Abstract
The availability of cadmium (Cd) in soils is an important factor affecting the safe production of crops. The application of certain soil amendments could reduce the soil Cd availability via the passivation of Cd. However, the passivation of Cd in alkaline soils is limited. Thus, different inorganic and organic amendments and their compound treatments were selected as passivators for reducing the Cd availability in a weakly alkaline farmland soil. The effects of different single and compound amendments on the soil pH and Cd availability, as well as the interactions between inorganic and organic components in immobilizing Cd, were evaluated. The results showed that the inorganic–organic compound amendments can considerably improve the Cd passivation efficiency in the weakly alkaline soil. Moreover, the inorganic and organic components in the compound amendments exerted different synergistic effects in Cd passivation. The manganese dioxide-based compound amendments showed the most remarkable synergistic effects, while the calcium–magnesium–phosphate fertilizer-based compound amendments displayed the weakest synergistic effects. The underlying mechanisms regarding the synergistic effects may be that the compound amendments enhanced the adsorption/specific adsorption, co-precipitation, and surface complexation of Cd in the alkaline soil. A more balanced recommendation for Cd immobilization in the weakly alkaline soil may be manganese dioxide-based compound amendments, given the synergistic effects and Cd immobilization capabilities of various compound materials. This study may provide a theoretical foundation for the passivation remediation of alkaline Cd-contaminated farmland soils by using inorganic–organic compound amendments.
1. Introduction
Cadmium (Cd) is one of the main contaminants affecting the farmland soil quality in China [1]. Approximately 7% of the soil in China is contaminated with cadmium [1]. Compared with other heavy metals, Cd has high mobility in soils and is easy to accumulate in edible crops such as cereal crops, fruits, and vegetables, thereby causing human health risks [2,3,4]. Cd may accumulate in the roots, stems, leaves, and grains of crops [5]. In situ passivation is currently one of the primary ways to reduce the availability of soil heavy metals because of its relatively high efficiency and consistent remediation performance, particularly in the case of acidic soils [6,7,8]. General passivation amendments include lime-based materials, phosphorus-containing materials, clay minerals, metal oxides, and organic materials [6,7,8,9,10,11], which mainly transform heavy metals into more chemically stable forms via precipitation, complexation, adsorption, and ion exchange, thereby reducing the bioavailability of heavy metals in soils [7].
Cd availability in alkaline soils is relatively low compared to acidic soils, resulting in limited Cd passivation effects of conventional amendments in alkaline soils [12,13,14,15]. A meta analysis study has shown that after the amendments’ application, there was a significant negative correlation between the soil-available Cd and pH in acidic soils (R2 = 0.43), while in neutral and alkaline soils, such a correlation was not significant (R2 is 0.017 and 0.016, respectively) [6], which indicated that the Cd immobilization mechanisms of soil amendments may be greatly varied depending on the soil acid–base properties. Simply adding alkaline materials as passivation amendments to alkaline farmland soils may cause over-alkalinization and the hardening of alkaline soils, thereby reducing the soil productivity [16,17]. Therefore, alkaline amendments alone may not be sufficient to passivate Cd in alkaline soils [18].
Inorganic amendments like manganese oxides, clay minerals, and phosphorus-containing materials could significantly reduce the availability of soil Cd to plants via mechanisms of adsorption/specific adsorption and co-precipitation reactions [7,18,19,20], whereas organic passivation materials such as biochar and humus substances may not only immobilize the soil Cd via mechanisms of adsorption and surface complexation reactions, but also serve as important nutrient sources for plants [2,7,21]. Previous studies have shown that compound amendments (i.e., a mixture of different amendments) have a greater impact on soil Cd immobilization than single amendments. Abdelrhman et al. [13] revealed that interactions between biochar and goethite contributed to the high Cd immobilization capacity of goethite-combined biochar in an alkaline paddy soil. Ge et al. [12] found that the combined application of phosphorus-modified hydrochar and zeolite significantly reduced the bioavailability of Cd, Cu, and Pb in weakly alkaline soils. These findings suggested that the combined application of inorganic and organic amendments may have synergistic effects on the passivation of Cd in alkaline soils, which might be a workable strategy to improve the Cd passivation efficiency in alkaline soils. Therefore, it is of utmost importance to systematically study the passivation performances and synergistic effects of inorganic–organic compound amendments in the remediation of alkaline Cd-contaminated soils [22].
However, there have not been many studies conducted to date to look into how compound amendments interactively affect the availability of Cd, particularly in alkaline soils. It is essential to fill these knowledge gaps. We hypothesized that the combined application of inorganic and organic amendments might have a higher Cd immobilization efficiency than the application alone. Hence, the goal of this study was to comprehensively assess the effects of various inorganic amendments (manganese dioxide, montmorillonite, attapulgite, and calcium–magnesium–phosphate fertilizer), organic amendments (cow manure biochar, straw ash, straw biochar, and humic acid), as well as their compound amendments on the soil pH and Cd availability in weakly alkaline Cd-contaminated farmland soil. The interactive effects of the compound materials in immobilizing the soil Cd were highlighted. This research may give a theoretical foundation for the passivation remediation of alkaline Cd-contaminated farmland soils.
2. Materials and Methods
2.1. Soil and Amendments
The experimental soil, which had a total Cd content of 0.28 mg·kg−1 and a carbonate content of 3.1% (CaCO3 eq.), was collected from the plough layer (0–20 cm) of a farmland in Jiangsu Province, China. The soil was cleaned of its debris and air-dried. Then, the soil samples were ground and passed through 10 mesh and 100 mesh sieves for use. A CdCl2 solution was added to the soil to create the Cd-contaminated soil, which was then incubated for 60 days. The total Cd amount in the prepared soil was 3.01 mg·kg−1; the soil pH value was 7.30, where the soil here was considered to be a weakly alkaline soil [20,23]; the content of alkaline hydrolysis nitrogen was 42.30 mg·kg−1; the content of available phosphorus was 11.21 mg·kg−1; the content of available potassium was 48.52 mg·kg−1; and the content of soil organic matter (SOM) was 20.12 g·kg−1.
The passivation amendments used in this study consisted of Inorganic materials and organic materials. Four types of inorganic materials were included, specifically, manganese dioxide (MnO2), montmorillonite (MMT), attapulgite (AT), and calcium–magnesium–phosphate fertilizer (CM). Four types of organic materials were used, i.e., cow manure biochar (CB), straw ash (SA), straw biochar (SB), and humic acid (HA). These amendments were widely used for the passivation of heavy metals in soils [7,19,21]. For the preparation of CB or SB, cow manure or wheat straw was placed in a muffle furnace and pyrolyzed under oxygen-limited circumstances at 400 °C for 4 h [24]. For the preparation of SA, wheat straw was dried in the sun for two days and burnt on the field [25]. hAs extracted from brown coals via the alkaline extraction method were used in this study [26]. All the amendments were dried at 105 °C for 2 h, ground to pass through a 0.15 mm sieve, and thoroughly homogenized before use [27]. The basic properties of soil amendments are shown in Table 1.

Table 1.
Basic properties of soil amendments.
2.2. Experimental Design
A total of 10 g of Cd-contaminated soil was weighed into a 50 mL centrifuge tube. Then, a certain proportion of amendment was added to the tube and mixed thoroughly with the soil followed by a 30-day incubation [27,28]. During the soil incubation, deionized water was regularly supplemented using the weighing method to maintain 70% of the field water capacity [20,28,29]. Seventy percent of the field water capacity was generally termed as drought-free stress [30,31]. The addition ratio of single amendments was set at 2%, 4%, and 8% (w/w) [27,28,32,33,34]. The inorganic–organic compound amendments were prepared by mixing the inorganic materials with organic materials in a 1:1 ratio, and the application rate of compound amendments was set at 2% + 2%, 4% + 4%, and 8% + 8% (w/w) [27,32,33,34]. A control treatment (without the addition of any amendment) was simultaneously included. Three replicates were set for each treatment. The detailed experimental design of single and compound soil amendments is shown in Table 2.

Table 2.
Experimental design and estimated application cost of single and compound soil amendments.
2.3. Analytical Methods
The pH of the passivation amendments was measured via the potentiometric method with water as the extract at a water-to-soil ratio of 2.5:1 (v/w) [35]. The SOM and total organic carbon of the organic amendments were determined calorimetrically using potassium dichromate oxidation [24]. Soil alkaline hydrolysis nitrogen was determined via the NaOH alkaline hydrolysis diffusion method [36]. Soil-available phosphorus was measured via the NaHCO3-extracting colorimetric method [37]. Soil-available potassium was analyzed via the NH4OAc-extracting flame spectrometry method [37]. The specific surface area of the amendments was measured via an ASAP 2460 system (Micromeritics Inc., Norcross, GA, USA) [28].
The total Cd content in the soil and passivation materials was measured via the HCl-HNO3-HF-HClO4 digestion method [38,39], the Cd recovery rate of which was above 95% for the standard reference material. Briefly, approximately 100 mg of the sample was digested with 3 mL of HCl, 1 mL of HNO3, 6 mL of HF, and 0.5 mL of HClO4 [39]. The digestive program consisted of two stages: stage 1 (10 min to reach 200 °C) and stage 2 (15 min at 200 °C). The digestion solutions were evaporated to near dryness after cooling and then dissolved in 1 mL of HNO3 and 20 mL of deionized water for further analysis.
After the 30-day incubation, the pH of the amended soil was immediately measured via the potentiometric method [35]. For the determination of available Cd in the soil after the 30-day incubation, a diethylenetriaminepentaacetic acid–calcium chloride–triethanolamine (DTPA-CaCl2-TEA) buffer solution was used as the extract [40]. The concentrations of DTPA, CaCl2, and TEA in the extract were 0.005, 0.01, and 0.1 mol·L−1, respectively. The pH of the extract was 7.30, and the ratio of soil to the extract was 1:2 (m/v). After extraction, the Cd content in the supernatant was determined via inductively coupled plasma mass spectrometry (ICP-MS, iCAP RQ, Thermo Fisher Scientific, Waltham, MA, USA). The RF power of the mass spectrometer is 1550 W, the cooling gas flow is 12 L/min, the auxiliary gas flow is 0.8 L/min, the atomizer gas flow is 1.2 L/min, and the sampling depth is 15 mm.
Reagent blanks were utilized to adjust the instrument readings in order to guarantee the dependability of the detection results. The analytical values were corrected using the standard soil (SRM 2586, National Institute of Standards and Technology, NIST, Gaithersburg, MD, USA), and triplicate and blank samples were utilized for quality control. The relative standard deviation (% RSD) was less than 5%, and the recovery rate was above 95%.
The soil Cd passivation ratio after the application of various amendments was calculated as follows:
where C0 was the soil-available Cd content in the control treatment, and C1 was the soil-available Cd content after application of passivation amendments.
Cd passivation ratio = [(C0 − C1)/C0] × 100%
2.4. Interactions between Inorganic and Organic Components of Compound Amendments in Passivation of Cd
The interaction equation [41] was used to quantitatively evaluate the interactive effects of inorganic and organic components of compound amendments in Cd passivation. The interaction equation was as follows:
where A represented the passivation ratio of the compound amendments, and B and C indicated the passivation ratio of the corresponding inorganic and organic components in the compound amendments when applied alone. E > 0, E < 0 and E = 0 denoted synergistic effects, antagonistic effects, and independent effects, respectively.
E = [A − Max (B, C)]/A × 100%
2.5. Data Analysis
Origin Pro 2021 was used for the data analysis and graphing. The LSD method was used to test the significance of different treatments. Different lowercase letters in the figures demonstrated significant differences among treatments at p < 0.05 level.
3. Results
3.1. Effects of Single Amendments on Soil pH and Cd Availability
The effects of single amendments on the soil pH and Cd availability are shown in Figure 1. Compared with the initial soil pH, MnO2, MMT, SB, and CB had minor effects on the soil pH. CM and SA significantly enhanced the soil pH, and the soil pH increased as the addition ratios rose. AT and HA could significantly reduce the soil pH, and the soil pH decreased as the addition ratios rose.
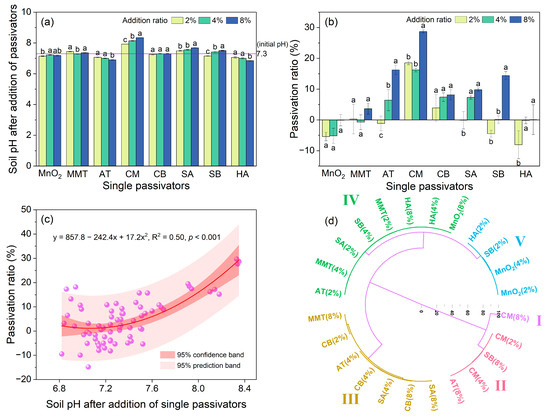
Figure 1.
Effects of different single passivators on soil pH (a) and soil Cd passivation ratio (b); nonlinear relationship between soil Cd passivation ratio and soil pH (c); and hierarchical cluster analysis based on Ward’s method (d). Different lowercase letters in figure (a,b) indicate significant difference between treatments with different addition ratios of passivators (p < 0.05). MnO2: manganese dioxide, MMT: montmorillonite, AT: attapulgite, CM: calcium–magnesium–phosphate fertilizer, CB: cow manure biochar, SA: straw ash, SB: straw biochar, HA: humic acid.
As shown in Figure 1b, the application of different single amendments exerted obviously different effects on Cd availability in the weakly alkaline soil. MnO2 and HA had no significant effects on Cd availability, and even increased the Cd availability at addition ratios of 2% or 4%. MMT also had little effect on Cd availability and could reduce the Cd availability by only 3.6% at 8%. The other five single amendments could reduce the soil Cd availability to varying degrees except for AT and SB at an addition ratio of 2%, and the Cd passivation ratios could be enhanced with the increase in addition ratios. At any treatment (2%, 4%, 8%), CM had the greatest impact on lowering the soil Cd availability with the highest passivation ratio of 28.7% at 8%. AT, CB, SA (addition ratios of 4% and 8%), and SB (addition ratio of 8%) exerted a certain effect on reducing the Cd availability, and the passivation ratios ranged from 6% to 14.4%. Generally, the single amendments had a limited ability to reduce the Cd availability in the weakly alkaline soil. The passivation performance of CM was better than that of other single amendments, while MnO2, MMT, and HA had little effect on the soil Cd availability.
As shown in Figure 1c, the nonlinear regression analysis showed that there was a nonlinear positive correlation between the soil pH and the Cd passivation ratio (R2 = 0.50) following the application of single amendments. Overall, as the soil pH increased, the Cd passivation ratios improved.
The passivation effects of various single amendments were divided into five categories (Figure 1d) via Ward’s minimum variance hierarchical cluster analysis method with Euclidean distance [42]. The Ward’s method minimizes the sum of squares of the distance between any two clusters that can develop at each stage of the cluster classification and uses an analysis of variance approach to measure the distances between clusters. A dendrogram is created as a result, which is a graphic representation of the linkage distance. The phenon line determines the number of clusters; thus, altering the phenon line’s position on the dendrogram alters the number of clusters [42]. The results indicated that the ratio of 8% CM showed the greatest passivation effect (Class I, passivation ratio 28.7%, soil pH 8.34). The ratios of 2% and 4% CM and 8% AT and SB displayed certain passivation effects (Class II, average passivation ratio 16.3%, average soil pH 7.62). The ratios of 2% CB, 4% AT, CB, and SA, and 8% MMT, CB, and SA showed slight passivation effects (Class III, mean passivation ratio 6.7%, mean soil pH 7.35).
The rest of the single amendments were almost ineffective on the soil Cd passivation (Class IV, average passivation ratio −0.2%, average soil pH 7.21) and even slightly enhanced the soil Cd availability (Class V, mean passivation ratio −5.8%, mean soil pH 7.15). In general, the passivation effects of CM, AT, and SB at high addition ratios were relatively apparent. Furthermore, the cluster analysis results also showed a trend that the average passivation ratio enhanced with the increase in the average soil pH.
3.2. Effects of Inorganic–Organic Compound Amendments on Soil pH and Cd Availability
The effects of inorganic–organic compound amendments on the soil pH and Cd availability are shown in Figure 2. All CM- and SA-based compound amendments significantly enhanced the soil pH, and the soil pH increased as the addition ratios rose (Figure 2a). At any addition ratio, the soil pH following the application of CM-based compound amendments was higher than that of MnO2-, MMT-, and AT-based compound materials. On the contrary, except for CM-HA compound amendments, HA-based compound materials could significantly reduce the soil pH, and the soil pH decreased as the addition ratios rose. In general, the influences of compound materials on the soil pH were related to the effects of the corresponding inorganic and organic components on the soil pH when applied alone. Compound amendments containing CM or SA could increase the soil pH, while compound materials including HA or AT may reduce the soil pH.
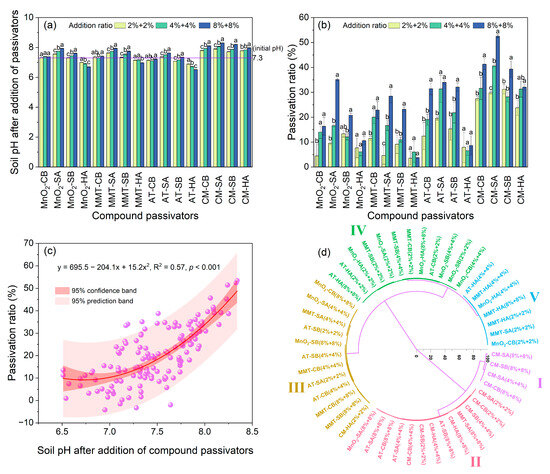
Figure 2.
Effects of different compound passivators on soil pH (a) and soil Cd passivation ratio (b); nonlinear relationship between soil Cd passivation ratio and soil pH (c); and hierarchical cluster analysis based on Ward’s method (d). Different lowercase letters in figure (a,b) indicate significant difference between treatments with different addition ratios of passivators (p < 0.05). MnO2: manganese dioxide, MMT: montmorillonite, AT: attapulgite, CM: calcium–magnesium–phosphate fertilizer, CB: cow manure biochar, SA: straw ash, SB: straw biochar, HA: humic acid.
As shown in Figure 2b, Cd availability in the weakly alkaline soil varied greatly depending on the types of compound amendments applied, with an overall trend indicating that the passivation ratios improved as the addition ratios increased. The CM-based compound materials (passivation ratio 23.8–52.5%) had the highest passivation capability when the organic component and the addition ratio were kept constant. With the same inorganic component and application ratio, the passivation ability of the HA-based compound amendments (passivation ratio 3.5–32.1%) was generally the weakest, while the passivation capacity of SA-based compound materials was overall the highest. In general, the Cd passivation effects of compound amendments were more pronounced than those of the corresponding single components when applied solely, and the maximum passivation ratio could reach 52.5%. It is worth noting that, whereas some single amendments may improve the soil Cd availability (Figure 1b), all types of compound amendments could reduce the soil Cd availability.
As shown in Figure 2c, the nonlinear regression analysis revealed that following the application of compound amendments, there was a nonlinear positive correlation between the soil pH and the Cd passivation ratio (R2 = 0.57). In general, the Cd passivation ratios improved as the soil pH increased.
The passivation effects of various compound amendments were classified into five groups via hierarchical cluster analysis based on Ward’s method (Figure 2d). The results showed that the passivation effects of Class I (average passivation ratio 43.4%, average soil pH 8.17) were the best, and they were all CM-based compound materials. The passivation effects of Class II (mean passivation ratio 31.1%, mean soil pH value 7.74), mainly containing CM- or SA-based compound amendments, were also relatively obvious.
Compound materials in Class III (average passivation ratio 19.7%, average soil pH 7.45) and Class IV (average passivation ratio 10.6%, average soil pH 7.19) had certain passivation effects, while compound amendments in Class V (mean passivation ratio 5.0%, mean soil pH 7.13), mainly including HA-based compound materials, exerted the weakest passivation effects. Although the Class IV and V compound amendments marginally reduced the soil pH, they all had passivation effects, implying that the inorganic and organic components in the compound materials may interact when immobilizing the soil Cd. Furthermore, the cluster analysis results revealed a tendency in which the mean passivation ratio enhanced as the mean soil pH increased.
3.3. Interactions between Inorganic and Organic Components of Compound Amendments in Passivation of Cd
The interaction equation [41] was used to quantitatively characterize the interactive effects of inorganic and organic components of compound amendments during the passivation of soil Cd. As shown in Figure 3, different synergistic effects were found between the inorganic and organic components of almost all compound materials except for AT-HA at a high addition ratio. The synergistic effects of various compound amendments followed the order of MnO2-based compound amendments (mean E value of 81.2%) > MMT-based compound amendments (mean E value of 70.0%) > AT-based compound amendments (mean E value of 55.3%) > CM-based compound amendments (mean E value of 37.2%). Among them, 2% + 2% MnO2-HA had the greatest synergistic effect (interaction E value 170.5%), and only 8% + 8% AT-HA exerted an antagonistic effect (interaction E value −88.4%). The interaction E values of the AT-based compound materials decreased with the increase in addition ratios. For other compound amendments with lower addition ratios, the synergistic effects became more noticeable.
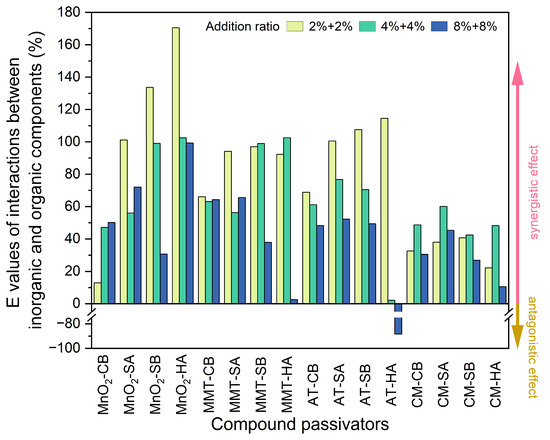
Figure 3.
Interactions between inorganic and organic components in different compound passivators. MnO2: manganese dioxide, MMT: montmorillonite, AT: attapulgite, CM: calcium–magnesium–phosphate fertilizer, CB: cow manure biochar, SA: straw ash, SB: straw biochar, HA: humic acid.
Even though CM-based compound materials had the greatest passivation effects, their synergistic effects were noticeably weakest, indicating that the Cd passivation effects of such compound amendments were closely related to the immobilization effects of the single components when used independently. The synergistic effects of MnO2-based compound materials were the highest, denoting that the Cd passivation effects of such compound amendments were significantly contributed by the interactions between MnO2 and the organic components.
4. Discussion
The availability of soil Cd has a significant impact on the safe production of crops, and soil pH is one of the major determinants of soil Cd availability. Soil pH has a crucial impact on the dissolution–precipitation, adsorption–desorption, and other reaction processes of Cd in the soil environment, and it could dramatically alter the chemical speciation, migration, and transformation capacities of Cd [7,12]. The passivation of heavy metals in soils can be caused by an elevation in the soil pH because it can raise the negative charges on the surfaces of soil colloids, improve the electrostatic adsorption of heavy metal ions, and increase the precipitates of heavy metal hydroxides and carbonates [12,22,28]. In this study, following the application of single amendments, the Cd passivation ratios generally enhanced with the increase in the soil pH. The effect of CM on improving the soil pH was the most noticeable. It also had the most obvious passivation effect among the eight single materials, with the highest passivation ratio of 28.7%. Such a passivation effect was comparable to the results of Luo et al. [19], in which the combination of CM and continuous flooding decreased the available content of Cd in soils by 28.57%, and the reports of Wang et al. [43], in which CM reduced the amount of rhizospheric DTPA-Cd in soils by 18–40%. CM is an alkaline material (Table 1) and includes soluble phosphates, which could raise the soil pH, facilitating the precipitation of Cd, and combine with Cd to generate insoluble metal orthophosphates via complexation or precipitation processes [44]. Therefore, CM generally exerted a relatively significant effect on the passivation remediation of heavy metal-contaminated soils [19,43]. Compared with CM, other single amendments in this study showed weaker effects on increasing the soil pH, and some materials such as AT and HA could reduce the soil pH. Therefore, except for CM, the ability of other single amendments to immobilize Cd was relatively limited, and some materials such as MnO2, MMT, and HA were almost ineffective for Cd passivation. Alternatively, the weak passivation effect of HA may not only be due to its low pH but also possibly because of its water solubility. On the one hand, the carboxyl and phenolic moieties of HA can combine with metal ions to form coordination complexes, the solubility of which depends on the ligands. On the other hand, HA might influence the generation of metal precipitates as hydroxides by altering the soil pH, which in turn influences the solubility of metals [45]. It should be noted that the grain size of the amendments might also affect the Cd passivation efficiency in soils [46]. Coarser amendments may possibly have a better Cd immobilization capacity in soils than the finer ones by improving the formation of more stable organic matter [46].
Previous studies have shown that the passivation effects of various amendments on soil heavy metals were not only related to the composition and properties of passivation materials, but also depended on the soil pH [6,15,28,47]. The Cd passivation effects were relatively poor in alkaline soils compared with those in acidic soils [15] because in alkaline soils, the change in Cd availability was less responsive to an increase in pH [47]. Jia et al. [28] found that in acidic soils, biochar-mediated changes in the soil pH had more significant effects on Cd bioavailability, while in alkaline soils, the strength of Cd–biochar interactions was more important for the Cd passivation efficiency. Specifically, the predominant mechanism for the interaction between biochar and Cd in acidic soils was found to be a cation exchange, whereas the mechanisms in alkaline soils were precipitation, the coordination effect, and a π–electron interaction [28]. Using meta-analysis, Wang et al. [6] found that the response of Cd availability to the soil pH may be quite different in acidic and alkaline soils following the application of passivation amendments. For example, in acidic soils, MMT and manganese oxides had apparent Cd passivation effects [7,48]. MMT could firmly adsorb soluble heavy metals on its surface or into the interlayer structure via physical, chemical, and ion exchange adsorption. Manganese oxide could exert strong specific adsorption effects on heavy metals and may thus significantly increase the proportion of reducible heavy metal components. However, for the weakly alkaline soil in this study, the application of these two amendments alone had almost no Cd passivation effects, which may be due to their insignificant effects on the soil pH or attributed to their relatively low adsorption or surface complexation capacity for Cd in the weakly alkaline soil [15,47,49,50].
For the passivation remediation of heavy metal-contaminated soils, the application of inorganic–organic compound materials may be another efficient strategy to improve the passivation effects of heavy metals [6,32,51]. In acidic soils, biochar-based or lime-based compound amendments were considerably more efficient in immobilizing heavy metals than the application of single materials [6,32,51]. For instance, Gao et al. [51] found that in an acidic soil, biochar associated with magnesium ferrite showed a better passivation ability in decreasing the bioavailable Cd than biochar or magnesium ferrite when applied alone. In this study, the application of inorganic–organic compound amendments also effectively improved the Cd passivation ability in the weakly alkaline soil [13,14,15,18,22]. Compared with the single amendments, the Cd passivation effects of inorganic–organic compound materials were all superior to those of the corresponding inorganic or organic components when applied alone [13,14,15,18,22]. Among them, CM-based compound materials displayed the best passivation effects [52,53], and the passivation ratio reached up to 52.5%, which was higher than or comparable to the Cd passivation ratio of 11.07% [29], 45.8% [54], and 29.5% [55] induced via various inorganic–organic compound amendments in alkaline or neutral soils.
Moreover, all kinds of compound amendments could immobilize the soil Cd [13,14,15,18,22]. Even if some compound materials reduced the soil pH, they still exhibited certain passivation effects, which demonstrated that the inorganic and organic components in the compound amendments may interact during the passivation of soil Cd [13,14,15,18,22,52,53]. The quantitative evaluation based on the interaction equation [41] denoted that the inorganic and organic components of the compound materials had varying degrees of synergistic effects during the Cd passivation in the weakly alkaline soil [13,15,18,22,52]. Although the passivation effects of the CM-based compound amendments were the most evident, their synergistic effects were the weakest, indicating that their Cd immobilization effects closely depended on the passivation effects of CM and the organic components when applied solely [44,52,53]. The CM displayed the greatest passivation ability among single amendments, resulting in the highest passivation capacities of the CM-based compound materials [19,43]. Notably, the synergistic effects of MnO2-based compound amendments were the most noticeable, underlying that the interactions between MnO2 and the organic components had a remarkable contribution to the Cd passivation ability of such compound materials [18,56].
When passivating heavy metals in alkaline soils, the synergistic effects of compound amendments may be attributed to different mechanisms due to the variations in inorganic and organic components [13,14,15,18,22,23]. Wang et al. [22] found that the biochar–zeolite–humus compound amendment had an excellent Cd passivation effect in weakly alkaline soils because it could convert the acid-soluble Cd to the Cd bound to the reducible fraction with a higher stability. In this study, MnO2-based compound amendments showed substantial synergistic effects on Cd immobilization, which may be ascribed to the similar Cd passivation mechanisms of manganese oxides and organic components, both of which may immobilize Cd via the adsorption or surface complexation [2,21,49]. Manganese oxides have large specific surface areas and abundant active specific adsorption sites on the surface [20,49]. Cd could be specifically adsorbed via the surface of manganese oxides by forming monodentate or bidentate inner layer complexes [20,49]. The tunnel structure of manganese oxides may also easily provide space for foreign ions [20,49]. Meanwhile, Cd could complex with the abundant carboxyl or hydroxyl groups on the surface of organic components in MnO2-based compound materials, thereby enhancing the adsorption and complexation of Cd in soils and improving the passivation effects [18,56]. Therefore, the excellent specific adsorption or surface complexation ability of MnO2 for Cd may substantially contribute to the greatest synergistic effects of MnO2-based compound amendments on Cd passivation. For clay mineral-based compound materials, i.e., MMT- and AT-based compound materials, the synergistic effects of clay minerals and organic components on Cd passivation may be attributed to the O-H groups and Si-O-Si of the compound amendments, strengthening the precipitation, electrostatic interactions, ion exchange, and surface complexation of Cd in soils [57,58]. The synergistic mechanisms of CM-based compound materials for Cd passivation may be that the abundant carboxyl or hydroxyl functional groups on the surface of organic components of the compound amendments enhanced the CM-induced co-precipitation reaction of Cd, promoted the formation of insoluble metal phosphates, or increased the proportion of organically bound Cd [53]. However, the weakest synergistic effects of CM-based compound materials on Cd passivation may be owing to the considerably different Cd immobilization mechanisms of CM and the organic components [2,19,21]. Specifically, CM could passivate the soil Cd mainly via the co-precipitation reaction mechanism, while organic components may immobilize Cd via the mechanisms of adsorption or surface complexation [2,19,21].
It is worth noting that for Cd passivation in the weakly alkaline soil, CM-based compound materials might not be a good option. Such amendments could greatly enhance the soil alkalinity (e.g., pH > 8), which may possibly lead to over-alkalinization and the hardening of alkaline soils, thereby reducing the soil productivity [17]. Moreover, CM-derived phosphate may be emitted into water bodies, which would result in eutrophication [44]. In addition, their weak synergistic effects on Cd immobilization might lead to a less stable Cd passivating effect in alkaline soils, thus reducing the long-term effectiveness of such compound amendments [59,60,61].
In this study, the synergistic effects of compound amendments with lower application rates were generally more evident, and the synergistic effect of 2% + 2% MnO2-HA was the most obvious. Henceforth, by changing the ratios of components (e.g., ranging from 3:1 to 1:3) in inorganic–organic compound amendments or increasing the diversity of inorganic and organic components, the synergistic effects of compound materials at low addition ratios on Cd immobilization in weakly alkaline soils may be strengthened, thereby improving the passivation effects and simultaneously reducing the application cost of compound amendments in fields [52,62]. For instance, iron oxides showed great adsorption and co-precipitation capabilities for Cd, and Cd could form stable internal complexes on the surface of iron oxides, which is attributed to the abundant specific adsorption sites and surface hydroxyl groups on iron oxides [20,63]. Thus, the application of the MnO2-HA compound amendment in combination with a certain proportion of iron oxides could improve the variety of components in compound materials, which may further enhance the Cd passivation effects and stability of compound amendments in weakly alkaline soils [62,64]. However, one should note that the immobilized soil Cd may be remobilized in some cases, such as soil acidification [65], earthworm-mediated nitrification and gut digestive processes [66], or different nitrogen fertilizer management [67], highlighting the importance of the management of Cd remobilization risks during long-term in situ immobilization [68].
5. Conclusions
In this study, the effects of various inorganic amendments, organic amendments, and their compound amendments on the soil pH and Cd availability were thoroughly assessed in weakly alkaline farmland soil. The Cd passivation ability could be significantly improved via inorganic–organic compound materials. Furthermore, the organic and inorganic components of the compound amendments had varying degrees of synergistic effects during the Cd passivation. The synergistic effects of the MnO2-based compound amendments were the strongest, while those of the CM-based compound amendments were the weakest. A more balanced recommendation for Cd immobilization in the weakly alkaline soil may be MnO2-based compound materials, given the synergistic effects and Cd immobilization capabilities of various compound materials. Nevertheless, this study has certain restrictions. On the one hand, the obtained results were derived from soil incubation tests, and it was found that soil incubation and the fields had quite different water management and climatic conditions. On the other hand, field application costs may possibly be improved via the relatively high application rates of amendments. Moreover, the synergistic mechanisms of compound materials for Cd passivation should be thoroughly investigated. Future studies should focus on exploring the underlying interactive mechanisms of compound amendments for Cd immobilization in alkaline soils, as well as conducting long-term field studies with relatively modest application dosages to validate the passivation efficiency and stability of soil amendments.
Author Contributions
Conceptualization, F.T., Q.H., L.L., X.L. and Y.G.; methodology, F.T., L.L., G.F. and G.S.; validation, F.T., L.L. and G.F.; formal analysis, F.T., Q.H., L.L., G.F., G.S. and X.L.; investigation, F.T., G.S. and Y.G.; resources, F.T., L.L., G.F., G.S., X.L. and Y.G.; writing—original draft preparation, F.T. and Q.H.; writing—review and editing, F.T., Q.H., L.L., G.F., G.S., X.L. and Y.G.; visualization, F.T., G.F., G.S. and X.L.; project administration, F.T., X.L. and Y.G.; funding acquisition, F.T. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Jiangsu Agricultural Science and Technology Independent Innovation Fund (CX(20)1010), and the National Natural Science Foundation of China (No. 41807140).
Data Availability Statement
All data analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, J.; Hu, R.; Zhao, C.; Wang, L.; Lei, M.; Guo, G.; Shi, H.; Liao, X.; Chen, T. Challenges and opportunities for improving the environmental quality of cadmium-contaminated soil in China. J. Hazard. Mater. 2023, 445, 130560. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.Q.; Liu, L.J.; Ling, Q.; Cai, Y.W.; Yu, S.J.; Wang, S.Q.; Fu, D.; Hu, B.W.; Wang, X.K. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Agrelli, D.; Duri, L.G.; Fiorentino, N.; Cozzolino, E.; Fagnano, M.; Adamo, P. Potentially Toxic Element Availability and Risk Assessment of Cadmium Dietary Exposure after Repeated Croppings of Brassica juncea in a Contaminated Agricultural Soil. Agronomy 2020, 10, 880. [Google Scholar] [CrossRef]
- Mubeen, S.; Ni, W.; He, C.; Yang, Z. Agricultural Strategies to Reduce Cadmium Accumulation in Crops for Food Safety. Agriculture 2023, 13, 471. [Google Scholar] [CrossRef]
- Yan, H.L.; Zhang, H.Z.F.; Hao, S.N.; Wang, L.Y.; Xu, W.X.; Mi, M.; Luo, Y.M.; He, Z.Y. Cadmium contamination in food crops: Risk assessment and control in smart age. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1643–1661. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, W.; Liang, X.; Xu, Y.; Wang, Y.; Huang, Q.; Li, L. Effects of exogenous additives on wheat Cd accumulation, soil Cd availability and physicochemical properties in Cd-contaminated agricultural soils: A meta-analysis. Sci. Total Environ. 2022, 808, 152090. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Duan, W.; Xie, M.; Dong, X. Heavy metal stabilization remediation in polluted soils with stabilizing materials: A review. Environ. Geochem. Health 2023, 45, 4127–4163. [Google Scholar] [CrossRef]
- Ullah, A.; Ma, Y.B.; Li, J.M.; Tahir, N.; Hussain, B. Effective Amendments on Cadmium, Arsenic, Chromium and Lead Contaminated Paddy Soil for Rice Safety. Agronomy 2020, 10, 359. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef]
- Chattha, M.U.; Arif, W.; Khan, I.; Soufan, W.; Chattha, M.B.; Hassan, M.U.; Ullah, N.; El Sabagh, A.; Qari, S.H. Mitigation of Cadmium Induced Oxidative Stress by Using Organic Amendments to Improve the Growth and Yield of Mash Beans [Vigna mungo (L.)]. Agronomy 2021, 11, 2152. [Google Scholar] [CrossRef]
- Lai, W.W.; Wu, Y.Y.; Zhang, C.N.; Dilinuer, Y.; Pasang, L.; Lu, Y.Q.; Wang, Y.H.; Chen, H.M.; Li, Z. Combination of Biochar and Phosphorus Solubilizing Bacteria to Improve the Stable Form of Toxic Metal Minerals and Microbial Abundance in Lead/Cadmium-Contaminated Soil. Agronomy 2022, 12, 1003. [Google Scholar] [CrossRef]
- Ge, Q.; Tian, Q.; Hou, R.; Wang, S. Combing phosphorus-modified hydrochar and zeolite prepared from coal gangue for highly effective immobilization of heavy metals in coal-mining contaminated soil. Chemosphere 2022, 291, 132835. [Google Scholar] [CrossRef] [PubMed]
- Abdelrhman, F.; Gao, J.; Ali, U.; Wan, N.; Hu, H. Assessment of goethite-combined/modified biochar for cadmium and arsenic remediation in alkaline paddy soil. Environ. Sci. Pollut. Res. Int. 2022, 29, 40745–40754. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, Z.; Zhang, Y.; Li, B.; Chen, C.; Shen, K. Effects of biochars combined with ferrous sulfate and pig manure on the bioavailability of Cd and potential phytotoxicity for wheat in an alkaline contaminated soil. Sci. Total Environ. 2021, 753, 141832. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Kang, X.; Zhang, J.; Sun, M.; Yu, J.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; et al. Effects of a novel Cd passivation approach on soil Cd availability, plant uptake, and microbial activity in weakly alkaline soils. Ecotoxicol. Environ. Saf. 2023, 253, 114631. [Google Scholar] [CrossRef]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/stabilization of soil heavy metals by alkaline industrial wastes: A critical review. Environ. Pollut. 2022, 312, 120094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xue, B.; Jiao, L.; Meng, X.; Zhang, L.; Li, B.; Sun, H. Preparation of ball-milled phosphorus-loaded biochar and its highly effective remediation for Cd- and Pb-contaminated alkaline soil. Sci. Total Environ. 2022, 813, 152648. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Sun, G.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L. Application of ferromanganese functionalized biochar simultaneously reduces Cd and Pb uptake of wheat in contaminated alkaline soils. Ecotoxicol. Environ. Saf. 2023, 257, 114930. [Google Scholar] [CrossRef]
- Luo, W.; Yang, S.; Khan, M.A.; Ma, J.; Xu, W.; Li, Y.; Xiang, Z.; Jin, G.; Jia, J.; Zhong, B.; et al. Mitigation of Cd accumulation in rice with water management and calcium-magnesium phosphate fertilizer in field environment. Environ. Geochem. Health 2020, 42, 3877–3886. [Google Scholar] [CrossRef]
- Lu, T.; Wang, W.; Liu, L.; Wang, L.; Hu, J.; Li, X.; Qiu, G. Remediation of cadmium-polluted weakly alkaline dryland soils using iron and manganese oxides for immobilized wheat uptake. J. Clean. Prod. 2022, 365, 132794. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Lin, Q.; Rashid, M.S.; He, Z.L.; Yang, X.E. Organic soil additives for the remediation of cadmium contaminated soils and their impact on the soil-plant system: A review. Sci. Total Environ. 2020, 707, 136121. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, T.; Liu, L.; Yang, X.; Li, X.; Qiu, G. Combined remediation effects of biochar, zeolite and humus on Cd-contaminated weakly alkaline soils in wheat farmland. Chemosphere 2022, 302, 134851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, P.; Ye, J.; Zhang, G.; Cai, Y. Simultaneous in-situ remediation and fertilization of Cd-contaminated weak-alkaline farmland for wheat production. J. Environ. Manag. 2019, 250, 109528. [Google Scholar] [CrossRef]
- Li, H.; Ren, R.; Zhang, H.; Zhang, G.; He, Q.; Han, Z.; Meng, S.; Zhang, Y.; Zhang, X. Factors regulating interaction among inorganic nitrogen and phosphorus species, plant uptake, and relevant cycling genes in a weakly alkaline soil treated with biochar and inorganic fertilizer. Sci. Total Environ. 2023, 905, 167280. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, N. Effect of crop residue ashes on sorption behavior of herbicides used in the succeeding crop in Indian soils. J. Environ. Sci. Health B 2020, 55, 630–645. [Google Scholar] [CrossRef] [PubMed]
- Skripkina, T.; Belokozenko, M.; Shatskaya, S.; Tikhova, V.; Lomovskiy, I. Concentrating rare earth elements in brown coal humic acids by mechanochemical treatment. RSC Adv. 2021, 11, 36016–36022. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Fu, P.; Li, Z.; Ma, W. The evaluation of in-site remediation feasibility of Cd-contaminated soils with the addition of typical silicate wastes. Environ. Pollut. 2020, 265, 114865. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Zeng, X.; Zhang, N.; Wen, J.; Liu, J.; Jiku, M.A.S.; Wu, C.; Su, S. The performance and mechanism of cadmium availability mitigation by biochars differ among soils with different pH: Hints for the reasonable choice of passivators. J. Environ. Manag. 2022, 312, 114903. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Zhao, P.; Huang, Z. Effects of composite environmental materials on the passivation and biochemical effectiveness of Pb and Cd in soil: Analyses at the ex-planta of the Pak-choi root and leave. Environ. Pollut. 2022, 309, 119812. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; ur Rehman, M.Z.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. Int. 2020, 27, 4958–4968. [Google Scholar] [CrossRef] [PubMed]
- Beatrice, A.; Varco, J.J.; Dygert, A.; Atsar, F.S.; Solomon, S.; Thirumalai, R.; Pittman, C.U.; Mlsna, T. Lead immobilization in simulated polluted soil by Douglas fir biochar-supported phosphate. Chemosphere 2022, 292, 133355. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Y.; Sun, Y.; Wang, L.; Liang, X.; Jia, H. Crayfish shell biochar for the mitigation of Pb contaminated water and soil: Characteristics, mechanisms, and applications. Environ. Pollut. 2021, 271, 116308. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Wang, P.; Mi, S.; Ali, A.; Liu, X.; Li, Y.; Guan, W.; Li, R.; Zhang, Z. Effects of crop straw and its derived biochar on the mobility and bioavailability in Cd and Zn in two smelter-contaminated alkaline soils. Ecotoxicol. Environ. Saf. 2019, 181, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Luo, D.; Yu, Y.; Yang, S.; Zhang, J.; Cao, G. Soil pH changes in a small catchment on the Chinese Loess Plateau after long-term vegetation rehabilitation. Ecol. Eng. 2022, 175, 106503. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.; Saeed, S.; Zhang, B.; Luo, M. The difference of soil properties between pure and mixed Chinese fir (Cunninghamia lanceolata) plantations depends on tree species. Glob. Ecol. Conserv. 2020, 22, e01009. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Xin, Y. Changes in and evaluation of surface soil quality in Populus × xiaohei shelterbelts in midwestern Heilongjiang province, China. J. For. Res. 2021, 32, 1221–1233. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, H.; Chu, H.; Hu, M.; Xu, T.; Xu, X.; He, Z. The potential ecological risk assessment of soil heavy metals using self-organizing map. Sci. Total Environ. 2022, 843, 156978. [Google Scholar] [CrossRef]
- Kazemi Moghaddam, V.; Latifi, P.; Darrudi, R.; Ghaleh Askari, S.; Mohammadi, A.A.; Marufi, N.; Javan, S. Heavy metal contaminated soil, water, and vegetables in northeastern Iran: Potential health risk factors. J. Environ. Health Sci. Eng. 2022, 20, 65–77. [Google Scholar] [CrossRef]
- Li, X.; Jia, R.; Lu, X.; Xu, Y.; Liang, X.; Shen, L.; Li, B.; Ma, C.; Wang, N.; Yao, C.; et al. The use of mercapto-modified palygorskite prevents the bioaccumulation of cadmium in wheat. J. Hazard. Mater. 2021, 417, 125917. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Y.; Liu, J.; Lu, S.; Guo, J.; Zhang, Z.; Zheng, X. Quantitative evaluation of the synergistic effect of biochar and plants on immobilization of Pb. J. Environ. Manag. 2022, 316, 115200. [Google Scholar] [CrossRef]
- Yang, J.; Ye, M.; Tang, Z.; Jiao, T.; Song, X.; Pei, Y.; Liu, H. Using cluster analysis for understanding spatial and temporal patterns and controlling factors of groundwater geochemistry in a regional aquifer. J. Hydrol. 2020, 583, 124594. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, X.; Lai, L.; Li, H.; Zhang, X.; Chen, H.; Xie, L. Phosphorus fertilization regimes and rates alter Cd extractability in rhizospheric soils and uptake in maize (Zea mays L.). Chemosphere 2022, 298, 134288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, D.; Yang, J.; Ye, Y.; Luo, J.; Zhou, X.; Yang, L.; Liu, Z. A combined passivator of zeolite and calcium magnesium phosphate fertilizer: Passivation behavior and mechanism for Cd (II) in composting. Environ. Res. 2023, 231, 116306. [Google Scholar] [CrossRef] [PubMed]
- Lodygin, E.D.; Alekseev, I.I.; Vasilevich, R.S.; Abakumov, E.V. Complexation of lead and cadmium ions with humic acids from arctic peat soils. Environ. Res. 2020, 191, 110058. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.; Yan, B.; Li, Y.; Bao, M. Additive grain-size: An innovative perspective to investigate the transformation among heavy metal and phosphorus fractions during aerobic composting. J. Environ. Manag. 2021, 292, 112768. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, B.; Peng, Y.; Gao, X.; Fan, B.; Chen, Q. A meta-analysis of heavy metal bioavailability response to biochar aging: Importance of soil and biochar properties. Sci. Total Environ. 2021, 756, 144058. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, J.; Yang, X.; Fu, Q.; Hu, H.; Huang, Q. Mineralization of organic matter during the immobilization of heavy metals in polluted soil treated with minerals. Chemosphere 2022, 301, 134794. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W.; Xie, F. Removal of Cadmium(II) by hydrated manganese dioxide: Behaviour and mechanism at different pH. Environ. Technol. 2023, 44, 3544–3562. [Google Scholar] [CrossRef]
- Zhao, C.C.; Yao, J.; Knudsen, T.S.; Liu, J.L.; Zhu, X.Z.; Ma, B.; Li, H.; Cao, Y.; Liu, B. Performance and mechanisms for Cd(II) and As(III) simultaneous adsorption by goethite-loaded montmorillonite in aqueous solution and soil. J. Environ. Manag. 2023, 330, 117163. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Y.; Zhou, Y.; Adeel, M.; Chen, Q. Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi (Brassica chinensis L.). J. Environ. Manag. 2019, 251, 109610. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, J.; Abbas, T.; Zhang, Q.; Cai, M.; Tahir, M.; Wu, D.; Di, H. Immobilization of exchangeable Cd in soil using mixed amendment and its effect on soil microbial communities under paddy upland rotation system. Chemosphere 2021, 262, 127828. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, L.; Wang, C.Q.; Zheng, S.Q.; Xiao, R.; Guo, Y. Effects of organic-inorganic amendments on the cadmium fraction in soil and its accumulation in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. Int. 2019, 26, 13762–13772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Nie, N.; Liu, K.; Li, Q.; Cui, H.; Du, H. Analog soil organo–ferrihydrite composites as suitable amendments for cadmium and arsenic stabilization in co-contaminated soils. Sci. Total Environ. 2023, 877, 162929. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, B.; Cui, H.; Yang, S.; Wang, Y.; Feng, Y.; Sun, X.; Feng, Y. Clay-hydrochar composites return to cadmium contaminated paddy soil: Reduced Cd accumulation in rice seed and affected soil microbiome. Sci. Total Environ. 2022, 835, 155542. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wei, W.; Xu, C.; Meng, Y.; Bai, W.; Yang, W.; Lin, A. Manganese-modified biochar for highly efficient sorption of cadmium. Environ. Sci. Pollut. Res. Int. 2020, 27, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.D.; Wang, X.; Wang, D.; Yang, Q.Q. Optimization and Modelling of Cd(II) Removal from Aqueous Solution with Composite Adsorbent Prepared from Alternanthera philoxeroides Biochar and Bentonite by Response Surface Methodology. Bioresources 2020, 15, 9413–9428. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Li, G.; Du, Q.; Yang, F. Preparation of montmorillonite modified biochar with various temperatures and their mechanism for Zn ion removal. J. Hazard. Mater. 2020, 391, 121692. [Google Scholar] [CrossRef]
- Qu, J.; Yuan, Y.; Zhang, X.; Wang, L.; Tao, Y.; Jiang, Z.; Yu, H.; Dong, M.; Zhang, Y. Stabilization of lead and cadmium in soil by sulfur-iron functionalized biochar: Performance, mechanisms and microbial community evolution. J. Hazard. Mater. 2022, 425, 127876. [Google Scholar] [CrossRef]
- He, Z.; Xu, Y.; Wang, W.; Yang, X.; Jin, Z.; Zhang, D.; Pan, X. Synergistic mechanism and application of microbially induced carbonate precipitation (MICP) and inorganic additives for passivation of heavy metals in copper-nickel tailings. Chemosphere 2023, 311, 136981. [Google Scholar] [CrossRef]
- Liu, Y.L.; Luo, H.Y.; Tie, B.Q.; Li, D.Y.; Liu, S.T.; Lei, M.; Du, H.H. The long-term effectiveness of ferromanganese biochar in soil Cd stabilization and reduction of Cd bioaccumulation in rice. Biochar 2021, 3, 499–509. [Google Scholar] [CrossRef]
- Tan, W.T.; Zhou, H.; Tang, S.F.; Zeng, P.; Gu, J.F.; Liao, B.H. Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Liu, C.; Ma, J.; Ouyang, X.; Weng, L.; Chen, Y.; Li, Y. Enhanced cadmium removal by biochar and iron oxides composite: Material interactions and pore structure. J. Environ. Manag. 2023, 330, 117136. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ding, C.; Zhou, Z.; Huang, G.; Wang, X. Stability of immobilization remediation of several amendments on cadmium contaminated soils as affected by simulated soil acidification. Ecotoxicol. Environ. Saf. 2018, 161, 164–172. [Google Scholar] [CrossRef]
- Wang, J.; Shi, L.; Liu, J.; Deng, J.; Zou, J.; Zhang, X.; Shen, Z.; Chen, Y. Earthworm-mediated nitrification and gut digestive processes facilitate the remobilization of biochar-immobilized heavy metals. Environ. Pollut. 2023, 322, 121219. [Google Scholar] [CrossRef]
- Wang, J.-F.; Li, W.-L.; Li, Q.-S.; Wang, L.-L.; He, T.; Wang, F.-P.; Xu, Z.-M. Nitrogen fertilizer management affects remobilization of the immobilized cadmium in soil and its accumulation in crop tissues. Environ. Sci. Pollut. Res. Int. 2021, 28, 31640–31652. [Google Scholar] [CrossRef]
- Zhang, D.; Ding, A.; Li, T.; Wu, X.; Liu, Y.; Naidu, R. Immobilization of Cd and Pb in a contaminated acidic soil amended with hydroxyapatite, bentonite, and biochar. J. Soils Sediments 2021, 21, 2262–2272. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).