Zinc Stress Alters Sugar Content in Rice Plants and the Reproduction and Trehalose Metabolism in Nilaparvata lugens
Abstract
1. Introduction
2. Materials and Methods
2.1. Zn2+ Treatment, Sampling, and Zn Detection
2.2. Insect Rearing, Sampling, and Fecundity Measurement
2.3. Determination of Sugar Content and Trehalase Activity in Rice Plants and N. lugens
2.4. Gene Expression Levels Pertaining to Trehalose Metabolism in N. lugens
2.5. Statistical Analyses
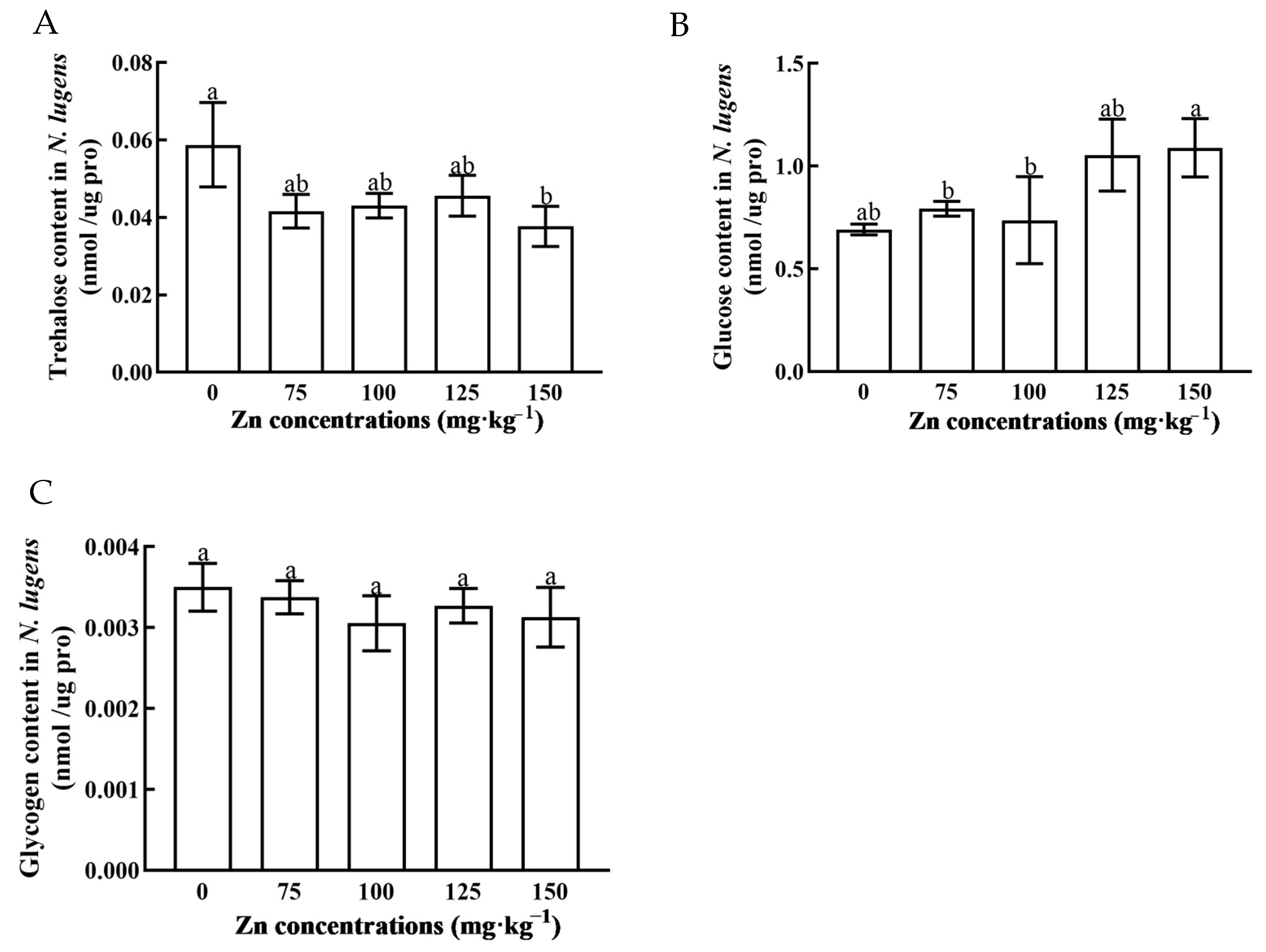
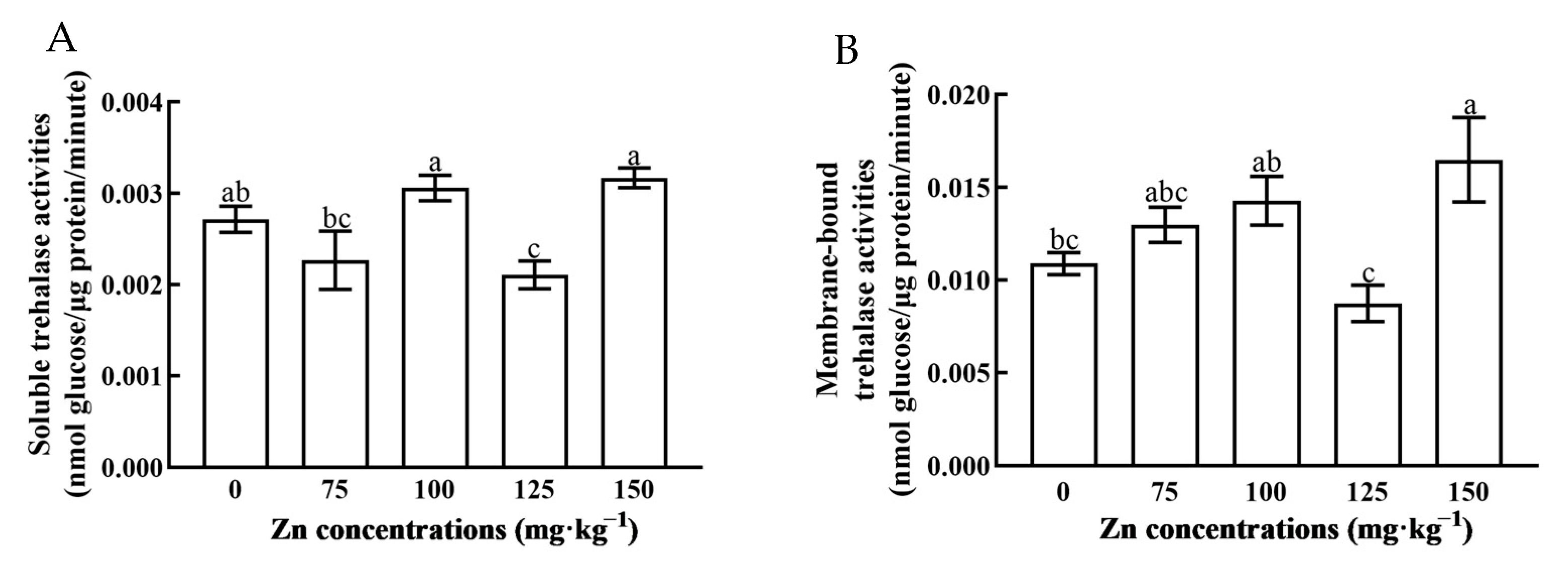
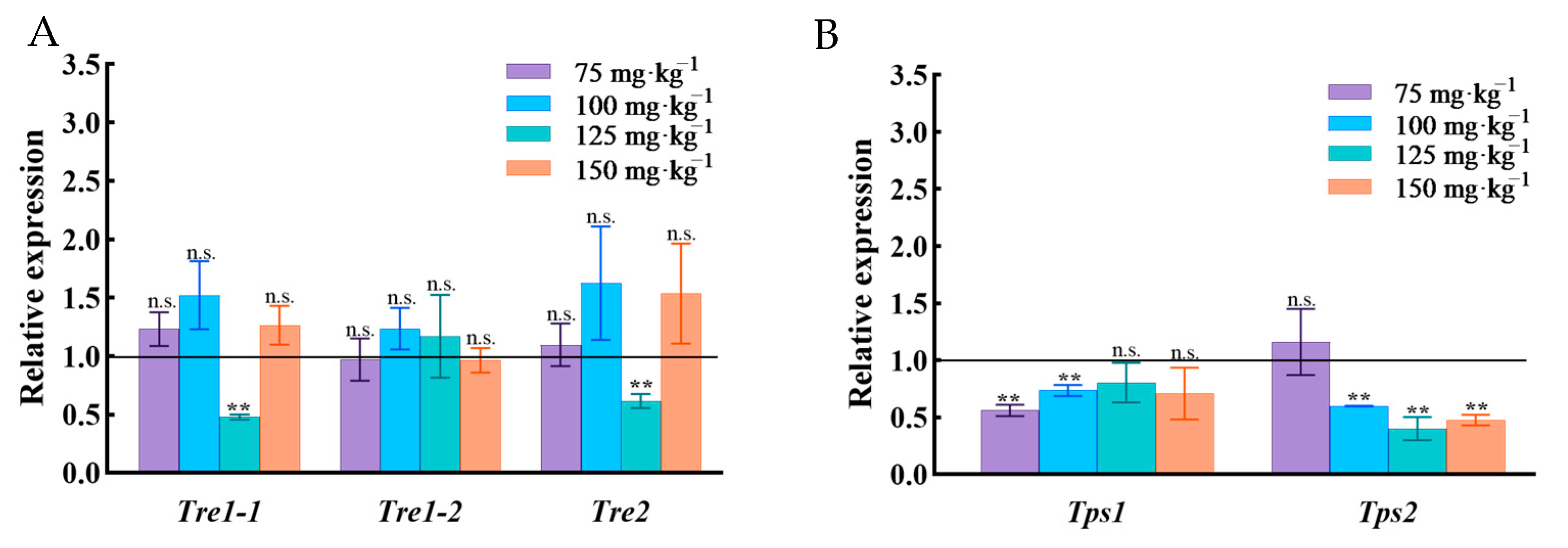
3. Results
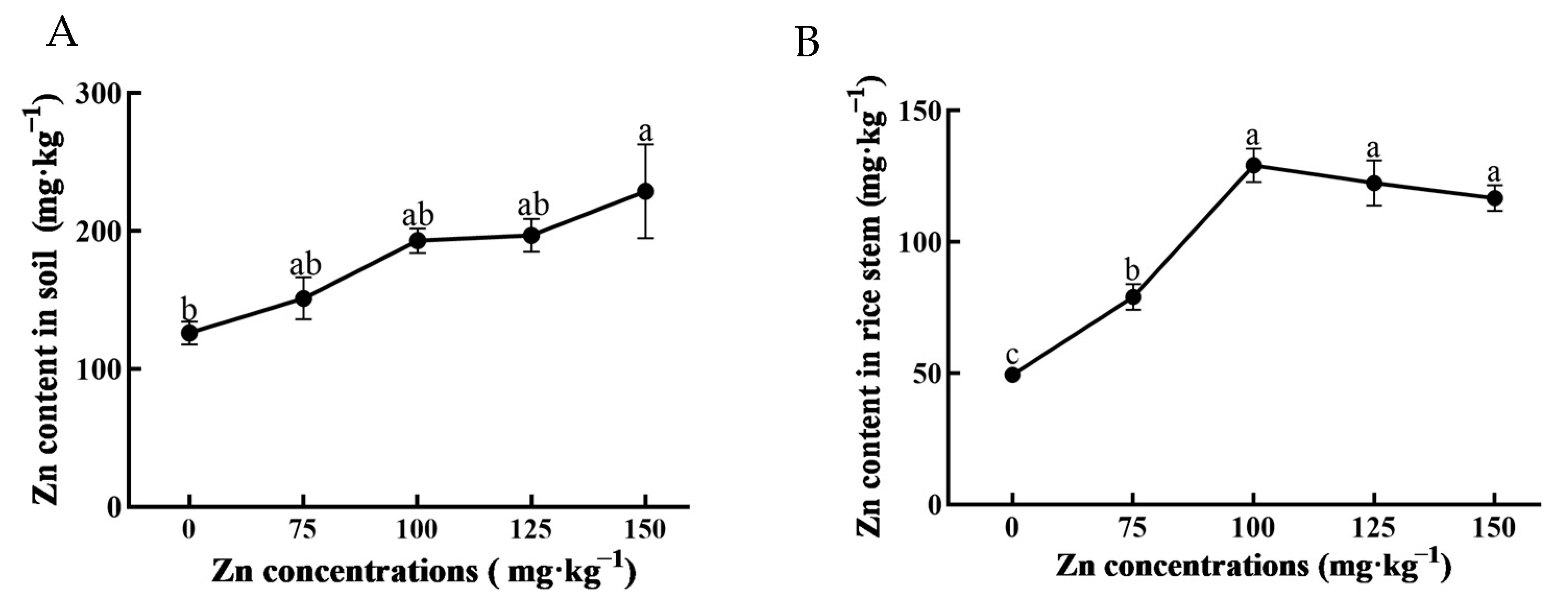
3.1. Zn Concentration in Soil and Rice Plants after Zn2+ Irrigation
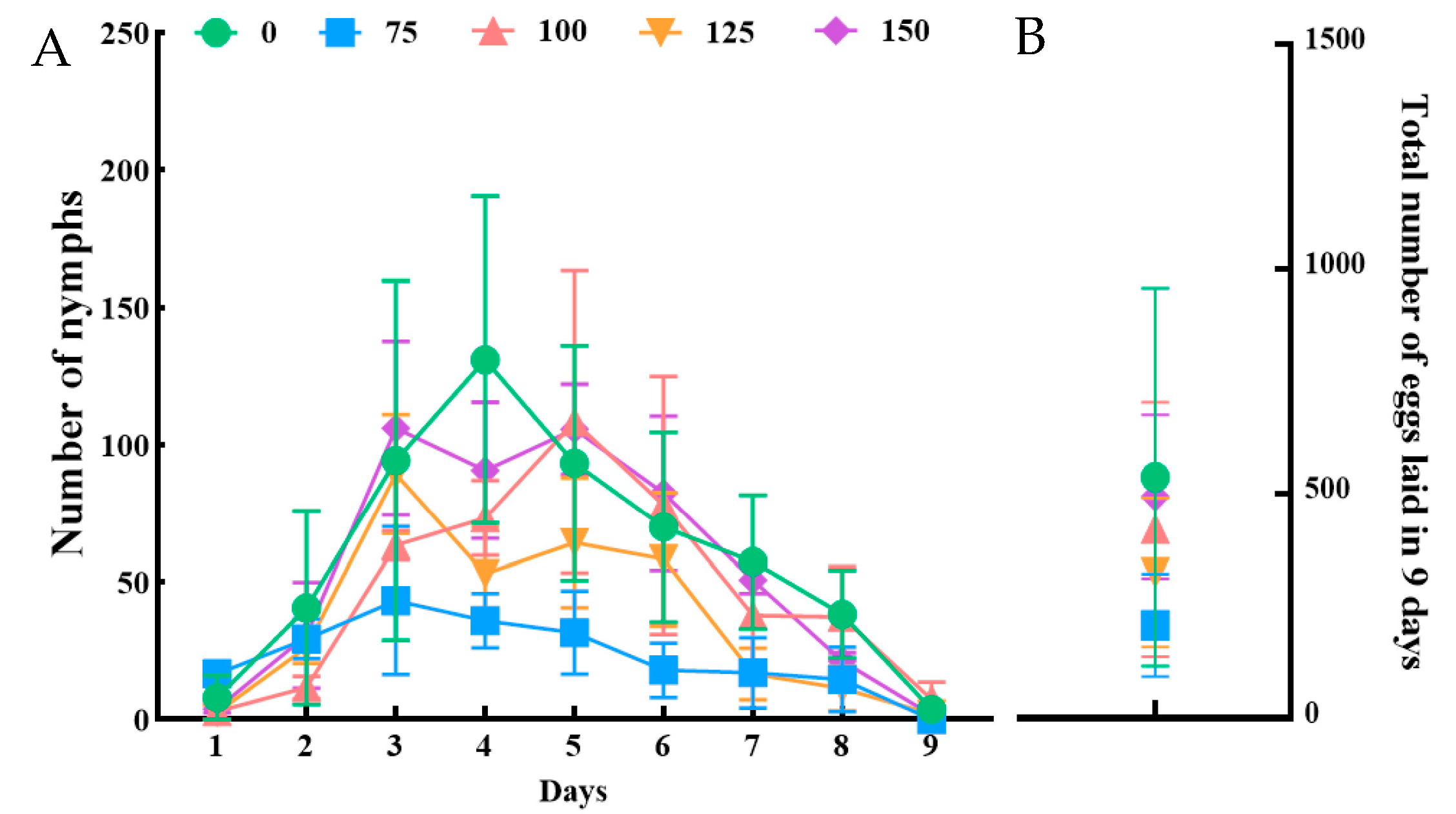
3.2. Nymph Numbers of N. lugens
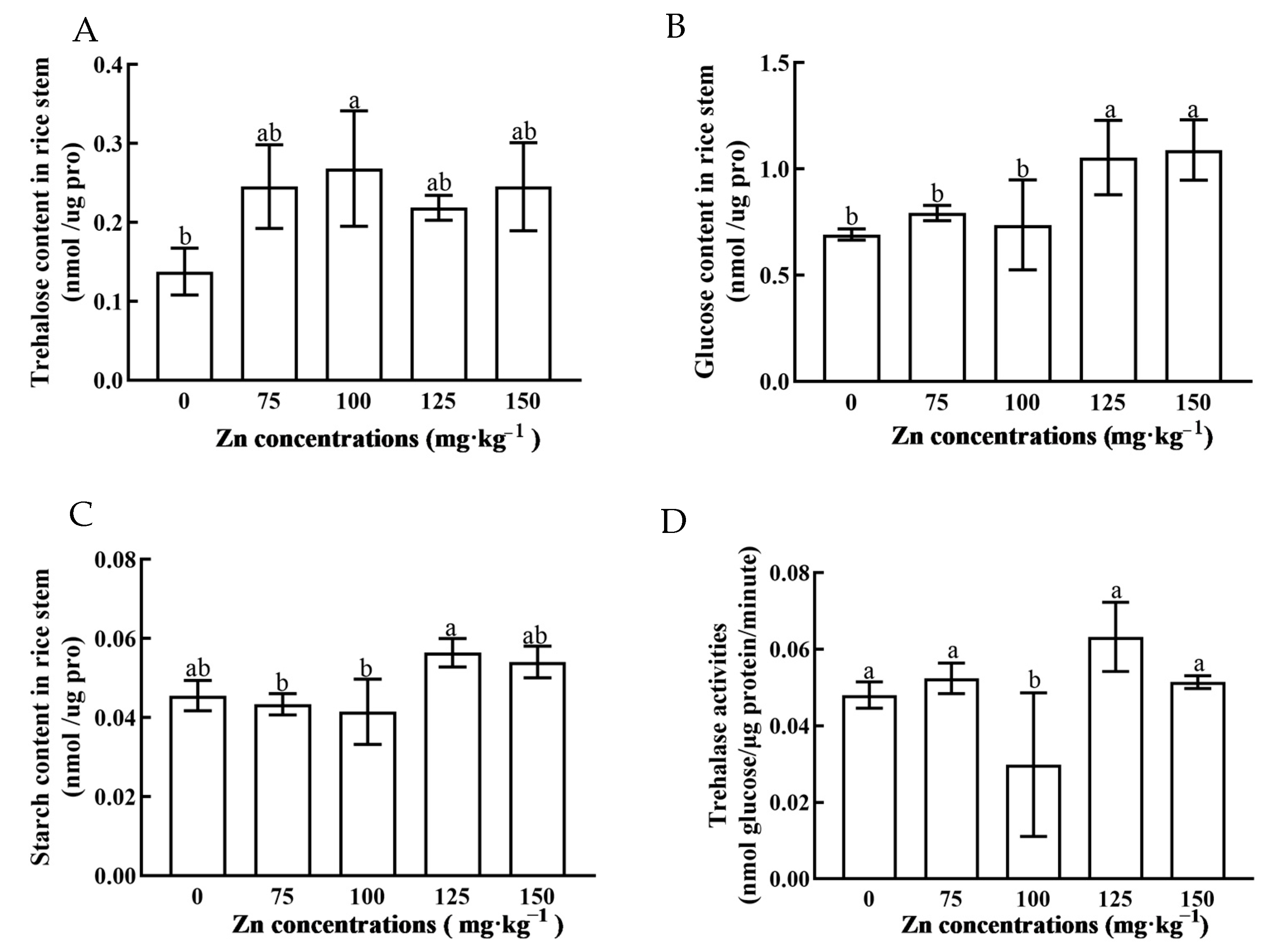
3.3. Sugar Content and Tre Activity in Rice Plants under Zn Stress
3.4. Comparison of Sugar Content in N. lugens Feeding on Zn-Stressed Rice
3.5. Tre Activity of N. lugens Feeding on Zn-Stressed Rice
3.6. Expression of Genes Related to Trehalose Metabolism in N. lugens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wing, R.A.; Purugganan, M.D.; Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, P.C.; Hu, L.; Ch, X.L.; Keller, M.A.; Chu, D. Host selection and adaptation of the invasive pest Spodoptera frugiperda to indica and japonica rice cultivars. Entomol. Gen. 2022, 42, 403–411. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Ann. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Wang, X.Q.; Li, P.R.; Tan, X.L.; Yang, X.Q. Diet-mediated effects of cadmium on the fitness-related traits and detoxification and antioxidative enzymes in the oriental armyworm, Mythimna separata. Entomol. Gen. 2020, 40, 407–419. [Google Scholar] [CrossRef]
- Baruah, S.G.; Ahmed, I.; Das, B.; Ingtipi, B.; Boruah, H.; Gupta, S.K.; Nema, A.K.; Chabukdhara, M. Heavy metal(loid)s contamination and health risk assessment of soil-rice system in rural and peri-urban areas of lower brahmaputra valley, northeast India. Chemosphere 2021, 266, 129150. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, X.; Ding, M.; Zhu, Y. Integrated analyses of miRNAome and transcriptome reveal zinc deficiency responses in rice seedlings. BMC Plant Biol. 2019, 19, 585. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Yuan, X.; Xue, N.; Han, Z. A meta-analysis of heavy metals pollution in farmland and urban soils in China over the past 20 years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef]
- Kaznina, N.M.; Titov, A.F.; Repkina, N.S.; Batova, Y.V. Effect of Zinc excess and low temperature on the IRT1 gene expression in the roots and leaves of barley. Dokl Biochem. Biophys. 2019, 487, 264–268. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Zhou, J.; Du, B.; Wang, Z.; Zhang, W.; Xu, L.; Fan, X.; Liu, X.; Zhou, J. Distributions and pools of lead (Pb) in a terrestrial forest ecosystem with highly elevated atmospheric Pb deposition and ecological risks to insects. Sci. Total Environ. 2019, 647, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Monchanin, C.; Devaud, J.M.; Barron, A.B.; Lihoreau, M. Current permissible levels of metal pollutants harm terrestrial invertebrates. Sci. Total Environ. 2021, 779, 146398. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.L.; Liu, S.; Tang, Q.Y.; Cheng, J.A. Heavy metal bioaccumulation and mobility from rice plants to Nilaparvata lugens (Homoptera: Delphacidae) in China. Environ. Entomol. 2014, 43, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, F.M.; Wang, M.Q. Transgenic expression of Bt in rice does not affect feeding behavior and population density of the brown planthopper, Nilaparvata lugens Stål (Hemiptera: Delphacidae). Entomol. Gen. 2018, 37, 35–45. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Zhang, Y.W.; Lin, X.D. Role of the transcription factor Taiman in moulting and ovarian development of Nilaparvata lugens. Entomol. Gen. 2021, 41, 169–177. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, L.; Dong, Q.; Chang, S.; Wen, K.; Jia, S.; Chu, Z.; Wang, H.; Gao, P.; Zhao, H.; et al. FRET-based glucose imaging identifies glucose signaling in response to biotic and abiotic stresses in rice roots. J. Plant Physiol. 2017, 215, 65–72. [Google Scholar] [CrossRef]
- Damien, M.; Llopis, S.; Desneux, N.; Van, B.J.; Le, L.C. How does floral nectar quality affect life history strategies in parasitic wasps? Entomol. Gen. 2020, 40, 147–156. [Google Scholar] [CrossRef]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Di, N.; Wang, S.; Ridsdill-Smith, J.; Chen, Y.F.; Harwood, J.D.; Zhang, K.; Liu, T. Nitrogen and plant growth regulator affect plant detoxification metabolism and tritrophic interactions among Triticum aestivum, Sitobion avenae and Aphelinus asychis. Entomol. Gen. 2021, 41, 369–384. [Google Scholar] [CrossRef]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Li, G.Y.; Liu, Y.K.; Luo, Y.J.; Xu, C.D.; Li, C.; Tang, B. Regulation of carbohydrate metabolism by trehalose-6-phosphate synthase 3 in the brown planthopper, Nilaparvata lugens. Front. Physiol. 2020, 11, 575485. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant, J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chen, X.; Li, Y.; Pan, B.Y.; Wang, S.G.; Dai, H.J.; Wang, S.; Tang, B. Effects of changing temperature on the physiological and biochemical properties of Harmonia axyridis larvae. Entomol. Gen. 2020, 40, 229–241. [Google Scholar] [CrossRef]
- Gross, J.; Gallinger, J.; Görg, L.M. Interactions between phloem-restricted bacterial plant pathogens, their vector insects, host plants, and natural enemies, mediated by primary and secondary plant metabolites. Entomol. Gen. 2022, 42, 185–215. [Google Scholar] [CrossRef]
- Liu, Y.K.; Xu, W.X.; Xu, J.J.; Zheng, S.R.; Yan, J.J.; Ding, Y.T.; Shen, B.; Tang, B. Brown planthopper infestations alter sugar metabolism in the rice plant as well as brown planthopper. Physiol. Entomol. 2021, 46, 167–178. [Google Scholar] [CrossRef]
- Yue, L.; Kang, K.; Zhang, W. Metabolic responses of brown planthoppers to IR56 resistant rice cultivar containing multiple resistance genes. J. Insect Physiol. 2019, 113, 67–76. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, M.; Dziurka, K. Insight into mechanisms of multiple stresses tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, Z.; Sun, K.; Huang, B.; Stanley, D.; Song, Q.S. The antibiotic jinggangmycin increases brown planthopper (BPH) fecundity by enhancing rice plant sugar concentrations and BPH insulin-like signaling. Chemosphere 2020, 249, 126463. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, L.Y.; Yang, H.L.; Wang, H.J.; Zhou, M.; Wang, S.G.; Tang, B. Study on the effect of wing bud chitin metabolism and its developmental network genes in the brown planthopper, Nilaparvata lugens, by knockdown of TRE Gene. Front. Physiol. 2017, 8, 750. [Google Scholar] [CrossRef]
- Ding, Y.J.; Li, G.Y.; Xu, C.D.; Wu, Y.; Zhou, Z.S.; Wang, S.G.; Li, C. Regulatory functions of Nilaparvata lugens GSK-3 in energy and chitin metabolism. Front. Physiol. 2020, 11, 518876. [Google Scholar] [CrossRef] [PubMed]
- Livaka, K.J.; Schmittgenb, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Deng, S.; Tan, D.; Long, J.; Lei, M. Heavy metal distribution, translocation, and human health risk assessment in the soil-rice system around Dongting Lake area, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 17655–17665. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhang, H.; Wang, Y.; Zheng, L. Accumulation and distribution characteristics of zinc and cadmium in the hyperaccumulator plant Sedum plumbizincicola. Bull. Environ. Contam. Toxicol. 2014, 93, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef]
- Jiang, D.; Yan, S. Effects of Cd, Zn or Pb stress in Populus alba berolinensis on the development and reproduction of Lymantria dispar. Ecotoxicology 2017, 26, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.D.; Wang, G.Z.; Li, S.J.; He, J.F. Heavy metal exposure reduces hatching success of Acartia pacifica resting eggs in the sediment. J. Environ. Sci. 2007, 19, 733–737. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, S.; Pan, B.; Liu, Y.; Li, Y.; Wang, S.; Tang, B. Effects of zinc acquired through the plant-aphid-ladybug food chain on the growth, development and fertility of Harmonia axyridis. Chemosphere 2020, 259, 127497. [Google Scholar] [CrossRef]
- Liu, H.; Su, X.Y.; Sun, Z.; Wang, C.; Shi, J.H.; Foba, C.N.; Jin, H.; Wang, M.Q. Nitrogen and plant pathogens alter rice plant volatiles mediating host location behavior of Nilaparvata lugens and its parasitoid Anagrus nilaparvatae. Entomol. Gen. 2022, 42, 549–557. [Google Scholar] [CrossRef]
- Duman, F. Effects of exogenous glycinebetaine and trehalose on lead accumulation in an aquatic plant (Lemna gibba L.). Int. J. Phytoremediation 2011, 13, 492–497. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M.; Tran, L.S. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 2015, 5, 11433. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, R.; Mheidat, M.; Abdo, N.; Tadros, M.; Al-Eitan, L.; Al-Hadid, K. Effect of lead on the physiological, biochemical and ultrastructural properties of Leucaena leucocephala. Plant Biol. 2019, 21, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Martínez Nicolás, J.J.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Berni, B.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, L.; Du, Z.; Sun, X.; Amombo, E.; Fan, J.; Fu, J. Effects of cadmium exposure on growth and metabolic profile of bermudagrass [Cynodon dactylon (L.) Pers]. PLoS ONE 2014, 9, e115279. [Google Scholar] [CrossRef] [PubMed]
- Ševčíková, H.; Mašková, P.; Tarkowská, D.; Mašek, T.; Lipavská, H. Carbohydrates and gibberellins relationship in potato tuberization. J. Plant Physiol. 2017, 214, 53–63. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Wei, Y.; Ding, Y.; Wang, Q.; Wang, S.; Tang, B.; Wang, S. Effects of long-term cadmium exposure on trehalose metabolism, growth, and development of Aedes albopictus (Diptera: Culicidae). Ecotoxicol. Environ. Saf. 2020, 204, 111034. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, Y.; Lu, Y.; Zhou, M.; Wang, S.; Wang, S. The Effect of different dietary sugars on the development and fecundity of Harmonia axyridis. Front. Physiol. 2020, 11, 574851. [Google Scholar] [CrossRef]
- Huang, J.H.; Lee, H.J. RNA interference unveils functions of the hypertrehalosemic hormone on cyclic fluctuation of hemolymph trehalose and oviposition in the virgin female Blattella germanica. J. Insect Physiol. 2011, 57, 858–864. [Google Scholar] [CrossRef]
- Lu, K.; Wang, Y.; Chen, X.; Zhang, X.; Li, W.; Cheng, Y.; Li, Y.; Zhou, J.; You, K.; Song, Y.; et al. Adipokinetic hormone receptor mediates trehalose homeostasis to promote vitellogenin uptake by oocytes in Nilaparvata lugens. Front. Physiol. 2019, 9, 1904. [Google Scholar] [CrossRef]
- Shen, X.N.; Ji, S.X.; Liu, W.X.; Guo, J.Y.; Lü, Z.C.; Wan, F.H. Molecular characteristics of three cold resistance genes and their roles in temperature stress response in two Bemisia tabaci cryptic species. Entomol. Gen. 2021, 41, 317–328. [Google Scholar] [CrossRef]
- Fraga, A.; Ribeiro, L.; Lobato, M.; Santos, V.; Silva, J.R.; Gomes, H.; da Cunha Moraes, J.L.; de Souza Menezes, J.; de Oliveira, C.J.; Campos, E.; et al. Glycogen and glucose metabolism are essential for early embryonic development of the red flour beetle Tribolium castaneum. PLoS ONE 2013, 8, e65125. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, J.; Guo, Y.; Ma, E. Molecular cloning and mRNA expression of heat shock protein genes and their response to cadmium stress in the grasshopper Oxya chinensis. PLoS ONE 2015, 10, e0131244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, M.; Shen, Q.; Liu, X.; Shi, Z.; Wang, S.; Tang, B. Functional characterization of three trehalase genes regulating the chitin metabolism pathway in rice brown planthopper using RNA interference. Sci. Rep. 2016, 6, 27841. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, L.; Shen, Q.; Xie, G.; Wang, S.; Tang, B. Knockdown of two trehalose-6-phosphate synthases severely affects chitin metabolism gene expression in the brown planthopper Nilaparvata lugens. Pest Manag. Sci. 2017, 73, 206–216. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| NlTre1-1 | CCTCGGCTCTATTCGTTC | ACCGCTTGACCAGTGAGA |
| NlTre1-2 | GATCGCACGGATGTTTA | AATGGCGTTCAAGTCAA |
| NlTre2-2 | CGTGCCAGGTGGACGGTTTA | ATGGGAGCGAGCAGAGGGAG |
| NlTps1 | AAGACTGAGGCGAATGGT | AAGGTGGAAATGGAATGTG |
| NlTps2 | GACAGGCGGTTGAAGAAGA | CAGTAGTCGCTGATGTGGAA |
| Actin | CCGAGATTTGACCGATTAC | GGTTGCCATTTCCTGTTC |
| Zn2+ Concentrations | Regression Type | ||
|---|---|---|---|
| Zn content in soil | Pearson correlation | 0.957 | Linear |
| Sig. | 0.005 | ||
| R2 | 0.917 | ||
| N | 5 | ||
| Regression equation | |||
| Zn content in rice stem | Model | Growth curve | Curvilinear |
| Sig. | 0.028 | ||
| R2 | 0.842 | ||
| Regression equation | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-K.; Xu, C.-D.; Zheng, X.-S.; Chao, L.; Zhou, Y.-F.; Li, G.-Y.; Wu, Y.; Bai, X.-L.; Zhou, T.; Tang, B.; et al. Zinc Stress Alters Sugar Content in Rice Plants and the Reproduction and Trehalose Metabolism in Nilaparvata lugens. Agronomy 2023, 13, 73. https://doi.org/10.3390/agronomy13010073
Liu Y-K, Xu C-D, Zheng X-S, Chao L, Zhou Y-F, Li G-Y, Wu Y, Bai X-L, Zhou T, Tang B, et al. Zinc Stress Alters Sugar Content in Rice Plants and the Reproduction and Trehalose Metabolism in Nilaparvata lugens. Agronomy. 2023; 13(1):73. https://doi.org/10.3390/agronomy13010073
Chicago/Turabian StyleLiu, Yong-Kang, Cai-Di Xu, Xu-Song Zheng, Lei Chao, Yan-Fei Zhou, Guo-Yong Li, Yan Wu, Xue-Lian Bai, Ting Zhou, Bin Tang, and et al. 2023. "Zinc Stress Alters Sugar Content in Rice Plants and the Reproduction and Trehalose Metabolism in Nilaparvata lugens" Agronomy 13, no. 1: 73. https://doi.org/10.3390/agronomy13010073
APA StyleLiu, Y.-K., Xu, C.-D., Zheng, X.-S., Chao, L., Zhou, Y.-F., Li, G.-Y., Wu, Y., Bai, X.-L., Zhou, T., Tang, B., & Xu, H.-X. (2023). Zinc Stress Alters Sugar Content in Rice Plants and the Reproduction and Trehalose Metabolism in Nilaparvata lugens. Agronomy, 13(1), 73. https://doi.org/10.3390/agronomy13010073