademacher, T.; Lampel, J.; Eudes, F.; Vitale, A.; Stoger, E. The Induction of Recombinant Protein Bodies in Different Subcellular Compartments Reveals a Cryptic Plastid-Targeting Signal in the 27-kDa γ-Zein Sequence. Front. Bioeng. Biotechnol. 2014, 2, 67. [Google Scholar] [CrossRef][Green Version]
- Phan, H.T.; Hause, B.; Hause, G.; Arcalis, E.; Stoger, E.; Maresch, D.; Altmann, F.; Joensuu, J.; Conrad, U. Influence of elastin-like polypeptide and hydrophobin on recombinant hemagglutinin accumulations in transgenic tobacco plants. PLoS ONE 2014, 9, e99347. [Google Scholar] [CrossRef]
- Mainieri, D.; Marrano, C.A.; Prinsi, B.; Maffi, D.; Tschofen, M.; Espen, L.; Stöger, E.; Faoro, F.; Pedrazzini, E.; Vitale, A. Maize 16-kD γ-zein forms very unusual disulfide-bonded polymers in the endoplasmic reticulum: Implications for prolamin evolution. J. Exp. Bot. 2018, 69, 5013–5027. [Google Scholar] [CrossRef]
- Hegedus, D.D.; Baron, M.; Labbe, N.; Coutu, C.; Lydiate, D.; Lui, H.; Rozwadowski, K. A strategy for targeting recombinant proteins to protein storage vacuoles by fusion to Brassica napus napin in napin-depleted seeds. Protein Expr. Purif. 2014, 95, 162–168. [Google Scholar] [CrossRef]
- Yang, L.; Hirose, S.; Takahashi, H.; Kawakatsu, T.; Takaiwa, F. Recombinant protein yield in rice seed is enhanced by specific suppression of endogenous seed proteins at the same deposit site. Plant Biotechnol. J. 2012, 10, 1035–1045. [Google Scholar] [CrossRef]
- Kurokawa, S.; Kuroda, M.; Mejima, M.; Nakamura, R.; Takahashi, Y.; Sagara, H.; Takeyama, N.; Satoh, S.; Kiyono, H.; Teshima, R.; et al. RNAi-mediated suppression of endogenous storage proteins leads to a change in localization of overexpressed cholera toxin B-subunit and the allergen protein RAG2 in rice seeds. Plant Cell Rep. 2014, 33, 75–87. [Google Scholar] [CrossRef]
- Kinney, A.J.; Jung, R.; Herman, E.M. Cosuppression of the alpha subunits of beta-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies. Plant Cell. 2001, 13, 1165–1178. [Google Scholar]
- Tada, Y.; Utsumi, S.; Takaiwa, F. Foreign gene products can be enhanced by introduction into low storage protein mutants. Plant Biotechnol. J. 2003, 1, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, L.; Zhang, W.; He, H.; Yang, J.; Xing, G.; Guo, D.; Du, Q.; Qian, X.; Yao, Y.; et al. Robust RNAi-mediated resistance to infection of seven potyvirids in soybean expressing an intron hairpin NIb RNA. Transgenic Res. 2017, 26, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xing, G.; Niu, L.; He, H.; Guo, D.; Du, Q.; Qian, X.; Yao, Y.; Li, H.; Zhong, X.; et al. Improved oil quality in transgenic soybean seeds by RNAi-mediated knockdown of GmFAD2-1B. Transgenic Res. 2018, 27, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.Y.; Song, Q.; Jia, G.; Specht, J.E.; Hyten, D.L.; Costa, J.; Cregan, P.B. A genome-wide association study of seed protein and oil content in soybean. BMC Genomics 2014, 15, 1. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Herman, E.M. Proteome rebalancing in soybean seeds can be exploited to enhance foreign protein accumulation. Plant Biotechnol. J. 2008, 6, 832–842. [Google Scholar] [CrossRef]
- Gutiérrez, S.P.; Saberianfar, R.; Kohalmi, S.E.; Menassa, R. Protein body formation in stable transgenic tobacco expressing elastin-like polypeptide and hydrophobin fusion proteins. BMC Biotechnol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Coates, R.J.; Young, M.T.; Scofield, S. Optimising expression and extraction of recombinant proteins in plants. Front. Plant Sci. 2022, 13, 1074531. [Google Scholar] [CrossRef]
- Iida, S.; Amano, E.; Nishio, T. A rice (Oryza sativa L.) mutant having a low content of glutelin and a high content of prolamine. Theor. Appl. Genet. 1993, 87, 374–378. [Google Scholar] [CrossRef]
- Iida, S.; Kusaba, M.; Nishio, T. Mutants lacking glutelin subunits in rice: Mapping and combination of mutated glutelin genes. Theor. Appl. Genet. 1997, 94, 177–183. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Hirose, S.; Yasuda, H.; Takaiwa, F. Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol. 2010, 154, 1842–1854. [Google Scholar] [CrossRef]
- He, W.; Wang, L.; Lin, Q.; Yu, F. Rice seed storage proteins: Biosynthetic pathways and the effects of environmental factors. J. Integr. Plant Biol. 2021, 63, 1999–2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.Y.; Yoon, U.H.; Lim, S.H.; Kim, Y.M. Effects of reduced prolamin on seed storage protein composition and the nutritional quality of rice. Int. J. Mol. Sci. 2013, 14, 17073. [Google Scholar] [CrossRef] [PubMed]
- Takaiwa, F.; Yang, L.; Wakasa, Y.; Ozawa, K. Compensatory rebalancing of rice prolamins by production of recombinant prolamin/bioactive peptide fusion proteins within ER-derived protein bodies. Plant Cell Rep. 2018, 37, 209–223. [Google Scholar] [CrossRef] [PubMed]


| Transgenes | Lines | Immature Seeds | Mature Seeds | ||
|---|---|---|---|---|---|
| % TSP | Mean ± SD | % TSP | Mean ± SD | ||
| Gyless/zein-GFP | G45 | 2.83 ± 0.11 | 2.69 ± 0.20 A | 3.15 ± 0.21 | 3.49 ± 0.33 A |
| G54 | 2.78 ± 0.21 | 3.80 ± 0.26 | |||
| G59 | 2.46 ± 0.26 | 3.51 ± 0.11 | |||
| Cgyless/zein-GFP | F24 | 2.72 ± 0.27 | 2.80 ± 0.12 A | 3.22 ± 0.25 | 4.49 ± 1.80 A |
| F32 | 2.89 ± 0.10 | 5.76 ± 1.36 | |||
| Zein-GFP | DG44 | 1.20 ± 0.22 B | 2.08 ± 0.21 B | ||
| Gyless/GFP-ELP | A44 | 1.84 ± 0.11 | 2.30 ± 0.55 A | 2.78 ± 0.24 | 4.31 ± 1.50 A |
| A58 | 2.90 ± 0.49 | 4.37 ± 0.89 | |||
| A59 | 2.14 ± 0.17 | 5.78 ± 1.58 | |||
| Cgyless/GFP-ELP | J41 | 2.96 ± 0.04 | 3.04 ± 0.08 A | 3.66 ± 0.14 | 5.03 ± 1.9 A |
| J47 | 3.04 ± 0.02 | 5.74 ± 1.22 | |||
| J53 | 3.12 ± 0.28 | 5.69 ± 0.41 | |||
| GFP-ELP | DE9 | 0.30 ± 0.12 B | 0.88 ± 0.19 B | ||
| Gyless/GFP | B14 | 1.03 ± 0.27 | 1.02 ± 0.08 A | 1.94 ± 0.84 | 1.53 ± 0.37 A |
| B43 | 0.94 ± 0.15 | 1.21 ± 1.1 | |||
| B66 | 1.09 ± 0.18 | 1.45 ± 0.98 | |||
| Unfused GFP | DF8 | 0.08 ± 0.03 B | 0.57 ± 0.18 B | ||
| Transgenes | Lines | Total Protein (%) | Total Oil (%) | Protein/Oil Ratio |
|---|---|---|---|---|
| Gyless/zein-GFP | G45 | 40.76 ± 0.15 | 18.61 ± 0.15 | 2.19 |
| G54 | 41.55 ± 0.25 | 18.72 ± 0.04 | 2.22 | |
| G59 | 41.56 ± 0.17 | 19.15 ± 0.22 | 2.17 | |
| Cgyless/zein-GFP | F24 | 40.74 ± 0.73 | 19.58 ± 0.05 | 2.08 |
| F32 | 40.60 ± 0.06 | 19.80 ± 0.30 | 2.05 | |
| Gyless/GFP-ELP | A44 | 39.93 ± 0.65 | 19.97 ± 0.62 | 2.00 |
| A58 | 41.19 ± 1.18 | 18.55 ± 1.01 | 2.22 | |
| A59 | 41.72 ± 0.44 | 18.12 ± 0.24 | 2.30 | |
| Cgyless/GFP-ELP | J41 | 41.61 ± 0.65 | 20.00 ± 0.12 | 2.08 |
| J47 | 41.16 ± 0.77 | 19.88 ± 0.43 | 2.07 | |
| J53 | 41.08 ± 0.35 | 19.94 ± 0.78 | 2.06 | |
| Gyless/GFP | B14 | 40.25 ± 0.04 | 18.90 ± 0.07 | 2.13 |
| B43 | 40.24 ± 1.07 | 19.35 ± 0.44 | 2.08 | |
| B66 | 41.15 ± 0.39 | 17.89 ± 0.11 | 2.30 | |
| Non-transformed control | P03 | 40.60 ± 0.02 | 19.71 ± 0.14 | 2.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, Y.; Xing, G.; Wei, J.; Niu, L.; Zhao, Q.; Cai, Q.; Zhong, X.; Yang, X. Increased Accumulation of Recombinant Proteins in Soybean Seeds via the Combination Strategy of Polypeptide Fusion and Suppression of Endogenous Storage Proteins. Agronomy 2023, 13, 2680. https://doi.org/10.3390/agronomy13112680
Yang J, Zhang Y, Xing G, Wei J, Niu L, Zhao Q, Cai Q, Zhong X, Yang X. Increased Accumulation of Recombinant Proteins in Soybean Seeds via the Combination Strategy of Polypeptide Fusion and Suppression of Endogenous Storage Proteins. Agronomy. 2023; 13(11):2680. https://doi.org/10.3390/agronomy13112680
Chicago/Turabian StyleYang, Jing, Yuanyu Zhang, Guojie Xing, Jia Wei, Lu Niu, Qianqian Zhao, Qinan Cai, Xiaofang Zhong, and Xiangdong Yang. 2023. "Increased Accumulation of Recombinant Proteins in Soybean Seeds via the Combination Strategy of Polypeptide Fusion and Suppression of Endogenous Storage Proteins" Agronomy 13, no. 11: 2680. https://doi.org/10.3390/agronomy13112680
APA StyleYang, J., Zhang, Y., Xing, G., Wei, J., Niu, L., Zhao, Q., Cai, Q., Zhong, X., & Yang, X. (2023). Increased Accumulation of Recombinant Proteins in Soybean Seeds via the Combination Strategy of Polypeptide Fusion and Suppression of Endogenous Storage Proteins. Agronomy, 13(11), 2680. https://doi.org/10.3390/agronomy13112680






