Diversity and Heterosis of Leaf Anatomical Traits in Backcross 1 (BC1) Derived from Interspecific Hybridization between Commercial Cane (Saccharum spp. Hybrid) and Wild Type (S. spontaneum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design and Cultural Practices
2.3. Data Collection
2.4. Statistical Analysis
3. Results
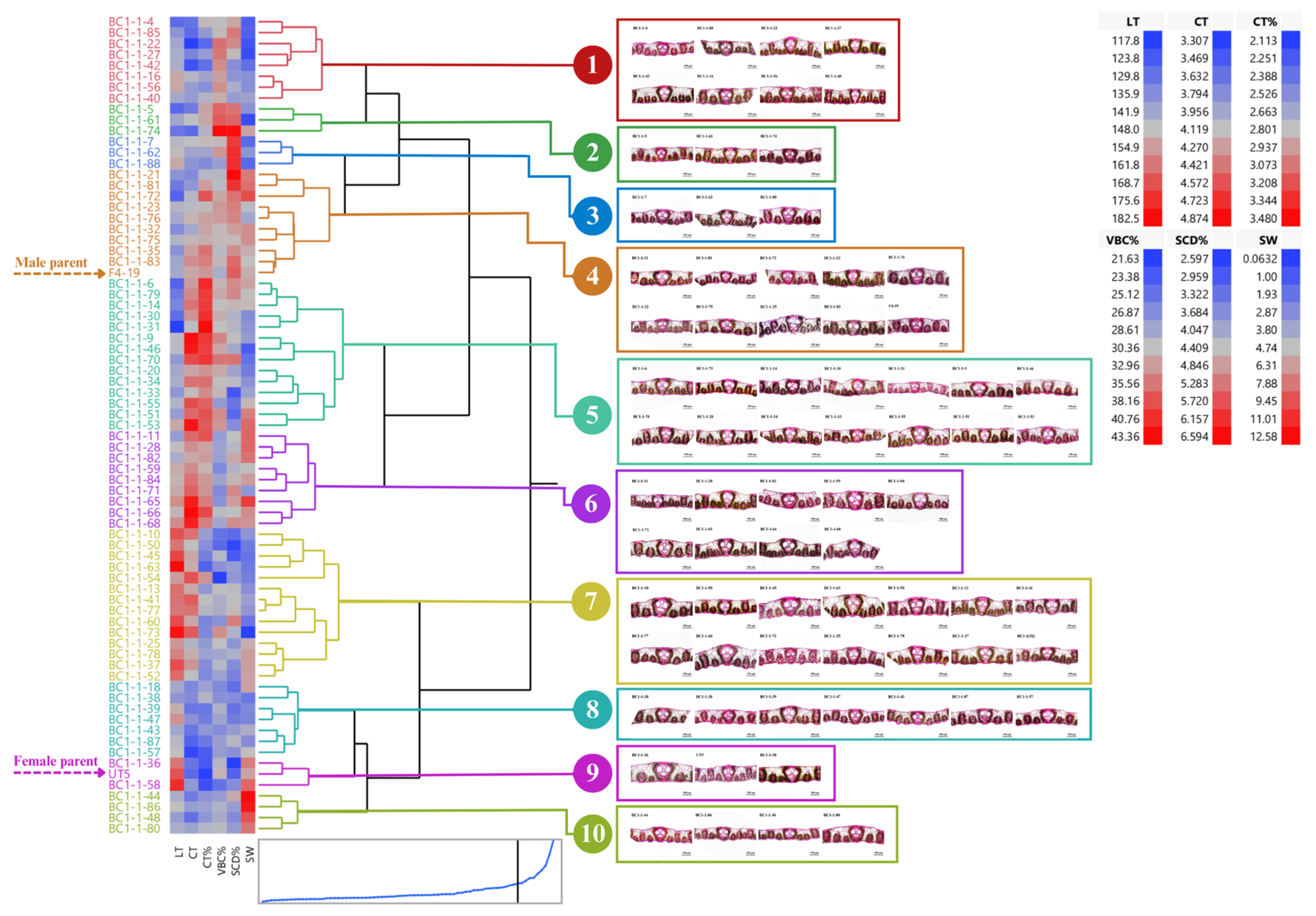
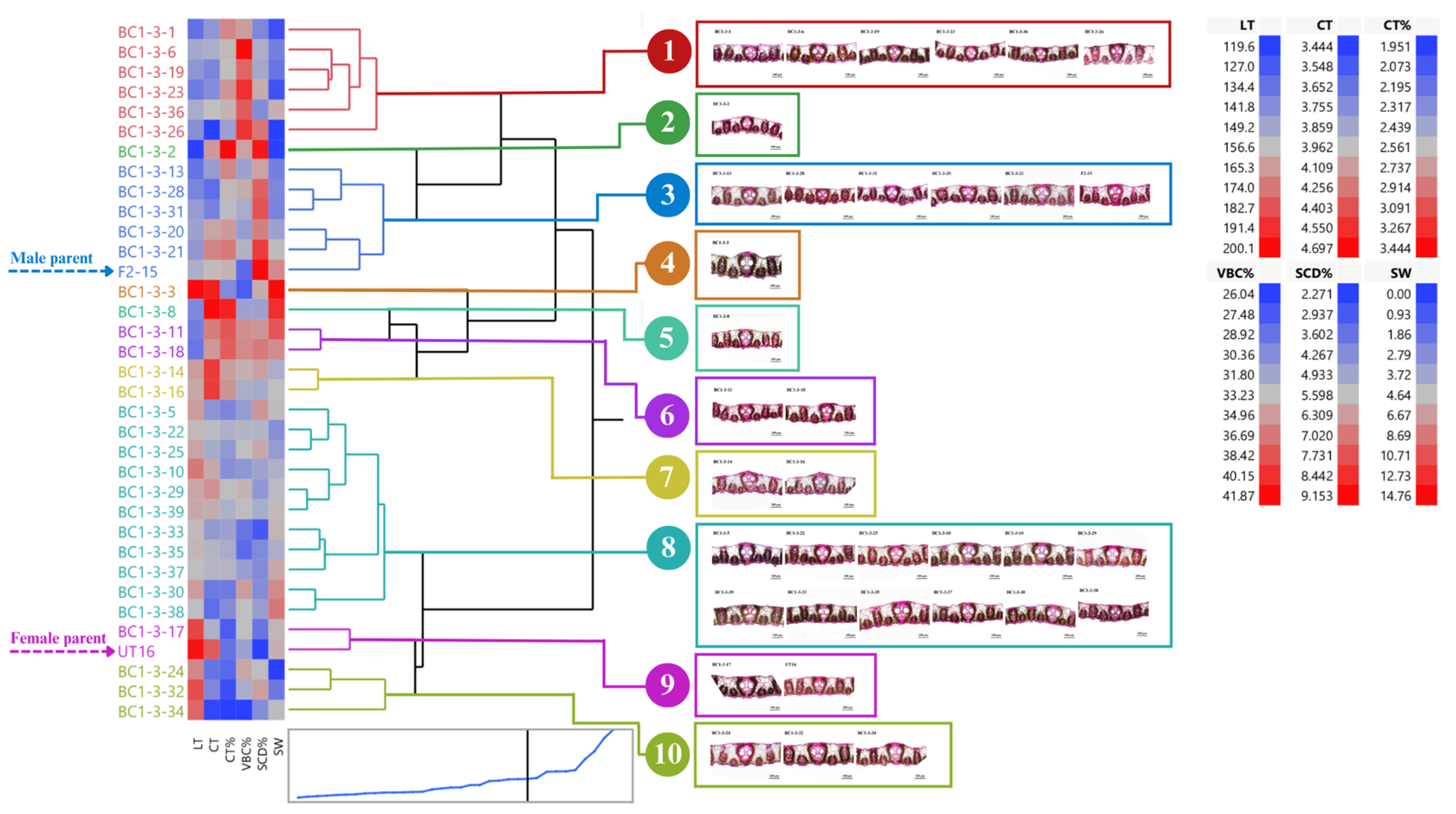
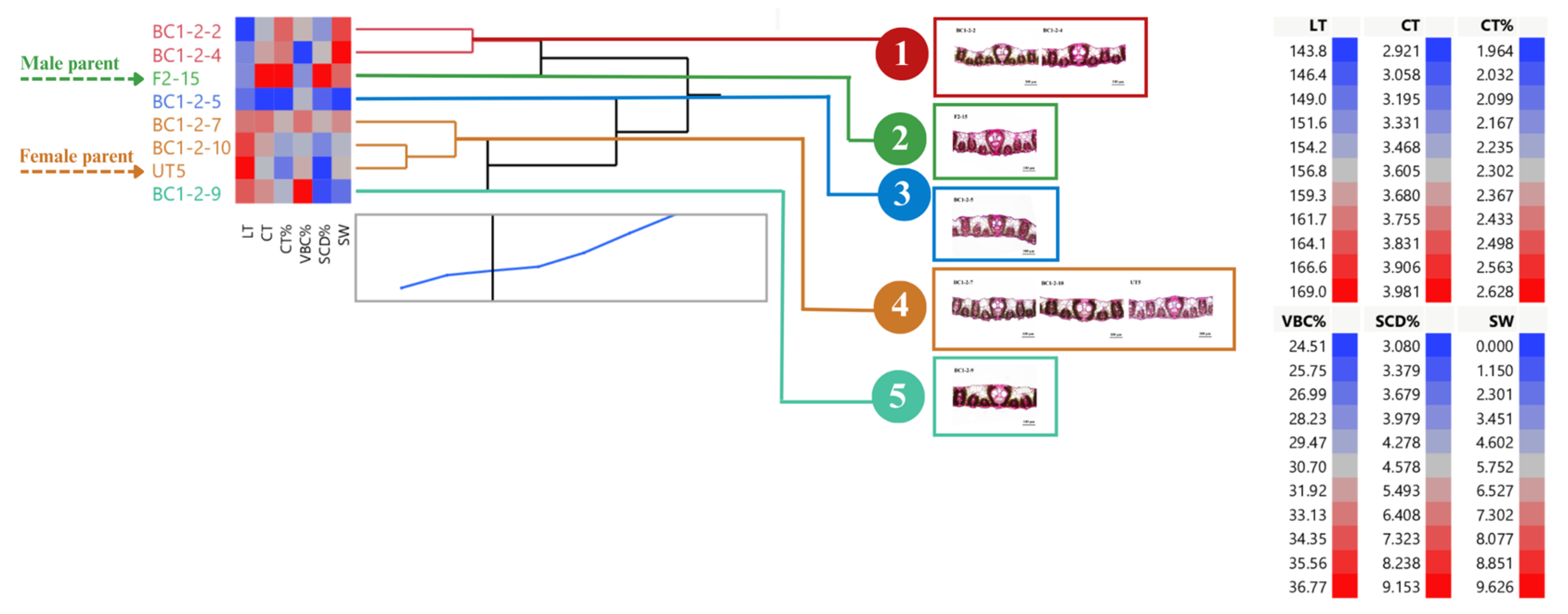
3.1. Distribution and Diversity of Sugarcane Interspecific Hybrid Backcross 1 (BC1) within Populations Regarding Leaf Anatomy
3.2. Performance of BC1 Clones on Stalk Weight and Leaf Anatomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budeguer, F.; Enrique, R.; Perera, M.F.; Racedo, J.; Castagnaro, A.P.; Noguera, A.S.; Welin, B. Genetic transformation of sugarcane, current status and future prospects. Front. Plant Sci. 2021, 12, 768609. [Google Scholar] [CrossRef]
- Mohan, C. Genome editing in sugarcane: Challenges ahead. Front. Plant Sci. 2016, 7, 1542. [Google Scholar] [CrossRef]
- UN Comtrade. World Cane Sugar Export’s Statistics. 2019. Available online: https://comtrade.un.org/data/ (accessed on 25 August 2019).
- Office of the Cane and Sugar Board. Production Report. 2019. Available online: http://www.ocsb.go.th/th/home/index.php (accessed on 20 February 2020).
- Pipitpukdee, S.; Attavanich, W.; Bejranonda, S. Climate Change Impacts on Sugarcane Production in Thailand. Atmosphere 2020, 11, 408. [Google Scholar] [CrossRef]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araújo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Hussain, B.; Khan, Z.; Ozturk, N.Z.; Ullah, N. From Genetics to Functional Genomics: Improvement in Drought Signaling and Tolerance in Wheat. Front. Plant Sci. 2015, 6, 1012. [Google Scholar] [CrossRef]
- Li, C.; Nong, Q.; Solanki, M.K.; Liang, Q.; Xie, J.; Liu, X.; Li, Y.; Wang, W.; Yang, L.; Li, Y. Differential expression profiles and pathways of genes in sugarcane leaf at elongation stage in response to drought stress. Sci. Rep. 2016, 6, 25698. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Filho, J.B.M.; Carena, M.J. Breeding Plans. In Quantitative Genetics in Maize Breeding, 3rd ed.; Carena, M.J., Hallauer, A.R., Filho, J.B.M., Eds.; Springer: New York, NY, USA, 2010; Volume 6, pp. 577–653. [Google Scholar] [CrossRef]
- Alwala, S.; Kimbeng, C.A.; Veremis, J.C.; Gravois, K.A. Identification of molecular markers associated with sugar-related traits in a Saccharum interspecific cross. Euphytica 2009, 167, 127–142. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Lin, Y.; Fu, C.; Liu, S.; Deng, Z. Unexpected inheritance pattern of Erianthus arundinaceus chromosomes in the intergeneric progeny between Saccharum spp. and Erianthus arundinaceus. PLoS ONE 2014, 9, e110390. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, X.; Chai, J.; Ding, X.; Li, X.; Huang, Y. Chromosome-specific painting unveils chromosomal fusions and distinct allopolyploid species in the Saccharum complex. New Phytol. 2022, 233, 1953–1965. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Khurshid, H.; Esh, A.; Wu, C.; Wang, Q.; Piperidis, N. Past and recent advances in sugarcane cytogenetics. Crop J. 2023, 11, 1–8. [Google Scholar] [CrossRef]
- Paterson, A.H.; Wang, X.; Li, J.; Tang, H. Ancient and recent polyploidy in monocots. In Polyploidy and Genome Evolution, 1st ed.; Soltis, P., Soltis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–108. [Google Scholar] [CrossRef]
- Bremer, G. Problems in breeding and cytology of sugar cane. Euphytica 1961, 10, 59–78. [Google Scholar] [CrossRef]
- Reyes-Valdés, M.H. A Model for Marker-Based Selection in Gene Introgression Breeding Programs. Crop Sci. 2000, 40, 91–98. [Google Scholar] [CrossRef]
- Chatterjee, J.; Dionora, J.; Elmido-Mabilangan, A.; Wanchana, S.; Thakur, V.; Bandyopadhyay, A.; Brar, D.S.; Quick, W.P. The evolutionary basis of naturally diverse rice leaves anatomy. PLoS ONE 2016, 11, e0164532. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Li, H.; Chang, C. Evolutionary insight of plant cuticle biosynthesis in bryophytes. Plant Signal. Behav. 2021, 16, 1943921. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The plant cuticle: Old challenges, new perspectives. J. Exp. Bot. 2017, 68, 5251–5255. [Google Scholar] [CrossRef]
- Zhou, X.; Jenks, M.A.; Liu, J.; Liu, A.; Zhang, X.; Xiang, J.; Zou, J.; Peng, Y.; Chen, X. Overexpression of transcription factor OsWR2 regulates wax and cutin biosynthesis in rice and enhances its tolerance to water deficit. Plant Mol. Biol. Rep. 2014, 32, 719–731. [Google Scholar] [CrossRef]
- Liu, L.L.; Deng, Y.Q.; Dong, X.X.; Wang, C.F.; Yuan, F.; Han, G.L.; Wang, B.S. ALDH2C4 regulates cuticle thickness and reduces water loss to promote drought tolerance. Plant Sci. 2022, 323, 111405. [Google Scholar] [CrossRef]
- Li, L.; Shi, Z.Y.; Li, L.; Shen, G.Z.; Wang, X.Q.; An, L.S.; Zhang, J.L. Overexpression of ACL1 (abaxially curled leaf 1) increased bulliform cells and induced abaxial curling of leaf blades in rice. Mol. Plant. 2010, 3, 807–817. [Google Scholar] [CrossRef]
- Matschi, S.; Vasquez, M.F.; Bourgault, R.; Steinbach, P.; Chamness, J.; Kaczmar, N.; Gore, M.A.; Molina, I.; Smith, L.G. Structure-function analysis of the maize bulliform cell cuticle and its potential role in dehydration and leaf rolling. Plant Direct. 2020, 4, e00282. [Google Scholar] [CrossRef]
- Latif, A.; Ying, S.; Cuixia, P.; Ali, N. Rice curled its leaves either adaxially or abaxially to combat drought stress. Rice Sci. 2023, 30, 405–416. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A.; Hassiotou, F.; Veneklaas, E.J. Stomatal crypts have small effects on transpiration: A numerical model analysis. Plant Physiol. 2009, 151, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.R.; Cui, H.; Callahan, H.; Scoffoni, C.; John, G.P.; Bartlett, M.K.; Burge, D.O.; Sack, L. Evolution of leaf structure and drought tolerance in species of Californian Ceanothus. Am. J. Bot. 2018, 105, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Taratima, W.; Ritmaha, T.; Jongrungklang, N.; Maneerattanarungroj, P.; Kunpratum, N. Effect of stress on the leaf anatomy of sugarcane cultivars with different drought tolerance (Saccharum officinarum, Poaceae). Rev. Biol. Trop. 2020, 68, 1159–1170. [Google Scholar] [CrossRef]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Cenchrus ciliaris L., from the Salt Range, Pakistan against drought stress. Pak. J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Jumkudling, S.; Songsri, P.; Taratima, W.; Jongrungklang, N. Diversity and distribution of anatomical characteristics involved with drought resistance of inter-specific (Saccharum spp. hybrid × S. spontaneum) sugarcane F1 hybrid population. Sugar Tech 2022, 24, 1342–1356. [Google Scholar] [CrossRef]
- Dermail, A.; Suriharn, B.; Chankaew, S.; Sanitchon, J.; Lertrat, K. Hybrid prediction based on SSR-genetic distance, heterosis and combining ability on agronomic traits and yields in sweet and waxy corn. Sci. Hortic. 2020, 259, 108817. [Google Scholar] [CrossRef]
- Dermail, A.; Lübberstedt, T.; Suwarno, W.B.; Chankaew, S.; Lertrat, K.; Ruanjaichon, V.; Suriharn, K. Combining ability of tropical × temperate maize inducers for haploid induction rate, R1-nj seed set, and agronomic traits. Front. Plant Sci. 2023, 14, 1154905. [Google Scholar] [CrossRef]
- Trentin, H.U.; Yavuz, R.; Dermail, A.; Frei, U.K.; Dutta, S.; Lübberstedt, T. A Comparison between Inbred and Hybrid Maize Haploid Inducers. Plants 2023, 12, 1095. [Google Scholar] [CrossRef]
- Mendes de Paula, T.O.; Brasileiro, B.P.; Cursi, D.E.; Freitas, E.G.; dos Santos, J.M.; Resende, M.D.V.; Kimbeng, C.; Pereira Barbosa, M.H. Establishment of gene pools for systematic heterosis exploitation in sugarcane breeding. Agron. J. 2020, 112, 3847–3858. [Google Scholar] [CrossRef]
- Khonghintaisong, J.; Songsri, P.; Jongrungklang, N. Understanding growth rate patterns among different drought resistant sugarcane cultivars during plant and ratoon crops encountered water deficit at early growth stage under natural field conditions. Agronomy 2021, 11, 2083. [Google Scholar] [CrossRef]
- White, W.H.; Viator, R.P.; Dufrene, E.O.; Dalley, C.D.; Richard, E.P.; Tew, T.L. Re-evaluation of sugarcane borer (Lepidoptera: Crambidae) bioeconomics in Louisiana. Crop Prot. 2008, 27, 1256–1261. [Google Scholar] [CrossRef]
- Sobhakumari, V.P.; Mathew, S.D. Effect of Hybrid Vigor on Callus Induction and Regeneration of Sugarcane. Cytologia 2009, 74, 71–77. [Google Scholar] [CrossRef]
- D’Hont, A.; Ison, D.; Alix, K.; Roux, C.; Glaszmann, J.C. Determination of basic chromosome numbers in the genus Saccharum by physical mapping of ribosomal RNA genes. Genome 1998, 41, 221–225. [Google Scholar] [CrossRef]
- Feng, M.; Zhao, J.; Li, S.; Wei, N.; Kuang, B.; Yang, X. Molecular genetic mechanisms of heterosis in sugarcane cultivars using a comparative transcriptome analysis of hybrids and ancestral parents. Agronomy 2023, 13, 348. [Google Scholar] [CrossRef]
- Garsmeur, O.; Droc, G.; Antonise, R.; Grimwood, J.; Potier, B.; Aitken, K.; Jenkins, J.; Martin, G.; Charron, C.; Hervouet, C.; et al. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nat. Commun. 2018, 9, 2638. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, A.K.; Singh, A. Wide hybridization. In Plant Breeding and Cultivar Development, 3rd ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 159–178. [Google Scholar] [CrossRef]
- Cuadrado, A.; Acevedo, R.; Moreno Diaz De La Espina, S.; Jouve, N.; De La Torre, C. Genome remodelling in three modern S. officinarum × S. spontaneum sugarcane cultivars. J. Exp. Bot. 2004, 55, 847–854. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Han, J.; Zhang, Y.; Ma, S.; Yu, G.; Wang, Z.; Wang, K. Species-specific abundant retrotransposons elucidate the genomic composition of modern sugarcane cultivars. Chromosoma 2020, 129, 45–55. [Google Scholar] [CrossRef]
- Ming, R.; Liu, S.C.; Moore, P.H.; Irvine, J.E.; Paterson, A.H. QTL analysis in a complex autopolyploid: Genetic control of sugar content in sugarcane. Genome Res. 2001, 11, 2075–2084. [Google Scholar] [CrossRef]
- Zhou, M. General and specific combining ability for cane yield and implications for sugarcane breeding. Crop Sci. 2021, 61, 539–551. [Google Scholar] [CrossRef]
- Verma, P.S.; Singh, S.B. Heterosis in relation to per-se performance and effects of general combining ability in sugarcane. Sugar Tech 2004, 6, 181–185. [Google Scholar] [CrossRef]
- McIntyre, C.; Jackson, P. Low level of selfing found in a sample of crosses in Australian sugarcane breeding programs. Euphytica 2001, 117, 245–249. [Google Scholar] [CrossRef]
- Zhang, F.J.; Zhang, K.K.; Du, C.Z.; Li, J.; Xing, Y.X.; Yang, L.T.; Li, Y.R. Effect of drought stress on anatomical structure and chloroplast ultrastructure in leaves of sugarcane. Sugar Tech 2015, 17, 41–48. [Google Scholar] [CrossRef]
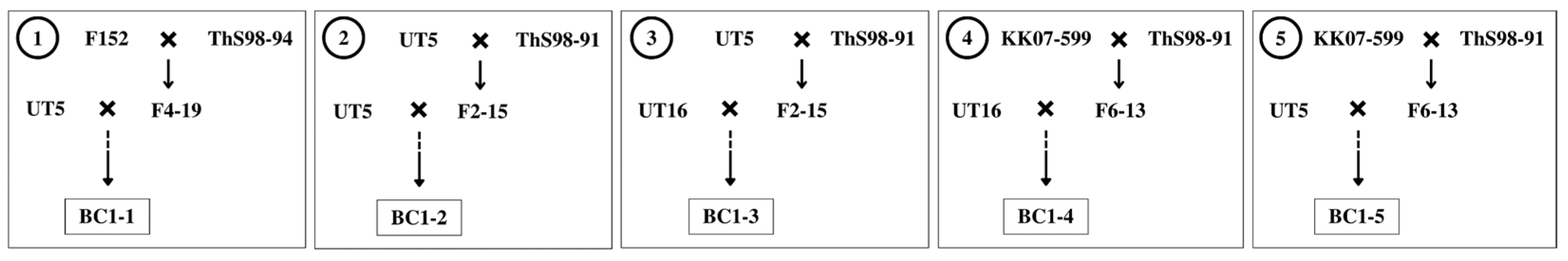
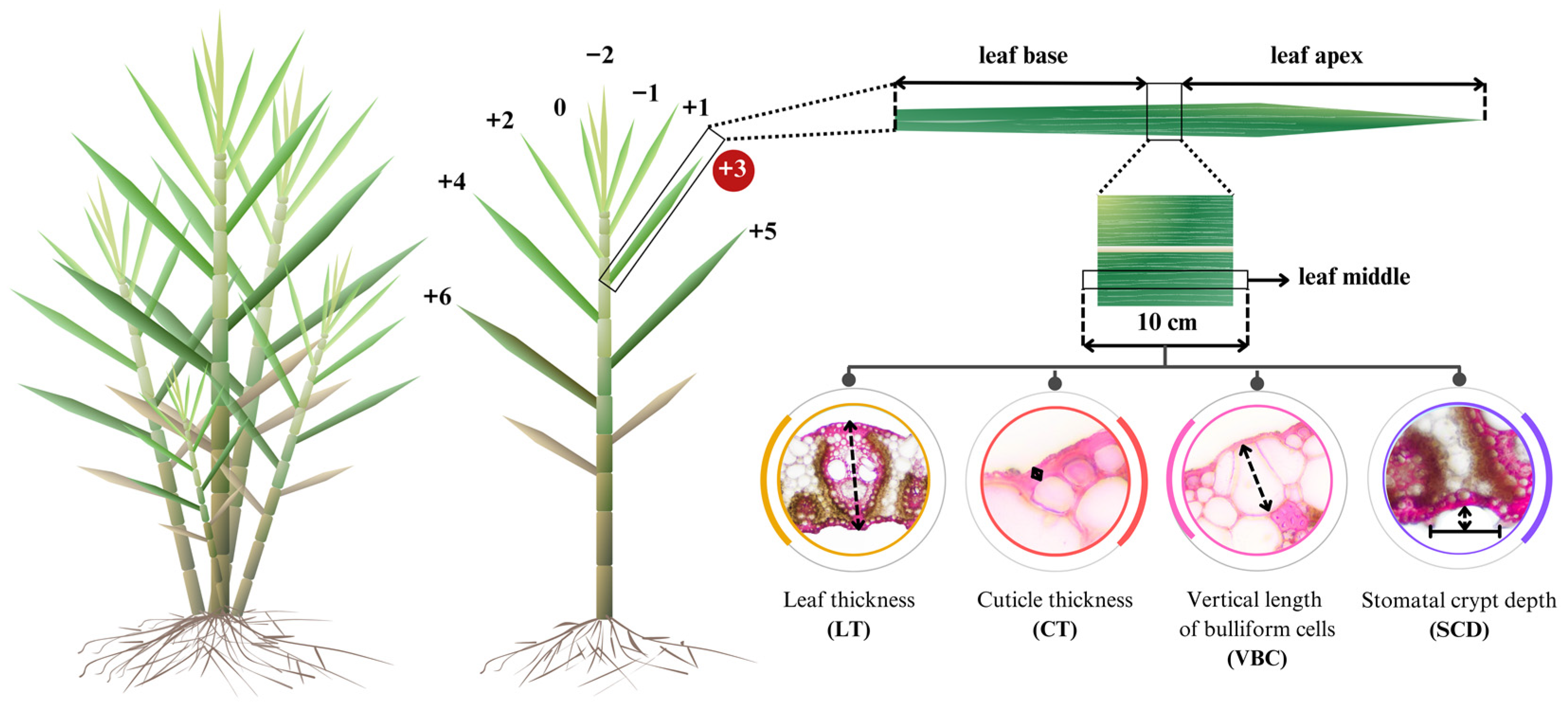
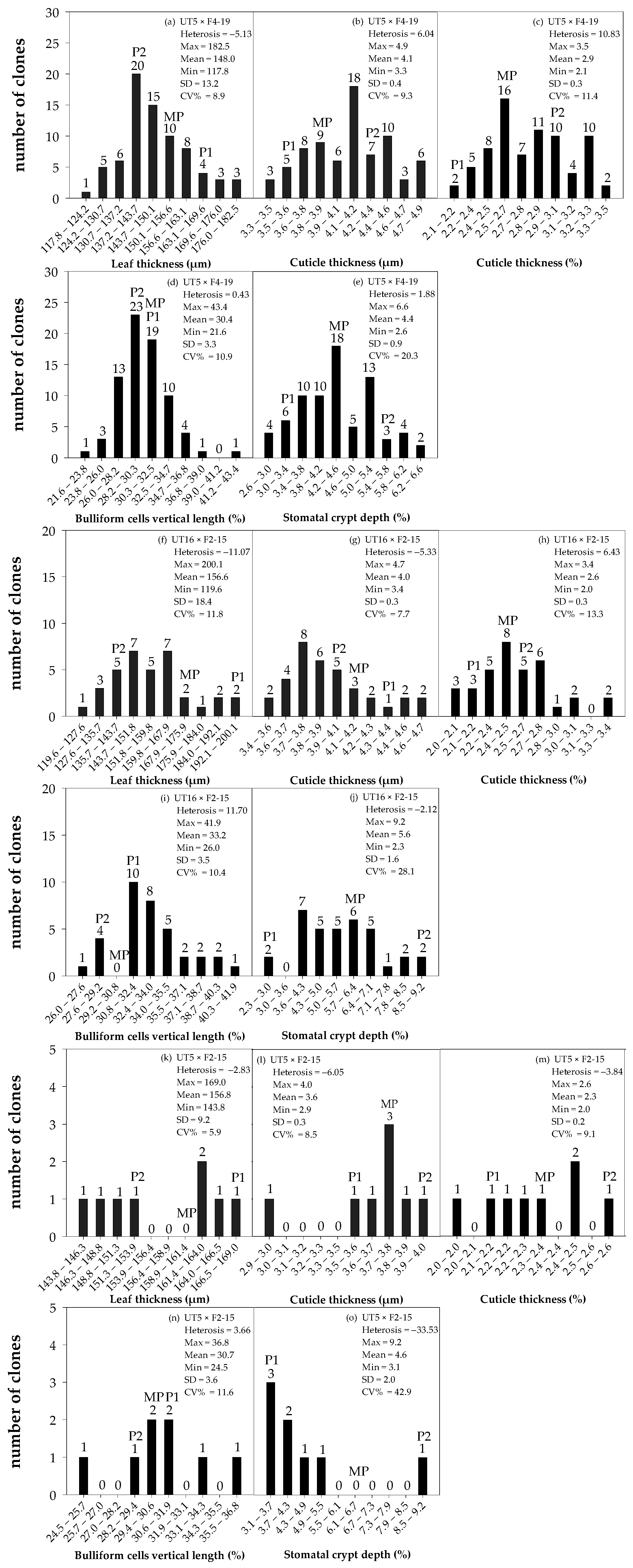
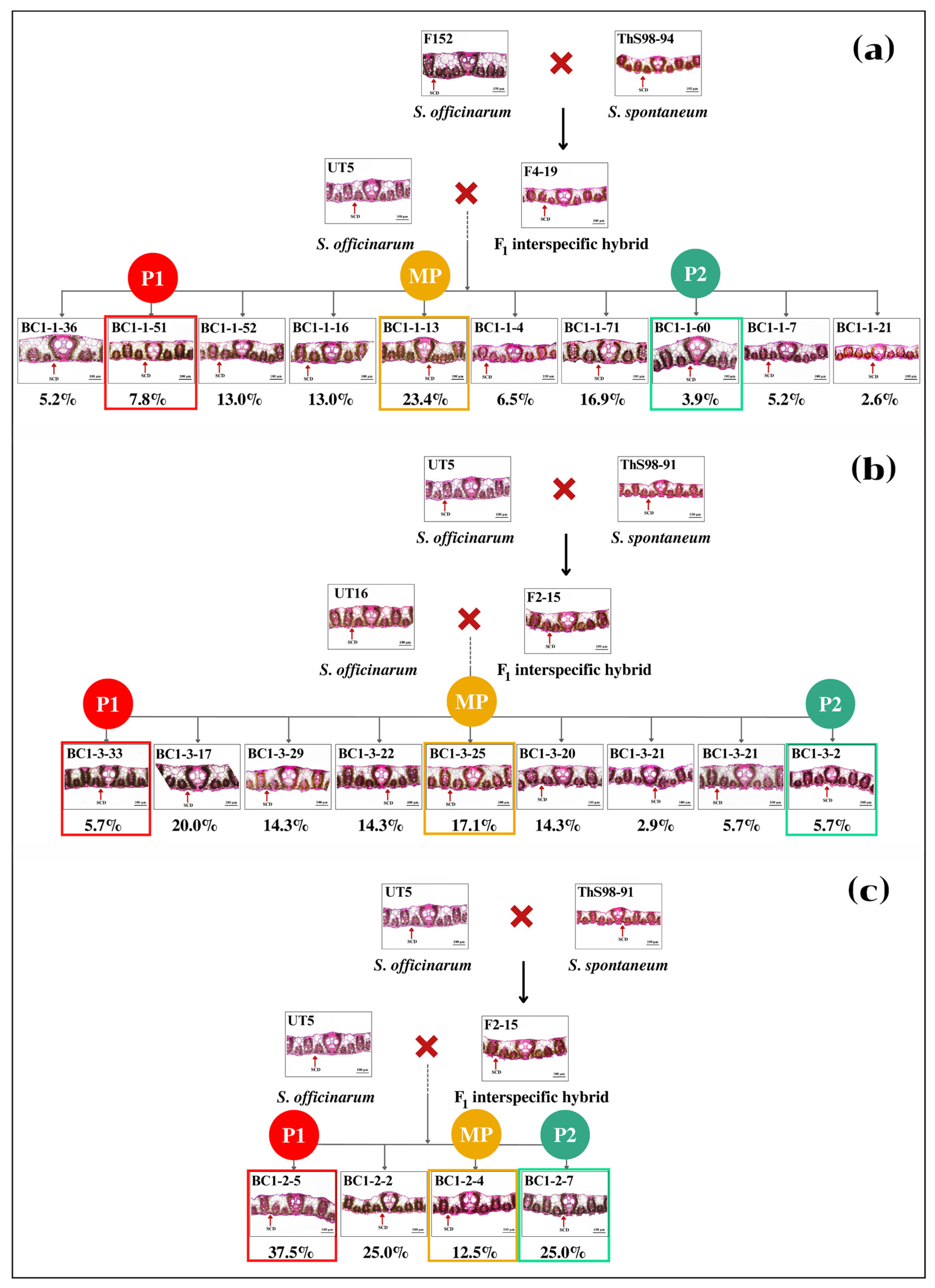
| Entry | Genotype | Source | Phenotype |
|---|---|---|---|
| 1 | UT5 | 87-2-1033 op. | upright clump shape, high sweetness, small stalk diameter |
| 2 | UT16 | 03-2-395 op. | high sweetness, large stalk diameter |
| 3 | F4-19 | F152 × ThS98-94 | high fiber |
| 4 | F2-15 | UT5 × ThS98-91 | high fiber |
| 5 | F6-13 | KK07-599 × ThS98-91 | high fiber |
| 6 | F152 | H3-8560 × PT 43–52 | upright clump shape, high sweetness, small stalk diameter, small number of stalks per clump |
| 7 | KK07-599 | 90-2-043 × UT6 | upright clump shape, high sweetness, large stalk diameter, high yield, low tillering |
| 8 | ThS98-91 | S. spontaneum | high fiber, high number of tillers and stalks per clump, low sweetness, tiny stalk diameter |
| 9 | ThS98-94 | S. spontaneum | high fiber, high number of tillers and stalks per clump, low sweetness, tiny stalk diameter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiangwiset, K.; Dermail, A.; Piwpuan, N.; Songsri, P.; Jongrungklang, N. Diversity and Heterosis of Leaf Anatomical Traits in Backcross 1 (BC1) Derived from Interspecific Hybridization between Commercial Cane (Saccharum spp. Hybrid) and Wild Type (S. spontaneum). Agronomy 2023, 13, 2457. https://doi.org/10.3390/agronomy13102457
Wiangwiset K, Dermail A, Piwpuan N, Songsri P, Jongrungklang N. Diversity and Heterosis of Leaf Anatomical Traits in Backcross 1 (BC1) Derived from Interspecific Hybridization between Commercial Cane (Saccharum spp. Hybrid) and Wild Type (S. spontaneum). Agronomy. 2023; 13(10):2457. https://doi.org/10.3390/agronomy13102457
Chicago/Turabian StyleWiangwiset, Kanlayanee, Abil Dermail, Narumol Piwpuan, Patcharin Songsri, and Nakorn Jongrungklang. 2023. "Diversity and Heterosis of Leaf Anatomical Traits in Backcross 1 (BC1) Derived from Interspecific Hybridization between Commercial Cane (Saccharum spp. Hybrid) and Wild Type (S. spontaneum)" Agronomy 13, no. 10: 2457. https://doi.org/10.3390/agronomy13102457
APA StyleWiangwiset, K., Dermail, A., Piwpuan, N., Songsri, P., & Jongrungklang, N. (2023). Diversity and Heterosis of Leaf Anatomical Traits in Backcross 1 (BC1) Derived from Interspecific Hybridization between Commercial Cane (Saccharum spp. Hybrid) and Wild Type (S. spontaneum). Agronomy, 13(10), 2457. https://doi.org/10.3390/agronomy13102457









