Short-term Response of Greenhouse Gas Emissions from Precision Fertilization on Barley
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. In-Field Estimation of Nitrogen Requirements Using Proximal Sensing
2.3. Soil GHGs Emission Measurements and Flux Estimation
2.4. Crop Analysis and Nitrogen Fate within the Soil-Plant System
2.5. Statistical Analysis
3. Results and Discussions
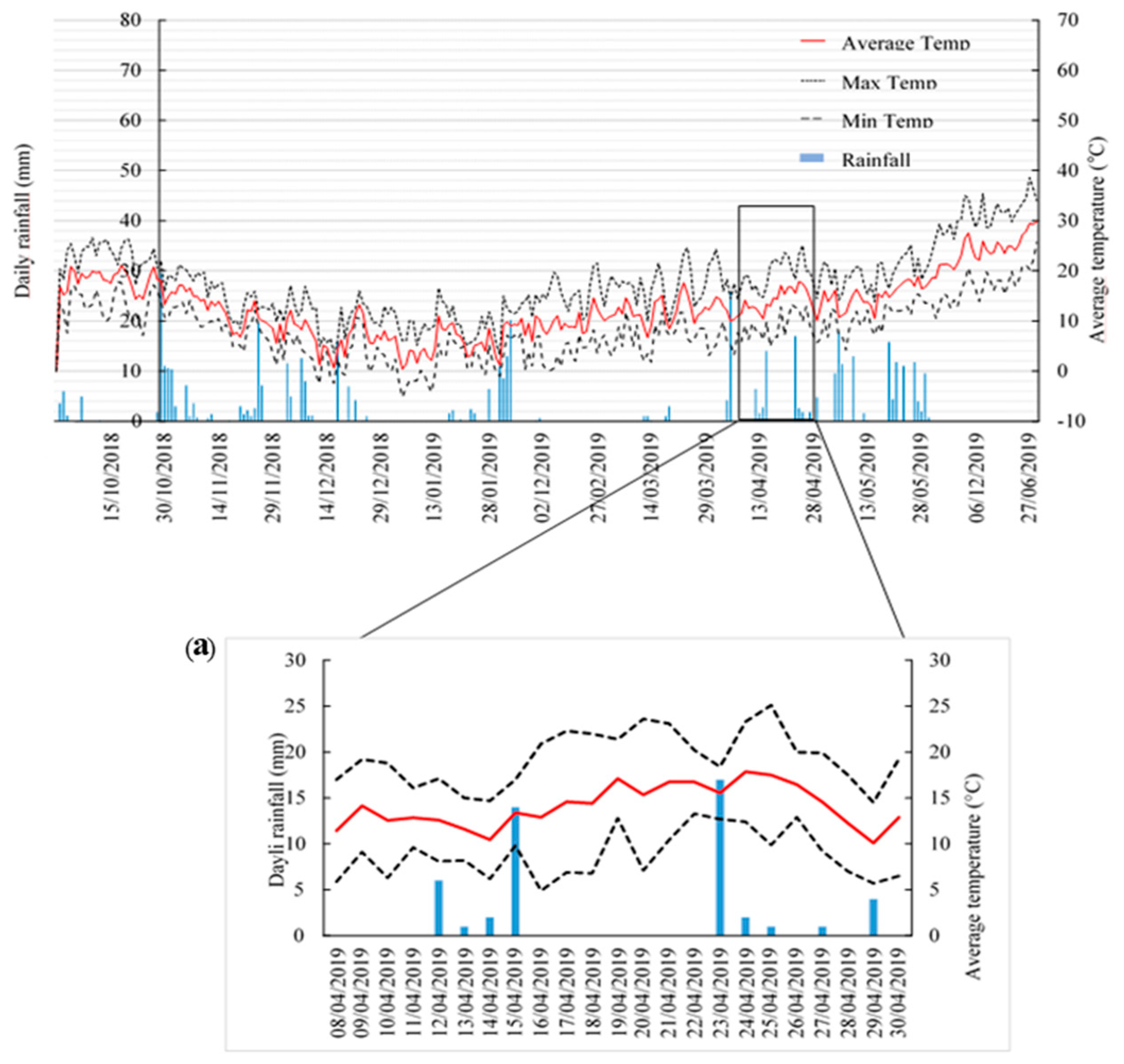
3.1. Meteorological Conditions during the Study Period
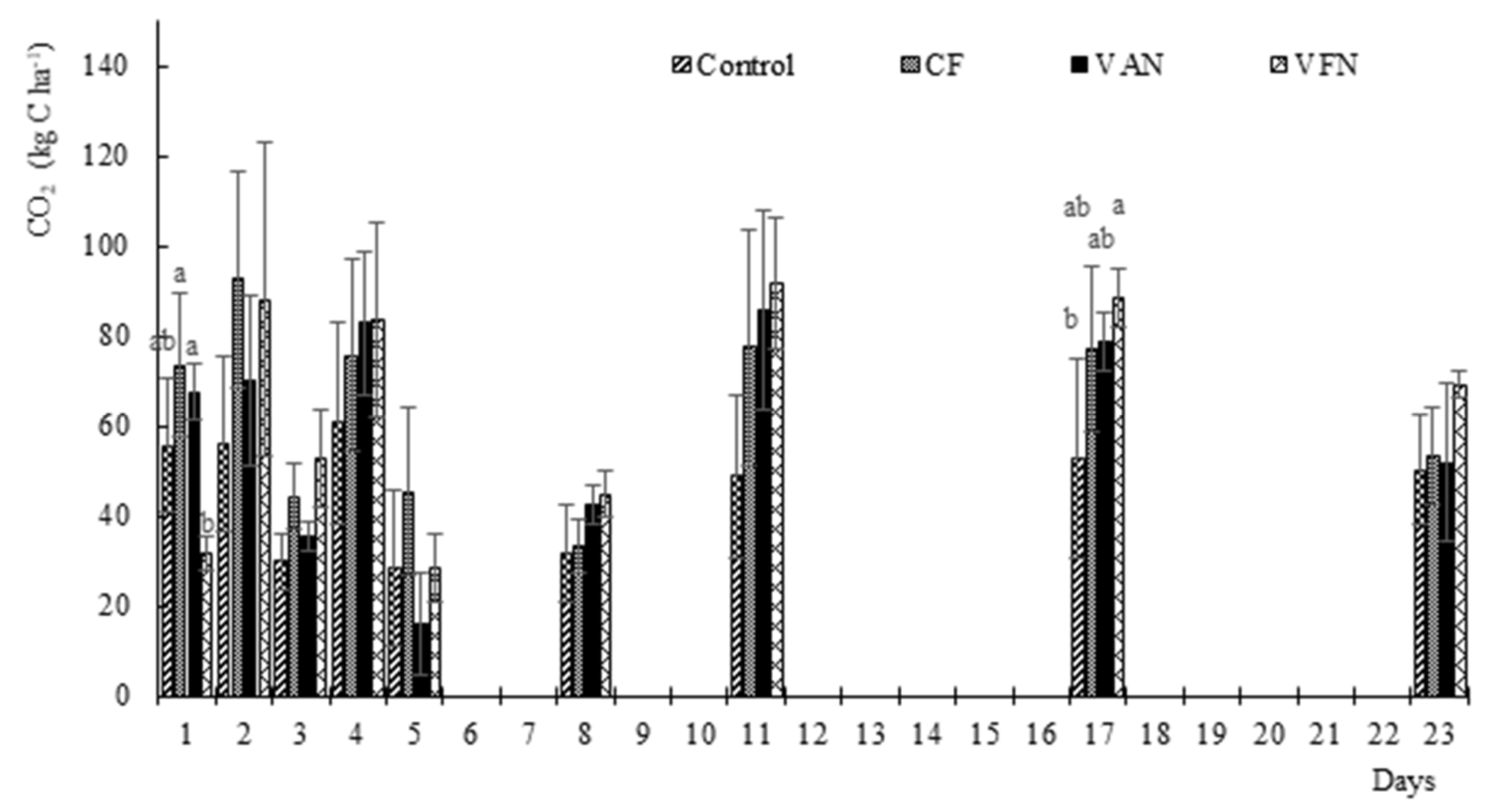
3.2. Carbon Dioxide Fluxes
3.3. Methane Fluxes
3.4. Nitrous Oxide Fluxes
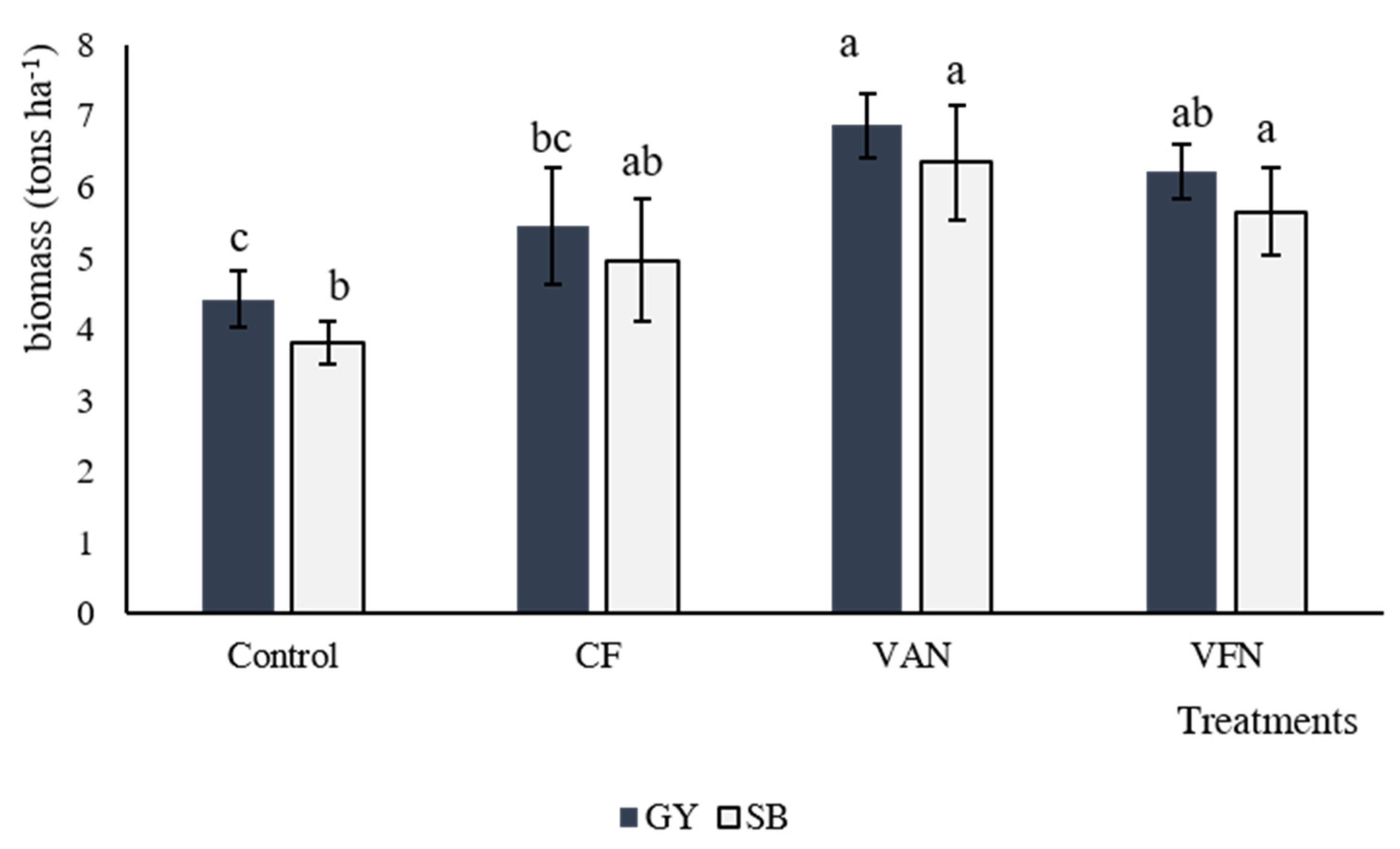
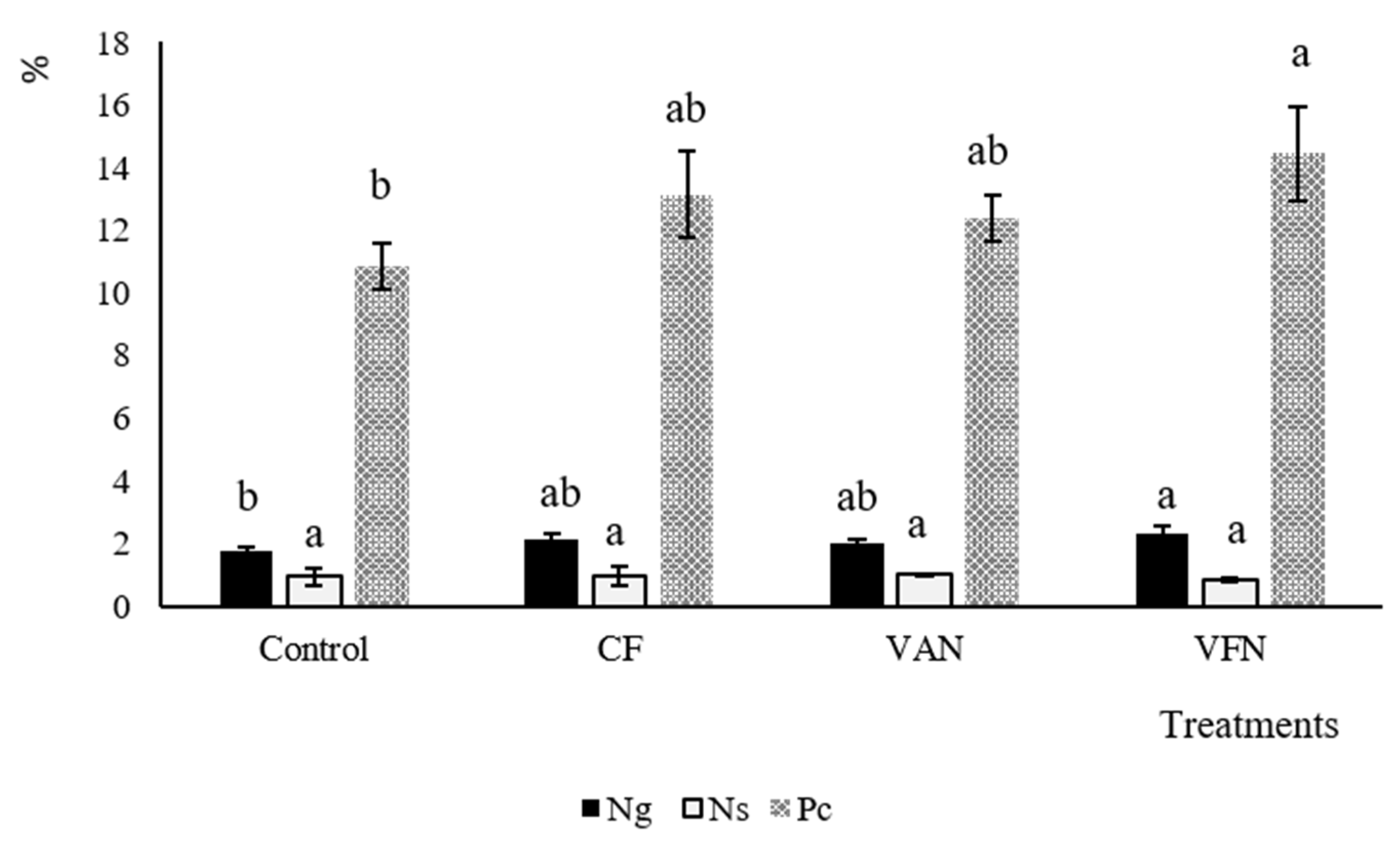
3.5. Crop Yield and N Content under Different Fertilizer Sources and Rates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Beatty, P.H.; Good, A.G. Future prospects for cereals that fix nitrogen. Science 2011, 333, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.A.; Wimalawansa, S.J. Agrochemical-related environmental pollution: Effects on human health. Glob. J. Biol. Agric. Health Sci. 2014, 3, 72–83. [Google Scholar]
- de Vries, W.; Schulte-Uebbing, L.; Kros, H.; Cees Voogd, J.; Louwagie, G. Spatially explicit boundaries for agricultural nitrogen inputs in the European Union to meet air and water quality targets. Sci. Total Environ. 2021, 786, 147283. [Google Scholar] [CrossRef] [PubMed]
- EU. 2021. Available online: https://ec.europa.eu/food/horizontal-topics/farm-fork-strategy_it (accessed on 7 July 2021).
- Sainju, U.M.; Ghimire, R.; Pradhan, G.P. Nitrogen Fertilization I: Impact on Crop, Soil, and Environment. In Nitrogen Fixation; Rigobelo, E.C., Serra, A.P., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Xia, L.L.; Xia, Y.Q.; Li, B.L.; Wang, J.Y.; Wang, S.W.; Zhou, W.; Yan, X.Y. Integrating agronomic practices to reduce greenhouse gas emissions while increasing the economic return in a rice-based cropping system. Agric. Ecosyst. Environ. 2016, 231, 24–33. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef]
- Mach, K.J.; Planton, S.; von Stechow, C.; IPCC. Annex II: Glossary. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; pp. 117–130. Available online: https://www.ipcc.ch/site/assets/uploads/2018/05/SYR_AR5_FINAL_full_wcover.pdf (accessed on 8 July 2021).
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; Intergovernmental Panel on Climate Change, IGES: Hayama, Japan, 2006; Available online: http://www.ipcc-nggip.iges.or.jp/public/2006gl/index.html (accessed on 7 July 2021).
- Zhou, W.; Ji, H.; Zhu, J.; Zhang, Y.-P.; Sha, L.-Q.; Liu, Y.-T.; Zhang, X.; Zhao, W.; Dong, Y.-x.; Bai, X.-L.; et al. The effects of nitrogen fertilization on N2O emissions from a rubber plantation. Sci. Rep. 2016, 6, 28230. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Chang, N.J.; Zhai, Z.; Li, H.; Wang, L.G.; Deng, J. Impacts of nitrogen management and organic matter application on nitrous oxide emissions and soil organic carbon from spring maize fields in the North China Plain. Soil Tillage Res. 2020, 196, 104441. [Google Scholar] [CrossRef]
- Liu, L.; Greaver, T.L. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecol. Lett. 2009, 12, 1103–1117. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Correction: Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 2019, 14, e0216006. [Google Scholar] [CrossRef]
- Verdi, L.; Napoli, M.; Santoni, M.; Dalla Marta, A.; Ceccherini, M.T. Soil carbon dioxide emission flux from organic and conventional farming in a long-term experiment in Tuscany. In Proceedings of the IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Portici, Italy, 24–26 October 2019; pp. 85–89. [Google Scholar]
- Dan, J.G.; Krüger, M.; Frenzel, P.; Conrad, R. Effect of a late season urea fertilization on methane emission from a rice field in Italy. Agric. Ecosyst. Environ. 2001, 83, 191–199. [Google Scholar] [CrossRef]
- Venterea, R.T.; Burger, M.; Spokas, K.A. Nitrogen oxide and methane emissions under varying tillage and fertilizer management. J. Environ. Qual. 2005, 34, 1467–1477. [Google Scholar] [CrossRef]
- Tedone, L.; Ali, S.A.; De Mastro, G. Optimization of nitrogen in durum wheat in the Mediterranean climate: The agronomical aspect and greenhouse gas (GHG) emissions. Nitrogen Agric.-Updates 2017, 8, 131–162. [Google Scholar]
- Fabbri, C.; Mancini, M.; Dalla Marta, A.; Orlandini, S.; Napoli, M. Integrating satellite data with a Nitrogen Nutrition Curve for precision top-dress fertilization of durum wheat. Eur. J. Agron. 2020, 120, 126148. [Google Scholar] [CrossRef]
- Roy, T.; George, K.J. Precision farming: A step towards sustainable, climate-smart agriculture. In Global Climate Change: Resilient and Smart Agriculture; Springer: Singapore, 2020; pp. 199–220. [Google Scholar]
- Rütting, T.; Aronsson, H.; Delin, S. Efficient use of nitrogen in agriculture. Nutr. Cycl. Agroecosyst. 2018, 110, 1–5. [Google Scholar] [CrossRef]
- Basso, B.; Fiorentino, C.; Cammarano, D.; Schulthess, U. Variable-rate nitrogen fertilizer response in wheat using remote sensing. Precis. Agric. 2015, 17, 168–182. [Google Scholar] [CrossRef]
- Van Loon, J.; Speratti, A.B.; Govaerts, B. Precision for smallholder farmers: A small-scale-tailored variable-rate fertilizer application kit. Agriculture 2018, 8, 48. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different responses of various chlorophyll meters to increasing nitrogen supply in sweet pepper. Front. Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef]
- Aranguren, M.; Castellón, A.; Aizpurua, A. Crop Sensor-Based In-Season Nitrogen Management of Wheat with Manure Application. Remote Sens. 2019, 11, 1094. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal Optical Sensors for Nitrogen Management of Vegetable Crops: A Review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Darra, N.; Psomiadis, E.; Kasimati, A.; Anastasiou, A.; Anastasiou, E.; Fountas, S. Remote and Proximal Sensing Derived Spectral Indices and Biophysical Variables for Spatial Variation Determination in Vineyards. Agronomy 2021, 11, 741. [Google Scholar] [CrossRef]
- Yousfi, S.; Peira, J.F.M.; De La Horra, G.R.; Ablanque, P.V.M. Remote Sensing: Useful Approach for Crop Nitrogen Management and Sustainable Agriculture. In Sustainable Crop Production; IntechOpen: London, UK, 2019. [Google Scholar]
- Shaver, T.M.; Khosla, R.; Westfall, D.G. Evaluation of two crop canopy sensors for nitrogen variability determination in irrigation maize. Precis. Agric. 2011, 12, 892–904. [Google Scholar] [CrossRef]
- Yao, Y.K.; Miao, Y.X.; Huang, S.Y.; Gao, L.; Ma, X.B.; Zhao, G.M.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K.; et al. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef]
- Raun, W.; Solie, J.; May, J.; Zhang, H.; Kelly, J.; Taylor, R. Nitrogen Rich Strips for Wheat, Corn and Other Crops; Publication E-1022; Oklahoma State University Extension: Stillwater, OK, USA, 2010; Available online: http://www.nue.okstate.edu/Index_Publications/Nstrip%20brochure.pdf (accessed on 7 July 2021).
- Morari, F.; Zanella, V.; Sartori, L.; Visioli, G.; Berzaghi, P.; Mosca, G. Optimising durum wheat cultivation in North Italy: Understanding the effects of site-specific fertilization on yield and protein content. Precis. Agric. 2018, 19, 257–277. [Google Scholar] [CrossRef]
- Soto, I.; Barnes, A.; Balafoutis, A.; Beck, B.; Sánchez, B.; Vangeyte, J.; Fountas, S.; Van der Wal, T.; Eory, V.; Gómez-Barbero, M. The Contribution of Precision Agriculture Technologies to Farm Productivity and the Mitigation of Greenhouse Gas Emissions in the EU; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Verdi, L.; Kuikman, P.J.; Orlandini, S.; Mancini, M.; Napoli, M.; Dalla Marta, A. Does the use of digestate to replace mineral fertilizers have less emissions of N2O and NH3? Agric. For. Meteorol. 2019, 269–270, 112–118. [Google Scholar] [CrossRef]
- Fan, J.; McConkey, B.; Wang, H.; Janzen, H. Root distribution by depth for temperate agricultural crops. Field Crops Res. 2016, 189, 68–74. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Mullen, R.W.; Freeman, K.W.; Thomason, W.E.; Lukina, E.V. Improving nitrogen use-efficiency in cereal grain production with optical sensing and variable-rate application. J. Agron. 2002, 94, 815–820. [Google Scholar] [CrossRef]
- Foster, A.; Atwell, S.; Dunn, D. Sensor-based Nitrogen Fertilization for Midseason Rice Production in Southeast Missouri. Crops Soils 2017, 51, 48–55. [Google Scholar] [CrossRef]
- Parkin, T.B.; Venterea, R.T. Chamber-Based Trace Gas Flux Measurements. In Sampling Protocols; Follet, R.F., Ed.; USDA-ARS: Washington, DC, USA, 2010; pp. 3.1–3.39. [Google Scholar]
- Adeyemi, O.; Keshavarz-Afshar, R.; Jahanzad, E.; Battaglia, M.L.; Luo, Y.; Sadeghpour, A. Effect of Wheat Cover Crop and Split Nitrogen Application on Corn Yield and Nitrogen Use Efficiency. Agronomy 2020, 10, 1081. [Google Scholar] [CrossRef]
- Halvorson, A.; Bartolo, M. Nitrogen Source and Rate Effects on Irrigated Corn Yields and Nitrogen-Use Efficiency. J. Agron. 2014, 106, 681–693. [Google Scholar] [CrossRef]
- Prakasha, G.; Mudalagiriyappa Ramachandrappa, B.K.; Nagaraju Hanumanthappa, D.C.; Sathish, A. Factor productivity, nitrogen use efficiency and economics of maize under different precision nitrogen management practices. Int. J. Chem. Stud. 2018, 6, 869–873. [Google Scholar]
- Van Groenigen, J.W.; Velthof, G.L.; Oenema, O.; Van Groenigen, K.J.; Van Kessel, C. Towards an agronomic assessment of N2O emissions: A case study for arable crops. Eur. J. Soil Sci. 2010, 61, 903–913. [Google Scholar] [CrossRef]
- Januskaitiene, I.; Kacienė, G. The effect of foliar spray fertilizers on the tolerance of Hordeum vulgare to UV-B radiation and drought stress. Cereal Res. Commun. 2017, 45, 390–400. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Y.; Cai, Z.; Yagi, K.; Zheng, X. Soil respiration under maize crops: Effects of water, temperature, and nitrogen fertilization. Soil Sci. Soc. Am. J. 2007, 71, 944–951. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Silvola, J.; Alm, J.; Ahlholm, U.; Nykänen, H.; Martikainen, P.J. The contribution of plant roots to CO2 fluxes from organic soil. Biol. Fertil. Soils 1996, 23, 126–131. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Y.; Xu, X.; Ruan, H.; Wang, J. Temperature sensitivity of soil organic carbon mineralization along an elevation gradient in the Wuyi Mountains, China. PLoS ONE 2013, 8, e53914. [Google Scholar] [CrossRef]
- Carbonell-Bojollo, R.; Veroz-Gonzalez, O.; Ordoñez-Fernandez, R.; Moreno-Garcia, M.; Basch, G.; Kassam, A.; Repullo-Ruiberriz de Torres, M.A.; Gonzalez-Sanchez, E.J. The Effect of Conservation Agriculture and Environmental Factors on CO2 Emissions in a Rainfed Crop Rotation. Sustainability 2019, 11, 3955. [Google Scholar] [CrossRef]
- Verdi, L.; Mancini, M.; Ljubojevic, M.; Orlandini, S.; Dalla Marta, A. Greenhouse gas and ammonia emissions from soil: The effect of organic matter and fertilisation method. Ital. J. Agron. 2018, 13, 260–266. [Google Scholar] [CrossRef]
- Kimura, M.; Asai, K.; Watanabe, A.; Murase, J.; Kuwatsuka, S. Suppression of methane fluxes from flooded paddy soil with rice plants by foliar spray of nitrogen fertilizers. Soil Sci. Plant Nutr. 1992, 38, 735–740. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; Liu, H.; Zheng, X.; Lu, F.; Wang, S.; Li, Z.; Liu, G.; Fu, B. Responses of CH4 and N2O fluxes to land-use conversion and fertilization in a typical red soil region of southern China. Sci. Rep. 2017, 7, 1057. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Bonilla, D.; Cantero-Martínez, C.; Bareche, J.; Arrúe, J.L.; Álvaro-Fuentes, J. Soil carbon dioxide and methane fluxes as affected by tillage and N fertilization in dryland conditions. Plant Soil 2014, 381, 111–130. [Google Scholar] [CrossRef]
- Yue, P.; Li, K.H.; Gong, Y.M.; Hu, Y.K.; Mohammat, A.; Christie, P.; Liu, X.J. A five-year study of the impact of nitrogen addition on methane uptake in alpine grassland. Sci. Rep. 2016, 6, 32064. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.; Tian, H.; Liu, D.; Lu, C.; Xu, X.; Zhang, L.; Yang, G.; Wan, Z. Effect of continued nitrogen enrichment on greenhouse gas emissions from a wetland ecosystem in the Sanjiang Plain, Northeast China: A 5 year nitrogen addition experiment. J. Geophys. Res. Biogeosci. 2013, 118, 741–751. [Google Scholar] [CrossRef]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Geochemestry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q.; Zhao, J.; Chen, Y.; Wang, H.; Ma, J.; Zou, P.; Bao, L. Restricted nitrous oxide emissions by ammonia oxidizers in two agricultural soils following excessive urea fertilization. J. Soils Sediments 2020, 20, 1502–1512. [Google Scholar] [CrossRef]
- Schils, R.L.M.; van Groenigen, J.W.; Velthof, G.L.; Kuikman, P.J. Nitrous oxide emissions from multiple combined applications of fertiliser and cattle slurry to grassland. Plant Soil 2008, 310, 89–101. [Google Scholar] [CrossRef]
- Jones, S.K.; Famulari, D.; Di Marco, C.F.; Nemitz, E.; Skiba, U.M.; Rees, R.M.; Sutton, M.A. Nitrous oxide emissions from managed grassland: A comparison of eddy covariance and static chamber measurements. Atmos. Meas. Tech. 2011, 4, 2179–2194. [Google Scholar] [CrossRef]
- Davidson, E.A.; Matson, P.A.; Brooks, P.D. Nitrous oxide emission controls and inorganic nitrogen dynamics in fertilized tropical agricultural soils. Soil Sci. Soc. Am. J. 1996, 60, 1145–1152. [Google Scholar] [CrossRef]
- Bouwman, A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosyst. 1996, 46, 57–70. [Google Scholar] [CrossRef]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Colaço, A.; Bramley, R. Do crop sensors promote improved nitrogen management in grain crops? Field Crops Res. 2018, 218, 126–140. [Google Scholar] [CrossRef]
- Fabbri, C.; Napoli, M.; Verdi, L.; Mancini, M.; Orlandini, S.; Dalla Marta, A. A Sustainability Assessment of the Greenseeker N Management Tool: A Lysimetric Experiment on Barley. Sustainability 2020, 12, 7303. [Google Scholar] [CrossRef]
- Du, X.; Xi, M.; Kong, L. Split application of reduced nitrogen rate improves nitrogen uptake and use efficiency in sweet potato. Sci. Rep. 2019, 9, 14058. [Google Scholar] [CrossRef]
- Vizzari, M.; Santaga, F.; Benincasa, P. Sentinel 2-Based Nitrogen VRT Fertilization in Wheat: Comparison between Traditional and Simple Precision Practices. Agronomy 2019, 9, 278. [Google Scholar] [CrossRef]
- Valkama, E.; Salo, T.; Esala, M.; Turtola, E. Nitrogen balances and yields of spring cereals as affected by nitrogen fertilization in northern conditions: A meta-analysis. Agric. Ecosyst. Environ. 2013, 164, 1–13. [Google Scholar] [CrossRef]
- Tubaña, B.S.; Arnall, D.B.; Walsh, O.; Chung, B.; Solie, J.B.; Girma, K.; Raun, W.R. Adjusting midseason nitrogen rate using a sensor-based optimization algorithm to increase use efficiency in corn (Zea mays L.). J. Plant Nutr. 2008, 31, 1393–1419. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Zhang, H.; Schroder, J.; Zhang, F.; Jia, L.; Cui, Z.; Li, R.; Chen, X.; Raun, W.R. In-Season Optical Sensing Improves Nitrogen Use Efficiency for Winter Wheat. Soil Sci. Soc. Am. J. 2009, 73, 1566–1574. [Google Scholar] [CrossRef]
- Hoseinlou, S.; Ebadi, A.; Ghaffari, M.; Mostafaei, E. Nitrogen Use Efficiency Under Water Deficit Condition in Spring Barley. Int. J. Agron. Plant Prod. 2013, 4, 3681–3687. [Google Scholar]
- Glass, A.D.S. Nitrogen Use Efficiency of Crop Plants: Physiological Constraints upon Nitrogen Absorption, Critical Reviews. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K. A Review of Methods to Improve Nitrogen Use Efficiency in Agriculture. Sustainability 2018, 10, 51. [Google Scholar] [CrossRef]
- Sen, S.; Chalk, P.M. Stimulation of root growth and soil nitrogen uptake by foliar application of urea to wheat and sunflower. J. Agric. Sci. 1996, 126, 127–135. [Google Scholar] [CrossRef]
- Walsh, O.S.; Christiaens, R.J.; Montana, A.P. Foliar-Applied Nitrogen Fertilizers in Spring Wheat Production; MT WERA-103 Committee; State University, Western Triangle Agricultural Research Center (WTARC): Conrad, MT, USA, 2013; Volume 5, pp. 3–5. [Google Scholar]
- Kara, B. Influence of late-season nitrogen application on grain yield, nitrogen use efficiency and protein content of wheat under Isparta ecological conditions. Turk. J. Field Crops 2010, 15, 1–6. [Google Scholar]
- Bly, A.G.; Woodard, H.J. Foliar nitrogen application timing influence on grain yield and protein concentration of hard red winter and spring wheat. J. Agron. 2003, 95, 335–338. [Google Scholar] [CrossRef]
- Blandino, M.; Marinaccio, F.; Reyneri, A. Effect of late-season nitrogen fertilization on grain yield and on flour rheological quality and stability in common wheat, under different production situations. Ital. J. Agron. 2016, 11, 107–113. [Google Scholar] [CrossRef]


| Unit | ||
|---|---|---|
| Total N | % | 0.152 ± 0.014 |
| Clay | % | 23.1 |
| Sand | % | 46.8 |
| Silt | % | 30.1 |
| Bulk density | g/cm3 | 1.08 |
| Treatment (1) | Cumulative CO2 Flux (kg C ha−1) | Cumulative CH4 Flux (kg C ha−1) | Cumulative N2O Flux (kg N ha−1) |
|---|---|---|---|
| Control | 1066 ± 359.52 b | 5.42 ± 0.65 a | 0.08 ± 0.03 c |
| CF | 1624 ± 268.78 a | 4.22 ± 0.10 b | 0.25 ± 0.08 ab |
| VAN | 1455 ± 145.60 a | 4.52 ± 0.90 ab | 0.33 ± 0.05 a |
| VFN | 1632 ± 178.22 a | 4.22 ± 0.76 b | 0.20 ± 0.10 b |
| Treatment | Nup (kg ha−1) | Nsur (kg ha−1) | PE (kg kg−1) | AE (kg kg−1) | PFP (kg kg−1) | RE (%) | |
|---|---|---|---|---|---|---|---|
| Nrate + Source | Control | 76 ± 2.71 b | −76 ± 2.71 b | 57 ± 4.86 | - | - | - |
| CF | 115 ± 30.56 b | 40 ± 21.94 a | 48 ± 6.31 | 8 ± 6.34 b | 36 ± 6.34 b | 0.26 ± 0.20 b | |
| VAN | 135 ± 9.70 a | −106 ± 21.21 b | 50 ± 1.59 | 81 ± 6.34 a | 228 ± 18.04 a | 2.01 ± 0.70 a | |
| VFN | 143 ± 16.08 a | −113 ± 21.92 b | 43 ± 5.35 | 60 ± 6.34 a | 207 ± 15.79 a | 2.26 ± 0.73 a | |
| Nrate | 0 | 76 ± 2.71 c | −76 ± 2.71 b | 57 ± 4.86 | - | - | - |
| 35 | 140 ± 19.72 a | −110 ± 19.72 b | 47 ± 5.75 | 70 ± 19.10 a | 218 ± 19.10 a | 2.13 ± 0.65 a | |
| 150 | 109 ± 21.94 b | 40 ± 21.94 a | 48 ± 6.31 | 8 ± 6.34 b | 36 ± 6.34 b | 0.26 ± 0.20 b | |
| ANOVA | Nrate + Source | ** | *** | NS | *** | *** | ** |
| Nrate | *** | *** | NS | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, C.; Dalla Marta, A.; Napoli, M.; Orlandini, S.; Verdi, L. Short-term Response of Greenhouse Gas Emissions from Precision Fertilization on Barley. Agronomy 2023, 13, 96. https://doi.org/10.3390/agronomy13010096
Fabbri C, Dalla Marta A, Napoli M, Orlandini S, Verdi L. Short-term Response of Greenhouse Gas Emissions from Precision Fertilization on Barley. Agronomy. 2023; 13(1):96. https://doi.org/10.3390/agronomy13010096
Chicago/Turabian StyleFabbri, Carolina, Anna Dalla Marta, Marco Napoli, Simone Orlandini, and Leonardo Verdi. 2023. "Short-term Response of Greenhouse Gas Emissions from Precision Fertilization on Barley" Agronomy 13, no. 1: 96. https://doi.org/10.3390/agronomy13010096
APA StyleFabbri, C., Dalla Marta, A., Napoli, M., Orlandini, S., & Verdi, L. (2023). Short-term Response of Greenhouse Gas Emissions from Precision Fertilization on Barley. Agronomy, 13(1), 96. https://doi.org/10.3390/agronomy13010096








