Genome-Wide Identification of the Phytocyanin Gene Family and Its Potential Function in Salt Stress in Soybean (Glycine max (L.) Merr.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Authentication of Candidate GmPC Genes in Soybean
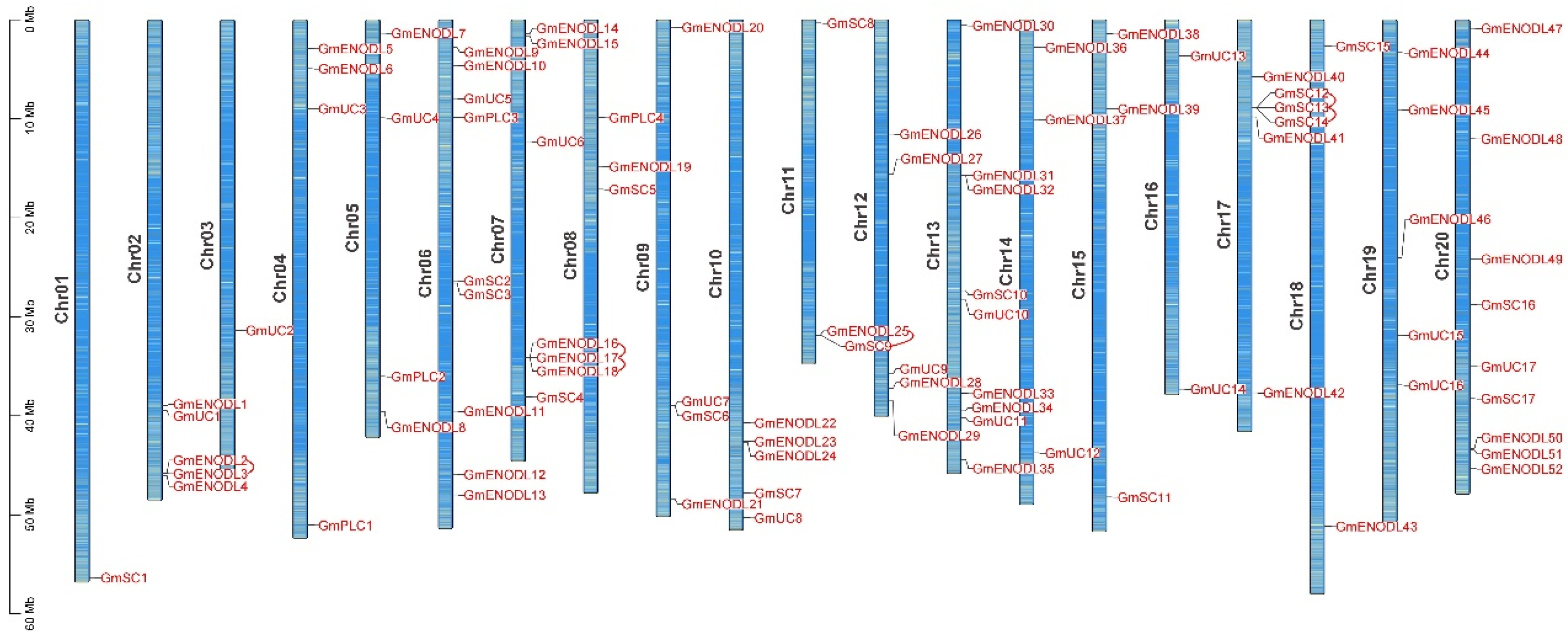
2.2. Characterization of Chromosomal Location, Structure, and Conserved Domain Distribution of GmPCs
2.3. Phylogenetic Analysis and Classification
2.4. GmPC Gene Duplication and Synteny Analyses
2.5. Promoter Cis-Regulatory Elements Analysis of GmPCs
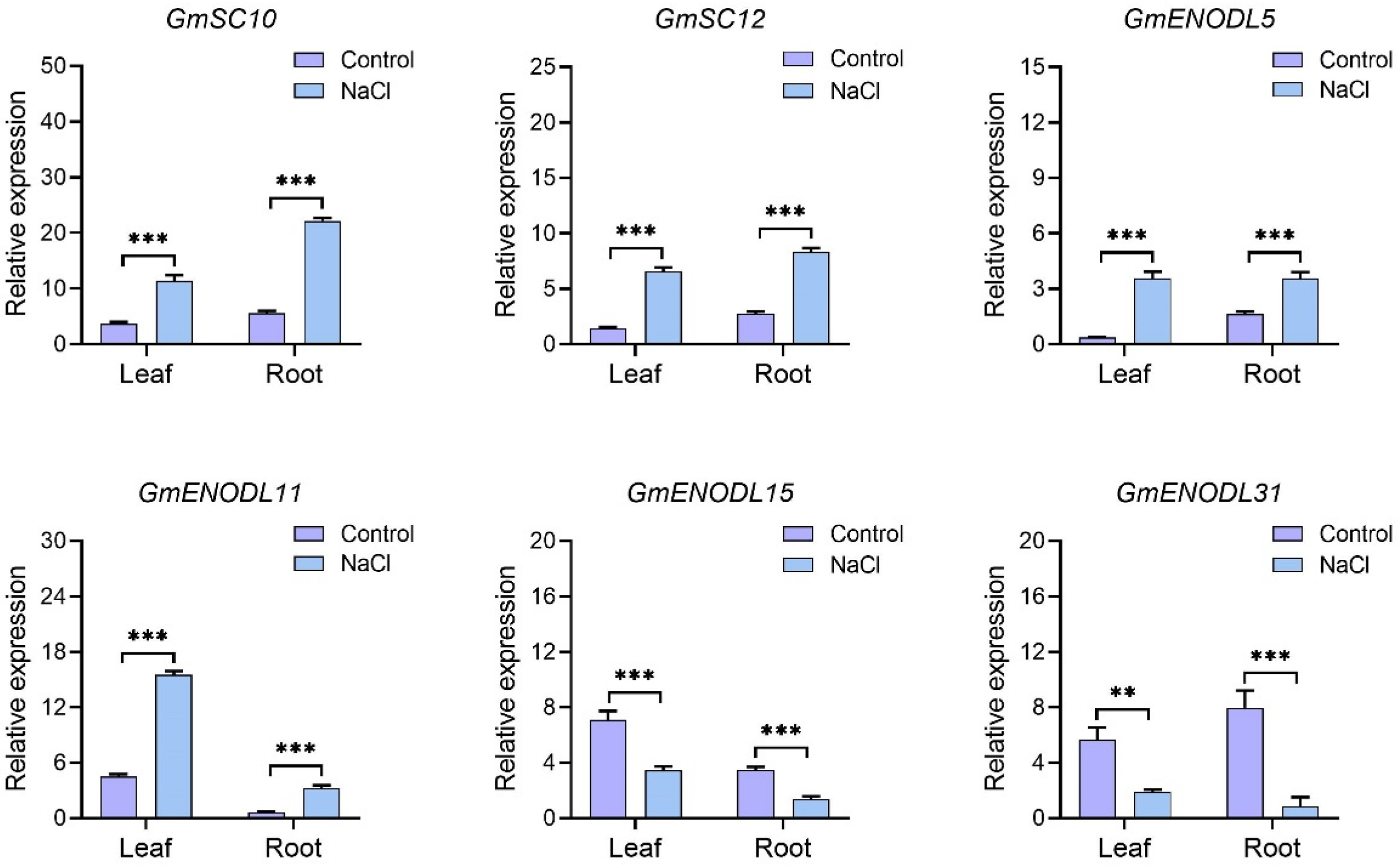
2.6. GmPCs Expression Analysis in Tissues and under Salt Stress
2.7. RNA Isolation and Reverse Transcription Quantitative PCR (RT-qPCR) Analysis
3. Results
3.1. Identification of PC Gene Family in Soybean
3.2. The Structure, Conserved Domain, and Motif Analyses of the GmPCs
3.3. Phylogenetic and Synteny Analyses of GmPCs
3.4. Analysis of Cis-Regulatory Elements in GmPCs
3.5. Expression Analysis of GmPCs in Different Tissues of Soybean
3.6. Co-Expression Network Analysis and Expression Profiles of GmPCs in Response to Saline Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giri, A.V.; Anishetty, S.; Gautam, P. Functionally specified protein signatures distinctive for each of the different blue copper proteins. BMC Bioinform. 2004, 5, 127. [Google Scholar] [CrossRef]
- Ruan, X.M.; Luo, F.; Li, D.D.; Zhang, J.; Liu, Z.H.; Xu, W.L.; Huang, G.Q.; Li, X.B. Cotton BCP genes encoding putative blue copper-binding proteins are functionally expressed in fiber development and involved in response to high-salinity and heavy metal stresses. Physiol. Plant 2011, 141, 71–83. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Asami, T.; Suzuki, Y. Genome-wide identification, structure and expression studies, and mutant collection of 22 early nodulin-like protein genes in Arabidopsis. Biosci. Biotechnol. Biochem. 2009, 73, 2452–2459. [Google Scholar] [CrossRef]
- Nersissian, A.M.; Immoos, C.; Hill, M.G.; Hart, P.J.; Williams, G.; Herrmann, R.G.; Valentine, J.S. Uclacyanins, stellacyanins, and plantacyanins are distinct subfamilies of phytocyanins: Plant-specific mononuclear blue copper proteins. Protein Sci. 1998, 7, 1915–1929. [Google Scholar] [CrossRef]
- Dong, J.; Kim, S.T.; Lord, E.M. Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol. 2005, 138, 778–789. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.C.; Zhang, J.P.; Yu, Y.; Zhou, Y.F.; Feng, Y.Z.; Yang, Y.W.; Lei, M.Q.; He, H.; Lian, J.P.; et al. Rice UCL8, a plantacyanin gene targeted by miR408, regulates fertility by controlling pollen tube germination and growth. Rice 2018, 11, 60. [Google Scholar] [CrossRef]
- Zhang, Y.C.; He, R.R.; Lian, J.P.; Zhou, Y.F.; Zhang, F.; Li, Q.F.; Yu, Y.; Feng, Y.Z.; Yang, Y.W.; Lei, M.Q.; et al. OsmiR528 regulates rice-pollen intine formation by targeting an uclacyanin to influence flavonoid metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 727–732. [Google Scholar] [CrossRef]
- Reyt, G.; Chao, Z.; Flis, P.; Salas-González, I.; Castrillo, G.; Chao, D.Y.; Salt, D.E. Uclacyanin Proteins Are Required for Lignified Nanodomain Formation within Casparian Strips. Curr. Biol. 2020, 30, 4103–4111. [Google Scholar] [CrossRef]
- Greene, E.A.; Erard, M.; Dedieu, A.; Barker, D.G. MtENOD16 and 20 are members of a family of phytocyanin-related early nodulins. Plant Mol. Biol. 1998, 36, 775–783. [Google Scholar] [CrossRef]
- Vijn, I.; Yang, W.C.; Pallisgård, N.; Ostergaard Jensen, E.; van Kammen, A.; Bisseling, T. VsENOD5, VsENOD12 and VsENOD40 expression during Rhizobium-induced nodule formation on Vicia sativa roots. Plant Mol. Biol. 1995, 28, 1111–1119. [Google Scholar] [CrossRef]
- Saji, S.; Saji, H.; Sage-Ono, K.; Ono, M.; Nakajima, N.; Aono, M. Phytocyanin-encoding genes confer enhanced ozone tolerance in Arabidopsis thaliana. Sci. Rep. 2022, 12, 21204. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, J.; Wang, Z.; Chen, B.; Zhang, X.; Zhang, D.; Sun, Z.; Wu, J.; Ke, H.; et al. GhENODL6 Isoforms from the Phytocyanin Gene Family Regulated Verticillium Wilt Resistance in Cotton. Int. J. Mol. Sci. 2022, 23, 2913. [Google Scholar] [CrossRef]
- Wu, H.; Shen, Y.; Hu, Y.; Tan, S.; Lin, Z. A phytocyanin-related early nodulin-like gene, BcBCP1, cloned from Boea crassifolia enhances osmotic tolerance in transgenic tobacco. J. Plant Physiol. 2011, 168, 935–943. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Lv, Y.; Ding, L. Comparative analysis of the phytocyanin gene family in 10 plant species: A focus on Zea mays. Front. Plant Sci. 2015, 6, 515. [Google Scholar] [CrossRef]
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, H.; Liu, Z.; Zhao, J. The phytocyanin gene family in rice (Oryza sativa L.): Genome-wide identification, classification and transcriptional analysis. PLoS ONE 2011, 6, e25184. [Google Scholar] [CrossRef]
- Luo, S.; Hu, W.; Wang, Y.; Liu, B.; Yan, H.; Xiang, Y. Genome-wide identification, classification, and expression of phytocyanins in Populus trichocarpa. Planta 2018, 247, 1133–1148. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.; Wang, Y.; Yang, J.; Wei, G.; Chou, M. Identification of Phytocyanin Gene Family in Legume Plants and their Involvement in Nodulation of Medicago truncatula. Plant Cell Physiol. 2019, 60, 900–915. [Google Scholar] [CrossRef]
- Bilal Tufail, M.; Yasir, M.; Zuo, D.; Cheng, H.; Ali, M.; Hafeez, A.; Soomro, M.; Song, G. Identification and Characterization of Phytocyanin Family Genes in Cotton Genomes. Genes 2023, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kong, F. Soybean. Curr. Biol. 2022, 32, R902–R904. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, X.; Wang, W.; Ma, J.; Wei, D.; He, P.; Pampolino, M.; Johnston, A. Estimating nutrient uptake requirements for soybean using QUEFTS model in China. PLoS ONE 2017, 12, e0177509. [Google Scholar] [CrossRef] [PubMed]
- Muncan, J.; Jinendra, B.M.S.; Kuroki, S.; Tsenkova, R. Aquaphotomics Research of Cold Stress in Soybean Cultivars with Different Stress Tolerance Ability: Early Detection of Cold Stress Response. Molecules 2022, 27, 744. [Google Scholar] [CrossRef]
- Rasheed, A.; Raza, A.; Jie, H.; Mahmood, A.; Ma, Y.; Zhao, L.; Xing, H.; Li, L.; Hassan, M.U.; Qari, S.H.; et al. Molecular Tools and Their Applications in Developing Salt-Tolerant Soybean (Glycine max L.) Cultivars. Bioengineering 2022, 9, 495. [Google Scholar] [CrossRef]
- Wang, K.; Bu, T.; Cheng, Q.; Dong, L.; Su, T.; Chen, Z.; Kong, F.; Gong, Z.; Liu, B.; Li, M. Two homologous LHY pairs negatively control soybean drought tolerance by repressing the abscisic acid responses. New Phytol. 2021, 229, 2660–2675. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, R.; Wang, L.; Cui, R.; Zhang, H.; Che, Z.; Hu, D.; Chu, S.; Jiao, Y.; Yu, D.; et al. GmEIL4 enhances soybean (Glycine max) phosphorus efficiency by improving root system development. Plant Cell Environ. 2023, 46, 592–606. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Finn, R.; Clements, J.; Eddy, S. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Cell-PLoc 2.0: An improved package of web-servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler-Bauer, A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinform. 2020, 69, e90. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.; Frank, M.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Severin, A.J.; Woody, J.L.; Bolon, Y.-T.; Joseph, B.; Diers, B.W.; Farmer, A.D.; Muehlbauer, G.J.; Nelson, R.T.; Grant, D.; Specht, J.E. RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010, 10, 160. [Google Scholar] [CrossRef]
- Lu, L.; Wei, W.; Tao, J.J.; Lu, X.; Bian, X.H.; Hu, Y.; Cheng, T.; Yin, C.C.; Zhang, W.K.; Chen, S.Y.; et al. Nuclear factor Y subunit GmNFYA competes with GmHDA13 for interaction with GmFVE to positively regulate salt tolerance in soybean. Plant Biotechnol. J. 2021, 19, 2362–2379. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, L.; Chang, X.; Wang, X.; Li, G.; Chen, S.; Jin, S. Overexpression of LpCPC from Lilium pumilum confers saline-alkali stress (NaHCO3) resistance. Plant Signal Behav. 2022, 17, 2057723. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Zhao, Z.; Bai, Y.; Yang, Q.; Gan, Y.; Wang, W.; Li, C.; Wang, J.; Cai, Y. Salt Tolerant Gene 1 contributes to salt tolerance by maintaining photosystem II activity in maize. Plant Cell Environ. 2023. [CrossRef] [PubMed]
- Li, J.; Gao, G.; Zhang, T.; Wu, X. The putative phytocyanin genes in Chinese cabbage (Brassica rapa L.): Genome-wide identification, classification and expression analysis. Mol. Genet. Genom. 2013, 288, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.; Schlueter, J.; Doyle, J. Paleopolyploidy and gene duplication in soybean and other legumes. Curr. Opin. Plant Biol. 2006, 9, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Hajibarat, Z.; Saidi, A.; Zeinalabedini, M.; Gorji, A.M.; Ghaffari, M.R.; Shariati, V.; Ahmadvand, R. Genome-wide identification of StU-box gene family and assessment of their expression in developmental stages of Solanum tuberosum. J. Genet. Eng. Biotechnol. 2022, 20, 25. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, S.; Xu, L.; Zhu, L.; Wang, D.; Liu, Y.; Liu, S.; Hao, Z.; Lu, Y.; Yang, L.; et al. Genome-wide identification of the Liriodendron chinense WRKY gene family and its diverse roles in response to multiple abiotic stress. BMC Plant Biol. 2022, 22, 25. [Google Scholar] [CrossRef]
- Jeffares, D.C.; Penkett, C.J.; Bähler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef]
- Roy, S.W.; Penny, D. A very high fraction of unique intron positions in the intron-rich diatom Thalassiosira pseudonana indicates widespread intron gain. Mol. Biol. Evol. 2007, 24, 1447–1457. [Google Scholar] [CrossRef]
- Wang, X.Y.; Paterson, A.H. Gene conversion in angiosperm genomes with an emphasis on genes duplicated by polyploidization. Genes 2011, 2, 1–20. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, N.; Song, W.; Yin, G.; Qin, Y.; Yan, Y.; Hu, Y. Soybean (Glycine max) expansin gene superfamily origins: Segmental and tandem duplication events followed by divergent selection among subfamilies. BMC Plant Biol. 2014, 14, 93. [Google Scholar] [CrossRef]
- Navarro, A.; Barton, N. Chromosomal speciation and molecular divergence--accelerated evolution in rearranged chromosomes. Science 2003, 300, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, L.Y.; Dai, J.X.; Wang, Y.; Lin, D. The NAC-type transcription factor CaNAC46 regulates the salt and drought tolerance of transgenic Arabidopsis thaliana. BMC Plant Biol. 2021, 21, 11. [Google Scholar] [CrossRef]
- Schmitz, R.; Grotewold, E.; Stam, M. Cis-regulatory sequences in plants: Their importance, discovery, and future challenges. Plant Cell 2022, 34, 718–741. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, B.; Sasaki, K.; Matsumoto, H.; Nakashima, S. Functions of two genes in aluminium (Al) stress resistance: Repression of oxidative damage by the AtBCB gene and promotion of efflux of Al ions by the NtGDI1gene. J. Exp. Bot. 2005, 56, 2661–2671. [Google Scholar] [CrossRef]
- Poon, S.; Heath, R.L.; Clarke, A.E. A chimeric arabinogalactan protein promotes somatic embryogenesis in cotton cell culture. Plant Physiol. 2012, 160, 684–695. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhang, J.; Li, H.; Zhang, G.; Hu, D.; Zhang, D.; Xu, X.; Yang, Y.; Huang, Z. Genome-Wide Identification of the Phytocyanin Gene Family and Its Potential Function in Salt Stress in Soybean (Glycine max (L.) Merr.). Agronomy 2023, 13, 2484. https://doi.org/10.3390/agronomy13102484
Wang L, Zhang J, Li H, Zhang G, Hu D, Zhang D, Xu X, Yang Y, Huang Z. Genome-Wide Identification of the Phytocyanin Gene Family and Its Potential Function in Salt Stress in Soybean (Glycine max (L.) Merr.). Agronomy. 2023; 13(10):2484. https://doi.org/10.3390/agronomy13102484
Chicago/Turabian StyleWang, Li, Jinyu Zhang, Huici Li, Gongzhan Zhang, Dandan Hu, Dan Zhang, Xinjuan Xu, Yuming Yang, and Zhongwen Huang. 2023. "Genome-Wide Identification of the Phytocyanin Gene Family and Its Potential Function in Salt Stress in Soybean (Glycine max (L.) Merr.)" Agronomy 13, no. 10: 2484. https://doi.org/10.3390/agronomy13102484
APA StyleWang, L., Zhang, J., Li, H., Zhang, G., Hu, D., Zhang, D., Xu, X., Yang, Y., & Huang, Z. (2023). Genome-Wide Identification of the Phytocyanin Gene Family and Its Potential Function in Salt Stress in Soybean (Glycine max (L.) Merr.). Agronomy, 13(10), 2484. https://doi.org/10.3390/agronomy13102484





