Exploring the Root Morphological Traits of Diverse-Origin Cultivated Soybean
Abstract
:1. Introduction
2. Materials and Methods
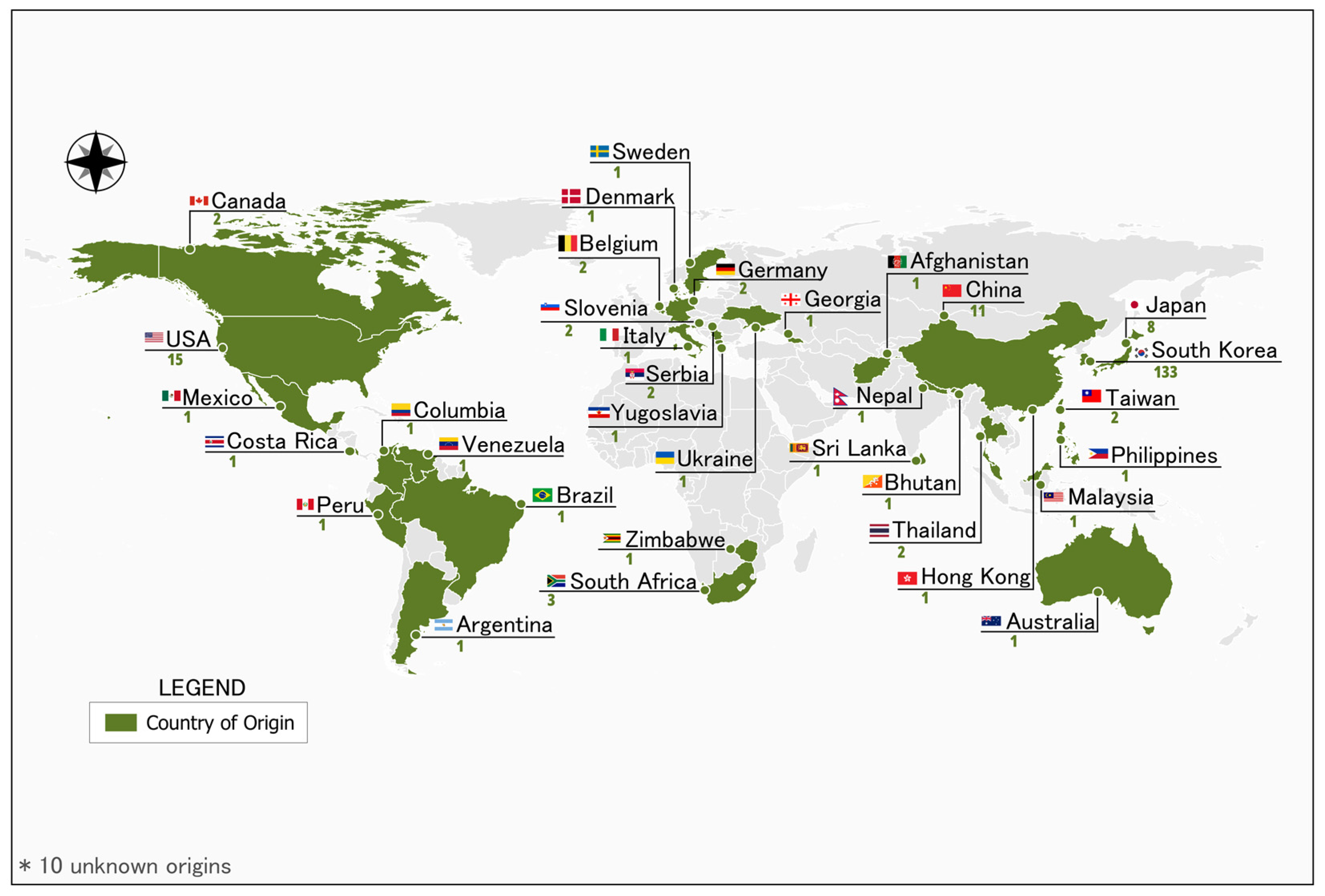
2.1. Plant Materials
2.2. Experimental Details
2.3. Data Collection
2.4. Statistical Analysis
3. Results
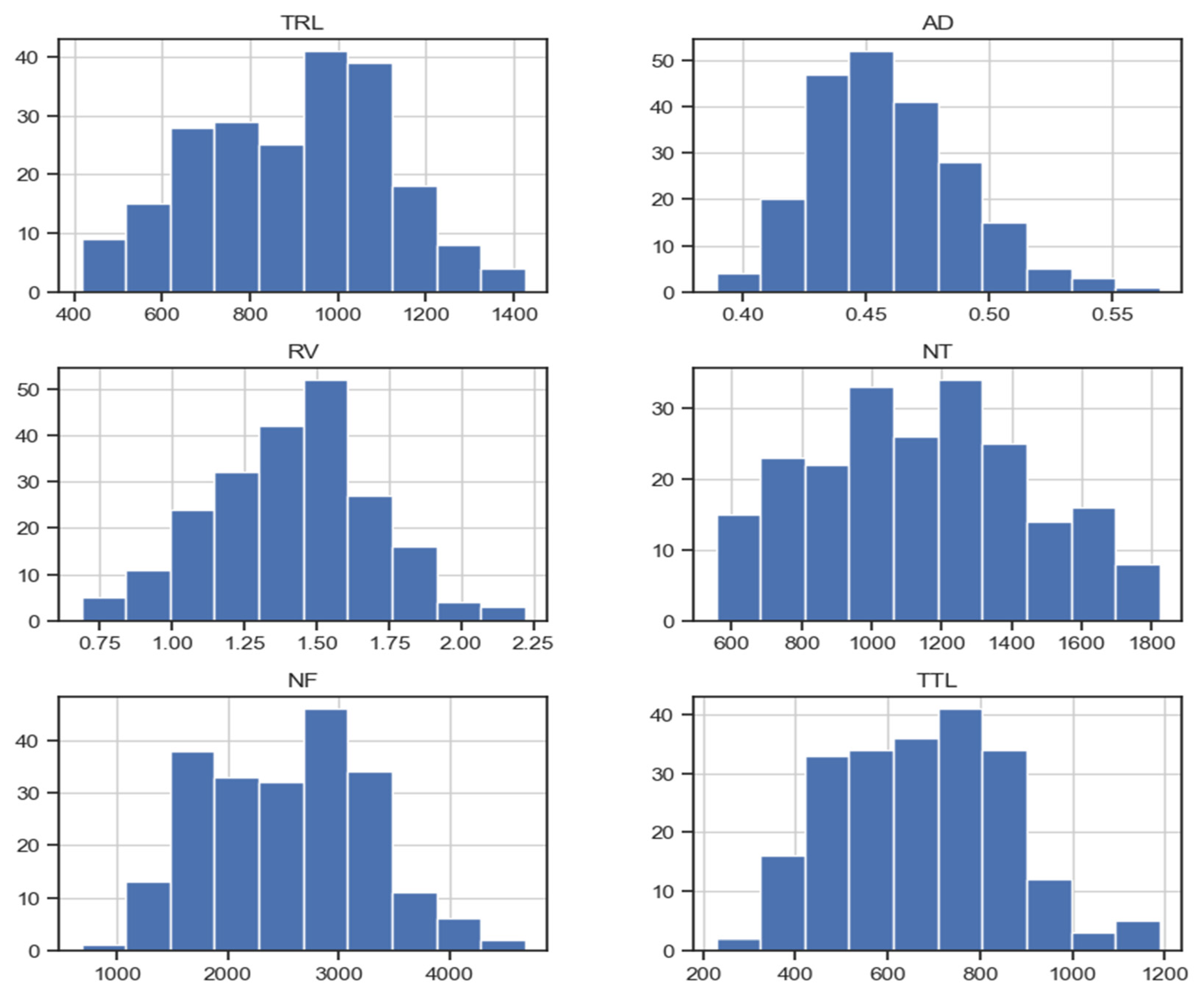
3.1. Variation Assessment: Descriptive Statistics, ANOVA, and Varieties Ranking
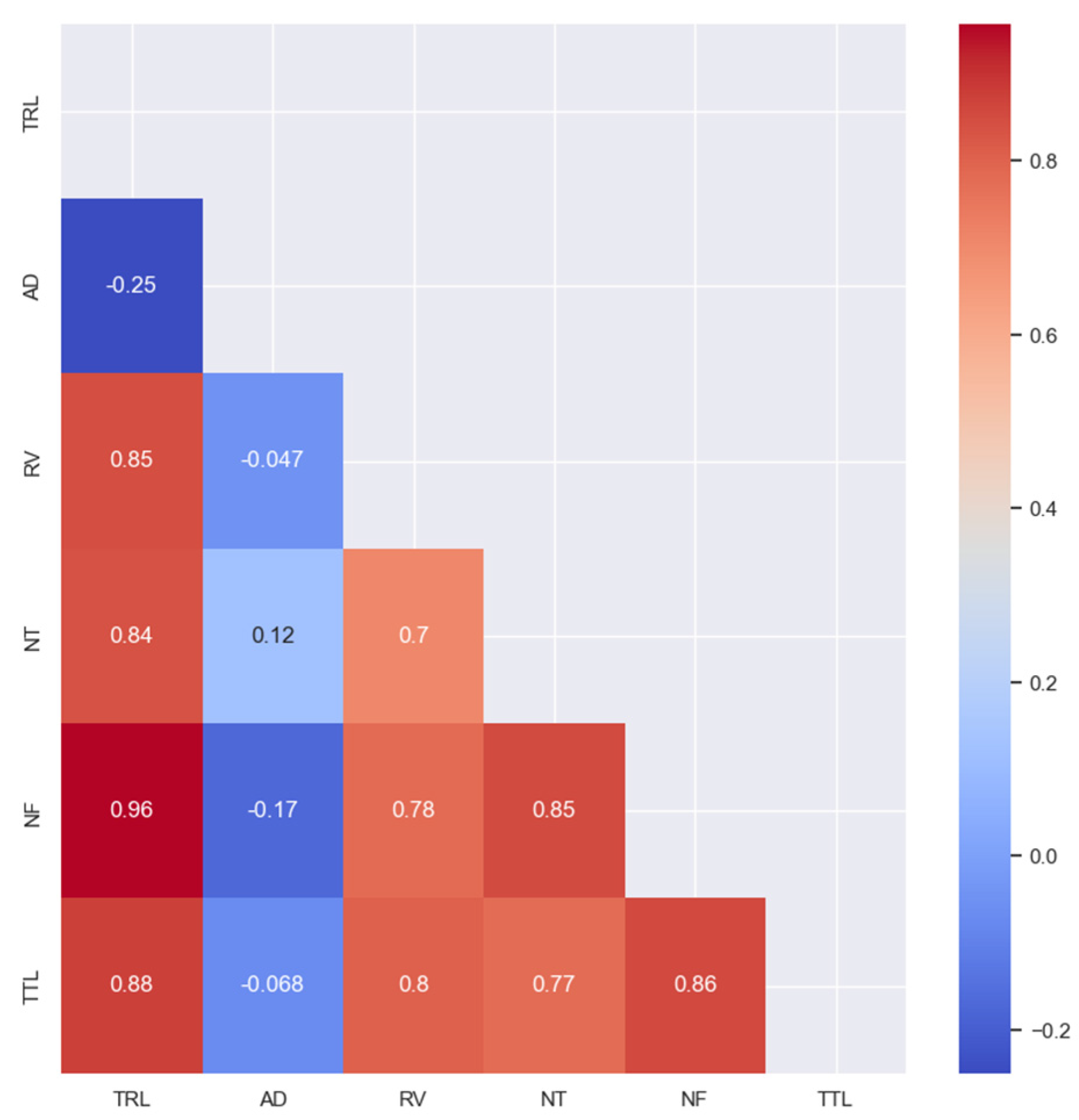
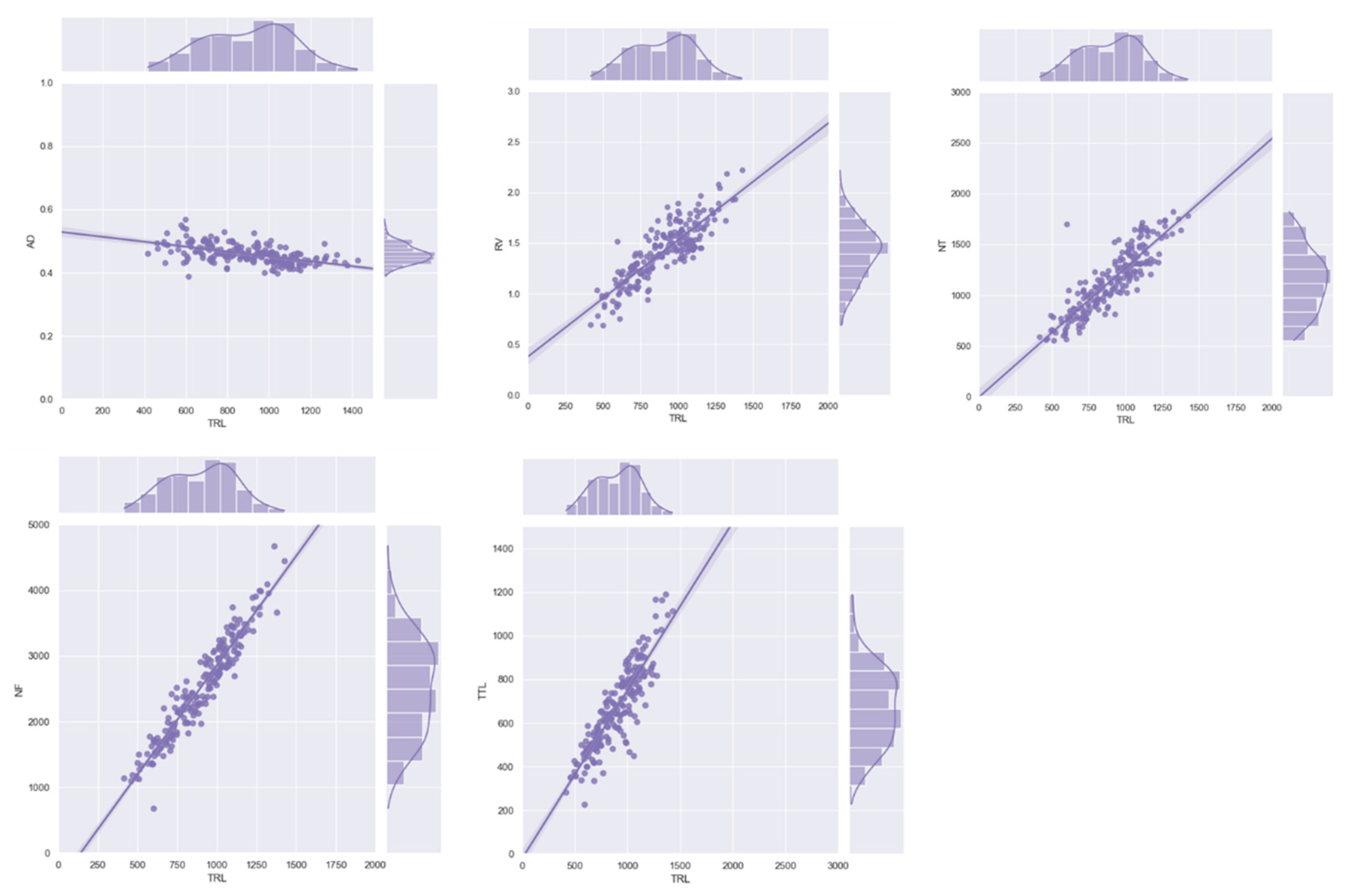
3.2. Correlation and Distribution Analysis
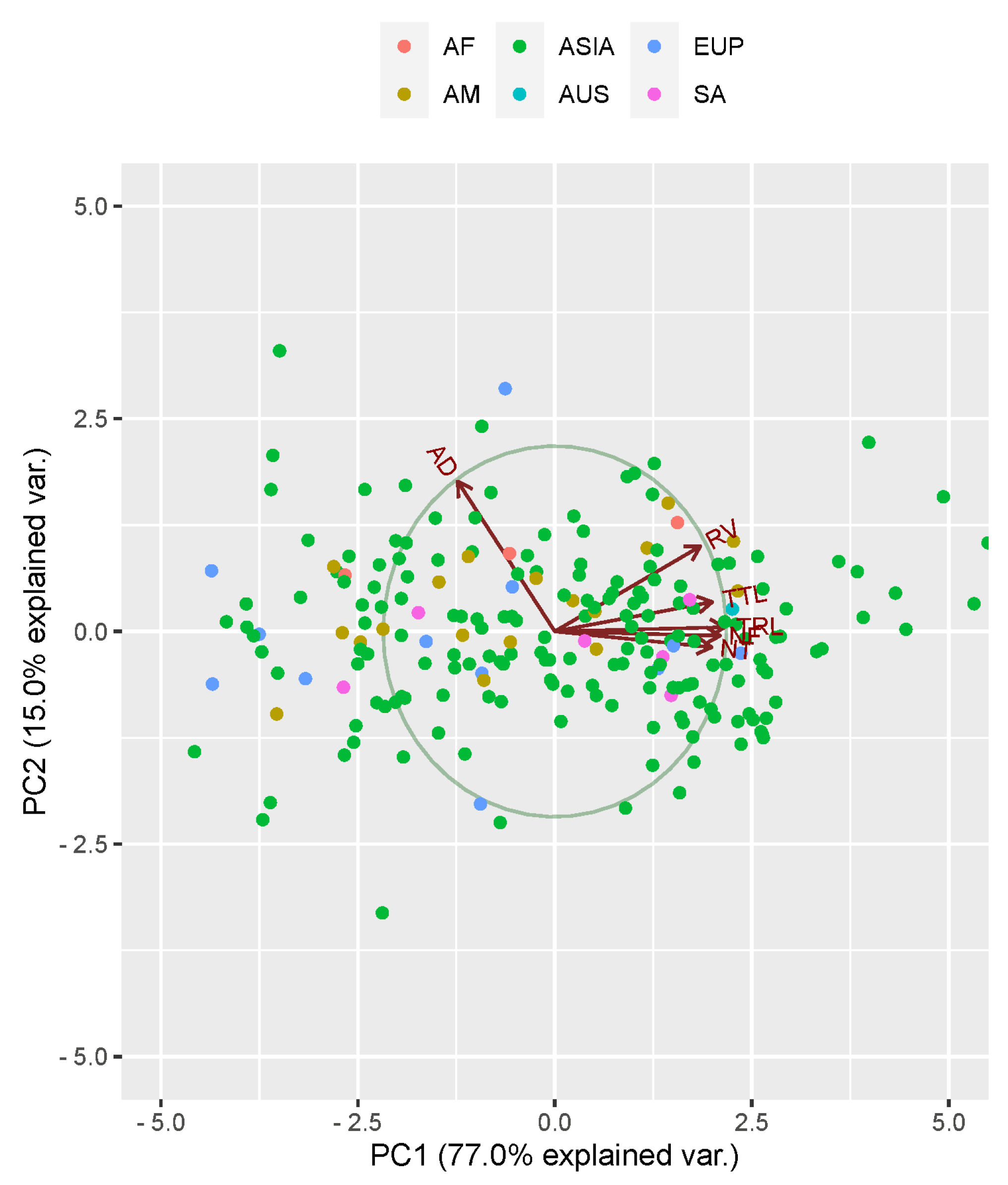
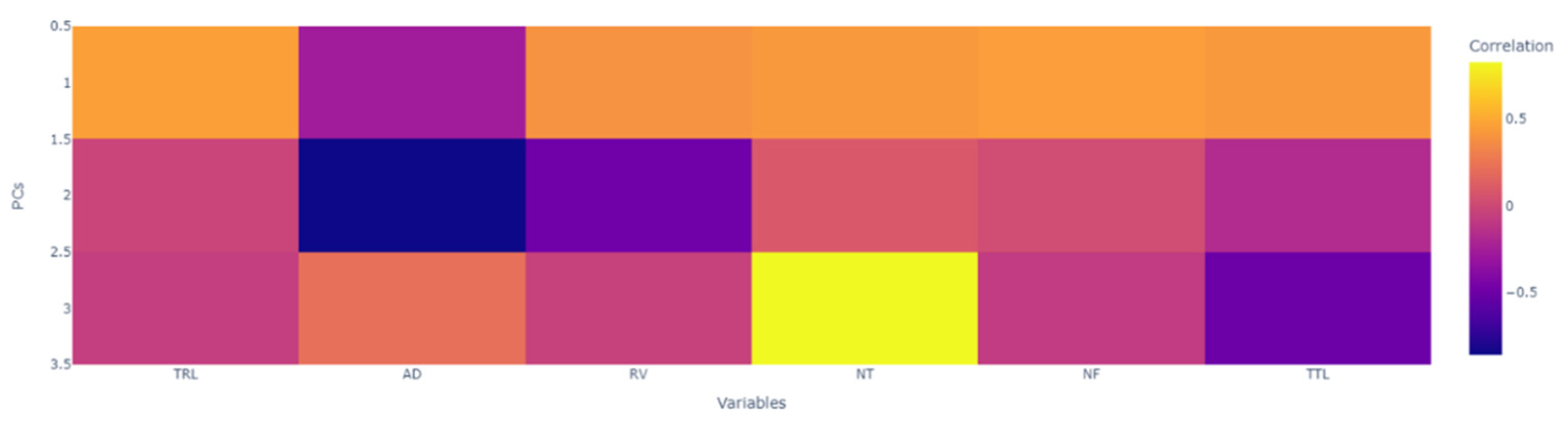
3.3. Principal Component Analysis
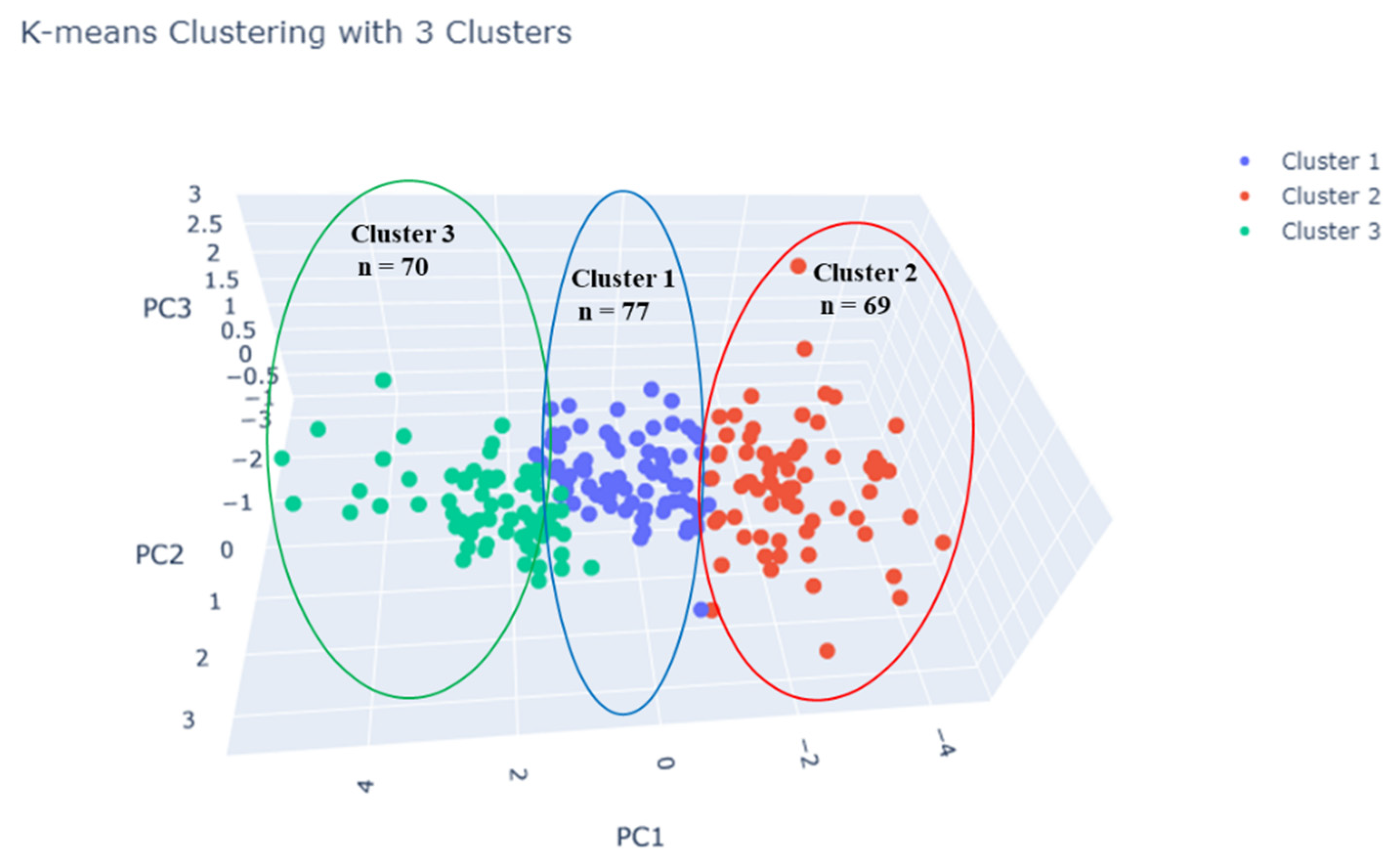
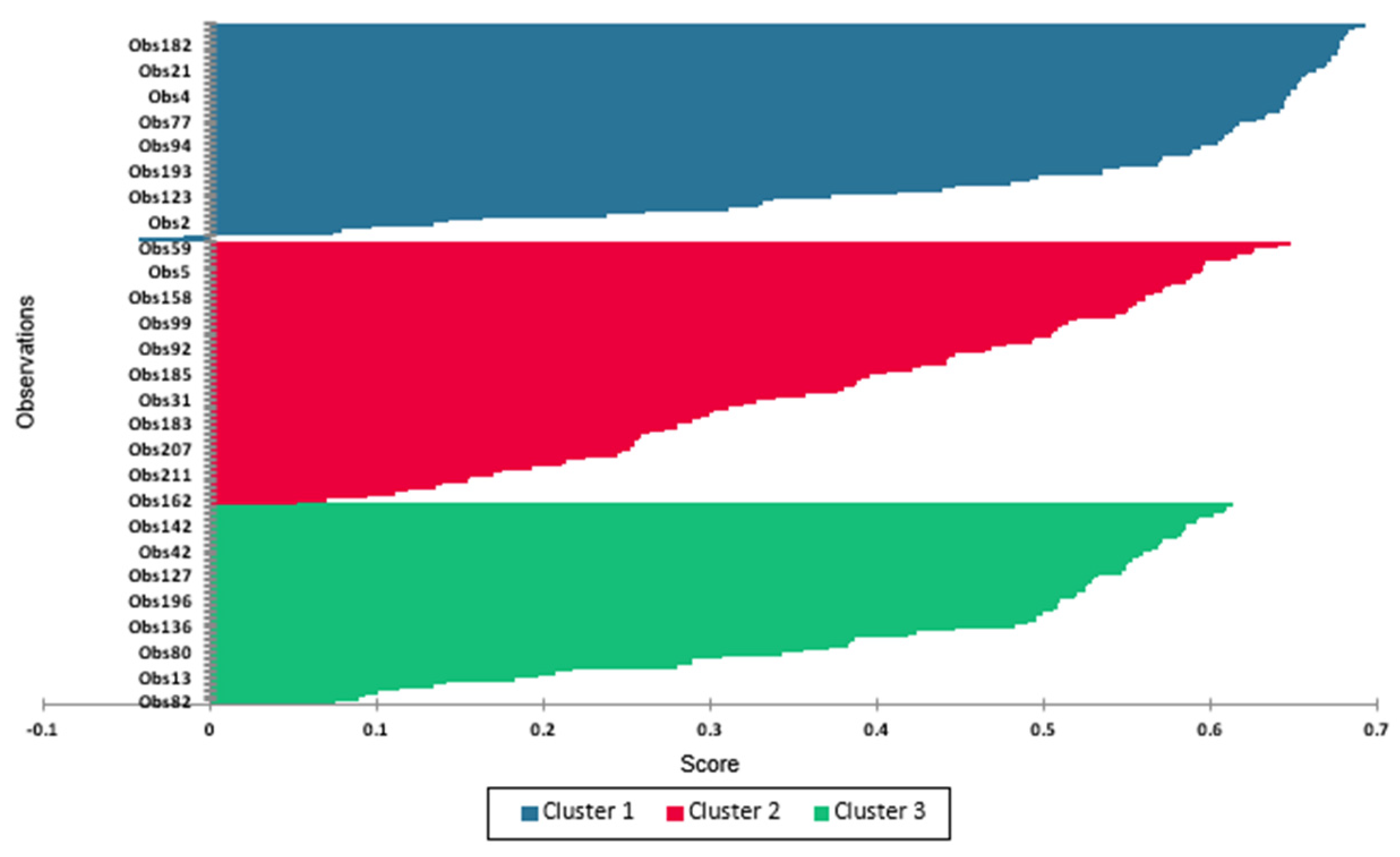
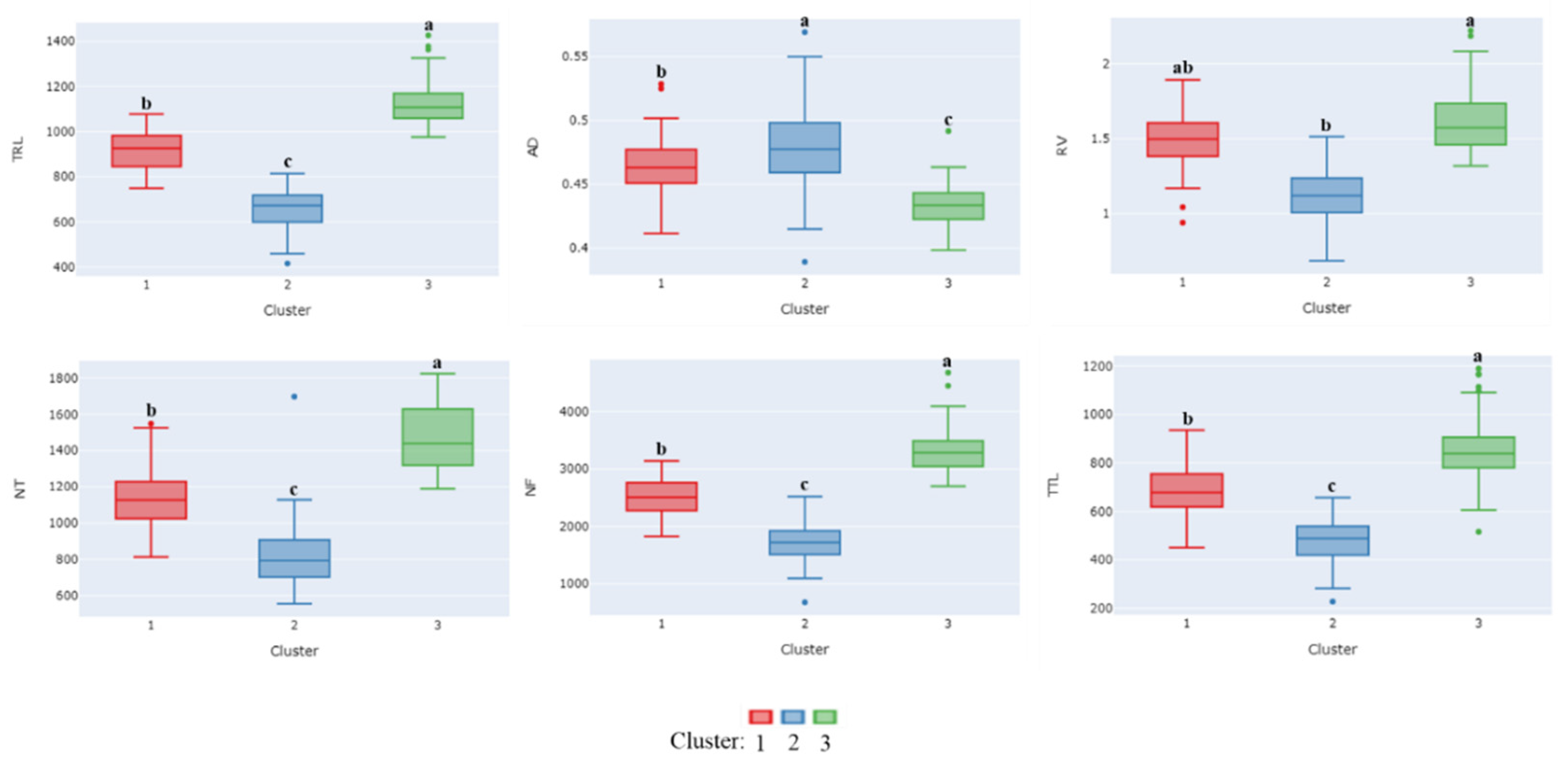
3.4. Cluster Analysis of Root Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, J.; Mooney, H.; Wu, W.; Tang, H.; Tong, Y.; Xu, Z.; Huang, B.; Cheng, Y.; Yang, X.; Wei, D.; et al. Importing food damages domestic environment: Evidence from global soybean trade. Proc. Natl. Acad. Sci. USA 2018, 115, 5415–5419. [Google Scholar] [CrossRef]
- Staniak, M.; Szpunar-Krok, E.; Kocira, A. Responses of soybean to Selected Abiotic Stresses—Photoperiod, Temperature and Water. Agriculture 2023, 13, 146. [Google Scholar] [CrossRef]
- Kotecki, A.; Lewandowska, S. Studia na Uprawą Soi Zwyczajnej (Glycine max (L.) Merrill) w Południowo-Zachodniej Polsce; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2020; pp. 54–79. [Google Scholar]
- Święcicki, W.; Chudy, M.; Żuk-Gołaszewska, K. Rośliny strączkowe w projektach badawczych Unii Europejskiej (Grain legumes in the UE framework programmes). Zesz. Probl. Postępów Nauk. Rol. 2007, 522, 55–65. [Google Scholar]
- Bellaloui, N.; Bruns, H.A.; Abbas, H.K.; Mengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic Reduction of Anti-nutritional Factors in Fermenting Soybeans by Lactobacillus plantarum Isolates from Fermenting Cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Anderson, E.J.; Ali, M.L.; Beavis, W.D.; Chen, P.; Clemente, T.E.; Diers, B.W.; Graef, G.L.; Grassini, P.; Hyten, D.L.; McHale, L.K.; et al. Soybean [Glycine max (L.) Merr.] breeding: History, improvement, production and future opportunities. In Advances in Plant Breeding Strategies: Legumes; Springer International Publishing: Cham, Switzerland, 2019; Volume 7, pp. 431–516. [Google Scholar]
- Michałek, S.; Borowski, E. Seed germination and seedling growth of the Polish soybean (Glycine max (L.) Merr.) cultivars in drought conditions. Biul. IHAR 2002, 195–202. [Google Scholar]
- Hou, G.; Ablett, G.R.; Pauls, K.P.; Rajcan, I. Environmental effects on fatty acid levels in soybean seed oil. J. Am. Oil. Chem. Soc. 2006, 83, 759–763. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.; Ma, Y.; Li, S.; Dong, S.; Zu, W. Transcriptome profilling analysis characterized the gene expression patterns responded to combined drought and heat stresses in soybean. Comput. Biol. Chem. 2018, 77, 413–429. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants 2019, 25, 667–681. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, R.; Sharma, M.K.; Sharma, M.P.; Satpute, G.K.; Garg, S.; Singla-Pareek, S.L.; Pareek, A. Signaling cross talk between biotic and abiotic stress responses in soybean. In Abiotic and Biotic Stresses in Soybean Production; Oxford Academic Press: Oxford, UK, 2016; Volume 1, pp. 27–52. [Google Scholar]
- Wijewardana, C.; Reddy, K.; Alsajri, F.; Irby, J.; Krutz, J.; Golden, B. Quantifying soil moisture deficit effects on soybean yield and yield component distribution patterns. Irrig. Sci. 2018, 36, 241–255. [Google Scholar] [CrossRef]
- Bengough, A.G.; Mullins, C.E. Mechanical impedance to root growth: A review of experimental techniques and root growth responses. J. Soil Sci. 1990, 41, 341–358. [Google Scholar] [CrossRef]
- Tu, J.; Tan, C. Effect of soil compaction on growth, yield and root rots of white beans in clay loam and sandy loam soil. Soil Biol. Biochem. 1991, 23, 233–238. [Google Scholar] [CrossRef]
- Yu, L.X.; Ray, J.D.; O’Toole, J.C.; Nguyen, H.T. Use of wax_petrolatum layers for screening rice root penetration. Crop Sci. 1995, 35, 684–687. [Google Scholar] [CrossRef]
- Barraclough, P.; Weir, A. Effects of a compacted subsoil layer on root and shoot growth, water use and nutrient uptake of winter wheat. J. Agric. Sci. 1988, 110, 207–216. [Google Scholar] [CrossRef]
- Busscher, W.; Sojka, R.; Doty, C. Residual effects of tillage on coastal plain soil strength1. Soil Sci. 1986, 141, 144–148. [Google Scholar] [CrossRef]
- Kim, S.H.; Subramanian, P.; Hahn, B.S.; Ha, B.K. High-Throughput Phenotypic Characterization and Diversity Analysis of soybean Roots (Glycine max L.). Plants 2022, 11, 2017. [Google Scholar] [CrossRef] [PubMed]
- Fried, H.G.; Narayanan, S.; Fallen, B. Characterization of a soybean (Glycine max L. Merr.) germplasm collection for root traits. PLoS ONE 2018, 13, e0200463. [Google Scholar] [CrossRef]
- Carter, T. Breeding for drought tolerance in soybean-where do we stand. In World Soybean Research Conference IV; Asociacion Agrentina de la Soja: Rosario, Argentina, 1989. [Google Scholar]
- Gaur, P.M.; Krishnamurthy, L.; Kashiwagi, J. Improving drought-avoidance root traits in chickpea (Cicer arietinum L.)-current status of research at ICRISAT. Plant Prod. Sci. 2008, 11, 3–11. [Google Scholar] [CrossRef]
- Ao, J.; Fu, J.; Tian, J.; Yan, X.; Liao, H. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Funct. Plant Biol. 2010, 37, 304–312. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, X.; Mei, M.; Yan, X.; Liao, H. QTL analysis of root traits as related to phosphorus efficiency in soybean. Ann. Bot. 2010, 106, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J. Root Architecture and Plant Productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Du, J.; Guo, X.; Wen, W.; Gu, S.; Wang, J.; Fan, J. Crop Phenomics: Current Status and Perspectives. Front. Plant Sci. 2019, 10, 714. [Google Scholar] [CrossRef]
- Waisel, Y.; Eshel, A.; Kafkafi, U. Plant-Roots–The Hidden Half, 3rd ed.; Marcel Decker: New York, NY, USA, 2002; pp. 775–776. [Google Scholar]
- Poorter, H.; Nagel, O.W. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- Eshel, A.; Beeckman, T. Plant Roots: The Hidden Half; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; Devoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Ali, O.; Sarker, A.; Nielsen, N.E.; Rahman, M.M. Genetic variation in root traits and nutrient acquisition of lentil genotypes. J. Plant Nutr. 2006, 29, 643–655. [Google Scholar] [CrossRef]
- Tayade, R.; Kim, S.; Tripathi, P.; Choi, Y.D.; Yoon, J.B.; Kim, Y. High-Throughput Root Imaging Analysis Reveals Wide Variation in Root Morphology of Wild Adzuki bean (Vigna angularis) Accessions. Plants 2022, 11, 405. [Google Scholar] [CrossRef]
- Attipoe, J.Q.; Khan, W.; Tayade, R.; Steven, S.; Islam, M.S.; Lay, L.; Ghimire, A.; Kim, H.; Sereyvichea, M.; Propey, T.; et al. Evaluating the Effectiveness of Calcium Silicate in Enhancing soybean Growth and Yield. Plants 2023, 12, 2190. [Google Scholar] [CrossRef]
- Freschet, G.T.; Roumet, C.; Comas, L.H.; Weemstra, M.; Bengough, A.G.; Rewald, B.; Bardgett, R.D.; De Deyn, G.B.; Johnson, D.; Klimešová, J.; et al. Root traits as drivers of plant and ecosystem functioning: Current understanding, pitfalls and future research needs. New Phytol. 2021, 232, 1123–1158. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Kucharski, J.M.; Zadworny, M.; Adams, T.S.; Koide, R.T. Linking root traits to nutrient foraging in arbuscular mycorrhizal trees in a temperate forest. New Phytol. 2015, 208, 114–124. [Google Scholar] [CrossRef]
- Tripathi, P.; Kim, Y. Investigation of root phenotype in soybeans (Glycine max L.) using imagery data. J. Crop Sci. Biotechnol. 2022, 25, 233–241. [Google Scholar] [CrossRef]
- Dayoub, E.; Lamichhane, J.R.; Schoving, C.; Debaeke, P.; Maury, P. Early-Stage Phenotyping of Root Traits Provides Insights into the Drought Tolerance Level of soybean Cultivars. Agronomy 2021, 11, 188. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in water-limited ecosystems. J. Ecol. 2002, 90, 480–494. [Google Scholar] [CrossRef]
- Desmae, H.; Jordan, D.R.; Godwin, I.D. Geographic patterns of phenotypic diversity in sorghum (Sorghum bicolor (L.) Moench) landraces from North Eastern Ethiopia. Afr. J. Agric. Res. 2016, 11, 3111–3122. [Google Scholar]
- Liu, S.; Begum, N.; An, T.; Zhao, T.; Xu, B.; Zhang, S.; Deng, X.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; et al. Characterization of Root System Architecture Traits in Diverse soybean Genotypes Using a Semi-Hydroponic System. Plants 2021, 10, 2781. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Palta, J.; Prasad, P.V.V.; Siddique, K.H.M. Phenotypic variability in bread wheat root systems at the early vegetative stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Byrne, P.; Reid, S.; Bauerle, W.; McKay, J.; Haley, S. Root traits contributing to drought tolerance of synthetic hexaploid wheat in a greenhouse study. Euphytica 2016, 207, 213–224. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Rewald, B.; Leuschner, C. Belowground competition in a broad-leaved temperate mixed forest: Pattern analysis and experiments in a four-species stand. Eur. J. For. Res. 2009, 128, 387–398. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, G.; Zhang, C. Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Funct. Plant Biol. 2001, 28, 1055–1061. [Google Scholar] [CrossRef]
| TRL | AD | RV | NT | NF | TTL | |
|---|---|---|---|---|---|---|
| Mean | 899.44 | 0.46 | 1.42 | 1140.82 | 2522.23 | 672.67 |
| Std Dev | 212.33 | 0.03 | 0.28 | 308.25 | 738.25 | 183.97 |
| Min | 415.73 | 0.39 | 0.69 | 554.67 | 678.11 | 227.56 |
| Max | 1426.24 | 0.57 | 2.22 | 1824.17 | 4677.83 | 1189.26 |
| Kurtosis | –0.69 | 0.64 | −0.03 | −0.79 | −0.48 | −0.22 |
| Skewness | −0.07 | 0.59 | −0.08 | 0.12 | 0.10 | 0.22 |
| Traits | Source | DF | Type III SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|---|
| TRL | var. | 215 | 89,031,898.74 | 414,101.85 | 28.22 | <0.0001 |
| rep. | 2 | 2973.04 | 1486.52 | 0.10 | 0.9037 | |
| AD | var. | 215 | 0.92931836 | 0.00432241 | 8.02 | <0.0001 |
| rep. | 2 | 0.0088429 | 0.00442145 | 8.20 | 0.0003 | |
| RV | var. | 215 | 99.2070554 | 0.46142816 | 15.69 | <0.0001 |
| rep. | 2 | 0.20860586 | 0.10430293 | 3.55 | 0.0297 | |
| NT | var. | 215 | 156,362,761.30 | 727,268.70 | 14.24 | <0.0001 |
| rep. | 2 | 361,778.50 | 180,889.20 | 3.54 | 0.0298 | |
| NF | var. | 215 | 1,199,371,126.00 | 5,578,470.00 | 20.64 | <0.0001 |
| rep. | 2 | 44,006.00 | 22,003.00 | 0.08 | 0.9218 | |
| TTL | var. | 215 | 66,205,864.10 | 307,934.25 | 12.44 | <0.0001 |
| rep. | 2 | 15,965.85 | 7982.92 | 0.32 | 0.7244 |
| Ranking | IT Number | TRL (cm) | Origin | IT Number | AD (mm) | Origin | IT Number | RV (cm3) | Origin | IT Number | NT | Origin | IT Number | NF | Origin | IT Number | TTL (cm) | Origin | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Top | 1 | IT115491 | 1426.24 | KOR | IT156262 | 0.57 | KOR | IT115491 | 2.22 | KOR | IT143347 | 1824.17 | KOR | IT154621 | 4677.80 | KOR | IT154621 | 1189.26 | KOR |
| 2 | IT154397 | 1377.52 | KOR | IT156253 | 0.55 | KOR | IT143347 | 2.18 | KOR | IT115491 | 1781.75 | KOR | IT115491 | 4449.20 | KOR | IT160105 | 1165.76 | KOR | |
| 3 | IT154621 | 1363.06 | KOR | IT156195 | 0.54 | KOR | IT154724 | 2.08 | KOR | IT154621 | 1752.33 | KOR | IT23848 | 4093.50 | KOR | IT143347 | 1165.42 | KOR | |
| 4 | IT143347 | 1325.75 | KOR | PUNGSAN | 0.54 | KOR | IT143169 | 2.05 | KOR | IT154724 | 1741.33 | KOR | IT160105 | 4002.30 | KOR | IT115491 | 1113.54 | KOR | |
| 5 | IT23848 | 1317.62 | KOR | IT153915 | 0.53 | KOR | IT24681 | 1.97 | USA | IT153901 | 1734.50 | KOR | IT143169 | 3987.80 | KOR | IT154397 | 1096.73 | KOR | |
| 6 | IT143169 | 1276.06 | KOR | IT23071 | 0.53 | SRB | IT154397 | 1.93 | KOR | IT25384 | 1723.17 | KOR | IT143347 | 3960.20 | KOR | IT154724 | 1090.54 | KOR | |
| 7 | IT153901 | 1268.40 | KOR | IT158073 | 0.52 | SRB | IT154621 | 1.93 | KOR | IT101015 | 1715.50 | KOR | IT154613 | 3909.30 | KOR | IT23848 | 1030.22 | KOR | |
| 8 | IT154724 | 1267.25 | KOR | IT143161 | 0.52 | KOR | IT142818 | 1.89 | KOR | PUNGSAN | 1697.33 | KOR | IT141627 | 3888.90 | KOR | IT153901 | 1020.78 | KOR | |
| 9 | IT160105 | 1267.23 | KOR | IT161990 | 0.52 | KOR | IT153901 | 1.89 | KOR | IT153722 | 1688.67 | KOR | IT154724 | 3746.10 | KOR | IT142811 | 992.27 | KOR | |
| 10 | IT154613 | 1241.95 | KOR | IT143039 | 0.51 | KOR | IT142807 | 1.88 | KOR | IT160105 | 1680.67 | KOR | IT141865 | 3741.00 | KOR | IT141865 | 985.56 | UNK | |
| 11 | IT25384 | 1232.20 | KOR | IT156190 | 0.51 | KOR | IT23071 | 1.86 | SRB | IT161435 | 1672.00 | CHN | IT25384 | 3725.00 | KOR | IT161443 | 973.60 | AUS | |
| Bottom | 1 | IT25668 | 415.73 | KOR | IT146110 | 0.39 | TWN | IT22891 | 0.69 | UNK | IT161474 | 554.67 | UNK | PUNGSAN | 678.10 | KOR | IT103951 | 227.56 | KOR |
| 2 | IT153844 | 459.19 | KOR | IT108929 | 0.40 | KOR | IT25668 | 0.69 | KOR | IT153844 | 558.33 | KOR | IT153844 | 1094.80 | KOR | IT25668 | 281.99 | KOR | |
| 3 | IT158006 | 464.94 | BEL | IT101256 | 0.40 | KOR | IT146110 | 0.76 | TWN | IT156262 | 565.67 | KOR | IT158073 | 1127.00 | SRB | IT158063 | 336.35 | ZAF | |
| 4 | IT157861 | 492.51 | KOR | IT24570 | 0.41 | KOR | IT158006 | 0.78 | BEL | IT158006 | 572.33 | BEL | IT158049 | 1140.50 | SWE | IT24099 | 338.58 | KOR | |
| 5 | IT158049 | 492.62 | SWE | IT104789 | 0.41 | KOR | IT24099 | 0.82 | KOR | IT25668 | 586.83 | KOR | IT25668 | 1141.80 | KOR | IT153844 | 349.26 | KOR | |
| 6 | IT22891 | 499.08 | UNK | IT143192 | 0.41 | JPN | IT141790 | 0.87 | KOR | IT156253 | 598.50 | KOR | IT157861 | 1181.90 | KOR | IT158006 | 352.37 | BEL | |
| 7 | IT141790 | 507.78 | KOR | IT101015 | 0.41 | KOR | IT103951 | 0.87 | KOR | IT21686 | 610.33 | USA | IT158006 | 1190.30 | BEL | IT158073 | 356.97 | SRB | |
| 8 | IT158073 | 508.28 | SRB | IT115505 | 0.41 | KOR | IT21665 | 0.89 | CHN | IT156278 | 629.50 | BTN | IT161474 | 1275.00 | UNK | IT141790 | 360.16 | KOR | |
| 9 | IT161474 | 509.85 | UNK | IT153834 | 0.41 | KOR | IT158073 | 0.90 | SRB | IT22074 | 630.00 | UNK | IT156278 | 1337.70 | BTN | IT22891 | 361.49 | UNK | |
| 10 | IT156278 | 524.50 | BTN | IT24147 | 0.41 | KOR | IT21686 | 0.90 | USA | IT103951 | 638.17 | KOR | IT156262 | 1352.20 | KOR | IT21649 | 369.70 | HKG | |
| 11 | IT24099 | 560.90 | KOR | IT23005 | 0.41 | DNK | IT158004 | 0.93 | BEL | IT158073 | 649.00 | SRB | IT103951 | 1356.00 | KOR | IT21686 | 369.80 | USA |
| Eigenvalues | Explained Variance | Cumulative Variance | |
|---|---|---|---|
| PC1 | 4.644 | 0.770 | 0.770 |
| PC2 | 0.900 | 0.149 | 0.919 |
| PC3 | 0.206 | 0.034 | 0.953 |
| PC4 | 0.107 | 0.021 | 0.974 |
| PC5 | 0.122 | 0.014 | 0.988 |
| PC6 | 0.027 | 0.012 | 1.000 |
| Traits | Principal Component | ||
|---|---|---|---|
| (Eigenvectors) | |||
| 1 | 2 | 3 | |
| TRL | 0.460 | −0.024 | −0.057 |
| AD | −0.265 | −0.852 | 0.216 |
| RV | 0.394 | −0.486 | −0.043 |
| NT | 0.425 | 0.092 | 0.828 |
| NF | 0.448 | 0.023 | −0.078 |
| TTL | 0.426 | −0.171 | −0.507 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, W.; Amos, S.A.; Islam, M.S.; Ghimire, A.; Lay, L.; Kim, Y. Exploring the Root Morphological Traits of Diverse-Origin Cultivated Soybean. Agronomy 2023, 13, 2666. https://doi.org/10.3390/agronomy13102666
Khan W, Amos SA, Islam MS, Ghimire A, Lay L, Kim Y. Exploring the Root Morphological Traits of Diverse-Origin Cultivated Soybean. Agronomy. 2023; 13(10):2666. https://doi.org/10.3390/agronomy13102666
Chicago/Turabian StyleKhan, Waleed, Stanley Ahamefula Amos, Mohammad Shafiqul Islam, Amit Ghimire, Liny Lay, and Yoonha Kim. 2023. "Exploring the Root Morphological Traits of Diverse-Origin Cultivated Soybean" Agronomy 13, no. 10: 2666. https://doi.org/10.3390/agronomy13102666
APA StyleKhan, W., Amos, S. A., Islam, M. S., Ghimire, A., Lay, L., & Kim, Y. (2023). Exploring the Root Morphological Traits of Diverse-Origin Cultivated Soybean. Agronomy, 13(10), 2666. https://doi.org/10.3390/agronomy13102666









