1. Introduction
Economic loss caused by insects is one of the main problems in the postharvest storage of commodities. Prevention measures are far more crucial in the framework of integrated pest management (IPM) for products that are stored than curative measures since the arthropods that die from the utilization of curative treatments and stay in the stored products continue to pose a phytosanitary issue. Indian meal moth,
Plodia interpunctella Hübner (Lepidoptera: Pyralidae) is one of the most important pests of stored products, causing direct (qualitative and quantitative) product losses and indirect economic costs through pesticide inputs and consumer constraints [
1,
2]. A number of comprehensive studies have referred to
P. interpunctella polyphagia as a major pest of raw and processed human and animal food like grain, legume and oil seeds, dried fruits and nuts, soy bean meal commodities, garlic bulbs, and a diversity of stored confectionary products (pasta, cookies, bread, chocolate, etc.) [
3,
4,
5]. When feeding on seeds, the larvae prefer the germ [
6,
7], which makes this pest particularly harmful for many categories of seeds [
4]. Even though sunflower seeds are considered resilient to storage pests they have an easily cracked shell, which makes them susceptible to secondary storage pests including
P. interpunctella [
4].
Management of
P. interpunctella is shifting from an insecticide-based system to an integrated approach, especially for the protection of sunflower seeds (the pollinator’s side effects), due to a severe ban on conventional insecticides. Directive 2009/128/EC of the EU Commission refers to the sustainable use of pesticides, aiming to restrict the use of synthetic products and promote the use of alternative, environmentally friendly strategies in pest management. Natural plant-derived products, or botanicals, represent a potent, safe, effective, eco-friendly alternative to conventional pesticides [
8,
9,
10,
11]. Plants make up a large reservoir of chemical structures with pesticidal activity due to the presence of naturally occurring secondary metabolites, which are a part of their self-defense system [
12,
13,
14]. The biological activity of plant-based compounds against insects (insecticidal, antifeedant, repellent) has been widely reported in the literature and is a research area that is gaining in relevance. In the first decades of the 21st century, there has been an expansion in the scientific productivity of entomologists and related scientists in identifying new plant materials that are biologically active against “old” and common storage pests [
15]. However, only a few commercially available plant-based insecticides for the control of postharvest pests have a wider application potential [
16,
17]. A move toward “green chemistry” and a constant need for developing new crop protection tools with novel modes of action make the discovery and commercialization of botanical products an attractive and profitable pursuit for new bioactive compounds [
17].
The protection of high-value seeds is crucial for plant production. However, the majority of conventional pesticides that served as sunflower seed protectants are now banned, which mandates the development of new bio-rational preparations. The number of studies related to the potential of botanicals as seed protectants is increasing [
15]. However, prior to their use, it is necessary to test their influence on germination, vitality, and vigor—the phytotoxic effects. Li et al. [
18] referred to allelopathy, an ecological mechanism in which plants provide themselves with a competitive advantage through biochemical pathways. Plants can influence the growth and development of other plant species in an inhibitory or stimulatory way through the release of secondary metabolites into the environment [
18]. These compounds can be released by root exudation, leaching, volatilization, and decomposition of plant organs in the soil [
19,
20]. Secondary metabolites with allelopathic potential (allelochemicals) directly stimulate or inhibit metabolic processes in other plants or indirectly interfere with soil microorganisms, nutrient cycling dynamics and availability. In agricultural production the influence of allelopathy has been demonstrated between crops, between crops and weeds, but also between weeds and weeds [
21]; thus, allelochemicals can successfully be used as bioherbicides [
19].
Hemp (
Cannabis sativa L.) has been cultivated for thousands of years and is one of the oldest crop plants known to humans [
22]. It has a wide range of applications in the production of fiber, ropes, textiles, paper, oils, construction materials, and for bioremediation, etc. [
23,
24]. Hemp seeds are a good source of essential polyunsaturated fatty acids and highly digestible proteins (a rich source of all essential amino acids), and the whole plant (especially the inflorescence) is rich in secondary metabolite compounds such as phytocannabinoids, terpenoids, and phenylpropanoids [
24,
25]. The most important phytocannabinoids in industrial hemp are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which, in synergy with the hemp essential oil compounds
α-pinene, D-limonene, and linalool, express anti-inflammatory, antibacterial, antidepressant, immunostimulative, sedative, locally anesthetic, and anticonvulsant activities [
24]. Additionally, hemp is reported to express insecticidal and repellent activity toward hematophagous and phytophagous insect pests. The most comprehensive report on the biological activity of hemp was presented by McPartland [
26]. The author studied hemp as a pest repellent and a pesticide in a variety of formulations and as a companion crop to deter insects, nematodes, fungi, and weedy plants, while pure cannabinoids reportedly inhibited or killed bacteria, fungi, and insects. Several more studies showed that industrial hemp could be used in pest control (pathogens, invertebrates, and weeds) and also could have inhibitory or stimulatory allelopathic effects on some field crops [
27,
28,
29]. However, there are no findings related to the bioactive potential of ethanolic hemp extract either against
P. interpunctella or on its effect on sunflower seeds. Literature references to the effects of industrial hemp extracts on beneficial or pest insects and other plant species are also very limited. Therefore, the aims of the present study were to (i) evaluate the bioactivity of industrial hemp extract against
P. interpunctella and (ii) to determine its phytotoxic effect on sunflower seeds, in order to determine if it can be used as a grain protectant. To achieve this goal, the chemical composition of industrial hemp extract was analyzed, the biological activity on
P. interpunctella was assessed in different bioassays (repellency, fumigant, contact and contact-digestive toxicity, effect on insects’ development), and the phytotoxic activity of the extract, based on the physiological and biochemical parameters of sunflower seedlings, was evaluated.
2. Materials and Methods
The analysis was performed on the industrial (fiber) hemp variety ‘Helena’ supplied by the Alternative Crops Department, Institute of Field and Vegetable Crops, Novi Sad, Serbia. Plants used for the analysis were grown in soilless culture in peat-growing media and harvested in the flowering phase, and only flowering tops were used. Industrial hemp flowering tops were air-dried at 40 °C till constant weight and then ground to a fine powder using a laboratory mill. The powder (1 g) was extracted with three organic solvents, 50 mL of 70% (v/v) methanol, 70% (v/v) ethanol, or 70% (v/v) acetone, during 24 h in the dark, stirring periodically using a sterile rod. All solvents were produced by CARLO ERBA Reagents GmbH (Milan, Italy). The extracts were centrifuged, vacuum-filtered through the sintered glass funnel, and kept refrigerated until the analysis. The chemical analysis encompassed the determination of the content of total phenols, total tannins, total flavonoids, and several antioxidant tests. All spectrophotometric analyses (Thermo Scientific Evolution 220 spectrophotometer, Madison, WI, USA) were performed in triplicate.
2.1. Chemical Analysis of Industrial Hemp Extract
Phenolic compounds: The total phenolic (TP) and total tannin (TT) contents were determined using the Folin–Ciocalteu colorimetric method [
30]. Total phenols were determined at 720 nm using a UV/VIS spectrophotometer. The TT content was determined after the removal of tannins by their adsorption on an insoluble PVPP (polyvinylpolypyrrolidone) matrix. The calculated values were subtracted from the TP content by measuring the absorbance of the reaction mixture at 720 nm using a UV/VIS spectrophotometer [
30]. The total flavonoids (TFs) were estimated according to the method described by Saha et al. [
31] at 415 nm using a spectrophotometer against a blank. The data for TP, TT, and TF were expressed as mg quercetin equivalents/g dry weight (mg QE/g).
Antioxidant tests: Scavenging of free radicals was tested in a DPPH (2,2-diphenyl-1-picrylhydrazyl) acetone solution at 517 nm [
32]. The ferric-reducing antioxidant power (FRAP) assay was carried out according to the procedure described by Spiegel et al. [
33]. The ABTS (2,2′azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt) assay was based on a method described by Kalinowska et al. [
34] by measuring the absorbance of the reaction mixture at 734 nm. A reducing power assay (total reduction capacity, TRC) was performed using the method of Saha et al. [
31] and absorbance was measured at 700 nm. The reduction of molybdenum (VI) to molybdenum (V) by the plant extracts was used to assess the total antioxidant activity (TAA) following the method described by Kalaskar and Surana [
35]. The standard curve for antioxidant tests (DPPH, FRAP, ABTS, TRC, and TAA) was plotted using trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) solution, and the results are expressed as mg trolox equivalents per g of the dry plant material (mg TE/g).
The nitro blue tetrazolium (NBT) test or superoxide dismutase-mimetic (SOD-mimetic) activity was assayed according to the slightly modified method of Kalaskar and Surana [
35] by measuring its ability to inhibit the photochemical reduction of NBT chloride. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.8), 75 μM NBT, 0.1 mM EDTA, 13 μM L-methionine, 2 μM riboflavin, and 30 μL of the enzyme extract. It was kept under a fluorescent lamp for 10 min, and then the absorbance was read at 560 nm. One unit of the SOD activity was defined as the amount of enzyme required to inhibit the reduction of NBT by 50%. The activity of the enzyme is expressed as IU SOD/g dry plant material. All chemicals used for chemical analysis were produced by Merck (Darmstad, Germany).
Phenolic compounds were also analyzed by the rapid resolution High-Performance Liquid Chromatography
(HPLC/DAD) method [
36], on an Eclipse XDB-C18 1.8 μm, 4.6 × 50 mm column Agilent 1200 series device (Agilent Technologies, Santa Clara, CA, USA) with a diode array detector (DAD). The binary mobile phase consisting of A (methanol) and B (1% formic acid in water (
v/v)) at the flow rate of 1.0 mL/min was used. Gradient elution was applied to efficiently separate the compounds: starting with 10% A; 0–10 min, 10–25% A; 10–20 min, 25–60% A; 20–30 min, 60–70% A. The injection volume was 5 μL and the column temperature was 30 °C. External calibration was applied to calculate the concentration of each phenolic compound.
2.2. Rearing of Plodia interpunctella and Biological Activity Assays
The
P. interpunctella culture originated from the laboratory population reared for ~50 generations at the Institute of Field and Vegetable Crops, Novi Sad, Serbia. Insects were reared in a thermostat chamber at 28 ± 1 °C, relative humidity (RH) 60 ± 5%, and a photoperiod of 14:10 (L:D) on a standard laboratory diet (SLD), according to Silhacek and Miller [
37]. The SLD consisted of ground dog meal (10%), rolled oats (4%), white cornmeal (26%), whole wheat flour (23%), wheat germ (2%), brewers’ yeast (5%), glycerol (16%), and honey (14%). One hundred (100) pairs of one-day-old adult males and females in copuli were isolated with an entomological aspirator from the containers for mass rearing and placed into empty 1 L glass jars where the females laid eggs. Before the experiment, the eggs were observed under a binocular microscope to eliminate those with obvious deformities. Only undamaged one-day-old eggs were used.
The biological activity of industrial hemp ethanolic extract was assessed in fumigant, contact, contact-digestive, and repellency tests. Also, the effect of the extract on the insects’ development was monitored. Acetone has been reported to cause significant mortality in the eggs, larvae, and adults of different insect species while ethanol is less harmful to test organisms, and therefore it was further used in the present study.
The fumigant activity test was performed according to Brito et al. [
38] in glass vials (10 mL) with slight modifications. Filter paper cuts (ø 5 mm diameter) treated with 1 µL of the test solution were placed under the vial cover and covered with a fine mesh so the insects did not come in contact with the treated paper. Ten larvae (L
3–4) were put inside the vial and the cap was closed. Mortality was recorded after 1, 2, 6, 12, and 24 h. The experiment was performed in six replicates.
The contact toxicity was assessed according to a method described by Kouninki et al. [
39] with slight modifications. In glass tubes, previously “rinsed” with plant extracts and air-dried, 10 larvae (L
3–4) were inserted. The tubes were sealed with parafilm and placed in a horizontal position. The mortality was recorded after 24, 48, and 72 h of exposure and expressed as % (percent) of dead and paralyzed larvae out of the total number. The experiment was performed in six replicates.
The contact-digestive test was set as a “No-choice” test, according to Obeng-Ofori et al. [
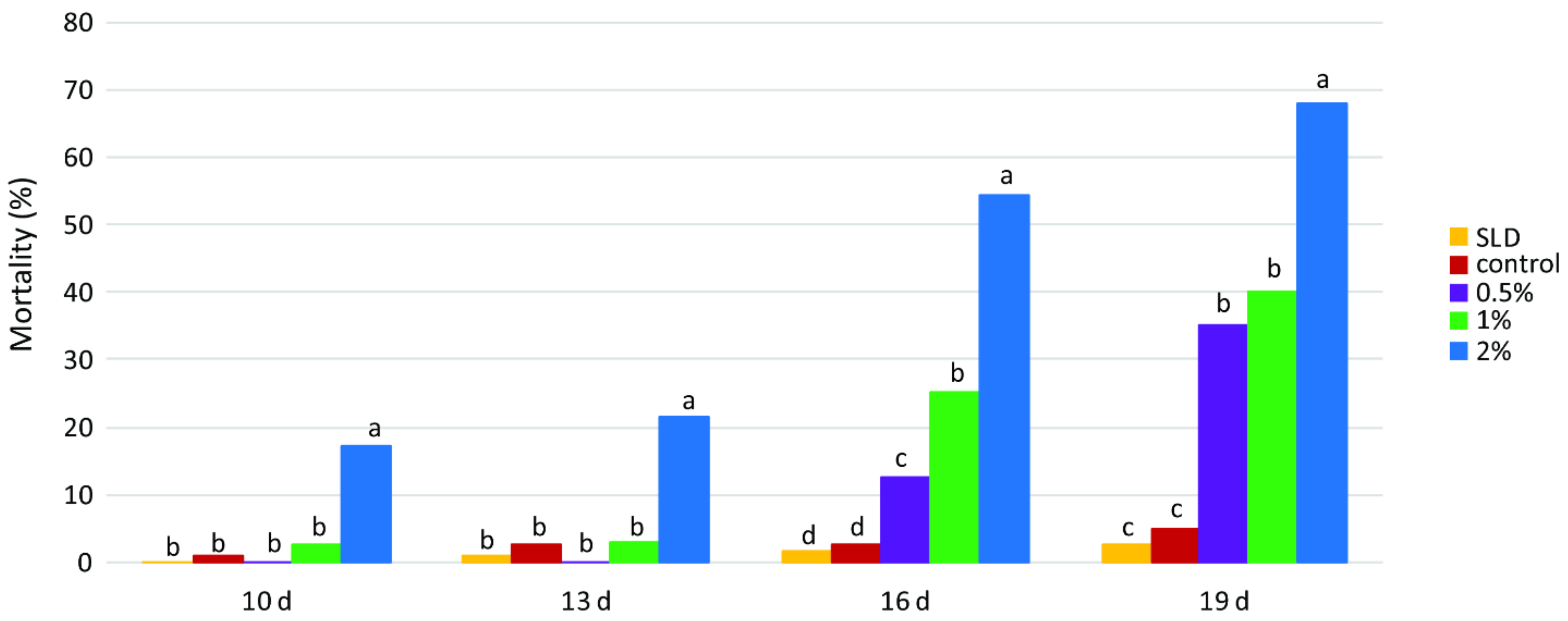
40]. A total of 100 g of sunflower seeds were treated with the ethanol extract of industrial hemp (0.5%, 1%, and 2%) and ethanol (72%) as the control, at a rate of 1 mL of extract per 100 g of seeds. The treated kernels were left to air dry for 2 h at room temperature, and after drying, the amount was divided into four equal portions representing four replicates (25 g of grains per replicate) and placed in Petri dishes along with 50 one-day-old eggs. The mortality was evaluated every three days starting from day 10, when the young larvae became visible, until the last larva pupated.
The effect of hemp extract on P. interpunctella development was assessed based on the following parameters: mean developmental duration (MDD, duration from egg to adult), number of emerged moths, female fecundity, and moth lifespan. The developmental parameters were assessed for the specimens from the contact-digestive test. After the adults emerged, young moths from the same experimental group were paired (4 pairs from each group, where possible) and isolated in separate glass tubes to copulate. Each day, the number of laid eggs per pair was counted, aiming to determine the fecundity, defined as the total number of laid eggs per female. After the copulation and oviposition, moth pairs were monitored to determine the lifespan of each specimen. The number of dead moths was counted every 24 h. The experiment was set in four replicates.
The repellency of the hemp extract was evaluated using a Y-tube olfactometer. Filter paper cuts were treated with 1 µL of the test solutions and ethanol as the control. The treated and control cuts were air-dried under laboratory conditions and placed in a glass vial attached to the end of each olfactometer arm. Ten
P. interpunctella larvae (L
3–4) were introduced at the central arm and sealed with parafilm. Each treatment was replicated four times, and the number of present larvae in each arm was recorded after 2, 4, 6, and 12 h. The repellency was interpreted based on the Repellency Index (RI; Equation (1)) calculated as follows:
where RI = repellency index; C = number of insects present on the control half-disk; T = number of insects present on the treated half-disk.
Based on the RI, repellent activity was categorized as:
RI < 20%—no repellency (–),
50% > RI ≥ 20%—slight repellency (+),
70% > RI ≥ 50%—medium repellency (++),
RI ≥ 70%—strong repellency (+++).
2.3. Germination Energy and Germination
Germination energy (GE, %) and germination (G, %) were determined in a standard laboratory test recommended by the International Seed Testing Association-ISTA [
41]. Filter paper was used as a substrate. On the filter paper moistened with distilled water, 100 treated sunflower seeds per replicate were placed. The seeds were treated with ethanolic hemp extracts in Erlenmeyer glass (1 mL of extract per 100 g of seeds) and placed on a rotary shaker to provide uniform coverage of each seed. After the treatment, the seeds were left to air dry at a room temperature. The filter paper with seeds was incubated in a climate chamber at 25 ± 1 °C, relative humidity 95%, at constant light. The GE was recorded after 4 days and G after 10 days. The experiment was set in four replicates.
2.4. Biochemical Analysis of Seedlings
Phenolic compounds and antioxidant capacity: Five grams of whole sunflower seedlings was homogenized with 10 mL of 70% methanol solution. After 24 h, seedling extracts were centrifuged and filtered. The extracts were used for determination of TP and TT content and antioxidant capacity with three different assays (DPPH, ABTS, and FRAP) according to the procedures described previously for determination of these parameters in the dry material of industrial hemp.
Enzyme extraction: Fresh plant material (2 g) of whole sunflower seedlings was crushed and homogenized in 10 mL of phosphate buffer (0.1 M, pH 7.0). Homogenates were centrifuged for 20 min at 10,000×
g and then filtered. The clear supernatants were used for biochemical assays, for the determination of oxidative stress parameters, the activity of antioxidative enzymes, the activity of enzymes of phenolic metabolism, and the intensity of lipid peroxidation. Protein content in homogenates was determined using bovine serum albumin as a protein standard [
42]. Lipid peroxidation (LP) was measured at 532 nm using the thiobarbituric acid (TBA) test [
43]. The total amount of TBA-reactive substances was given as nmol malondialdehyde equivalents (MDA)/mg protein. Catalase (CAT) (EC 1.11.1.6) activity was determined according to Sarker and Oba [
44]. The decomposition of H
2O
2 was noted as a decrease in absorbance at 240 nm. Superoxide dismutase (SOD) (EC 1.15.1.1) activity was assayed according to the method of Mandal et al. [
43] by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT) chloride. Peroxidase (EC 1.11.1.7) activity was measured at 436 nm using pyrogallol (pyrogallol peroxidase-POD) as substrates according to Morkunas and Gmerek [
45] with modifications [
46]. Phenylalanine ammonia lyase (PAL) (EC 4.3.1.24) activity was measured according to Şirin et al. [
47] by measuring the absorbance of
trans-cinnamic acid at 290 nm. Polyphenol oxidase (PPO) (EC 1.10.3.1) activity was determined at 420 nm as by Xylia et al. [
48]. The activity of the enzymes is expressed in the international units (IU)/mg of proteins. Chemicals used for biochemical analysis of seedlings were produced by Merck (Darmstad, Germany).
2.5. Statistical Analysis
Results were first tested with an ANOVA and when significant differences existed between treatments, the post hoc test was applied. Data were processed using one-way ANOVA, to test the differences in the content of phenolic compounds and antioxidant capacity depending on the solvent; parameters were monitored in biological activity assays (larval mortality, MDD, moth emergence, fecundity, and moth longevity), and Duncan’s multiple range test was applied; differences between GE and G (transformed to arcsine prior to statistical analysis) and differences among the content of phenolic compounds, antioxidant activity, enzyme activity, and the intensity of lipid peroxidation in sunflower seedlings, depending on the treatment (extract concentration) were measured. All tests were performed at the level of significance of 95% using the statistical software SPSS 17, Version 17.
4. Discussion
This work aimed to assess the biological activity of industrial hemp (variety Helena) extract on
P. interpunctella, in regard to the biochemical composition of the extract, as well as its influence on sunflower seed vigor parameters (GE and G) and the antioxidative stress of seedlings. The problems concerning the use of synthetic chemicals for insect pest management have imposed an urgent need for the introduction of natural, plant-derived insecticides in plant protection programs [
11], and industrial hemp can be a promising candidate [
28]. Recent investigations have demonstrated that the application of different hemp products (hemp oil, hempseed oil, or hempseed cake) improves the performance and bone strength of animals, increases milk yield, and enriches egg fatty acid profiles. Cannabidiol use as a non-psychoactive compound manifested positive results in alleviating and/or preventing pain, oxidation, inflammation processes, and anxiety in different animal species and in humans [
49].
Recently, there has been a growing interest in hemp (
Cannabis sativa L.; Rosids: Cannabaceae) and its extracts for medicinal and commercial purposes [
50]. Hemp represents a rich source of different bioactive compounds, as proven previously in a number of studies. According to the available literature, the most well-known bioactive substances belonging to the class of polyphenols synthesized in the hemp plant are flavones: orientin, vitexin, luteolin, and apigenin [
24,
51], as well as the specific prenyl-flavones canflavin A and B, which are luteolin derivatives [
52]. In general, plant flavones play a role in regulating auxin transport, as well as various roles in plant–pathogen interaction, UV protection, and plant pigmentation [
53]. As reported by Moore et al. [
54], terpenoids belong to a class of compounds that includes both hydrocarbon terpenes and their oxygenated derivatives. Hemp plants biosynthesize about 140 terpenoids, mostly monoterpenoids (C
10H
16 templates) and sesquiterpenoids (C
15H
24 templates). Three terpenoids produced by hemp plants, limonene, linalool, and pinene, are marketed as insecticides [
54]. Collectively, terpenoids constitute the plant’s essential oil (EO, also known as volatile oil). Other classes of natural products are minor constituents of hemp, such as flavonoids (quercetin, apigenin, orientin, kaempferol, canniflavone, cannflavin), phenols (eugenol, cannabispiradienone), polyphenols (cannabispirone, canniprene, tannins), phytosterols (campesterol, stigmasterol, β-sitosterol), amines (piperidine), lignanamides (cannabisin A-G), and fatty acids in seeds. Many of these compounds exhibit repellency toward harmful insects such as
Culex pipiens fatigans,
Aedes quadrimaculatus,
Aedes aegypti [
26,
54,
55],
Culex quinquefasciatus, and
Spodoptera littoralis [
55].
Several studies have reported on the insecticidal and repellent activity of cannabis-based preparations and their use against human and agricultural phytophagous arthropods, although very little is known about the mechanism of cannabinoid action [
26,
55]. It is not clearly understood which constituents confer the insecticidal function, the inhibitory mechanisms, and/or the possible synergistic interactions with other metabolites (e.g., terpenoid aldehydes) in the defense mechanisms. In the up-to-date literature, information about the biological activity of hemp on storage pests is scarce. Therefore, this work presents a comprehensive study on different aspects of industrial hemp biological activity, including its effect on
P. interpunctella, as one of the most important pests of stored sunflower seeds. In the present work, the hemp extract demonstrated certain insecticidal activity against the larvae of
P. interpunctella and caused the reduction in female fecundity, even when the lowest concentration (0.5%) was applied. Similar results were presented by other authors. Mantzoukas et al. [
12] mentioned cannabidiol (CBD) oil as the most promising substance in this plant, which exhibits potential insecticidal effect against 4th instar larvae of
Tribolium confusum,
Oryzaephilus surinamensis, and
P. interpunctella, on wheat, rice, and corn seeds, respectively. In comparison with the control, the results showed clear dose-dependent pesticidal activity and 100% mortality at the highest dose (90 mg/mL) examined. Moreover, the overall survival time of the tested
P. interpunctella larvae was also considerably shorter than that of the control group, while the treatments also produced considerably fewer offspring in the tested insects. This is in accordance with the results of our study, where ethanolic hemp extract caused 42.5% mortality of
P. interpunctella larvae after 72 h of exposure. There are different reports on hemp activity against phytophagous pests. Park et al. [
56] investigated the defensive role of CBD in a feeding preference assay with tobacco hornworm (
Manduca sexta). The larvae showed a feeding preference toward the hemp tissue containing low CBD over high CBD, while the larvae avoided the high CBD diet. The results of the mentioned work indicate CBD’s defensive role against pest insects, which suggests its possible use as an insecticide and repellent. Sharma et al. [
57] tested the larvicidal effects of hemp extract against the tobacco cutworm, (
Spodoptera litura) and the cabbage worm (
Pieris brassicae) and confirmed the insecticidal effect of water hemp extracts. The authors also assessed the ovicidal activity of leaf extracts against the diamondback moth (
Plutella xylostella), which caused a slight reduction in egg hatching. Sharma et al. [
58] tested 2% ethanol leaf extracts against the potato tuber moth (
Phthorimaea operculella), which caused a reduction in egg hatching by 13.7%. Pavela [
59] reported on the significant efficacy of CBD oil against
Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae) larvae and several other non-lepidopteran pests. Benelli et al. [
25] reported that the essential oil from inflorescences of industrial hemp cv. Felina 32 was highly toxic to
Myzus persicae aphids (LC
50 of 3.5 mL/L) and
Musca domestica flies (43.3 μg/adult), while the toxicity was moderate toward
Spodoptera littoralis larvae (152.3 μg/larva). All presented literature results indicate a higher resilience of lepidopteran larvae to hemp extracts, compared to other pest species, which was also proven in the present study, since the mortality of
P. interpunctella was not very high in contact toxicity tests (0–42.5%).
The present work was also carried out to evaluate any possible inhibitory effects of industrial (fiber) hemp ethanolic extracts on sunflower seed vigor. The hemp extract did not negatively affect seed vigor parameters. Quite the opposite, hemp extracts at 2% concentration stimulated the GE of sunflower seeds. The results differed significantly from those reported by Arafat et al. [
60] and Sarkar et al. [
61]. These authors mentioned that depending on the source plant and the concentrations used, the botanical pesticides have no or slight allelopathic effect on crops. However, several studies demonstrated that hemp extracts can decrease the germination parameters of different plant species, especially allelochemicals detected in the hemp shoot that may be responsible for the inhibition of the metabolic activities of lupine, rape, rye, wheat [
27],
Lactuca sativa [
62], and the weed
Parthenium hysterophorus [
63] seeds. The authors emphasized that phytotoxicity was directly proportional to the concentration of applied extracts. According to the presented results, the industrial hemp extract, regardless of the applied concentration, did not have a negative effect on seed vigor, based on the GE and G values. Moreover, the highest concentration had a simulative effect on the G and/or GE of the tested seeds.
The results obtained from the germination assay are related to those from the biochemical analysis of sunflower seedlings. Specifically, although the hemp extract disrupted the activity of the enzymes of the phenolic compounds metabolism, increased PPO activity, and decreased PAL activity, it did not affect the overall content of total phenolic compounds and the germination of sunflower seedlings. The stress induction of phenylpropanoid biosynthesis involves increases in the steady-state transcript levels for various biosynthetic enzymes. The transcription of lupine (
Lupinus luteus L.) chalcone synthase and isoflavone synthase increased with time and concentration, indicating a defensive reaction to the allelopathic action of hemp extracts, while a small increase of 5% in the PAL transcription level was found with the 3 h and 5% hemp extracts [
27]. Not all allelochemicals can induce oxidative stress in target plants. Some of them can act as antioxidants. The fact that an allelochemical can act as an antioxidant or prooxidant depends on different factors: chemical structure, concentration of allelochemicals, and sensitivity of the acceptor plant [
64,
65]. Supposing that allelochemicals act as prooxidants to target plant species, their action leads to modification of reactive oxygen species (ROS) production and metabolism, which is associated with the degradation of cell compounds (proteins, lipids, and nucleic acids) and structures (mainly biomembranes). ROS production in plant tissues and organs is steady during normal metabolic activity but can be drastically increased during stressed conditions and induce oxidative stress. Many plants are reported to increase the level of antioxidants, both enzymatic and non-enzymatic, in response to biotic or abiotic environmental stresses [
64,
66]. Allelochemicals can cause oxidative damage in target plants and induce so-called allelopathic stress by producing a high amount of ROS during response to environmental stresses, as evidenced by the enhanced activity of ROS-scavenging enzymes and higher intensity of lipid peroxidation [
65,
67].
Different phenolic compounds are ascribed significant roles in plant antioxidant responses and development, pigment, and lignin biosynthesis. Plants exhibit increased synthesis of phenolic compounds such as phenolic acids, tannins, or flavonoids under abiotic and biotic stress conditions. The increase in the plant’s resistance is correlated with the function of phenolics in plants, principally consisting of their ROS-scavenging ability, the capacity to protect the plant from excessive light, and/or acting as signal molecules [
68,
69]. Phenolic compounds are also contributors to the increased antioxidant capacity of treated plants. Treatment of sunflower seeds with hemp ethanolic extracts did not induce changes in the content of total phenolics, total tannins, and antioxidant activity in seedlings of treated plants. The biosynthesis of phenolic compounds and related antioxidant capacity of seedling extracts were suppressed with the application of hemp extracts of lower concentrations (0.5% and 1%) on sunflower seeds.
SOD scavenges the superoxide radicals (O
2−), highly reactive free radicals, by converting them into hydrogen peroxide (H
2O
2). Although H
2O
2 is also a toxic molecule, it can be further reduced to H
2O by CAT in the peroxisomes and a few other enzymes [
66]. Peroxidase (POD) is believed to play an important role in the oxidation of phenolics to form lignin, the cross-linking of hydroxyl proline-rich glycol proteins in plant cell walls, auxin catabolism, and the production and breakdown of hydrogen peroxide and other reactive oxygen species. POD can exhibit simultaneous oxidant and anti-oxidant capabilities and is an important factor in the integrated defense response of plants to a variety of stresses [
70]. An increase in antioxidative enzyme activity has been reported in other studies on the allelochemical mode of action on different target plant species: Bromus [
65,
69,
71,
72,
73,
74]. However, from our results, it can be inferred that hemp extracts possess some allelochemicals that might have significantly increased and/or decreased the antioxidant enzyme and phenolic metabolism activity in sunflower seedlings. Similar results with other extracts and plant species were reported previously:
Calotropis procera [
70,
72].
For various plant species under allelopathic stress, a significant increase in MDA content and LP intensity is reported [
68,
73]. The results of this study revealed that the industrial hemp extract affected LP in the sunflower seedlings. The significantly higher accumulation of MDA in sunflower seedlings treated with the hemp extracts points to the fact that stress provoked by allelopathic substances was strong enough to induce LP.